Abstract
Purpose
Previous studies have indicated that OxS (oxidative stress) may appear as a possible reason for poor ART outcome. Our aim was to study OxS levels in both partners of couples seeking Assisted reproduction Technology (ART).
Methods
Altogether 79 couples were recruited. Oxidative DNA damage (8-OHdG) and lipid peroxidation (8-EPI) were measured, and clinical background and ART outcomes were recorded.
Results
Both OxS markers accurately reflected clincal conditions with prominent negative effects attributable to genital tract infections, endometriosis, uterine myoma and smoking. Furthermore, the level of OxS was also affected by partner’s state of health. The highest 8-EPI levels were detected in both partners when biochemically detectable pregnancies did not develop into clinically detectable pregnancies (in women, 97,8 ± 16,7 vs 72.9 ± 22,9, p = 0.007; in men, 89.6 ± 20,4 vs 72,1 ± 22,6, p = 0.049).
Conclusions
To conclude, high grade systemix OxS in both partners may negatively affect the maintenance and outcome of pregnancy. Applying the detection of OxS in ART patients may select patients with higher success rate and/or those who require antioxidant therapy. This would lead to improvement of ART outcome as well as natural fertility.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-015-0466-6) contains supplementary material, which is available to authorized users.
Keywords: 8-EPI (8-iso Prostaglandin F2α), 8-OHdG (8-hydroxy-2’-deoxyguanosine), ART (assisted reproductive technique), Infertility, OxS (oxidative stress), ROS (reactive oxygen species)
Introduction
The medical definition of infertility is the failure to conceive following 12 months of unprotected intercourse [1]. Infertility may occur due to problems with the female, male or both partners. ART (assisted reproductive technique) includes a range of methods used to circumvent human fertility [2]. Despite many advances in ART, outcome is often unsuccessful: according to European data collected from 36 countries, the clinical pregnancy rates per embryo transfer were 33 % for IVF (in vitro fertilization) and 32 % for ICSI (intracytoplasmic sperm injection) procedure [3]. The frequent reasons for unsuccessful ART outcome are poor quality of germ cells or embryos, genital tract infections, uterine pathologies or endometrial receptivity or unsuitable ART method [4–10].
Some studies have indicated that OxS (oxidative stress) may appear as a possible reason for poor ART outcome. High seminal ROS (reactive oxygen species) level is associated with impaired sperm fertilizing ability and lower pregnancy rates after IVF. Also, negative association has been observed with embryo development to the blastocyst stage after ICSI [11]. Oxidative DNA damage may impair embryo development, cause miscarriage and birth defects in the offspring [6, 12]. Reason for elevated OxS level may be a general or reproductive disease [13].
Since OxS is a possible cause of poor ART outcome, there is a need for introducing inexpensive and effective assays to identify OxS that can be easily conduct in any infertility clinic. To date, one of the most common markers for assessment of systemic OxS is 8-isoprostanes (8-iso Prostaglandin F2α or 8-EPI), the byproducts of lipid peroxidation that is excreted by urine [14]. 8-hydroxy-2’-deoxyguanosine (8-OHdG) is a common marker of oxidative DNA damage [15].
Aim of this research was to detect OxS levels (applying detection of 8-EPI and 8-OhdG) in couples attending ART procedure as well as to examine the relationship between OxS level, clinical parameters of both partners and also ART outcome.
Material and methods
Study group
A total 79 couples undergoing either IVF (58 couples) or ICSI (21 couples) procedure at Nova Vita Clinic (Tallinn, Estonia) in 2011–2012 were enrolled. Before ART procedure was carried out, sexually transmitted infections (Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Trichomonas vaginalis) were tested and treated in Nova Vita Clinic. The presence of ureaplasmas (Ureaplasma urealyticum, Ureaplasma parvum), HSV (herpes simplex virus), HCV (hepatitis C virus) and Treponema pallidum antibodies were also detected. Gram stained vaginal smears were microscopically examined to assess vaginal candidiasis and bacterial vaginosis [16]. The analysis of semen was performed according to WHO guidelines [17]. Inflammatory prostatitis was assessed by the neutrophil concentration in semen applying the WHO normative 106 WBC/ml [18] as well as lowered level of neutrophils 0,2 × 106 WBC/ml [19].
None of the subjects had used prostaglandins during the last week. Table S1 describes the study group and represents the lifestyle and clinical background of both partners.
Sample collection for biochemical analyses
The samples were collected in Nova Vita Clinic on the day of follicular puncture. Urine samples of both partners were collected into a sterile collection tube and frozen at −20 °C within 1 h. Semen samples were obtained after urinating and were self-collected into a sterile collection tubes in a private room after washing the glans penis with warm water. After ejaculation, the semen was incubated at 37 °C for 25–45 min for liquefaction, seminal plasma was obtained by centrifugation of semen 3000 g × 15 min and frozen at −20 °C.
Biochemical analyses
Oxidative DNA damage (8-OHdG) and lipid peroxidation (8-EPI) levels were detected from different body fluids: 8-OHdG from seminal plasma, women’s urine and 8-EPI from urine of both partners.
The level of systemic OxS marker 8-EPI was detected in urine from all patients (79 men and 79 women) enrolled in this study using the previously described method [20]. This assay is a competitive enzyme-linked immunoassay (ELISA) for determining levels of 8-EPI in biological samples (BIOXYTECH 8-IsoprostaneAssay, Cat. No. 21019; Oxis International, Inc., Portland, OR, USA). The urinary concentrations of isoprostanes were corrected by urinary creatinine concentrations to account for the differences in renal excretory function.
Local OxS level was measured by using oxidative DNA damage marker 8-OHdG in seminal plasma. Furthermore, to assess the systemic OxS, 8-OHdG was also detected in urine from 50 couples. 8-OHdG was detected by using a commercial ELISA kit and following the manufacturer’s instructions (Trevigen HT 8-oxo-dG ELISA Kit II, Cat. No. 4380-192-K; Trevigen, Inc., Gaithersburg, MD, USA).
Statistical analysis
Statistical analyses were performed with the use of SigmaPlot 12.0 (Systat Software Inc, IL, USA) (Jandel Scientific, San Rafael, CA, USA) and Excel (Microsoft Corp., Redmond, OR, USA) software programs. The study groups were compared with t-test (in case of normal distribution) and Mann–Whitney rank sum test (in case of nonparametric distribution) as well as Fisher Exact test. In addition, a multiple regression model was constructed to uncover the significant effects of known clinical parameters and oxidative stress level on ART outcome. Stata IC/12.1 (StataCorp LP, USA) software program was used for correlation analyses and 2-Way ANOVA. Statistical significance was assumed at p < 0.05 level for all parameters.
Ethical considerations
Participation in the study was voluntary. Informed written consent was obtained from the patients. The study was approved by the Ethics Review Committee on Human Research of the University of Tartu.
Results
Clinical data of the study subjects are presented in Table S1. Sperm motility and semen volume were normal in all 79 male partners while sperm concentration remained below the WHO limit in 4 men and neutrophil concentration in semen was elevated in 23 male partners. 55 women had been pregnant, previous IVF procedures were done for 34 women and ICSI for 9 women. Twelve men of 79 had at least one child with current partner, 11 men with previous sexual partner and one man with both partners (Table S1).
8-EPI and 8-OHdG levels in infertile couples
8-EPI mean value among all 79 women was higher compared to male partners (p = 0.009; Table 1). A strong positive correlation in systemic OxS marker 8-EPI levels among couples was found (r = 0.42, p < 0.001), indicating a significant association between the partners’ organisms.
Table 1.
OxS levels in urine and seminal plasma
| Systemic OxS | Local OxS | ||
|---|---|---|---|
| Urine (women) | Urine (men) | Seminal plasma | |
| 8-EPI (ng/mmol creatinine) | 78.2 (±23.1)1 | 69.6 (±21.0)1 | |
| 8-OHdG (nM) | 434.3 (±200.0) | 429.1 (±142.1) | 296.9 (±128.3) |
1 p = 0.009
8-EPI (8-iso Prostaglandin F2α), 8-OHdG (8-hydroxy-2’-deoxyguanosine)
The data are presented as mean (±SD)
The urinary levels of 8-OHdG were detected in 50 couples
The mean values of 8-OHdG level are shown in Table 1. Unlike with 8-EPI, urinary 8-OHdG levels between men and women were not statistically significant. A positive correlation between urinary 8-OHdG and 8-EPI in female partners was seen (ρ = 0.32, p < 0.022). Systemic oxidative DNA damage (8-OHdG, measured in urine) was significantly higher compared to local oxidative DNA damage, measured in seminal plasma (p < 0,001).
Association between clinical background and OxS markers
We revealed association between women’s clinical background and oxidative DNA damage (8-OHdG; Table S2). Women with mycoplasmosis, candidiasis, salpingo-oophoritis, endometriosis and/or uterine myoma had higher 8-OHdG level compared to the women without known diseases. Men whose partners had genital tract infections, showed significantly higher 8-OHdG levels compared to men, whose partners were without known diseases. Subsequent 2-Way ANOVA analysis confirmed that men, whose sexual partner had bacterial vaginosis, had significantly higher oxidative DNA damage in seminal plasma due to the women’s state of health (p < 0.0005). Furthermore, men whose partners had salpingo-oophoritis had also higher lipid peroxidation (8-EPI) levels compared to men, whose partners were without known diseases.
Table S3 shows OxS levels of both partners according to the male partner’s state of health. Men who had infections (prostatitis, inflammation of foreskin or glans penis, others) showed significantly higher levels of 8-EPI compared to normozoospermic men without infections. Furthermore, women with infectious partners had significantly higher 8-EPI levels compared to women whose male partners did not have infections. Men with infections had also higher 8-OHdG levels compared to men without known diseases (p = 0,007).
Seminal plasma 8-OHdG level was higher among smokers compared to non-smokers (344,2 ± 193,7 vs 282,7 ± 99,6, p = 0,037), similar tendency was revealed for 8-EPI level (77,9 ± 24,3 vs 67,2 ± 19,8, p = 0,029).
Association between ART outcome and OxS markers
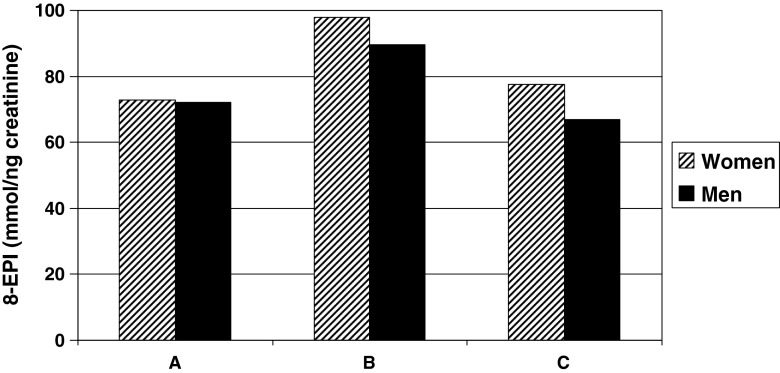
After collecting the study material for current research, ART procedures (IVF or ICSI) were performed. No pregnancies after IVF or ICSI procedures occurred among 63.3 % of patients (n = 50), 8.8 % (n = 7) of pregnancies were detectable only by pregnancy test (biochemical method) and 27.9 % (n = 22) of pregnancies were detectable also by ultrasound (clinical method). The lowest 8-EPI levels were seen when pregnancy was detectable by clinical method (Fig. 1). We also found that the 8-EPI levels were below the suggested reference level (50 ng/mmolCr) in 23 % of the women with clinical pregnancy while only in 7 % in the other women (p = 0.10, Fisher exact test). A multiple regression analysis was subsequently performed to uncover the effects of clinical parameters and oxidative stress level on ART outcome. The association between OxS and ART outcome was confirmed in case of both female (p = 0.018) and male partners (p = 0.022). It also revealed association of ART outcome with some clinical parameters of female partners (ovulation cycle disturbances, p = 0.013; smoking, borderline significance p = 0.060) and male partners (age, p = 0.005; chronic diseases in anamnesis, p = 0.027; myco- or ureaplasmosis in anamnesis, p = 0.011; prostatitis, borderline significance p = 0.099).
Fig. 1.
Association of ART outcome with lipid peroxidation levels (8-EPI) in couples. a – pregnancy was detected by both methods (n = 22), b – pregnancy was detected by biochemical method only (n = 7), c – no pregnancy (n = 50). 8-EPI (8-iso Prostaglandin F2α), ART (assisted reproductive technique)
Discussion
Our study uncovered negative effect of both partners’ oxidative stress on maintenance and outcome of pregnancy. A strong positive correlation between the partners’ OxS levels was seen. We also showed that both OxS markers – 8-OHdG in seminal plasma and female urine, and urinary 8-EPI levels of both partners reflected the clinical conditions very well, therefore both can be considered as suitable markers for estimating the OxS in infertile couples, and 8-EPI may have a potential for predicting ART success rates.
A net of pro-oxidants and the potency of an antioxidant defense system are normally balanced in the body. Low levels of reactive oxygen species (ROS) are needed for maintaining several biofunctions like intracellular messaging, growth, cellular differentation, phagocytosis and immune response while ROS abundance may have deleterious effects on cellular functions. Any imbalance in favour of the pro-oxidants potentially leading to damage of biomolecules has been termed ‘oxidative stress’ [21–24]. The correct balance between ROS and antioxidants has an important role also in reproductive processes such as folliculogenesis, ovarian steroidogenesis, oocyte maturation, ovulation, corpus luteum formation and function, luteolysis, germ cell function, embryogenesis, embryonic implantation, maintenance of pregnancy and beginning of parturition. OxS is known to be one of the possible factors causing infertility by affecting oocyte quality, fertilization, early embryo development, implantation and pregnancy rates [25–28]. Endometriosis has been shown to be associated with higher lipid peroxidation and DNA damage [29] while bacterial vaginosis with high grade OxS levels and adverse pregnancy outcome [30]. OxS-related DNA damage has been identified as a major contributor to poor ART outcomes, including impaired embryo development, miscarriage and birth defects in the offspring [6, 12].
In male reproductive tract, small quantities of ROS have also important role in sperm functioning by regulating capacitation, acrosome reaction, hyperactivation and fusion with the oocyte [31, 32]. At the same time high-grade OxS is associated with reduction of sperm quality and the level of antioxidants in seminal and blood plasma are significantly lower among infertile men compared to fertile men [15, 33]. Mammalian spermatozoa are particularly vulnerable to peroxidative damage, because they are endowed with more of polyunsaturated fatty acids (that are highly vulnerable to free radical attack), superoxide anion generating system in their membranes, less antioxidants and less repair capacity [34]. Oxidative DNA damage in human spermatozoa is known to be associated with a variety of adverse clinical outcomes affecting both reproductive efficiency and the health and wellbeing of the offspring [4, 35].
Encouragingly, the involvement of OxS in the aetiology of infertility has opened up new opportunities for therapeutic interventions involving the judicious administration of antioxidants. Antioxidant therapy is used as an alternative to expensive ART procedures or to improve ART outcome and improve natural fertility [36]. Antioxidant therapy includes either oral consumption or in vitro addition of antioxidants during ART procedure. While choosing antioxidant therapy, the dual function of ROS in the body must be taken into account. It should be also considered that the antioxidants work together as integrated antioxidative defence network system. Current antioxidant treatments, given irrespectively of clinically quantified deficiencies, may be potentially detrimental and overexposure to them is risky [37].
To get more information about the need and justification of antioxidant therapy we aimed to detect the level of OxS in both partners attending ART procedure. Oxidative DNA damage marker 8-OHdG was measured in urine and seminal plasma, and 8-EPI, marker of lipid peroxidation was measured in urine. The suggested reference values for urine 8-EPI are 10–50 ng/mmolCr while in case of serious infections these values are typically more than 100 ng/mmolCr. Our study subjects were not fully healthy but consecutive, thus having different health problems and underlying diseases that could contribute to their OxS levels (Table S1), therefore the mean values (78 for women, 69 for men) seem to be consistent.
We found higher level oxidative DNA damage in women with genital tract infections, candidiasis, salpingo-oophoritis, endometriosis and uterine myoma in comparison with the rest of women. Also the polymicrobial condition bacterial vaginosis showed association with higher 8-OHdG levels. According to the literature, OxS is common in women having candida-vaginitis. This fungal pathogen uses a complicated antioxidant defense mechanism to survive in high grade OxS environment [38]. Increased OxS levels were associated also with male genital tract inflammation as has been revealed also by previous studies [39, 40]. Other studies have shown that smokers have higher level OxS [41] and that was revealed also in our study. This is one of the mechanisms by which smoking may have an adverse impact on fertility and also ART outcome. In fact, many other factors may influence ART outcome and our study could not cover all of them, however, Table S1 describes many possible factors, both from female and male side.
Very interesting is the finding that OxS levels in men and women are in correlation and reflect the clinical conditions of both partners, indicating a significant bond between the partner’s organisms. The influence of partner’s state of health on OxS level in women organism has been previously described in one investigation only indicating that sexual intercourse with male partner having leukocytospermia increased the OxS level in women’s organism [42]. Another earlier study showed that sexual intercourse with men having prostatitis had an influence on the women’s genital tract microbiota [43]. In the present study we noted that the women whose sexual partner had prostatitis, inflammation of the foreskin or glans penis, had significantly higher 8-EPI levels compared with women whose men were without known diseases. An opposite relationship seems to exist also – men whose partners had genital tract infections, candidiasis, bacterial vaginosis or salpingo-oophoritis had higher 8-OHdG levels in seminal plasma compared to men whose partners were without known diseases. To date, there are no previous studies on the effects of vaginal microbiota on her partner, hence, supportive studies would be indispensable in the future.
Our most interesting finding was the association between ART outcome and 8-EPI levels of both partners: when pregnancy was detected by clinical method (in addition to biochemical method), the level of 8-EPI was significantly lower in both partners. Thus, high grade systemic OxS may negatively affect the maintenance and outcome of pregnancy. According to the literature, the quality of embryos that were achieved after ICSI procedure was higher in case of lower urinary 8-EPI levels and greater amount of antioxidants in blood plasma [44].
The weakness of current study was the absence of fertile control group and small study groups. As there are large number of markers and a lot of different technologies available for detecting OxS level in different body fluids, the confirmatory studies with bigger study group are necessary.
Conclusions
Oxidative stress levels in male and female partners’ organisms are significantly associated. Both 8-OHdG and 8-EPI are suitable markers for estimating the rate of OxS in infertile couples. High grade systemic OxS in both partners affects negatively the maintenance of pregnancy, therefore its testing in ART patients may select the patients with higher success rate and/or those who need antioxidant therapy. This would all in all lead to improvement of ART outcome as well as natural fertility.
Electronic supplementary material
(DOC 88 kb)
(DOC 42 kb)
(DOC 32 kb)
Acknowledgments
The present study was supported by Enterprise Estonia (Grant no. EU 30020), Estonian Ministry of Education and Research (Target Financing SF0180132s08, Institutional Research Funding IUT 20–42, Institutional Research Funding IUT 15–19 and Scientific Collection Financing KOGU-HUMB) and University of Tartu (Grant no. SARMBARENG).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Capsule
High grade systemic oxidative stress in both partners affects negatively the maintenance of pregnancy, therefore its testing in ART patients may select the patients with higher success rate and/or those who need antioxidant therapy.
References
- 1.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology. Fertil Steril. 2009;92:1520–4. [DOI] [PubMed]
- 2.National Health and Medical Research Council. Ethical guidelines on assisted reproductive technology. Available at: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/e28.pdf. Accessed 2 Feb 2014.
- 3.Ferraretti AP, Goossens V, de Mouzon J, Bhattacharya S, Castilla JA, Korsak V, et al. Assisted reproductive technology in Europe, 2008: results generated from European registers by ESHRE. Hum Reprod. 2012;27:2571–84. doi: 10.1093/humrep/des255. [DOI] [PubMed] [Google Scholar]
- 4.Loft S, Kold-Jensen T, Hjollund NH, Giwercman A, Gyllemborg J, Ernst E, et al. Oxidative DNA damage in human sperm influences time to pregnancy. Hum Reprod. 2003;18:1265–72. doi: 10.1093/humrep/deg202. [DOI] [PubMed] [Google Scholar]
- 5.Schulte RT, Ohl DA, Sigman M, Smith GD. Sperm DNA damage in male infertility: etiologies, assays, and outcomes. J Assist Reprod Genet. 2010;27:3–12. doi: 10.1007/s10815-009-9359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon L, Proutski I, Stevenson M, Jennings D, McManus J, Lutton D, et al. Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod BioMed Online. 2013;26:68–78. doi: 10.1016/j.rbmo.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Knox CL, Allan JA, Allan JM, Edirisinghe WR, Stenzel D, Lawrence FA, et al. Ureaplasma parvum and Ureaplasma urealyticum are detected in semen after washing before assisted reproductive technology procedures. Fertil Steril. 2003;80:921–9. doi: 10.1016/S0015-0282(03)01125-7. [DOI] [PubMed] [Google Scholar]
- 8.Li MQ, Jin LP. Ovarian stimulation for in vitro fertilization alters the protein profile expression in endometrial secretion. Int J Clin Exp Pathol. 2013;6:1964–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF–ET. Hum Reprod. 2006;2:3036–43. doi: 10.1093/humrep/del305. [DOI] [PubMed] [Google Scholar]
- 10.Fiedler K, Ezcurra D. Predicting and preventing ovarian hyperstimulation syndrome (OHSS): the need for individualized not standardized treatment. Reprod Biol Endocrinol. 2012;10:32. doi: 10.1186/1477-7827-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zorn B, Vidmar G, Meden-Vrtovec H. Seminal reactive oxygen species as predictors of fertilization, embryo quality and pregnancy rates after conventional in vitro fertilization and intracytoplasmic sperm injection. Int J Androl. 2003;26:279–85. doi: 10.1046/j.1365-2605.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 12.Meseguer M, Martínez-Conejero JA, O’Connor JE, Pellicer A, Remohí J, Garrido N. The significance of sperm DNA oxidation in embryo development and reproductive outcome in an oocyte donation program: a new model to study a male infertility prognostic factor. Fertil Steril. 2008;89:1191–9. doi: 10.1016/j.fertnstert.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montuschi P, Barnes P, Roberts LJ. Insights into oxidative stress: the isoprostanes. Curr Med Chem. 2007;14:703–17. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- 15.Cambi M, Tamburrino L, Marchiani S, Olivito B, Azzari C, Forti G, et al. Development of a specific method to evaluate 8-hydroxy, 2-deoxyguanosine in sperm nuclei: relationship with semen quality in a cohort of 94 subjects. Reproduction. 2013;145:227–35. doi: 10.1530/REP-12-0404. [DOI] [PubMed] [Google Scholar]
- 16.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO . WHO laboratory manual for the examination and processing of human semen. 5. Geneva: World Health Organization; 2010. [Google Scholar]
- 18.WHO, editor. Laboratory manual for examination of human semen and sperm–cervical mucus interaction. 4. New York: Cambridge University Press; 1999. [Google Scholar]
- 19.Punab M, Lõivukene K, Kermes K, Mändar R. The limit of leucocytospermia from the microbiological viewpoint. Andrologia. 2003;35:271–8. doi: 10.1111/j.1439-0272.2003.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 20.Kullisaar T, Songisepp E, Mikelsaar M, Zilmer K, Vihalemm T, Zilmer M. Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenecity in human subjects. Br J Nutr. 2003;90:449–56. doi: 10.1079/BJN2003896. [DOI] [PubMed] [Google Scholar]
- 21.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. New York: Oxford University Press; 1999. [Google Scholar]
- 22.Sies H. Oxidative stress: oxidants and antioxidants. London: Academic; 1991. [DOI] [PubMed] [Google Scholar]
- 23.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–79. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 24.Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A, et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–56. [PubMed] [Google Scholar]
- 25.Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42:1634–50. doi: 10.1016/j.biocel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Combelles CMH, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod BioMed Online. 2009;18:864–80. doi: 10.1016/S1472-6483(10)60038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizzo A, Roscino MT, Binetti F, Sciorsci RL. Roles of reactive oxygen species in female reproduction. Reprod Domest Anim. 2011;47:344–52. doi: 10.1111/j.1439-0531.2011.01891.x. [DOI] [PubMed] [Google Scholar]
- 28.Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25:287–99. doi: 10.1016/j.bpobgyn.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma I, Dhaliwal L, Saha S, Sangwan S, Dhawan V. Role of 8-iso-prostaglandin F2alpha and 25-hydroxycholesterol in the pathophysiology of endometriosis. Fertil Steril. 2010;94:63–70. doi: 10.1016/j.fertnstert.2009.01.141. [DOI] [PubMed] [Google Scholar]
- 30.Bogavac M, Lakic N, Simin N, Nikolic A, Sudji J, Bozin B. Bacterial vaginosis and biomarkers of oxidative stress in amniotic fluid. J Matern Fetal Neonatal Med. 2012;25:1050–4. doi: 10.3109/14767058.2011.614660. [DOI] [PubMed] [Google Scholar]
- 31.Kothari S, Thompson A, Agarwal A, du Plessis SS. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol. 2010;48:425–35. [PubMed] [Google Scholar]
- 32.de Lamirande E, Leclerc P, Gagnon C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol Hum Reprod. 1997;3:175–94. doi: 10.1093/molehr/3.3.175. [DOI] [PubMed] [Google Scholar]
- 33.Benedetti S, Tagliamonte MC, Catalani S, Primiterra M, Canestrari F, De Stefani S, et al. Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod BioMed Online. 2012;25:300–6. doi: 10.1016/j.rbmo.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Aitken RJ, Jones KT, Robertson SA. Reactive oxygen species and sperm function — in sickness and in health. J Androl. 2012;33:1096–106. doi: 10.2164/jandrol.112.016535. [DOI] [PubMed] [Google Scholar]
- 35.Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod. 2010;25:2415–26. doi: 10.1093/humrep/deq214. [DOI] [PubMed] [Google Scholar]
- 36.Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011;26:1628–40. doi: 10.1093/humrep/der132. [DOI] [PubMed] [Google Scholar]
- 37.Ménézo Y, Entezami F, Lichtblau I, Belloc S, Cohen M, Dale B. Oxidative stress and fertility: incorrect assumptions and ineffective solutions? Zygote. 2014;22(1):80–90. doi: 10.1017/S0967199412000263. [DOI] [PubMed] [Google Scholar]
- 38.Frohner IE, Bourgeois C, Yatsyk K, Majer O, Kuchler K. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol Microbiol. 2009;71:240–52. doi: 10.1111/j.1365-2958.2008.06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kullisaar T, Türk S, Punab M, Mändar R. Oxidative stress – cause or consequence of male genital tract disorders? Prostate. 2012;72:977–83. doi: 10.1002/pros.21502. [DOI] [PubMed] [Google Scholar]
- 40.Kullisaar T, Türk S, Punab M, Korrovits P, Kisand K, Rehema A, et al. Oxidative stress in leucocytospermic prostatitis patients: preliminary results. Andrologia. 2008;40:161–72. doi: 10.1111/j.1439-0272.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 41.Taha EA, Ez-Aldin AM, Sayed SK, Ghandour NM, Mostafa T. Effect of smoking on sperm vitality, DNA integrity, seminal oxidative stress, zinc in fertile men. Urology. 2012;80:822–5. doi: 10.1016/j.urology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Mändar R, Kullisaar T, Borovkova N, Punab M. Sexual intercourse with leucocytospermic men may be a possible booster of oxidative stress in female partners of infertile couples. Andrology. 2013;1:464–8. doi: 10.1111/j.2047-2927.2012.00052.x. [DOI] [PubMed] [Google Scholar]
- 43.Borovkova N, Korrovits P, Ausmees K, Türk S, Jõers K, Punab M, et al. Influence of sexual intercourse on genital tract microbiota in infertile couples. Anaerobe. 2011;17:414–8. doi: 10.1016/j.anaerobe.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 44.Velthut A, Zilmer M, Zilmer K, Kaart T, Karro H, Salumets A. Elevated blood plasma antioxidant status is favourable for achieving IVF/ICSI pregnancy. Reprod BioMed Online. 2013;26:345–52. doi: 10.1016/j.rbmo.2012.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 88 kb)
(DOC 42 kb)
(DOC 32 kb)