Abstract
Purpose
To quantify blastocyst morphologic parameters with a feasible and standardized tool, investigating their predictive value on implantation outcome.
Method
The study retrospectively analyzes 124 blastocysts from 75 patients. Quantitative measurements of blastocyst expansion, inner cell mass and trophoectoderm were taken using digital image analysis software.
Result(s)
Blastocysts areas were found to be ranging from 11626.2 up to 35076.4 μm2. The area of an early blastocyst is A ≤ 18500 μm2 with a mean diameter d = 140 ± 9 μm, and the area of an expanded blastocyst is A ≥ 24000 with d = 190 ± 9 μm. While blastocyst mean area was not related to implantation rate, more expanded blastocysts displayed a significantly higher implantation rate. Trophoectoderm cell number is a predictor of positive outcome: since a higher of cells (25.6 ± 11.3 vs 16.3 ± 12.8) `forming a tightly knit epithelium is prognostic of implantation potential. Conversely, inner cell mass size is significantly related to implantation only in expanded blastocysts (3122.7 ± 739.0 vs. 2978.1 ± 366.0 μm2).
Conclusion(s)
Evaluation of blastocyst morphology with a digital image system could be a valuable tool to standardize blastocyst grading based on quantitative parameters. Therefore, digital analysis may be helpful in identifying the best blastocyst to transfer.
Keywords: Blastocyst expansion, Implantation, Inner cell mass, Morphometry, Trophoectoderm
Introduction
Elective single-embryo transfer (eSET) is the only effective strategy to decrease multiple pregnancies after assisted reproduction [1] and in the last years researchers efforts have been directed towards developing reliable methods to choose the most viable embryo to transfer. Prolonged blastocyst culture assists the embryologist in the selection process. It has been reported that aneuploidy typically affects more than 50 % of blastocysts [2]. These abnormalities are a cause of early arrest of many developing embryos: therefore, cultivating embryos for a longer period may increase the chance of having chromosomally normal embryos [3]. In fact, it is believed that certain types of aneuploidies [2] or inappropriate expression of embryonic genome occurring from the 4–8 cell stage onwards [4] prevent development to blastocyst stage in a large portion of embryos. Moreover, extending embryo culture to the blastocyst stage and delaying transfer improves uterine and embryonic synchronicity; in fact, blastocyst transfer appears to provide higher implantation and live birth rates compared to cleavage stage embryos [5–8]. Though in the past few years powerful technologies have been introduced in the IVF field to improve embryo implantation potential, as Preimplantation Genetic Screening (PGS) or time-lapse technology [9, 10], a thorough blastocyst morphology evaluation is still necessary in those cases in which multiple day five embryos are available to transfer or when these sophisticated and expensive techniques are not available in the practical clinic.
Embryo evaluation based on morphological visual information obtained by the embryologist is subjected to inter and intra-observer variability [11]. Traditionally, blastocyst evaluation is based on morphological analysis. In 2011 the Istanbul consensus workshop on embryo assessment [12, 13] proposed an international consensus on embryo morphology assessment. The most widely used grading system is that of Gardner and Schoolcraft [5], based on the assessment of three parameters: blastocoele expansion and hatching status, size and compactness of the inner cell mass (ICM), and the cohesiveness and number of trophectoderm (TE) cells. Furthermore, this light microscopy observation requires limited time for blastocyst analysis so as not to compromise its quality. Even if continuous monitoring of human embryos is performed with time-lapse incubators, only timing of cleavage is considered and morphology isn’t a parameter of the compare/selection algorithm. Therefore, it would be desirable to find an objective and standardized method to assess blastocyst morphology in detail.
While automated image analysis of cleavage stage human embryos (Day 1 to 3 post-fertilization) has been the subject of several studies investigating cleavage stage embryos parameters as cell number, symmetry, multinucleation, fragmentation [14–18], few attempts at semi-automatic objective evaluation of the rather complex blastocyst structure have previously been made [19, 20].
Over the years, various quantitative but mostly qualitative analyses have been made to determine the impact of each blastocyst parameter on IVF outcome [21–27]. A detailed study was performed by Richter and colleagues in 2001 [28]. They analysed quantitatively the three major parameters of blastocyst morphology i.e. expansion, ICM size and shape and TE cell number and correlated their measurements to the implantation rate concluding that ICM only correlates to blastocyst viability; more recently Ahlstrom and colleagues suggested that the quality of TE morphology may be predictive of life birth [21]. Unfortunately, due to transfers of multiple blastocysts with heterogeneous features, the effect of morphology on implantation is still ambiguous. Based on these considerations our study aims to quantitatively determine blastocyst parameters (expansion, TE and ICM) with a digital image system and correlate them with implantation rate as a tool for an objective non-invasive embryo morphology selection.
Materials & methods
Patients
A retrospective morphometric analysis of blastocysts was undertaken in 75 patients (mean age 36.2 ± 3.6) who received a fresh autologous blastocyst transfer at our clinic, from June 2010 to April 2012. In order to precisely assess the implantation fate of each transferred embryo, this study included only patients who received a single embryo transfer (SET) or double embryo transfer (DET) with 0 or 100 % implantation, both elective and non-elective transfers. There were no exclusion criteria based on patients characteristics.
Seventyfive transfers met inclusion criteria: 27 patients received a SET and 48 a DET. Morphometric data from 124 blastocysts were included in the study according to the 0–100 % implantation rule. Thirty blastocysts ended up in implantation and twenty led to born babies.
Ovarian stimulation protocol, embryo culture and transfer
Controlled ovarian stimulation was induced using an agonist (Enantone, Takeda, Rome, Italy) or an antagonist (Cetrotide, Serono, Rome, Italy; or Orgalutran, Organon, Rome, Italy) and recombinant or urinary follicle-stimulating hormone (FSH) (Gonal-F, Serono, Rome, Italy; or Meropur, Ferring, Milan, Italy). A dose of 10,000 IU human chorionic gonadotrophin (hCG) (Gonasi, Amsa, Rome, Italy) or one ampoule of recombinant hCG (r-hCG; Ovitrelle, Serono, Rome, Italy) was administered when one or more follicles reached a maximum diameter of >23 mm. [29]. Oocyte collection was performed transvaginally, under ultrasound guidance, 36 h after hCG injection. Retrieved oocytes were rinsed and placed in Sydney IVF Fertilization Medium (Cook IVF, Brisbane, Australia) at 37 °C, 6 % CO2, 5 % O2 e 89 % N2 for at least 4 h. Fertilization was achieved by IVF or intracytoplasmic sperm injection (ICSI), following standard techniques. At 16–18 h after insemination (Day 1), normal fertilization was checked by the presence of two pronuclei and zygotes were placed into droplets of fresh Sidney IVF Cleavage Medium (Cook IVF, Brisbane, Australia). Day 3 embryos were transferred into Sydney IVF Blastocyst Medium (Cook IVF, Brisbane, Australia) and cultured until day 5. Embryo transfers were performed on day 5 and supernumerary blastocysts were cryopreserved using a vitrification protocol. Blastocysts were evaluated at 116 ± 1 h after insemination and selection for transfer was performed according to modified Gardner and Schoolcraft grading system by Cornell’s group [30]. In this new model a blastocyst is defined as having a blastocoel filling greater than half the volume of the embryo and should possess cells that suggest the formation of ICM, while embryos with blastocoels smaller than half the volume are considered to be cavitating morulas. This means that Score 3 from Gardner’s system is equal to Score 1 from Cornell’s grading. Blastocysts with slightly thinner zona pellucida due to growing of the embryo are graded with Score 2 and Score 3 is given to fully expanded blastocysts with thin zona. As Cornell’s system, there are four alphabetical grades (A–D) for ICM and four for TE, where D is the score for degenerative ICM or TE.
Digital image analysis and study outcomes
Digital images of blastocysts were taken using a Nikon optical microscope with Hoffmann modulation contrast at the same magnification of 200×. Each blastocyst was photographed in a sequence of at least 4 images on different focal planes by Pinnacle program. Pictures were analysed using the ImageJ program (http://rsbweb.nih.gov/ij/) after calibration with a graduated ruler.
For each blastocyst, the following final measurements were carried out: blastocyst internal area, ICM area, trophoectoderm cells number.
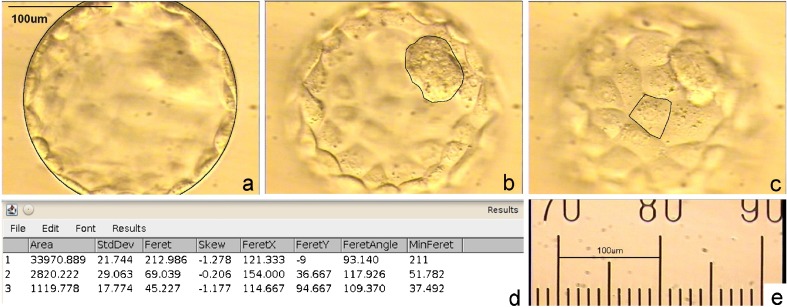
Blastocyst internal area, not including the zona pellucida, was calculated in the maximal cross-sectional image and was recorded together with ICM area. Blastocyst volume was calculated from internal area as V = 4/3*√ (A3/π) and the V/Vmin ratio between blastocyst volume (V) and the volume of the smallest blastocyst of the study group (Vmin), was calculated. Additionally, to evaluate TE cell number we hypothesized that the layer was composed of similar-sized cells. According to this model we measured a single TE cell area calculating the blastocyst/TE cell area ratio to obtain TE cell number. The relationship of these parameters with embryo implantation potential was analyzed; Implantation Rate is defined as the number of gestational sacs per transferred embryo (Fig. 1).
Fig. 1.
Representative images of A3 blastocysts at different focal planes used to analyse semi-automatic morphometric measurements. a internal blastocyst area, b ICM area, c trophoectoderm cell area. d Results panel of ImageJ software with area calculation. e 200× magnification of the ocular micrometer used to calibrate software measurement tool
Statistical analysis
All statistical analysis were performed by using Sigmaplot v11.0 (Systat Software). Values are expressed as the mean ± standard deviation (SD). Group variance was analyzed by ANOVA followed by Tukey-Kramer test for comparison between multiple groups. Chi-square test was used for percentages and Student’s t-test for means. Differences were considered significant when the P-value was ≤0.05.
Results
Blastocyst expansion
The morphometric measurements revealed that the mean area of this blastocysts study group was 21105.0 ± 5747.0 μm2 ranging from 11626.2 up to 35076.4 μm2. To evaluate the correlation between blastocyst area and implantation potential we compared the mean area of blastocyst that implanted with that did not but this difference wasn’t statistically significant (22702.4 ± 5658.7 vs 20595.2 ± 5710.6 μm2; p = 0.08).
According to the modified Gardner score system [30] based on three blastocyst expansion categories, we divided our blastocysts in 3 groups on the basis of their volume and calculated the V/Vmin ratio where V and Vmin are the volume of each blastocyst and the smallest one, respectively.
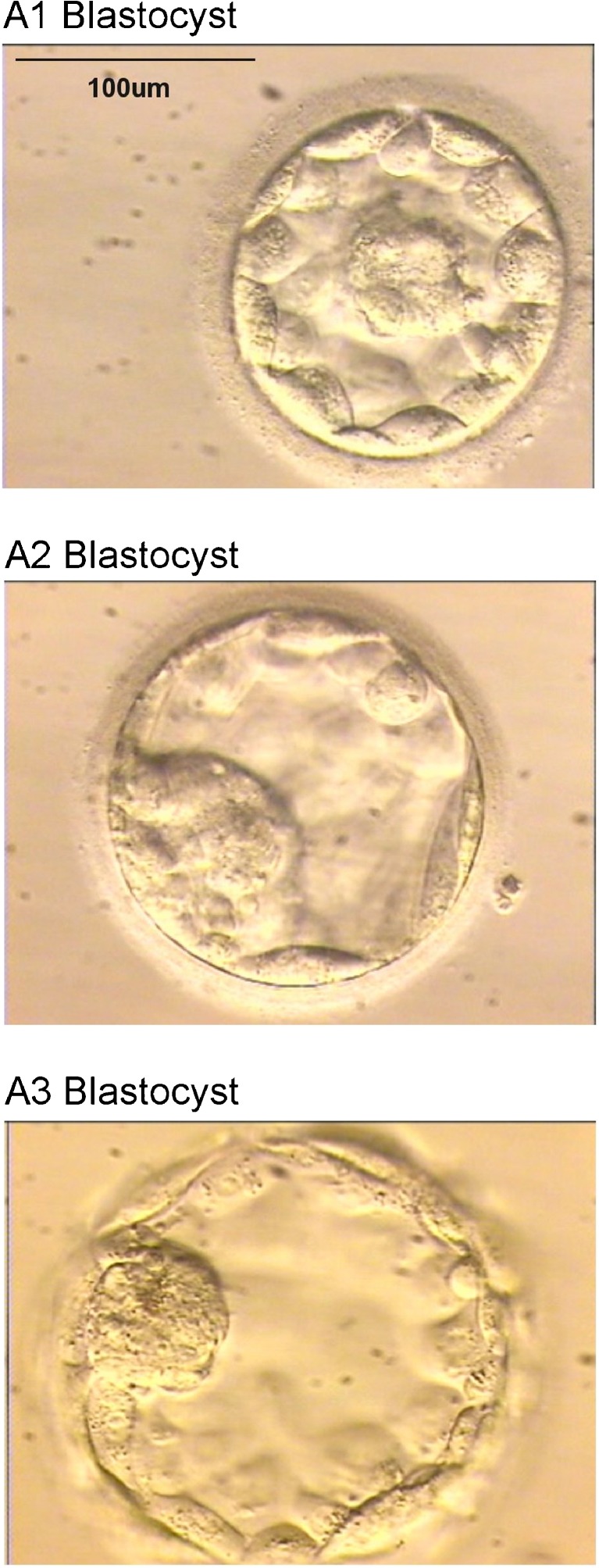
We found that the V/Vmin ratio ranged from 1 to 5.24, i.e. the most expanded blastocyst of the study was 5.24 fold the volume of an early blastocyst. A1 group includes blastocysts with V/Vmin ≤ 2, i.e. blastocysts whose volume doubled that of an early blastocyst. In A2 group the blastocyst volume is 2 up to 3 fold an early blastocyst, and in A3 group the volume is 3 fold an early blastocyst (Fig. 2). When implanting blastocysts areas were compared within the groups, differences in implantation rate were also not found.
Fig. 2.
Representative images of calculated blastocyst categories: A1 blastocyst: early blastocyst (blastocoel filling greater than half of the volume of the conceptus), but without overall increase in size more than half as compared to earlier stages. It should possess cells that suggest the formation of an inner cell mass. A2 blastocyst: a true blastocyst with slight expansion in overall size and some thinning of the zona pellucida. A3 blastocyst: full blastocyst with overall fully enlarged and a very thin zona pellucida
Nonetheless when IR comparison was performed between the three categories, expansion parameter was predictive of implantation potential.
This analyses let us characterize the area of an early blastocyst as A1 ≤ 18500 μm2 with a mean diameter d = 140 ± 9 μm, and the area of an expanded blastocyst as A3 ≥ 24000 μm2 with d = 190 ± 9 μm (Table 1).
Table 1.
Blastocyst expansion categories and cut-off area: diameter and area values are expressed as means plus or minus standard deviation
| Cut-off area (μm2) | Mean area ± SD (μm2) | Mean diameter ± SD (μm) | |
|---|---|---|---|
| A1 | <18500 | 15219 ± 1869 | 140 ± 8.6 |
| A2 | 18500–24000 | 21173 ± 1339 | 165 ± 5.2 |
| A3 | >24000 | 28132 ± 2720 | 190 ± 9.0 |
Of the 124 analysed blastocysts, 37.9 % fell in the A1 group, 30.6 % in the A2 and 31.5 % in the A3 group. The percentage of blastocysts that implanted in the three categories was 12.5 % (6/48), 29.7 % (11/37) and 33.3 % (13/ 39) respectively. A1 blastocysts implantation rate was statistically lower than A2 (IR = 12.5 vs 29.7 %, p = 0.04) and A3 blastocysts (IR = 12.5 vs. 33.3 %, p = 0.02) (Table 2).
Table 2.
Area blastocyst and Implantation Rate. Chi-square test was used for comparison of percentages
| Area (μm2) | TOT | Implanted | %IR | P | |
|---|---|---|---|---|---|
| A1 | <18500 | 48 | 6 | 12.5 | – |
| A2 | 18500–24000 | 37 | 11 | 29.7 | A1 vs. A2 p < 0.05* |
| A3 | >24000 | 39 | 13 | 33.3 | A1 vs. A3 p < 0.05* |
*Statistical differences were considered significant at p < 0.05
To further investigate blastocyst expansion parameter, we analysed the relationship between blastocyst area, implantation and patient age. As previously demonstrated [22], the proportion of early blastocysts increased with patient age (Table 3): 29.4 % (10/34) in women ≤34 years, 32.2 % (19/49) in women aged 35–38 and 51.6 % (16/31) in women ≥39 years. Though statistical significance was not reached due to small sample size, it’s probably worth mentioning a trend of a lower A1 blastocyst implantation rate (IR) in younger patients compared to older ones (10 vs 18,8 %), moreover in older patients the IR remained comparable in all three blastocyst categories, while it increased in A2 and A3 respectively in other patients (Table 3).
Table 3.
Blastocyst Implantation Rate (IR) according to expansion and patients age
| ≤34 | 35–38 | ≥39 | ||||
|---|---|---|---|---|---|---|
| % blastocyst | %IR | % blastocyst | %IR | % blastocyst | %IR | |
| A1 | 29.4 (10/34) | 10.0 | 32.2 (19/59) | 5.3 | 51.6 (16/31) | 18.8 |
| A2 | 50.0 (17/34) | 23.5 | 32.2 (19/59) | 31.6 | 22.6 (7/31) | 28.6 |
| A3 | 20.6 (7/34) | 42.9 | 35.6 (21/59) | 42.9 | 25.8 (8/31) | 12.5 |
Inner cell mass (ICM)
Inner cell mass was measured to find a possible correlation between its area and blastocyst implantation potential. Only visible ICMs were measured and those not detectable were considered as null value and not included in the mean. A total of 18 blastocysts hadn’t an observable ICM (14.6 %), 77.8 % of which were Area 1 blastocysts. Only one blastocyst without a distinguishable ICM implanted (5.5 %), but ended up in miscarriage.
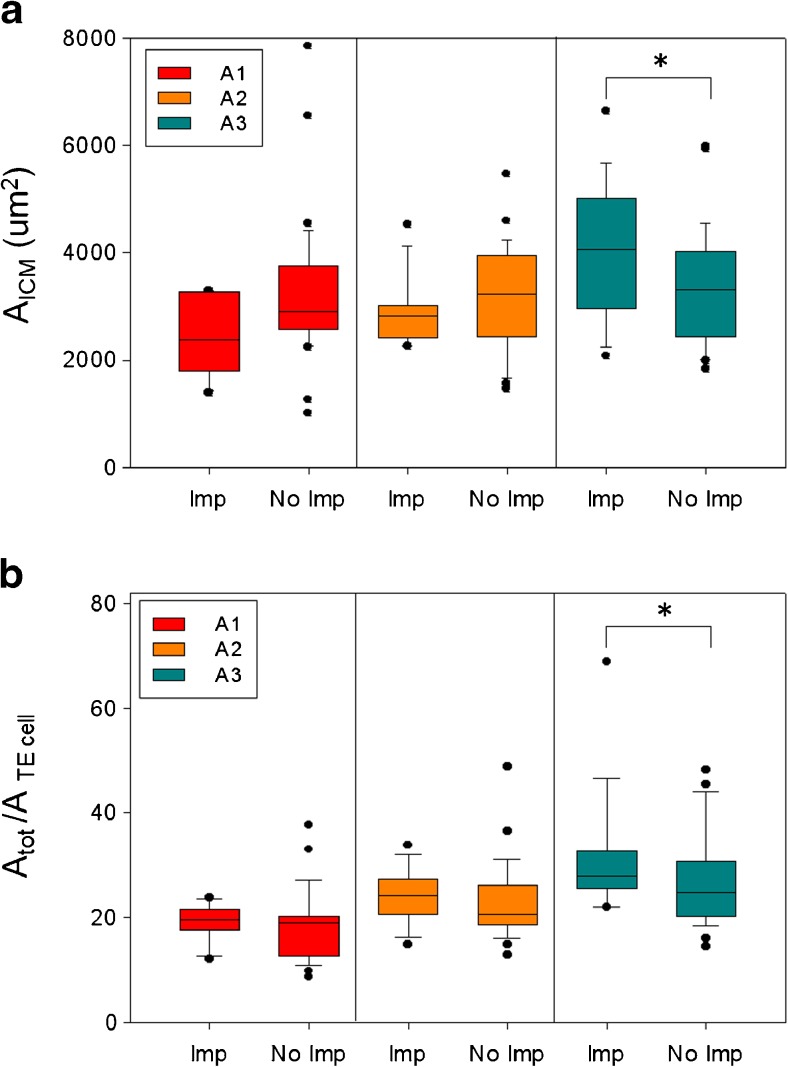
Mean AICM values were not statistically different in the three expansion categories (ranging from 2180.4 ± 1816.8 μm2 in A1 to 3460.1 ± 1433.3 μm2 in A3 blastocysts) and the ICM area alone did not show to be informative in predicting the implantation potential as no statistically significant difference was found between mean AICM of blastocysts which implanted and those which did not (3333.4 ± 1241.3 vs 3280.7 ± 1172.3 μm2). However when AICM was related to expansion, it showed a positive correlation with implantation in A3 blastocysts. Hence we can conclude that fully expanded blastocysts that implant display a statistically significant larger ICM area (3122.7 ± 739.0 vs 2978.1 ± 366.0 μm2; p = 0.047) (Fig. 3a).
Fig. 3.
ICM area, TE cell number and Implantation Rate. ICM area (a) and TE cell number (b) distributions in implanted (Imp) and not implanted (No Imp) blastocysts summarized as box plots. A1, A2 and A3 are the blastocyst expansion categories. A significant difference in ICM area of implanted vs. not implanted blastocysts exists in A3 blastocysts as well as in TE cell number (*p < 0,05). Box plots show the 10th, 25th, 50th (median), 75th, and 90th percentiles and outlier are plotted as individual points. Group variance was analysed by ANOVA followed by Tukey-Kramer test for comparison between multiple groups
Trophectoderm (TE)
To perform a quantitative evaluation, total internal blastocyst area was divided by a single epithelial cell area. The theoretical TE cell number in the maximal cross-section was determined. As for ICM evaluation, a value of 0 was assigned when TE cells were undetectable or degenerated. In this study, blastocysts with no distinct TE were 28 (22.8 %), with 22 A1 blastocysts. Only one of them gave an implant (3.6 %), followed by miscarriage. The analysis of blastocysts which implanted and those which did not, showed that, unlike ICM area, the overall TE cell number is higher in the implantation group (25.6 ± 11.3 vs 16.3 ± 12.8; p = 0.005). When TE cell number was analysed taking into consideration the different expansion categories a significant difference was found in the A3 category (31.9 ± 12.5 vs 26.2 ± 10.7; p = 0.04) (Fig. 3b).
Discussion
In recent years, the development of more physiological culture media and advances in laboratory practices have increased the routine use of human blastocyst cultures thus making it possible to choose the best single embryo to transfer. Currently, blastocyst evaluation relies on morphological system based on subjective observations. Quantitative analysis of embryo morphology could limit possible operator-dependent variability [11]. Actually, there is paucity of publications concerning automatic image analysis of human embryos [9, 10, 15], particularly at the blastocyst stage [17–19]. Hence the aim of our study was to establish a feasible and routinely applicable morphometric system to choose the most viable embryo to transfer. The first parameter measured in this study was the internal blastocyst expansion. Zona pellucida thickness (ZPT) is a highly variable embryo characteristic, affected by various factors such as maternal age, cause of infertility and ovarian hormonal stimulation [30, 31]. Therefore, we only measured the internal blastocyst area. Our results showed that the mean area of blastocysts that did implant and those that did not wasn’t statistically significant, in agreement with previous papers [28] suggesting that blastocysts of similar area have the same implantation potential. Since it has been largely documented in the literature that implantation potential depends on blastocyst expansion [27, 29], we divided our blastocysts in 3 groups according to their volume. We found that these 3 groups closely reflect the empiric score system established by Cornell [30] and based on the most efficient visual grading system by Gardner & Schoolcraft [5]. An early blastocyst, A1 group, was an initial stage of blastocyst development with A ≤ 18500 μm2; A2 was an intermediate category whose volume doubles that of an early blastocyst, with area ranging between 18000 and 24000 μm2 while A3, whose volume triples that of an early blastocyst, has A ≥ 24000 μm2. Again, the mean area of blastocysts within each group wasn’t statistically different between implanting and not implanting blastocyst, but our analyses of the relationship between expansion and implantation between groups, showed that A1 blastocysts implant less than A2 and A3 blastocysts (IR = 12.5 vs 29.7 and 33.3 p < 0.05), proving the correctness of our classification system, because positive correlation between blastocoele expansion and implantation is consistent with the data widely reported in the literature [20, 27, 29]. In this study only SET or DET with null or double implant were considered. We are aware that this inclusion criteria led to an artificial underestimation of our IVF Unit average implantation rate, since all DET transfers that ended up in an implantation failure were included in the study while DET transfers with one embryo implanted were excluded. Nevertheless our purpose was to correlate unambiguously blastocyst morphometric parameters to implantation fate. Interestingly, investigating the relationship between blastocyst expansion and patient age, we observed a negative linear relationship between patient age and time of embryonic development, and reduced blastocyst expansion seems to have a more limited implantation potential in young women compared to older ones. In fact, A1 blastocysts are frequent in aged women (≥39 years), where they represent 51.6 % of the total blastocysts formed, but tend to have a lower implantation rate when transferred to young women (18.8 % in women ≥39 years vs 10.0 % in women ≤34). So, probably, embryo development is compromised in advanced maternal age patients, but once the embryo forms it has an implantation potential. The occurrence of A1 blastocyst in younger patients may be considered a sign of a serious embryo impairment so that the embryo is not able to implant efficiently. This hypothesis should be further investigated since recent works [32] correlating embryo development and aneuploidy through time-lapse system, claim that a correlation exists between embryo delayed blastulation and aneuploidy. Our small sample size does not allow to draw conclusions, though the possibility that delayed blastocysts in older women may retain a residual implantation potential could be interesting.
In many IVF laboratories the ICM has been considered the most important predictor of pregnancy/live births, with the main rationale being that ICM contains the cells that will give rise to the growing fetus. In this study we measured the ICM area and correlated its dimension to outcome. The inner cell mass is a morphological feature that is not always immediately well perceived and its observation requires some adjustment of the microscope focus. For this reason, each blastocyst was photographed in a sequence of at least 4 images of different focal planes. A total of 18/124 blastocysts without an identifiable ICM was found (14.6 %), 14 of which were Area 1 blastocysts. This result confirmed the difficulty to recognize a well-defined ICM in early blastocysts, though only 14/49 early blastocysts hadn’t a discernible ICM. In this study only 1 A1 blastocyst without ICM implanted (5.5 %), followed by miscarriage.
In this study, the ICM area did not seem to be a suited measure to predict the implantation potential, because, as for expansion, AICM values were not statistically different in implanted vs. not implanted blastocysts. However when blastocysts were divided according to expansion, a significant difference appeared in A3 blastocyst category (Fig. 3a) where AICM were significantly larger (P < 0,05) with a mean value of 3122.7 ± 739.0 μm2. We found that ICM area increased along blastocyst expansion (A1 2211.1 ± 1896.3 μm2 and A2 2949.4 ± 1298.1 μm2) and this might account for the discrepancy between our results and that of previous studies reporting an overall significant larger ICM in implanting blastocysts [19]. Richter and colleagues [28] didn’t consider different expansion groups and our analysis showed that the proportion of A3 blastocysts considered in their study might positively influence the outcome thus inducing an overestimation of the ICM mean value.
In recent years, there was a revaluation of the importance of trophoectoderm quality in IVF outcome [20, 23]. Trophoectoderm cells develop into the placenta and their ability to invade the endometrium is the most important step not only for starting the complex process of implantation, but also for maintaining pregnancy and avoiding miscarriage [24]. In this study, trophoectoderm was evaluated as the theoretical TE cell number in the section of the focal plane of the image. As in ICM evaluation, also TE with degenerated cells or undetectable cells was considered as a null value. Blastocysts with degraded or indistinct trophoectoderm were 28 (22.6 %) mostly in Area 1 blastocysts; as in ICM evaluation, only one blastocyst gave an implant (3.6 %), followed by miscarriage. Probably because, as showed in a recent study by Alfarawati et al. [33], embryos with poorly developed trophoectoderm had a 2.5-fold greater probability of aneuploidy than those with good trophoectoderm grade. Where it was possible to carry out the TE measurement, we observed a positive correlation between trophoectoderm cell number and outcome. In particular, in A3 blastocysts, a higher number of trophoectoderm cells indicates a greater likelihood of implantation (31.9 ± 12.5 vs 26.2 ± 10.7; p < 0.05). The presence of many cells forming a tightly knit epithelium corresponds to the best grade of TE in Gardner’s evaluation. The possible mechanisms through which a higher grade of TE is a better predictor of live births outcome may be linked to improved TE function, for example, the role of secretion of hCG. So, higher grades of TE may be translated into more hCG producing cells and higher levels of hCG lead to stronger signaling capacity of the implanting blastocysts [21, 34].
In conclusion, our study showed that an objective evaluation of blastocyst morphology with a digital image system could be a valuable tool to select blastocysts based on quantitative parameters. In elective transfers it is preferable to choose a blastocyst with area ≥18500 μm2, with an elevated number of TE cells (25.6 ± 11.3) and a large well-defined ICM area (3122.7 ± 739.0 μm2). Interestingly the comparison of the calculated morphometric score with the morphologic one performed at the time of transfer revealed some bias of evaluation. We noticed a tendency towards an underestimation of blastocyst expansion: 4/38 blastocysts were considered A1 blastocysts while our measurements assigned them to the A2 group and 10/39 Type2 blastocysts were included in the A3 group. This could be of importance considering the difference in implantation potential of blastocysts belonging to the three categories and underlines the necessity of a standardized blastocyst parameters measurement.
One limitation of this study is the small sample size, also due to the laborious semi- automated image processing operations. The use of fully automated systems would be desirable to collect large data numbers in a fast and confident manner.
As Istanbul Consensus stated, if blastocyst morphological quality could be defined and validated [12, 13], it could be adopted as a standard in the comparison of clinical trials. The use of well-defined algorithms for the evaluation of embryo images would allow the development of automated tools to quantitatively determine blastocyst morphological features increasing collaborative research between laboratories worldwide.
Footnotes
Capsule Blastocyst quantitative measurements are a useful tool to standardize morphological grading and highly indicative of embryo implantation potential.
References
- 1.ESHRE. ESHRE ART fact sheet. http://www.eshre.eu/ESHRE/English/Guidelines-Legal/ART-factsheet/page.aspx/1061.
- 2.Fragouli J, Wells D. Aneuploidy in the human blastocyst. Cytogenet Genome Res. 2011;133:149–159. doi: 10.1159/000323500. [DOI] [PubMed] [Google Scholar]
- 3.Munne S. Chromosome Abnormalities and their relationship to morphology and development of human embryos. Reprod Biomed Online. 2006;12(2):234–254. doi: 10.1016/S1472-6483(10)60866-8. [DOI] [PubMed] [Google Scholar]
- 4.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332:459–461. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 5.Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. In: Janson R, Mortimer D, editors. Towards Reproductive Certainty: Infertility and Genetics Beyond 1999. Carnforth: Parthenon Press; 1999. pp. 378–388. [Google Scholar]
- 6.Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod. 1998;13:3434–3440. doi: 10.1093/humrep/13.12.3434. [DOI] [PubMed] [Google Scholar]
- 7.Blake DA, Farquhar CM, Johnson N, Proctor M. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database Syst Rev. 2007;4:CD002118. doi: 10.1002/14651858.CD002118.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod. 2008;23:91–99. doi: 10.1093/humrep/dem339. [DOI] [PubMed] [Google Scholar]
- 9.Kirkegaard K, Hindkjaer J, Grøndahl M, Kesmodel U, Ingerslev H. A randomized clinical trial comparing embryo culture in a conventional incubator with a time-lapse incubator. J Assist Reprod Genet. 2012;29:565–572. doi: 10.1007/s10815-012-9750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz M, Gadea B, Garrido N, Pedersen KS, Martinez M, Perez-Cano I, et al. Embryo quality, blastocyst and ongoing pregnancy rates in oocyte donation patients whose embryos were monitored by time-lapse imaging. J Assist Reprod Genet. 2011;28:569–573. doi: 10.1007/s10815-011-9549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendus AEB, Mayer JF, Shipley SK, Catherino WH. Interobserver and intraobserver variation in day 3 embryo grading. Fertil Steril. 2006;86:1608–1615. doi: 10.1016/j.fertnstert.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 12.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod Biomed Online. 2011;22:632–646. doi: 10.1016/j.rbmo.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 14.Hnida C, Engenheiro E, Ziebe S. Computer-controlled, multilevel, morphometric analysis of blastomere size as biomarker of fragmentation and multinuclearity in human embryos. Hum Reprod. 2004;19:288–293. doi: 10.1093/humrep/deh070. [DOI] [PubMed] [Google Scholar]
- 15.Ziebe S. Morphometric analysis of human embryos to predict developmental competence. Reprod Fertil Dev. 2013;26(1):55–64. doi: 10.1071/RD13296. [DOI] [PubMed] [Google Scholar]
- 16.Paternot G, Debrock S, De Neubourg D, D’Hooghe TM, Spiessens C. Semi-automated morphometric analysis of human embryos can reveal correlations between total embryo volume and clinical pregnancy. Hum Reprod. 2013;28(3):627–633. doi: 10.1093/humrep/des427. [DOI] [PubMed] [Google Scholar]
- 17.Beuchat A, Thevenaz P, Unser M, Ebner T, Senn A, Urner F, et al. Quantitative morphometrical characterization of human pronuclear zygotes. Hum Reprod. 2008;23:1983–1992. doi: 10.1093/humrep/den206. [DOI] [PubMed] [Google Scholar]
- 18.Santos Filho E, Noble JA, Wells D. A review on automatic analysis of human embryo microscope images. Open Biomed Eng J. 2010;4:170–177. doi: 10.2174/1874120701004010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos Filho ES, Noble JA, Poli M, Griffiths T, Emerson G, Wells D. A method for semi-automatic grading of human blastocyst microscope images. Hum Reprod. 2012;27:2641–2648. doi: 10.1093/humrep/des219. [DOI] [PubMed] [Google Scholar]
- 20.De Kock AD, Smuts MP, Madden JD, Rodriguez AJ, Chantilis SJ, Meintjes M. Digital image analysis of blastocysts. Morphometrics correlations with pregnancy outcome. Fertil Steril. 2006;86:S51–S52. doi: 10.1016/j.fertnstert.2006.07.141. [DOI] [Google Scholar]
- 21.Ahlstrom A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod. 2011;26:3289–3296. doi: 10.1093/humrep/der325. [DOI] [PubMed] [Google Scholar]
- 22.Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Blastocyst quality affects the success of blastocyst-stage embryo transfer. Fertil Steril. 2000;74:282–287. doi: 10.1016/S0015-0282(00)00645-2. [DOI] [PubMed] [Google Scholar]
- 23.Dokras A, Sargent IL, Barlow DH. Human blastocyst grading: an indicator of developmental potential? Hum Reprod. 1993;8:2119–2127. doi: 10.1093/oxfordjournals.humrep.a137993. [DOI] [PubMed] [Google Scholar]
- 24.Goto S, Kadowaki T, Tanaka S, Hashimoto H, Kokeguchi S, Shiotani M. Prediction of pregnancy rate by blastocyst morphological score and age, based on 1,488 single frozen-thawed blastocyst transfer cycles. Fertil Steril. 2011;95:948–952. doi: 10.1016/j.fertnstert.2010.06.067. [DOI] [PubMed] [Google Scholar]
- 25.Hill MJ, Richter KS, Heitmann RJ, Graham JR, Tucker MJ, Decherney AH, et al. Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertil Steril. 2013;99(5):1283–9. doi: 10.1016/j.fertnstert.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Honnma H, Baba T, Sasaki M, Hashiba Y, Ohno H, Fukunaga T, et al. Trophectoderm morphology significantly affects the rates of ongoing pregnancy and miscarriage in frozen-thawed single-blastocyst transfer cycle in vitro fertilization. Fertil Stertil. 2012;98:361–367. doi: 10.1016/j.fertnstert.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Zaninovic N, Berrios R, Clarke RN, Bodine R, Ye Z, Veeck LL. Blastocyst expansion, inner cell mass (ICM) formation, and trophectoderm (TM) quality: is one more important for implantation? Fertil Steril. 2001;76:S8. doi: 10.1016/S0015-0282(01)02038-6. [DOI] [Google Scholar]
- 28.Richter KS, Harris DC, Daneshmand ST, Shapiro BS. Quantitative grading of a human blastocyst: optimal inner cell mass size and shape. Fertil Steril. 2001;76:1157–1167. doi: 10.1016/S0015-0282(01)02870-9. [DOI] [PubMed] [Google Scholar]
- 29.Dal Prato L, Borini A, Trevisi MR, Bonu MA, Sereni E, Flamigni C. Effect of reduced dose of triptorelin at the start of ovarian stimulation on the outcome of IVF: a randomized study. Hum Reprod. 2001;16(7):1409–1414. doi: 10.1093/humrep/16.7.1409. [DOI] [PubMed] [Google Scholar]
- 30.Veeck LL, Zaninović N. An atlas of human blastocysts. New York: Parthenon Publishing Group; 2003. [Google Scholar]
- 31.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Thomas S. Large blastocyst diameter, early blastulation, and low preovulatory serum progesterone are dominant predictors of clinical pregnancy in fresh autologous cycles. Fertil Steril. 2008;90:302–309. doi: 10.1016/j.fertnstert.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 32.Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Hickman CF. Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod Biomed Online. 2013;26(5):477–485. doi: 10.1016/j.rbmo.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft WB, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95:520–524. doi: 10.1016/j.fertnstert.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Balaban B, Yakin K, Urman B. Randomized comparison of two different blastocyst grading systems. Fertil Steril. 2006;85:559–563. doi: 10.1016/j.fertnstert.2005.11.013. [DOI] [PubMed] [Google Scholar]