Abstract
Purpose
Morphological evaluation is currently considered the single most important predictive measure for assessing embryo quality. The aim of this study was to investigate whether cycles with at least one non-cleaved embryo (i.e., a 1-cell embryo on day 3) have different outcomes compared with cycles in which all embryos had cleaved by day 3.
Methods
All autologous IVF/ICSI cycles with a fresh day 3 transfer and without using a gestational carrier performed at our center between 1/1/2010 and 12/31/2011 were analyzed retrospectively. Those cycles with at least one non-cleaved embryo on day 3 were compared with all other autologous cycles that had l00 % cleaved embryos performed during the study period.
Results
Eight hundred and forty two cycles were included. Of them, 144 cycles comprised the non-cleaved group, and 698 cycles comprised the cleaved group. Cycles in the non-cleaved group had more oocytes retrieved (15.4 ± 7.1 vs. 12.5 ± 7.1, p < 0.001), more zygotes obtained (10.0 ± 5.3 vs. 7.9 ± 5.2, p = <0.001), but the embryos exhibited lower cleavage rates and higher rates of fragmentation and asymmetry compared with controls (p < 0.001). However, spontaneous abortion rates, ectopic pregnancies rates as well as delivery rates were similar between the two groups.
Conclusions
Our results show that the presence of a non-cleaved embryo on day 3 is associated with a more exuberant response to controlled ovarian stimulation as reflected by more oocytes retrieved. Despite the significant decrease in quality of the whole cohort in the non-cleaved group, implantation, delivery rates and number of embryos frozen were not adversely affected by the presence of a non-cleaved embryo.
Keywords: Non-cleaved embryo, Implantation, IVF, Cleaved embryos
Introduction
Normally developing human embryos cleave along a predictable timeline from the 2-cell to the 16-cell stage [1]. The zygote typically undergoes the first cleavage 20 to 27 h after insemination [2–4]. Using the evolving technology of time-lapse imaging, it has been postulated that time intervals between cleavages, rather than fixed time limits for cell division, can predict embryo viability [5–8] . However, a consensus remains that embryos that follow the “average” growth curve for the normal development of the early human embryo (i.e., reach the four-cell stage by 44 h, and the eight-cell stage by 68 h) are those of highest quality [7, 9, 10]. While recent studies have investigated the value of time-lapse imaging for embryo assessment and selection [reviewed by 6] standard morphological evaluation remains an important approach for evaluating embryo quality in IVF programs, particularly for those that lack time-lapse imaging systems. In addition, numerous studies have shown a direct correlation between the number of cells in a day 3 embryo and blastocyst formation [11–14], as well as implantation rates [15–21]. Indeed, several studies have shown that cleavage rates that are either too slow or too fast are associated with a negative impact on implantation rate [15, 18, 21]. Moreover, arrested zygotes and slow cleaving embryos (two cells on day 2 or 3) are associated with higher rates of chromosomal abnormalities [22].
To our knowledge, no data exist regarding whether an embryo arrested at the one-cell stage may reflect the overall quality of the rest of the embryos in the day 3 cohort. The aim of this study was to investigate whether embryo quality and/or clinical outcome in cycles with at least one non-cleaved embryo (i.e., a 1-cell embryo on day 3) differs from that of cycles in which all embryos had cleaved on day 3.
Materials and methods
This study was approved by the Partners’ Healthcare Institutional Review Board for chart review.
Study population
The medical records and the electronic data of all IVF patients who were treated at our tertiary, university-affiliated hospital, between 1/1/2010 and 12/31/2011 were retrospectively analyzed. IVF cycles (with or without ICSI) resulting in an embryo transfer were identified, with the following cycle types subsequently excluded: day 2 transfers, day 5 transfers, PGD cycles, oocyte donor cycles, gestational carrier cycles, cryopreservation cycles and IUI conversion. According to our program’s policy during this study period, most patients underwent day 3 embryo transfer. Day 2 embryo transfers were excluded as they were mainly offered to patients with decreased ovarian reserve. Day 5 transfers and PGD cycles were excluded as they were performed primarily for women with a high number of good quality day 3 embryos. Cycles were then screened for the presence of at least one non-cleaved embryo on day 3; these comprised the study group (henceforth referred to as the “non-cleaved group”). Remaining cycles were then considered for inclusion in the control group. However, only the first cycle for each patient that was performed in our program during this time period was included to ensure that each patient contributed to the study only once. The resulting control group is henceforth referred to as the “cleaved group”.
Ovarian stimulation protocols
Standard stimulation protocols were used as previously described [23, 24] with the long protocol most commonly used. Alternatively, micro flare or GnRH antagonist (GnRH-a) protocols were employed. Oocyte retrieval was performed 36 h after hCG trigger with 10,000 IU administered IM. Luteal progesterone supplementation was begun 1 day after retrieval with use of IM progesterone (locally compounded at one of two pharmacies: Village Fertility, Waltham, MA 02451 or Freedom Fertility, Byfield, MA 01922) or 2 days after retrieval when vaginal progesterone (8 % Crinone; Watson Pharmaceuticals, Parsippany, NJ, USA) was used [25]. Progesterone supplementation was continued until 10 weeks in patients who became pregnant.
Laboratory protocols
Gametes and embryos were maintained at 37 °C in a humidified atmosphere of 5 % CO2 in air as previously described [18, 26]. Oocytes were either inseminated within 4–6 h of retrieval or injected 3–5 h after retrieval. The fertilization check was performed 16–18 h after insemination or ICSI and zygotes having two pronuclei were cultured individually in 25 μl microdrops of Global medium (IVFOnline, Guelph, Ontario, Canada) supplemented with 5 % human serum albumin. Embryo morphology was assessed on day 3 between 66 and 69 h post-insemination/ICSI using standard criteria of number of blastomeres and extent of fragmentation and blastomere asymmetry [9, 18]. Fragmentation scores of 0 through 4 were assigned to each embryo, where scores correlated with 0, 1–9, 10–25, 26–50 % or >50 % fragmentation, respectively. Blastomere symmetry was graded using a numerical score of 1 through 3 according to uniformity in size and shape of the cells (Grade 1, 2 or 3 for perfect symmetry, moderate symmetry or severe asymmetry, respectively) [27].
Outcome variables assessed
Data collected from patient charts included demographic characteristics, cycle parameters including age, infertility diagnosis, stimulation protocol, day 3 FSH, peak E2 levels, number of oocytes retrieved, oocyte maturation status and fertilization rates. Quality of day 3 embryos was collected with good quality embryos defined as embryos having 7-8 cells on day 3, with <10 % of fragmentation. Clinical outcome data included implantation rate, pregnancy rate (both positive βhCG and clinical) as well as delivery rate. Implantation rate was defined as the number of gestational sacs detected by ultrasound 5 weeks after embryo transfer divided by the total number of embryos transferred. Chemical pregnancy was defined as a rise of βhCG and a subsequent spontaneous decline of βhCG with no detectable gestational sac. Clinical pregnancy was defined by the presence of a gestational sac and visualization of a fetal heart beat by ultrasound 5-6 weeks after embryo transfer. Miscarriage was defined as the absence of a fetal heart beat subsequent to this initial ultrasound. Therapeutic abortion was defined as either termination of the entire pregnancy or reduction of a fetus in a multiple pregnancy due to chromosomal abnormality or a major structural anomaly. Ectopic pregnancy was defined as visualization of a gestational sac, with or without fetal heartbeat, in the fallopian tube.
Statistical analysis
SPSS statistical software (Version 15) was utilized to perform the data analysis for this study. Differences in continuous variables between the study group and matched-pair control group were determined using the Wilcoxon Signed Rank test. Categorical variables were analyzed by Fisher’s exact test. In all cases, p < 0.05 was considered to be statistically significant.
Results
Patient characteristics
The characteristics of patients in the final dataset are shown in Table 1. Of the 842 cycles included, 144 cycles comprised the non-cleaved group, and 698 cycles comprised the cleaved group. Patients in the two study groups were of similar age (36.3 ± 3.8 y vs. 36.7 ± 4.1 y, respectively) but those in the non-cleaved group had undergone more IVF attempts (mean ± SD: 1.9 ± 1.2 vs. 1.6 ± 1.1, p < 0.004). There was no difference in the distribution of patients by fertility diagnosis or type of stimulation protocol used. Women in the non-cleaved group and had a lower day 3 FSH (7.4 ± 2.7 vs. 8.0 ± 2.9 mIU/ml, p = 0.016), a higher peak E2 (2243.8 ± 851.4 vs. 2002.5 + 865.1 pg/ml, p = 0.001) and more oocytes retrieved (15.4 ± 7.1 vs. 12.5 ± 7.1, p < 0.001) compared with the cleaved group. They also had a higher number of MII oocytes (13.4 ± 6.1 vs. 10.6 ± 6.2, p < 0.001) and, although the percentage of mature oocytes did not differ, the number of zygotes formed was significantly greater in the non-cleaved group (10.0 ± 5.3 vs. 7.9 ± 5.2, p = <0.001) despite no difference in the percentage of MII oocytes that formed 2pn embryos. No difference was observed between the two groups regarding the use of ICSI.
Table 1.
Characteristics of patients with or without at least one non-cleaved embryo on day 3
| Non-cleaved group (n = 144) | Cleaved group (n = 698) | p-value | |
|---|---|---|---|
| Patient age (y) | 36.3 ± 3.8 | 36.7 ± 4.1 | 0.240 |
| Stimulation protocol: | |||
| GnRH agonist (%) | 54.9 % | 53.0 % | 0.147 |
| Antagonist (%) | 20.8 % | 15.8 % | |
| Poor responders (%) | 24.3 % | 31.2 % | |
| Infertility causes: | |||
| Ovulatory dysfunction* | 6.3 % | 5.5 % | 0.575 |
| Male factor | 26 % | 21 % | |
| Diminished ovarian reserve | 11.1 % | 15.1 % | |
| Endometriosis | 6.3 % | 6.0 % | |
| Tubal factor | 9.7 % | 9.6 % | |
| Unexplained | 33.0 % | 31.0 % | |
| Other | 8.3 % | 12.5 % | |
| Day 3 FSH (mIU/ml) | 7.4 ± 2.7 | 8.0 ± 2.9 | 0.016 |
| Peak E2 (pg/ml) | 2243.8 ± 851.4 | 2002.5 ± 865.1 | <0.001 |
| Number of oocytes retrieved | 15.4 ± 7.1 | 12.5 ± 7.1 | <0.001 |
| No. MII | 13.4 ± 6.1 | 10.6 ± 6.2 | <0.001 |
| % MII/Total oocytes retrieved | 87.9 | 84.9 | 0.159 |
| ICSI (%) | 31.3 % | 34.4 % | 0.499 |
| No. 2pn | 10.0 ± 5.3 | 7.9 ± 5.2 | <0.001 |
| % 2pn/MII | 74.9 | 74.4 | 0.837 |
*Ovulatory dysfunction included PCOS, anovulation, oligo-ovulation
**Values represent n (%) or mean ± standard deviation
Incidence of non-cleaved embryos
Of the 7033 embryos from the 842 cycles included in the analysis, 161 were non-cleaved (2.4 %). These non-cleaved embryos were retrieved from 144 patients. One hundred and thirty patients had one non-cleaved embryo (130/144, 90.3 %), 12 patients had 2 non-cleaved embryos (8.3 %), one (1/144; 0.7 %) had 3 non-cleaved embryos and one (1/144, 0.7 %) had 4 non-cleaved embryos in the same cycle evaluated.
Day 3 embryo morphology
To assess any association between the presence of at least one non-cleaved embryo and quality of the remaining embryos in the cohort, we compared the morphology of the embryos that had cleaved between the two groups. We excluded the 1-cell embryos in this analysis as we were specifically interested in the morphological distributions of exclusively the sibling embryos that had cleaved.
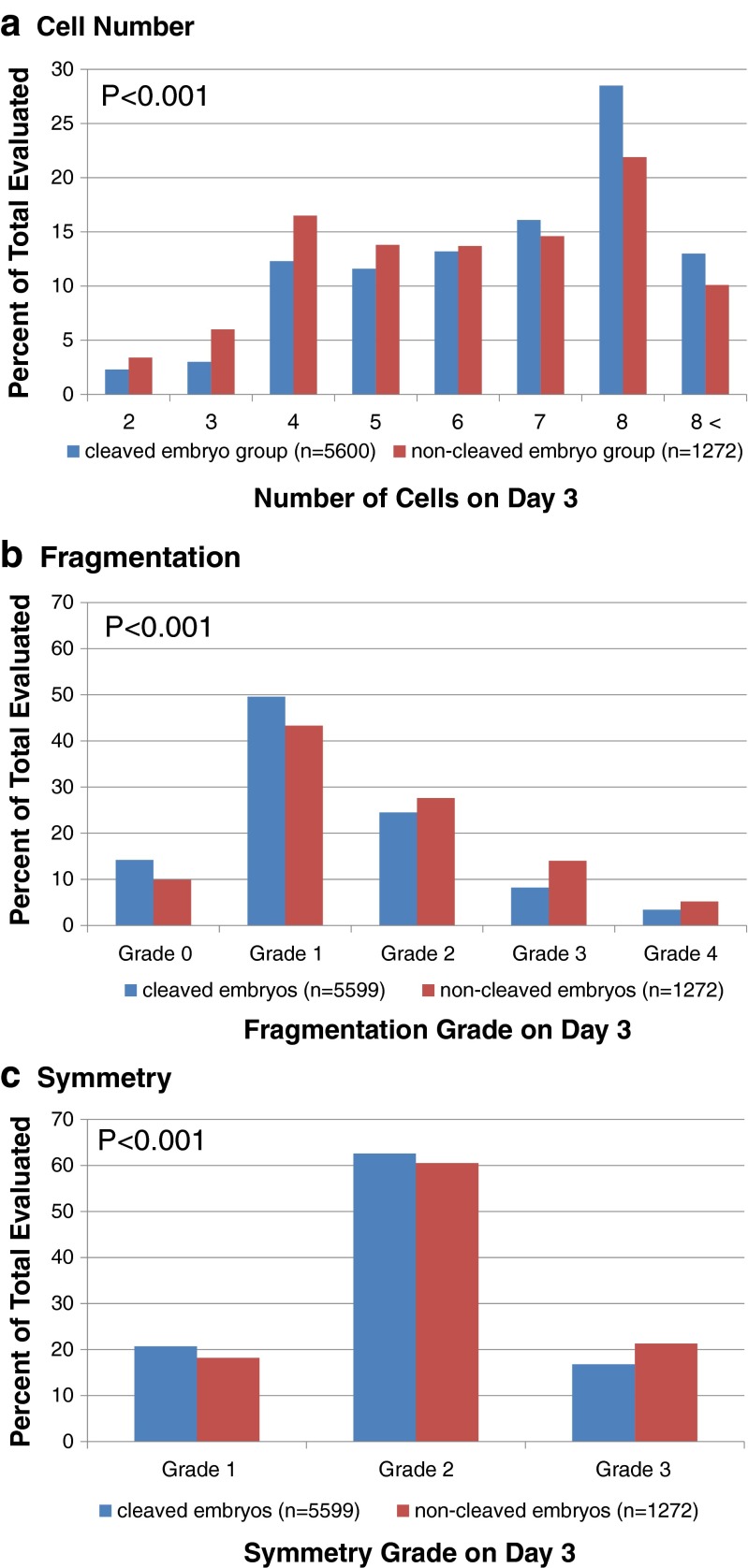
Significantly more embryos in those cohorts with at least one non-cleaved embryo had less than the expected number of 8-cells on day 3 compared with the control, cleaved group (p < 0.001); this trend was manifest by more embryos having < 6-cells, and correspondingly fewer having > 7-cells in the non-cleaved group (Fig. 1a). There were also significant differences between the two cohorts regarding the distribution of embryos according to both the extent of fragmentation (Fig. 1b; p < 0.001) and degree of symmetry (Fig. 1c; p < 0.001); a lower percentage of embryos exhibited low fragmentation grades (Grades 0 or 1; Fig. 1b) and a higher percentage had severe asymmetry (Grade 3; Fig 1c) in the non-cleaved cohort.
Fig. 1.
Distribution of day 3 embryos according to the number of cells (a); fragmentation grade (b); and symmetry grade (c)
Cycle outcomes
Cycle outcomes are shown in Table 2. The non-cleaved group had significantly fewer good quality embryos (2.0 ± 2.9 vs. 2.5 ± 3.0, p = 0.008, respectively), and there was a trend towards fewer embryos being frozen (1.5 ± 2.7 vs. 2.0 ± 3.3, p = 0.07). However, the groups did not differ for any of the clinical outcomes. Fifty two percent of the patients who had non-cleaved cycles conceived compared with 56 % that had cleaved cycles (p = 0.519). Clinical pregnancy rates were 36.1 and 43.0 % respectively (p = 0.138). Delivery rates as well as the abortion rates and ectopic rates were also similar in both groups.
Table 2.
Cycle outcome variables in cycles having at least one non-cleaved embryo on day 3 versus comparison controls (mean ± SD)
| Non-cleaved group (n = 144) | Cleaved group (n = 698) | p-value | |
|---|---|---|---|
| No. of ‘good-quality’ embryos | 2.0 ± 2.9 | 2.5 ± 3.0 | 0.008 |
| No. embryos transferred | 2.5 ± 1.1 | 2.5 ± 1.3 | 0.08 |
| No. embryos frozen | 1.5 ± 2.7 | 2.0 ± 3.3 | 0.07 |
| Implantation rate (% of embryos transferred) |
73/374 (22.2 %) |
444/1827 (28.9 %) |
0.052 |
| Ectopic (% of positive hCG) |
2/76 2.6 % |
4/391 1.0 % |
0.253 |
| Spontaneous abortions (% of clinical pregnancies) |
8/76 10.5 % |
64/391 16.4 % |
0.227 |
| Therapeutic abortions (% of clinical pregnancies) |
0/76 0 % |
5/391 1.3 % |
1.0 |
| Deliveries (% of total transfers) |
44/144 30.6 % |
231/698 33.1 % |
0.626 |
Values represent n (%) or mean ± standard deviation
Discussion
In this study with a dataset of 842 cycles from 842 women, we found that the presence of at least one non-cleaved embryo on day 3 was associated with more oocytes retrieved and 2pn embryos formed, but the overall quality of the other embryos in the day 3 cohort was significantly reduced compared with controls. Nevertheless, clinical outcomes were similar between the two groups.
Evaluation of embryos in vitro has undergone a dramatic change in the past few years with the introduction of time-lapse imaging. This novel technique enables acquisition of dynamic images of embryo development [6, 10, 28–30]. It has been proposed that the information acquired regarding the kinetics of embryo cleavage, either with or without use of standard static embryo morphology grading, will greatly improve successful selection of the most viable embryo for transfer. However, according to a recent systemic review [6], whether multiple morphokinetic evaluations of embryos using time-lapse imaging are superior to a single evaluation of embryo morphology before transfer remains currently unresolved, due to insufficient high-quality data. Moreover, there are publications showing correlations between blastocyst formation or implantation potential and standard morphological criteria on day 3, including not only cell number, but also fragmentation and blastomere symmetry [21, 31–33], despite the latter two features being dynamic. Therefore, the balance of available evidence emphasizes the importance of the current study, particularly as many IVF units are still using static morphological grading to assess embryo quality and selection.
It is well recognized that in the human, the first two to three cell divisions are regulated by the maternal genome [34]. Several studies have shown that a primary determinant of embryo viability is timing of onset of zygotic cleavage and entry into the first mitotic division [15, 35, 36]. Both animal and human studies have demonstrated that timely entry into the first cleavage (so called “early cleavage”) is associated with developmentally more competent embryos compared with late cleavage or no cleavage [37]. Indeed, embryos that cleave between 24 and 27 h after fertilization are more developmentally competent, resulting in higher blastocyst and implantation rates compared with those that cleave relatively later [7, 13, 38–44].
Recently, a retrospective study using time-lapse imaging data showed that extremely early cleavage of embryos (i.e., direct division from 2 cell to 3 cells in ≤5 h) was associated with significantly lower implantation rates compared with embryos that exhibited a normal cleavage pattern. The authors suggested that the short cell cycle was associated with incomplete DNA replication and might lead to impaired quality embryos due to unequal distribution of DNA to blastomeres [45]. In bovine, a higher frequency of chromosomal abnormalities was reported among embryos showing direct cleavage from one cell to 3 or 4 blastomeres [46].
In the present study, non-cleaved embryos occurred in cycles in which both peak estradiol levels and total number of oocytes retrieved were higher compared with controls. It is conceivable that the presence of non-cleaved embryos in the setting of higher oocyte yield is purely a fluke of sampling when there is a more exuberant response to ovarian stimulation. However, as these developmentally incompetent embryos were associated with cohorts of poorer quality, it would seem more likely that they arose from recruitment of smaller, less competent follicles containing oocytes of reduced cytoplasmic maturity and compromised ability to undergo entry into mitosis. There has been controversy regarding the effect of higher estradiol levels on oocyte and embryo quality. In mice, higher estradiol levels were reported to have a direct toxic effect on embryo quality and development in vitro [47], however the detrimental effects of higher estradiol levels on embryo development in the human have not been proven [48, 47]. Of interest, Balakier et al. recorded significantly higher E2 levels and a higher number of oocytes in cohorts that contained giant oocytes compared with controls [49].
We did not find that a non-cleaved embryo was associated with lower pregnancy rates or higher miscarriages rates. This observation may be explained by the availability of a sufficient number of top quality embryos in the non-cleaved group, thereby providing embryos for transfer that were of comparable developmental competency to the controls. The association between the number of oocytes retrieved and pregnancy rate is controversial. Some researchers have shown a correlation between the number of oocytes retrieved and higher pregnancy rates [50–52] whereas others argue that excessive response to ovarian stimulation might result in detrimental effects on oocyte and embryo quality and/or that supra-physiological estradiol levels may alter implantation [53, 54]. Of note, we observed a trend towards a lower number of embryos frozen among cohorts with non-cleaved embryos, although the numbers were not statistically significant.
In conclusion, our results show that cycles in which at least one zygote in a cohort fails to undergo the first mitotic division is associated with a more exuberant response to controlled ovarian stimulation as reflected by more oocytes retrieved and more zygotes obtained. Although the presence of a non-cleaved embryo was associated with a significant decrease in overall quality of the cohort, implantation and clinical pregnancy rates were not adversely affected. While it is possible that a negative association with clinical outcome is observed in cohorts having a significant percentage of non-cleaved embryos, the current dataset did not allow us to explore this possibility.
Taken together, our results show that clinicians can be reassured that this rare developmental aberration, at least in a single sibling embryo, appears not to be associated with reduced probability of a successful cycle. We hope that new insights investigating molecular and morphokinetic markers associated with oocyte and embryo quality using time-lapse imaging, will help to define the abnormalities underlying mitotic arrest at the zygote stage.
Acknowledgments
Ethical Statement
For this type of study formal consent is not required.
Footnotes
Capsule The presence of a non-cleaved embryo on day 3 is associated with a decrease in the cohort quality but does not impact the clinical outcome.
References
- 1.Edwards RG, Steptoe PC, Purdy JM. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol. 1980;87(9):737–56. doi: 10.1111/j.1471-0528.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- 2.Balakier H, Casper RF. A morphologic study of unfertilized oocytes and abnormal embryos in human in vitro fertilization. J In Vitro Fert Embryo Trans : IVF. 1991;8(2):73–9. doi: 10.1007/BF01138658. [DOI] [PubMed] [Google Scholar]
- 3.Capmany G, Taylor A, Braude PR, Bolton VN. The timing of pronuclear formation, DNA synthesis and cleavage in the human 1-cell embryo. Mol Hum Reprod. 1996;2(5):299–306. doi: 10.1093/molehr/2.5.299. [DOI] [PubMed] [Google Scholar]
- 4.Trounson AO, Mohr LR, Wood C, Leeton JF. Effect of delayed insemination on in-vitro fertilization, culture and transfer of human embryos. J Reprod Fertil. 1982;64(2):285–94. doi: 10.1530/jrf.0.0640285. [DOI] [PubMed] [Google Scholar]
- 5.Chamayou S, Patrizio P, Storaci G, Tomaselli V, Alecci C, Ragolia C, et al. The use of morphokinetic parameters to select all embryos with full capacity to implant. J Assist Reprod Genet. 2013;30(5):703–10. doi: 10.1007/s10815-013-9992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaser DJ, Racowsky C. Clinical outcomes following selection of human preimplantation embryos with time-lapse monitoring: a systematic review. Hum Reprod Update. 2014;20(5):617–31. doi: 10.1093/humupd/dmu023. [DOI] [PubMed] [Google Scholar]
- 7.Meseguer M, Herrero J, Tejera A, Hilligsoe KM, Ramsing NB, Remohi J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26(10):2658–71. doi: 10.1093/humrep/der256. [DOI] [PubMed] [Google Scholar]
- 8.Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28(10):1115–21. doi: 10.1038/nbt.1686. [DOI] [PubMed] [Google Scholar]
- 9.Alpha Scientists in Reproductive M. Embryology ESIGo The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 10.Herrero J, Meseguer M. Selection of high potential embryos using time-lapse imaging: the era of morphokinetics. Fertil Steril. 2013;99(4):1030–4. doi: 10.1016/j.fertnstert.2013.01.089. [DOI] [PubMed] [Google Scholar]
- 11.Boostanfar R, Jain JK, Slater CC, Tourgeman DE, Francis MM, Paulson RJ. The prognostic significance of day 3 embryo cleavage stage on subsequent blastocyst development in a sequential culture system. J Assist Reprod Genet. 2001;18(10):548–50. doi: 10.1023/A:1011953907332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrillo AJ, Lane B, Pridman DD, Risch PP, Pool TB, Silverman IH, et al. Improved clinical outcomes for in vitro fertilization with delay of embryo transfer from 48 to 72 hours after oocyte retrieval: use of glucose- and phosphate-free media. Fertil Steril. 1998;69(2):329–34. doi: 10.1016/s0015-0282(97)00499-8. [DOI] [PubMed] [Google Scholar]
- 13.Edwards RG, Purdy JM, Steptoe PC, Walters DE. The growth of human preimplantation embryos in vitro. Am J Obstet Gynecol. 1981;141(4):408–16. doi: 10.1016/0002-9378(81)90603-7. [DOI] [PubMed] [Google Scholar]
- 14.Jones GM, Trounson AO, Lolatgis N, Wood C. Factors affecting the success of human blastocyst development and pregnancy following in vitro fertilization and embryo transfer. Fertil Steril. 1998;70(6):1022–9. doi: 10.1016/s0015-0282(98)00342-2. [DOI] [PubMed] [Google Scholar]
- 15.Giorgetti C, Terriou P, Auquier P, Hans E, Spach JL, Salzmann J, et al. Embryo score to predict implantation after in-vitro fertilization: based on 957 single embryo transfers. Hum Reprod. 1995;10(9):2427–31. doi: 10.1093/oxfordjournals.humrep.a136312. [DOI] [PubMed] [Google Scholar]
- 16.Machtinger R, Racowsky C. Morphological systems of human embryo assessment and clinical evidence. Reprod Biomed Online. 2013;26(3):210–21. doi: 10.1016/j.rbmo.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Puissant F, Van Rysselberge M, Barlow P, Deweze J, Leroy F. Embryo scoring as a prognostic tool in IVF treatment. Hum Reprod. 1987;2(8):705–8. doi: 10.1093/oxfordjournals.humrep.a136618. [DOI] [PubMed] [Google Scholar]
- 18.Racowsky C, Combelles CM, Nureddin A, Pan Y, Finn A, Miles L, et al. Day 3 and day 5 morphological predictors of embryo viability. Reprod Biomed Online. 2003;6(3):323–31. doi: 10.1016/s1472-6483(10)61852-4. [DOI] [PubMed] [Google Scholar]
- 19.Steer CV, Mills CL, Tan SL, Campbell S, Edwards RG. The cumulative embryo score: a predictive embryo scoring technique to select the optimal number of embryos to transfer in an in-vitro fertilization and embryo transfer programme. Hum Reprod. 1992;7(1):117–9. doi: 10.1093/oxfordjournals.humrep.a137542. [DOI] [PubMed] [Google Scholar]
- 20.Van Royen E, Mangelschots K, De Neubourg D, Valkenburg M, Van de Meerssche M, Ryckaert G, et al. Characterization of a top quality embryo, a step towards single-embryo transfer. Hum Reprod. 1999;14(9):2345–9. doi: 10.1093/humrep/14.9.2345. [DOI] [PubMed] [Google Scholar]
- 21.Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod. 1997;12(7):1545–9. doi: 10.1093/humrep/12.7.1545. [DOI] [PubMed] [Google Scholar]
- 22.Magli MC, Gianaroli L, Ferraretti AP, Lappi M, Ruberti A, Farfalli V. Embryo morphology and development are dependent on the chromosomal complement. Fertil Steril. 2007;87(3):534–41. doi: 10.1016/j.fertnstert.2006.07.1512. [DOI] [PubMed] [Google Scholar]
- 23.Racowsky C, Ohno-Machado L, Kim J, Biggers JD. Is there an advantage in scoring early embryos on more than one day? Hum Reprod. 2009;24(9):2104–13. doi: 10.1093/humrep/dep198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skiadas CC, Jackson KV, Racowsky C. Early compaction on day 3 may be associated with increased implantation potential. Fertil Steril. 2006;86(5):1386–91. doi: 10.1016/j.fertnstert.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 25.Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein MD. Comparison of Crinone 8 % intravaginal gel and intramuscular progesterone supplementation for in vitro fertilization/embryo transfer in women under age 40: interim analysis of a prospective randomized trial. Fertil Steril. 2008;89(2):485–7. doi: 10.1016/j.fertnstert.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Machtinger R, Politch JA, Hornstein MD, Ginsburg ES, Racowsky C. A giant oocyte in a cohort of retrieved oocytes: does it have any effect on the in vitro fertilization cycle outcome? Fertil Steril. 2011;95(2):573–6. doi: 10.1016/j.fertnstert.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 27.Reichman DE, Jackson KV, Racowsky C. Incidence and development of zygotes exhibiting abnormal pronuclear disposition after identification of two pronuclei at the fertilization check. Fertil Steril. 2010;94(3):965–70. doi: 10.1016/j.fertnstert.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Cruz M, Gadea B, Garrido N, Pedersen KS, Martinez M, Perez-Cano I, et al. Embryo quality, blastocyst and ongoing pregnancy rates in oocyte donation patients whose embryos were monitored by time-lapse imaging. J Assist Reprod Genet. 2011;28(7):569–73. doi: 10.1007/s10815-011-9549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirkegaard K, Agerholm IE, Ingerslev HJ. Time-lapse monitoring as a tool for clinical embryo assessment. Hum Reprod. 2012;27(5):1277–85. doi: 10.1093/humrep/des079. [DOI] [PubMed] [Google Scholar]
- 30.Basile N, Vime P, Florensa M, Aparicio Ruiz B, Garcia Velasco JA, Remohi J, et al. The use of morphokinetics as a predictor of implantation: a multicentric study to define and validate an algorithm for embryo selection. Hum Reprod. 2014 doi: 10.1093/humrep/deu331. [DOI] [PubMed] [Google Scholar]
- 31.Sela R, Samuelov L, Almog B, Schwartz T, Cohen T, Amit A, et al. An embryo cleavage pattern based on the relative blastomere size as a function of cell number for predicting implantation outcome. Fertil Steril. 2012;98(3):650–6. doi: 10.1016/j.fertnstert.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 32.della Ragione T, Verheyen G, Papanikolaou EG, Van Landuyt L, Devroey P, Van Steirteghem A. Developmental stage on day-5 and fragmentation rate on day-3 can influence the implantation potential of top-quality blastocysts in IVF cycles with single embryo transfer. Reprod Biol Endocrinol : RB&E. 2007;5:2. doi:10.1186/1477-7827-5-2. [DOI] [PMC free article] [PubMed]
- 33.Scott L, Finn A, O’Leary T, McLellan S, Hill J. Morphologic parameters of early cleavage-stage embryos that correlate with fetal development and delivery: prospective and applied data for increased pregnancy rates. Hum Reprod. 2007;22(1):230–40. doi: 10.1093/humrep/del358. [DOI] [PubMed] [Google Scholar]
- 34.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332(6163):459–61. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 35.Edwards RG, Fishel SB, Cohen J, Fehilly CB, Purdy JM, Slater JM, et al. Factors influencing the success of in vitro fertilization for alleviating human infertility. J In Vitro Fert Embryo Trans : IVF. 1984;1(1):3–23. doi: 10.1007/BF01129615. [DOI] [PubMed] [Google Scholar]
- 36.Lechniak D, Pers-Kamczyc E, Pawlak P. Timing of the first zygotic cleavage as a marker of developmental potential of mammalian embryos. Reprod Biol. 2008;8(1):23–42. doi: 10.1016/s1642-431x(12)60002-3. [DOI] [PubMed] [Google Scholar]
- 37.Sakkas D, Shoukir Y, Chardonnens D, Bianchi PG, Campana A. Early cleavage of human embryos to the two-cell stage after intracytoplasmic sperm injection as an indicator of embryo viability. Hum Reprod. 1998;13(1):182–7. doi: 10.1093/humrep/13.1.182. [DOI] [PubMed] [Google Scholar]
- 38.Giorgetti C, Hans E, Terriou P, Salzmann J, Barry B, Chabert-Orsini V, et al. Early cleavage: an additional predictor of high implantation rate following elective single embryo transfer. Reprod Biomed Online. 2007;14(1):85–91. doi: 10.1016/s1472-6483(10)60768-7. [DOI] [PubMed] [Google Scholar]
- 39.Isom SC, Li RF, Whitworth KM, Prather RS. Timing of first embryonic cleavage is a positive indicator of the in vitro developmental potential of porcine embryos derived from in vitro fertilization, somatic cell nuclear transfer and parthenogenesis. Mol Reprod Dev. 2012;79(3):197–207. doi: 10.1002/mrd.22013. [DOI] [PubMed] [Google Scholar]
- 40.Lee MJ, Lee RK, Lin MH, Hwu YM. Cleavage speed and implantation potential of early-cleavage embryos in IVF or ICSI cycles. J Assist Reprod Genet. 2012;29(8):745–50. doi: 10.1007/s10815-012-9777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lian WL, Xin ZM, Jin HX, Song WY, Peng ZF, Sun YP. Effects of early-cleavage embryo transfer on in vitro fertilization-embryo transfer pregnancy outcomes. Clin Exp Obstet Gynecol. 2013;40(3):319–22. [PubMed] [Google Scholar]
- 42.Lundin K, Bergh C, Hardarson T. Early embryo cleavage is a strong indicator of embryo quality in human IVF. Hum Reprod. 2001;16(12):2652–7. doi: 10.1093/humrep/16.12.2652. [DOI] [PubMed] [Google Scholar]
- 43.Salumets A, Hyden-Granskog C, Makinen S, Suikkari AM, Tiitinen A, Tuuri T. Early cleavage predicts the viability of human embryos in elective single embryo transfer procedures. Hum Reprod. 2003;18(4):821–5. doi: 10.1093/humrep/deg184. [DOI] [PubMed] [Google Scholar]
- 44.Terriou P, Giorgetti C, Hans E, Salzmann J, Charles O, Cignetti L, et al. Relationship between even early cleavage and day 2 embryo score and assessment of their predictive value for pregnancy. Reprod Biomed Online. 2007;14(3):294–9. doi: 10.1016/s1472-6483(10)60870-x. [DOI] [PubMed] [Google Scholar]
- 45.Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escriba MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98(6):1458–63. doi: 10.1016/j.fertnstert.2012.07.1135. [DOI] [PubMed] [Google Scholar]
- 46.Somfai T, Inaba Y, Aikawa Y, Ohtake M, Kobayashi S, Konishi K, et al. Relationship between the length of cell cycles, cleavage pattern and developmental competence in bovine embryos generated by in vitro fertilization or parthenogenesis. J Reprod Dev. 2010;56(2):200–7. doi: 10.1262/jrd.09-097a. [DOI] [PubMed] [Google Scholar]
- 47.Valbuena D, Martin J, de Pablo JL, Remohi J, Pellicer A, Simon C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertil Steril. 2001;76(5):962–8. doi: 10.1016/s0015-0282(01)02018-0. [DOI] [PubMed] [Google Scholar]
- 48.Bianco K, Mahutte NG, Arici A, Sakkas D, Taylor HS. Effect of estradiol on oocyte development. Int J Gynaecol Obstet : Off Organ Int Fed Gynaecol Obstet. 2009;104(3):230–2. doi: 10.1016/j.ijgo.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balakier H, Bouman D, Sojecki A, Librach C, Squire JA. Morphological and cytogenetic analysis of human giant oocytes and giant embryos. Hum Reprod. 2002;17(9):2394–401. doi: 10.1093/humrep/17.9.2394. [DOI] [PubMed] [Google Scholar]
- 50.Cai Q, Wan F, Huang K, Zhang H. Does the number of oocytes retrieved influence pregnancy after fresh embryo transfer? PLoS One. 2013;8(2):e56189. doi: 10.1371/journal.pone.0056189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meniru GI, Craft IL. Utilization of retrieved oocytes as an index of the efficiency of superovulation strategies for in-vitro fertilization treatment. Hum Reprod. 1997;12(10):2129–32. doi: 10.1093/humrep/12.10.2129. [DOI] [PubMed] [Google Scholar]
- 52.Yih MC, Spandorfer SD, Rosenwaks Z. Egg production predicts a doubling of in vitro fertilization pregnancy rates even within defined age and ovarian reserve categories. Fertil Steril. 2005;83(1):24–9. doi: 10.1016/j.fertnstert.2004.05.096. [DOI] [PubMed] [Google Scholar]
- 53.Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26(7):1768–74. doi: 10.1093/humrep/der106. [DOI] [PubMed] [Google Scholar]
- 54.van der Gaast MH, Eijkemans MJ, van der Net JB, de Boer EJ, Burger CW, van Leeuwen FE, et al. Optimum number of oocytes for a successful first IVF treatment cycle. Reprod Biomed Online. 2006;13(4):476–80. doi: 10.1016/s1472-6483(10)60633-5. [DOI] [PubMed] [Google Scholar]