Abstract
Purpose
The incidence of aneuploidy in eggs from women of advanced reproductive age can exceed 60 %, making the mammalian egg a unique model system to study the mechanisms of chromosome segregation errors.
Methods
Here we applied a novel biophysical chromosome stretching approach to quantify mechanical stiffness of meiotic chromosomes in the mammalian egg and then documented how these properties changed in a mouse model of physiologic reproductive aging.
Results
We found significant differences in chromosome micromechanics, and thus in higher order chromosome structure, coincident with advanced reproductive age, a time that is also unequivocally associated with an increase in egg aneuploidy.
Conclusions
These findings have important implications for both reproductive and cancer biology where aneuploidy plays a central role in aging related disease states.
Keywords: Reproductive aging, Meiosis, Mammalian, Chromosome, Micromechanics
The female reproductive system is one of the first in the body to undergo overt signs of physiologic aging characterized by a marked decline in egg quality. Specifically, errors in chromosome segregation during meiosis increase as females age, with the incidence of aneuploidy in the egg rising from approximately 20 % in women under age 35 to 60 % after age 40 [1,2]. High fidelity transmittance of genetic material is essential during both mitosis and meiosis to ensure healthy daughter cells or gametes, respectively. Meiotic errors in the egg are associated with miscarriages, birth defects, and infertility [3,4]. Multiple molecular mechanisms have been examined to explain the age-associated increase in egg aneuploidy, including recombination errors leading to non-disjunction, improper spindle assembly and microtubule-kinetochore interactions, and insensitive spindle assembly checkpoints [1,5]. In addition, recent clinical findings in human eggs have demonstrated that many meiotic errors are due to premature separation of sister chromatids [6,7]. These clinical findings are consistent with observations that chromosome cohesion deteriorates with advanced reproductive age in mouse and human eggs [1,2,5]. Although age-related increases in aneuploidy are likely due to a variety of causes, the one common denominator is the chromosome itself. Age-associated changes in the higher order physical structure of meiotic chromosomes in the egg have never been investigated.
Micromechanical studies of chromosomes are a powerful tool to analyze overall chromosome organization in a quantitative way, through measurement of structural response to mechanical forces applied to the whole chromosome. Such properties can be measured and quantified by isolating single, native chromosomes and applying force using glass micropipettes [8–10]. The use of native chromosomes avoids the pitfalls of bulk isolation or fixation methods that may alter chromosome structure. This type of assay has been used successfully on chromosomes from non-mammalian cells (newt and Xenopus; [8]) as well as human cell lines [10]. Using this approach, this force measurement has been shown to be indicative of the protein makeup and to provide information on the structure of the chromosome [8,10]. This has lead to our current understanding that mitotic chromosomes are organized in a network of chromatin fibers interconnected by linker proteins [9,10]. Here, we translated this micromechanical assay to mammalian meiotic chromosomes, providing unique insight into the structural changes that occur with aging in mammalian oocytes.
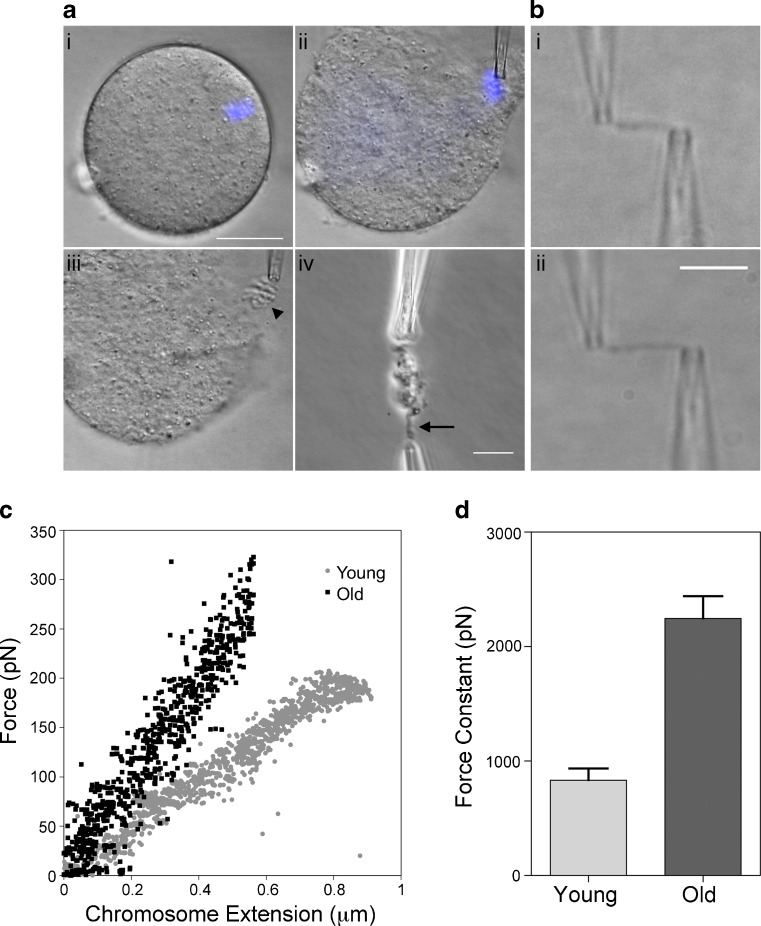
For this study, the mouse was used as a model system due to the limited supply of human eggs for research purposes. To first characterize the micromechanical properties of mammalian meiotic chromosomes, we isolated chromosomes from mouse eggs arrested at metaphase II of meiosis, at which stage the chromosomes are fully condensed and aligned on the metaphase plate of the bipolar spindle. The chromosomes of each egg were identified by phase contrast and isolated gently following a targeted disruption of the plasma membrane (Fig. 1a). The entire chromosome complement was released from the cell as a unit (Fig. 1aii), with the individual chromosomes attached by interchromosomal linkers [9]. From this tightly-associated chromosome network, an individual chromosome was isolated and attached to glass micropipettes at both ends (Fig. 1aiii–iv). To probe chromosome structure (Fig. 1b), we measured the force needed to stretch one meiotic chromosome. We found that meiotic chromosomes stretch linearly, such that as the distance stretched increased, the amount of force required to stretch the chromosome also increased (Fig. 1c). Based on this chromosome stretching data, we were able to calculate the force constant for a meiotic chromosome. The force constant is the force that would correspond to doubling the initial, relaxed length of the chromosome by extrapolation of the initial linear force-extension response. This quantity provides a length-independent characterization of chromosome stiffness (spring constant). We found that mouse meiotic chromosomes have an average force constant of 830 ± 100 pN, more than 2-fold greater than the previously reported measurement for human mitotic chromosomes (300 pN, HeLa cells, [10] and Fig. 1d).
Fig. 1.
Meiotic chromosomes can be isolated from eggs and exhibit significant age-associated differences in structure. a. Chromosomes were isolated from mouse eggs after removal of the zona pellucida. The spindle and chromosomes were identified by phase contrast microscopy, shown here stained with Hoescht 33342 (i–ii). Scale bar, 25 μm (ii). Chromosomes are removed from the egg as a cluster (iii, arrowhead). To isolate an individual chromosome, a free end was captured by a second glass pipette and pulled away from the cluster. Scale bar, 10 μm. (iv, arrow). b. A single chromosome can be removed from the cluster of chromosomes, held between two glass pipettes and stretched (i, unstretched; ii, stretched) Scale bar, 5 μm. c. After calibration of the floppy pipette, a force-extension curve can be generated from the relative positions of the pipette tips as recorded by the Labview software. The slope of the line is indicative of the spring constant of the chromosome; the spring constant for chromosomes from eggs from old animals is significantly different from those of young animals. Representative curves shown. d. The force constant of chromosomes from eggs of old animals is significantly higher than those from young animals. The mean force constant is 830 ± 100 pN for young and vs 2250 ± 190 pN for old animals. N = 8 chromosomes for each group. Each individual animal contributed a single chromosome for a total of 8 animals per experimental group. Error bars; SEM. P < 0.0001
Given the well-documented age-associated increase in aneuploidy in both human and mouse eggs, we were interested in investigating the micromechanical properties of meiotic chromosomes in a physiologic model of reproductive aging [1,5]. For these studies we used the CD1 mouse strain as our physiologic reproductive aging model; this strain has a well-documented increase in reproductive age-associated egg aneuploidy beginning as early as 12 months of age [11,12]. Here, we used animals that were 14–16 months of age to increase the population of eggs that were likely to be aneuploid, as the aneuploidy incidence at this age is 60 % [11,13]. Consistent with previous studies, we found that old mice had fewer fully-grown oocytes compared to young mice (14 ± 7 oocytes/mouse vs. 39 ± 16 oocytes/mouse; Table 1). We isolated metaphase chromosomes from eggs from mice of the two distinct age cohorts and performed chromosome stretching experiments as described to determine if age-associated differences in the micromechanical properties of meiotic chromosomes existed. We found a significant difference in the force constant of chromosomes between the two age groups, with meiotic chromosomes from reproductively old mice requiring more than 2.5 times the force needed to stretch them to twice their initial length compared to those from young mice (2250 ± 190 pN vs. 830 ± 100 pN, respectively; Fig. 1d). These results indicate that reproductive age is associated with an increase in the stiffness of meiotic chromosomes.
Table 1.
Oocyte collection and in vitro maturation data
| Cohort | Oocytes collected (Avg ± SD) | MII rate (Avg ± SD) |
|---|---|---|
| Young | 38.6 ± 15.9* | 69.8 ± 2.7 % |
| Old | 13.7 ± 7.2* | 73.5 ± 5.2 % |
*P < 0.0001, n = 8
In this study, we measured and quantified the micromechanical properties of chromosomes from mammalian eggs for the first time. Moreover, we demonstrate a significant increase in the stiffness of chromosomes in a population of mammalian eggs from reproductively aged mice [1]. Previous work in mitotic cells demonstrated that changes in the force constant of a chromosome are indicative of a change in structure [8,14]. Thus, this data suggests that there is appreciable structural change in the complex network of chromatin and proteins that makes up the chromosome, likely due to altered physiology caused by aging. The loss of chromosome-associated cohesin protein complexes is well documented to occur with age in mammalian eggs and generates a change in the relationship of the homologous chromosomes and the sister chromatids; however, it is not understood how this affects chromatin organization [5]. Due to the technical rigor of this method, only one chromosome can be isolated and stretched per oocyte. Moreover we cannot identify which specific chromosome is being analyzed. Nevertheless, by averaging the data from a number of chromosomes, we can infer changes that are occurring throughout the genome. Future studies will be aimed at elucidating the factor or factors responsible for the structural alterations that occur with reproductive aging and that are reflected in changes in micromechanical properties. An intriguing possibility is that the increasing stiffness we measured is a generalized feature of systemic aging. However, reproductive aging occurs earlier than aging of other organ systems. Additional studies on young and aged populations of somatic cells would be necessary to demonstrate if the effect that we have documented in meiotic chromosomes also occurs in mitotic chromosomes, indicating whether this phenomenon is a general aging effect or specific to reproductive aging. Taken together, our results demonstrate that there are global changes in chromosome structure with age, lending another important aspect to understanding age-related increases in aneuploidy.
Materials and methods
Animals
CD1 mice (6–8 weeks) female mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and either used after a brief equilibration period or housed until they reached advanced reproductive age. All mice were housed in a controlled barrier facility, provided food and water ad libitum, and were exposed to a 14 L/10D cycle with constant humidity and temperature. All animal experiments were approved by the Institutional Animal Use and Care Committee and were consistent with National Institutes of Health guidelines. A total of 8 animals per experimental group were used.
Oocyte collection and in vitro maturation
Fully-grown cumulus-enclosed oocytes were collected from large, antral follicles in the ovaries of naturally cycling animals at either 6–8 weeks of age (young) or 14–16 months (old) by puncturing large antral follicles and in vitro matured for 14–16 h as described previously, except we used the following IVM media: alpha MEM-Glutamax (Invitrogen) supplemented with 3 mg/mL bovine serum albumin (αMEM + BSA, Sigma-Aldrich) (Duncan, FE et al. BOR 2009). MII eggs with a visible polar body were selected for further experiments. Mature eggs were obtained following IVM rather than following induced ovulation because more oocytes can be retrieved by puncturing large antral follicles than can be obtained following ovulation (our unpublished results). This approach is critical to maximize the number of eggs that can be obtained from reproductively aged mice to perform the experiments described below.
Chromosome isolation and force measurement
Chromosome isolation and force measurement experiments were performed as previously described (Sun et al. Phys Biol. 2011, Kawamura et al. JCB 2010), with the following modifications needed to adapt the procedure to mammalian oocytes. Micromanipulation experiments were performed on the stage of an inverted microscope (IX-70; Olympus) using a 60 × 1.4 NA oil immersion objective as previously described (Sun et al. Phys Biol. 2011). We maintained the temperature of the slide containing the living cells at 30 °C using an objective heater (20–20 Technologies); maintaining the temperature at 30 °C rather than 37 °C improved cell viability and minimized media evaporation. Micromanipulation and was performed using glass micropipettes mounted on motorized micromanipulators (MP-285; Sutter Instrument Co.).
The zona pellucida was removed from MII eggs, selected as above, with acid tyrodes solution and allowed to recover for 30 min in αMEM + BSA before continuing with experiments. The eggs were then placed into L15 media supplemented with 3 mg/mL polyvinylpyrolidine (L15 + PVP) on a coverslip to which two 25 mm diameter rubber O-rings had been affixed with wax. The position of the spindle and chromosomes within the egg was determined by phase contrast microscopy (Fig. 1ai) and a solution of 0.05 % Triton X-100 in PBS (Thermo Fisher Scientific) was sprayed onto the plasma membrane in the highly localized region of the spindle with a micropipette in order to lyse the egg. As the cytoplasm, including the spindle and chromosomes, flowed out of the egg, one pipette was used to capture the cluster of chromosomes by aspiration (Fig. 1aiii, arrowhead). A second pipette was used to capture a free end of a single chromosome and to separate it from the group (Fig. 1aiv, arrow). We note that the cluster of chromosomes was connected by interchromosomal linkers; thus, a series of rapid pulling movements was required to separate a single chromosome from this group. Once a single chromosome was isolated from the group, the opposite end was captured by a second pipette (Fig. 1b, top panel). The two pipettes used to hold the isolated chromosome were pulled with a micropipette puller (P-97, Sutter Instrument Co.) to precise specifications such that one had a shorter, thicker taper rendering it less flexible, or “stiff” and the second had a longer, thinner taper rendering it more flexible, or “floppy”. The pipettes were cut with a microforge to have an opening of approximately 1–2 μm in diameter. Each experiment used a new floppy pipette, which was calibrated after each experiment to have a soft end deflection spring constant of approximately 130 pN μm−1, as previously (Poirier et al. 2000).
In order to measure the force constant of a chromosome, an isolated chromosome was repeatedly stretched by moving the stiff pipette. The stiff pipette was moved at a rate of 5 μm min−1, slowly enough to avoid viscoelastic effects. Each extension–relaxation measurement was repeated three to five times to ensure reproducibility. Data (i.e. pipette positions) was collected during the experiments by Labview image analysis software (National Instruments). The extension of the chromosome as well as the bending of the floppy pipette were determined by recording the positions of the stiff and floppy pipettes, respectively. The chromosome extension and force were calculated from the relative pipette positions using Labview.
Statistical analysis
Plotting of results and statistical analysis was done using GraphPad Prism (La Jolla, CA, USA). Force constant data were analyzed using an unpaired t-test. A P value of <0.05 was considered significant. Data from a total of 8 chromosomes from each group, one per animal, was used to generate the average.
Acknowledgments
This work was supported by grants from the National Institutes of Health (U54HD041857 to TKW and U54HD076188, U54CA143869 and R01GM105847 to JFM) and the National Science Foundation (NSF) (MCB-1022117 and DMR-1206868 to JFM).
Footnotes
Capsule Micromechanical properties were measured in chromosomes from the oocytes of young and old mice. Significant differences were seen in chromosomes from older mice, suggesting age-associated structural changes in chromosomes.
Contributor Information
Jessica E. Hornick, Email: j-hornick@northwestern.edu
Francesca E. Duncan, Email: fduncan@kumc.edu
Mingxuan Sun, Email: biomxsun@gmail.com.
Ryo Kawamura, Email: phyrk@nus.edu.sg.
John F. Marko, Email: john-marko@northwestern.edu
Teresa K. Woodruff, Phone: 312-503-2503, Email: tkw@northwestern.edu
References
- 1.Jones KT, Lane SIR. Molecular causes of aneuploidy in mammalian eggs. Development. 2013;140(18):3719–30. doi: 10.1242/dev.090589. [DOI] [PubMed] [Google Scholar]
- 2.Duncan FE, Hornick JE, Lampson MA, Schultz RM, Shea LD, Woodruff TK. Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell. 2012;11(6):1121–4. doi: 10.1111/j.1474-9726.2012.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassold T, Hunt P. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr. 2009;21(6):703–8. doi: 10.1097/MOP.0b013e328332c6ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt P, Hassold T. Female meiosis: coming unglued with age. Cur Biol. 2010;20(17):R699–702. doi: 10.1016/j.cub.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol. 2010;20(17):1522–8. doi: 10.1016/j.cub.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Cruz R, Brieño MA, Roig I, Grossmann M, Velilla E, Pujol A, et al. Dynamics of cohesin proteins REC8, STAG3, SMC1 beta and SMC3 are consistent with a role in sister chromatid cohesion during meiosis in human oocytes. Hum Reprod. 2010;25(9):2316–27. doi: 10.1093/humrep/deq180. [DOI] [PubMed] [Google Scholar]
- 7.Vialard F, Lombroso R, Bergere M, Gomes DM, Hammoud I, Bailly M, et al. Oocyte aneuploidy mechanisms are different in two situations of increased chromosomal risk: older patients and patients with recurrent implantation failure after in vitro fertilization. Fertil Steril. 2007;87(6):1333–9. doi: 10.1016/j.fertnstert.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura R, Pope LH, Christensen MO, Sun M, Terekhova K, Boege F, et al. Mitotic chromosomes are constrained by topoisomerase II-sensitive DNA entanglements. J Cell Biol. 2010;188(5):653–63. doi: 10.1083/jcb.200910085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marko JF. Micromechanical studies of mitotic chromosomes. Chromosom Res Int J Mol Supramol Evol Asp Chromosom Biol. 2008;16(3):469–97. doi: 10.1007/s10577-008-1233-7. [DOI] [PubMed] [Google Scholar]
- 10.Sun M, Kawamura R, Marko JF. Micromechanics of human mitotic chromosomes. Phys Biol. 2011;8(1):015003. doi: 10.1088/1478-3975/8/1/015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merriman JA, Jennings PC, McLaughlin EA, Jones KT. Effect of aging on superovulation efficiency, aneuploidy rates, and sister chromatid cohesion in mice aged up to 15 months. Biol Reprod. 2012;86(2):49. doi: 10.1095/biolreprod.111.095711. [DOI] [PubMed] [Google Scholar]
- 12.Yun Y, Lane SI, Jones KT. Premature dyad separation in meiosis II is the major segregation error with maternal age in mouse oocytes. Development. 2014;141(1):199–208. doi: 10.1242/dev.100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI, Choi MS, et al. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2010;48(3):937–43. doi: 10.1016/j.fct.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Poirier MG, Marko JF. Micromechanical studies of mitotic chromosomes. Curr Top Dev Biol. 2003;55:75–141. doi: 10.1016/S0070-2153(03)01002-0. [DOI] [PubMed] [Google Scholar]