Abstract
Objectives
MTHFR C677T and A1298C have been associated with the risk of preeclampsia (PE), but with conflicting results. We performed this meta-analysis to derive a more precise estimation of the association between MTHFR polymorphisms and PE.
Study design
An electronic search of PubMed and Chinese Biomedicine database was conducted to select studies for meta-analysis. 54 case controlled studies containing MTHFR C677T and A1298C gene polymorphisms were chosen, and odds ratio (OR) with confidence interval (CI) was used to assess the strength of this association.
Result
These studies evaluated 7398 cases and 11230 controls for MTHFR C677T. The overall results suggested that MTHFR C677T was associated with the risk of PE. (T vs. C: OR = 1.157, 95 % CI: 1.057-1.266, p=0.002; TT+CT vs. CC: OR=1.165, 95 % CI : 1.049-1.293, P = 0.004; TT vs. CT + CC: OR = 1.371, 95 % CI: 1.153-1.63, p < 0.001). We also evaluated 1103 cases and 988 controls for MTHFR A1298C but could not demonstrate an increased risk of PE for this polymorphism (p=0.667). A symmetric funnel plot, the Egger’s test (p = 0.819) suggested a lack of publication bias.
Conclusion
This meta-analysis supports the idea that MTHFR C677T genotype is associated with increased risk for PE, especially in the case of Asians and Caucasians.
Keywords: MTHFR C677T, A1298C, Polymorphism, Preeclampsia, Meta-analysis
Introduction
Preeclampsia (PE), characterized by the presence of a triad of signs involving high blood pressure, proteinuria and oedema after the 20th week of pregnancy, is one of the commonest and most serious complications of pregnancy [1]. This disease can progress to eclampsia (characterized by seizures as a sign of affection of the cerebral vessels), HELLP syndrome (hemolysis, elevated liver enzyme, low platelets) or disseminated intravascular coagulation. PE affects about 5–8 % of pregnancies, and it is still responsible for 10 to 15 % of maternal mortality [2, 3]. Although preeclampsia remains a significant source of maternal and perinatal mortality and morbidity, its etiology is not yet elucidated.
Nowadays, an association between hyperhomocysteinemia and preeclamptic patients has been reported [4–7]. Hyperhomocysteinemia lead to vascular and metabolic changes which have been associated as an established risk factor for endothelial disorders, such as arteriosclerosis and coronary artery disease, however, the underlying mechanisms remain unknown [8]. In previous studies, homocysteine concentration is increased in preeclampsia and it weakly and negatively correlates with plasma folate concentration [9, 10]. The increasing of homocysteine concentration in preeclampsia may be due to a C677T polymorphism in the MTHFR results in a reduced MTHFR enzyme activity, and subsequently elevated homocysteine levels.
The human MTHFR gene contains 11 exons, located on chromosome 1p36.3, and encodes methylenetetrahydrofolate reductase (MTHFR) key enzyme in folate and homocysteine metabolism. MTHFR catalyzes the biologically irreversible reduction of 5, 10-methylenetetrahydrofolate to 5-methyltetrahydrofolate. Folate is important as the substrate for 5-methyltetrahy, which acts as a methyl donor for the B12-dependent remethylation of homocysteine to methionine via the methionine synthase reaction. In the MTHFR enzyme, several single nucleotide polymorphisms including the two most important C677T and A1298C can affect folate and total homocysteine (tHcy) status.
Women with the MTHFR C677T and A1298C mutations displayed higher plasma HCy levels as compared to controls with normal genotype [11–13]. The MTHFR C677T, which involves a cytosine (C) to a thymine (T) substitution at position 677, changes an alanine to a valine in the enzyme. The C677T substitution increases thermolability of MTHFR and causes impaired folate binding and reduced activity of the MTHFR enzyme [14]. MTHFR C677T results in an increased requirement for folic acid to maintain normal homocysteine remethylation to methionine. MTHFR C677T is associated with decreased concentrations of folate in serum, plasma, and red blood cells , and mildly increased plasma total homocysteine (tHcy) concentration [15].
Because of the mild hyperhomocysteinaemia found in women with preeclampsia [9, 10], the MTHFR C677T polymorphism could be a genetic factor contributing to the pathophysiology preeclampsia. In preeclampsia, a strong heritable component has been demonstrated: women born of a preeclamptic pregnancy are themselves at increased risk of preeclampsia in their own pregnancies; men born of a preeclamptic pregnancy have an increased risk of fathering a preeclamptic pregnancy [16]. Genetic predisposition plays an important role in the development of preeclampsia, but attempts to show associations between MTHFR C677T and preeclampsia have produced widely divergent results [6, 13, 17–41]. Thus in the present study, we conducted a meta-analysis to quantitatively assess the associations between the MTHFR polymorphisms and preeclampsia.
Materials and methods
Publication search
We searched the PubMed and Chinese biomedicine databases for all articles on the association between MTHFR C677T/A1298C and preeclampsia risk(last search update,July 7,2014). The following key words were used :‘MTHFR ’ , ‘C677T’, ‘A1298C ’, ‘polymorphism’ and ‘preeclampsia’ or ‘pre-eclampsia ’. Case–control studies containing available genotype frequencies of C677T were chosen. Preeclampsia was defined as the development of hypertension and proteinuria (>300 mg urinary protein in 24 h) in women with no baseline proteinuria. Hypertension was defined as blood pressure ≥ 140/90 mmHg. Maternal age ranged from18 to 44 years. The control group comprised women with uncomplicated pregnancy admitted for natural childbirth or caesarean section, with normal-length pregnancy, blood pressure ≤120/ 80 mmHg, and without proteinuria. Of the studies with overlapping data published by the same author, only the most recent or complete study was included in this meta-analysis.
Statistic analysis
The genotype distribution of the control group was evaluated for agreement with the hardy-Weinberg equilibrium (HWE) using the χ2 test with a significant level of 0.05. Odds ratios (OR) with 95 % CIs were used to determine the strength of association between the MTHFR polymorphisms and PE risk. The pooled ORs for the risk associated with the MTHFR C677T genotype, additive genetic model (T vs. C), dominant model (TT + CT vs. CC), and recessive model (TT vs. CT+ CC) respectively. For MTHFR A1298C, the pooled ORs were performed for additive genetic model (C vs. A), dominant model (CC + CA vs. AA), and recessive model (CC vs. CA+ AA) respectively. Subgroup analyses were done by ethnicity. Heterogeneity assumption was evaluated by a chi-square based Q-test. A p value greater than 0.05 for the Q test indicated a lack of heterogeneity among the studies. Thus, the pooled OR estimate of each study was calculated by the fixed-effects model. Otherwise, the random-effects model was used [42, 43]. An estimate of the potential publication bias was examined by a Begg’s test (funnel plot method) and Egger’s linear regression test (P < 0.05 considered representative of statistical significance) [44]. All analyses were performed using Stata software (version 8.2; Stata Corporation, College Station, TX).
Result
Eligible studies
In this meta-analysis, we identified 54 studies on the association between MTHFR gene polymorphisms and preeclampsia (Fig. 1), including 7398/11222 cases/controls for MTHFR C677T (Table 1) and 1103 /988 cases/controls for MTHFR A1298C (Table 3, Table 4). The distribution of genotypes in the controls of the studies was in agreement with Hardy–Weinberg equilibrium, except for four studies [11, 33, 45, 46]. The search results were combined and duplicates were removed.
Fig. 1.
Flow chart of the literature search and article selection
Table 1.
The distribution of the MTHFR C677T genotypes for cases and controls
| Author | Publication year |
Country | Ethnicity | Case | Control | Pa | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | CC | CT | TT | |||||
| Chedraui, P.[11] | 2014 | Ecuador | Caucasian | 59 | 73 | 18 | 47 | 91 | 12 | 0 |
| Coral-Vazquez, R. M.[56] | 2013 | Mexico | Latino | 38 | 109 | 83 | 71 | 166 | 115 | 0.43 |
| Lykke, J. A[60] | 2012 | Denmark | Caucasian | 113 | 118 | 31 | 906 | 793 | 143 | 0.09 |
| Dissanayake, V. H.[61] | 2012 | Sri Lanka | Asian | 136 | 36 | 3 | 142 | 27 | 2 | 0.58 |
| Klai, S.[45] | 2011 | Tunisia | Caucasian | 22 | 20 | 2 | 61 | 39 | 0 | 0.02 |
| Mislanova, C.[12] | 2011 | Austria | Caucasian | 12 | 11 | 5 | 21 | 17 | 2 | 0.54 |
| Aggarwal, S.[62] | 2011 | India | Asian | 160 | 33 | 7 | 134 | 58 | 8 | 0.59 |
| Sun [55] | 2011 | China | Asian | 32 | 22 | 22 | 331 | 154 | 28 | 0.08 |
| Stiefel, P.[63] | 2009 | Spain | Caucasian | 157a | – | 27 | 113 | – | 21 | – |
| Shen, X.N[31] | 2009 | China | Asian | 12 | 35 | 14 | 30 | 21 | 9 | 0.12 |
| Wang, S.M[46] | 2008 | China | Asian | 6 | 19 | 17 | 13 | 40 | 11 | 0.04 |
| Zhang, X.Y[64] | 2008 | China | Asian | 22 | 21 | 7 | 29 | 8 | 3 | 0.05 |
| Muetze, S.[65] | 2008 | German | Caucasian | 30 | 34 | 7 | 35 | 29 | 15 | 0.06 |
| Canto, P.[18] | 2008 | Mexico | Latino | 36 | 66 | 23 | 61 | 131 | 82 | 0.53 |
| Stonek, F.[66] | 2007 | Austria | Caucasian | 9 | 14 | 2 | 669 | 573 | 155 | 0.06 |
| Nagy, B.[67] | 2007 | Hungary | Caucasian | 71 | 68 | 25 | 32 | 35 | 5 | 0.27 |
| Zhang, Z.H[54] | 2007 | China | Asian | 12 | 21 | 20 | 10 | 30 | 9 | 0.12 |
| Dusse, L. M.[68] | 2007 | Brazil | Latino | 16 | 12 | 2 | 46 | 31 | 6 | 0.81 |
| Jaaskelainen, E.[69] | 2006 | Finland | Caucasian | 78 | 43 | 12 | 64 | 42 | 6 | 0.79 |
| Demir, S. C.[70] | 2006 | German | Caucasian | 19 | 29 | 8 | 43 | 47 | 12 | 0.88 |
| Dalmaz, C. A.[19] | 2006 | Brazil | Latino | 31 | 27 | 17 | 76 | 51 | 18 | 0.05 |
| Mello, G.[71] | 2005 | Italy | Caucasian | 729a | – | 79 | 793 | – | 15 | – |
| Driul, L.[72] | 2005 | Italy | Caucasian | 34 a | – | 5 | 57 | – | 7 | – |
| Davalos, I. P.[20] | 2005 | Mexico | Latino | 13 | 14 | 6 | 24 | 27 | 11 | 0.48 |
| Also-Rallo, E.[26] | 2005 | Spain | Caucasian | 78 | 59 | 20 | 63 | 75 | 19 | 0.64 |
| Yilmaz, H.[73] | 2004 | Turkey | Caucasian | 29 | 28 | 7 | 24 | 17 | 6 | 0.30 |
| Williams, M. A[74] | 2004 | Peru | Latino | 37 | 61 | 25 | 62 | 85 | 30 | 0.92 |
| Perez-Mutul, J.[75] | 2004 | Mexico | Latino | 33 | 66 | 49 | 36 | 80 | 61 | 0.30 |
| Pegoraro, R. J.[36] | 2004 | South Africa | Asian | 464 | 76 | 2 | 298 | 38 | 2 | 0.52 |
| De Maat, M. P.[41] | 2004 | Netherlands | Caucasian | 78 | 59 | 20 | 63 | 75 | 19 | 0.64 |
| Fabbro, D.[76] | 2003 | Italy | Caucasian | 44 a | – | 8 | 68 | – | 12 | – |
| Prasmusinto, D.[22] | 2002 | German | Caucasian | 7 | 7 | 1 | 12 | 15 | 7 | 0.57 |
| Prasmusinto, D.[22] | 2002 | Croatian | Caucasian | 11 | 12 | 2 | 18 | 15 | 5 | 0.51 |
| Prasmusinto, D.[22] | 2002 | Indonesian | Asian | 34 | 6 | 1 | 22 | 5 | 0 | 0.60 |
| Morrison, E. R.[29] | 2002 | Scotland | Caucasian | 169 | 193 | 42 | 81 | 66 | 17 | 0.52 |
| D’Elia, A. V.[77] | 2002 | Italy | Caucasian | 52 a | – | 6 | 65 | – | 9 | – |
| Watanabe, H.[78] | 2001 | Japan | Asian | 40 | 59 | 34 | 89 | 103 | 32 | 0.80 |
| Raijmakers, M. T.[30] | 2001 | Scotland | Caucasian | 72 | 74 | 21 | 205 | 162 | 36 | 0.62 |
| Livingston, J. C.[37] | 2001 | USA | Caucasian | 66 | 34 | 10 | 61 | 27 | 7 | 0.12 |
| Lachmeijer, A. M.[13] | 2001 | Netherlands | Caucasian | 66 | 94 | 14 | 58 | 51 | 11 | 0.96 |
| Kim, Y. J.[31] | 2001 | USA | Caucasian | 131 | 117 | 33 | 167 | 152 | 41 | 0.47 |
| Kaiser, T.[54] | 2001 | Australia | Caucasian | 71 | 66 | 19 | 37 | 31 | 11 | 0.29 |
| Alfirevic, Z.[64] | 2001 | UK | Caucasian | 56 a | – | 7 | 42 | – | 2 | – |
| Rigo, J., Jr.[33] | 2000 | Hungary | Caucasian | 46 | 66 | 8 | 42 | 53 | 6 | 0.04 |
| Rajkovic, A.[79] | 2000 | USA | Caucasian | 142 | 28 | 1 | 151 | 32 | 0 | 0.19 |
| Li, K.[80] | 2000 | China | Asian | 9 | 30 | 18 | 5 | 16 | 3 | 0.09 |
| Laivuori, H.[81] | 2000 | Finland | Caucasian | 64 | 45 | 4 | 56 | 40 | 7 | 0.97 |
| Kupferminc, M. J.[82] | 2000 | Israel | Caucasian | 48 a | – | 15 | 114 | – | 12 | – |
| Kobashi, G.[23] | 2000 | Japan | Asian | 25 | 40 | 8 | 83 | 99 | 33 | 0.7 |
| Kaiser, T.[83] | 2000 | Australia | Caucasian | 65 | 68 | 14 | 46 | 49 | 14 | 0.87 |
| Powers, R. W.[84] | 1999 | USA | Caucasian | 35 | 49 | 15 | 54 | 46 | 14 | 0.39 |
| O’Shaughnessy, K. M.[35] | 1999 | UK | Caucasian | 138 | 114 | 31 | 51 | 37 | 12 | 0.2 |
| Kupferminc, M. J.[23] | 1999 | Israel | Caucasian | 27 a | – | 7 | 101 | – | 9 | – |
| Chikosi, A. B.[85] | 1999 | South Africa. | Asian | 86 | 18 | 1 | 97 | 13 | 0 | 0.51 |
| Sohda, S.[5] | 1997 | Japan | Asian | 19 | 32 | 16 | 38 | 49 | 11 | 0.42 |
| Grandone, E.[6] | 1997 | Italy | Caucasian | 66 a | – | 28 | 105 | – | 24 | – |
aCC+CT
Table 3.
The distribution of the MTHFR A1298C variant for cases and controls
| Author | Publication year |
Country | Ethnicity | Case | Control | Pa | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| AA | AC | CC | AA | AC | CC | |||||
| Chedraui, P.[11] | 2014 | Ecuador | Caucasian | 100 | 27 | 23 | 110 | 39 | 1 | |
| Dissanayake, V. H.[61] | 2012 | SriLanka | Asian | 71 | 89 | 13 | 76 | 83 | 12 | |
| Klai, S.[45] | 2011 | Tunisia | Caucasian | 40 | 0 | 4 | 61 | 39 | 0 | |
| Pegoraro, R. J.[36] | 2004 | South Africa | Asian | 426 | 110 | 6 | 263 | 67 | 8 | |
| Lachmeijer, A. M.[13] | 2001 | Netherlands | Caucasian | 18 | 22 | 7 | 45 | 64 | 11 | |
| Kaiser, T.[83] | 2000 | Australia | Caucasian | 53 | 81 | 13 | 44 | 53 | 12 | |
Table 4.
ORs and 95 % CI for PE and the MTHFR A1298C polymorphism under different genetic models
| genetice model | population | pooled OR [95 % CI] p | Heterogeneity p-value |
Publication Bias | |
|---|---|---|---|---|---|
|
p-value Begg |
p-value Egger |
||||
| Additive (C vs. A) |
Caucasian | 1.074[0.642–1.798]0.785 | 0.004 | 0.497 | 0.374 |
| Asian | 0.988[0.795–1.229]0.917 | 0.399 | 0.317 | – | |
| Overall | 1.067[0.795–1.431]0.667 | 0.009 | 0.748 | 0.256 | |
| Dominant (C-carriers vs.AA) |
Caucasian | 0.821[0.417–1.619]0.57 | 0.004 | 0.042 | 0.037 |
| Asian | 1.023[0.788–1.327]0.865 | 0.5 | 0.317 | – | |
| Overall | 0.965[0.679–1.371]0.844 | 0.02 | 0.108 | 0 | |
| Recessive (CC vs. A-carriers) |
Caucasian | 3.755[0.748–18.852]0.108 | 0.001 | 0.174 | 0.068 |
| Asian | 0.759[0.335–1.717]0.507 | 0.217 | 0.317 | – | |
| Overall | 1.729[0.662–4.52]0.264 | 0.001 | 0.335 | 0 | |
Meta-analysis
Differences in allelic distribution by ethnicity could be partially responsible for the observed differences in the association between MTHFR C677T and preeclampsia. The results of the association between the MTHFR C677T polymorphism and preeclampsia and the heterogeneity test are shown in Table 2. The association was most pronounced for MTHFR C677T (Additive model: OR = 1.157, 95 % CI: 1.057-1.266, p=0.002; dominant model:OR = 1.165, 95 % CI : 1.049-1.293, p=0.004 ; Recessive model: OR = 1.371,95 % CI : 1.153-1.63, p < 0.001). The positive association was driven by a Caucasian recessive model (OR = 0.282, 95 % CI: 1.048-1.567,p = 0.015) and an Asian recessive model (OR = 2.078, 95 % CI: 1.368-3.157, p=0.001, Fig.2). For MTHFR A1298C gene polymorphism and its association with increased the risk for preeclampsia, the additive (p = 0.667), dominant (p = 0.844) and recessive models (p = 0.264) for MTHFR A1298C produced no significant associations overall.
Table 2.
ORs and 95 % CI for PE and the MTHFR C677T polymorphism under different genetic models
| genetice model | population | pooled OR [95 % CI] p | Heterogeneity p-value |
Publication Bias | |
|---|---|---|---|---|---|
|
p-value Begg |
p-value Egger |
||||
| Additive (T vs. C) |
Caucasian | 1.07[0.993–1.152]0.075 | 0.752 | 0.547 | 0.502 |
| Asian | 1.477[1.175–1.857]0.001 | P < 0.001 | 0.826 | 0.74 | |
| Latino | 1.026[0.797–1.32]0.844 | 0.038 | 0.188 | 0.53 | |
| Overall | 1.157[1.057–1.266]0.002 | P < 0.001 | 0.569 | 0.885 | |
| Dominant (T-carriers vs.CC) |
Caucasian | 1.099[0.995-1.213]0.063 | 0.41P < 0.001 | 0.378 | 0.921 |
| Asian | 1.465[1.086-1.977]0.013 | p<0.001 | 0.956 | 0.414 | |
| Latino | 1.081[0.876-1.333]0.469 | 0.411 | 0.881 | 0.947 | |
| Overall | 1.165[1.049-1.293]0.004 | p=0.004 P < 0.001 | 0.441 | 0.819 | |
| Recessive (TT vs. C-carriers) |
Caucasian | 1.282[1.048-1.567]0.015 | 0.002 | 0.865 | 0.553 |
| Asian | 2.078[1.368-3.157]0.001 | P = 0.001 | 0.547 | 0.394 | |
| Latino | 1.033[0.755-1.414]0.837 | 0.093 | 0.881 | 0.888 | |
| Overall | 1.371[1.153-1.63]p<0.001 | P < 0.001 | 1.000 | 0.383 | |
Fig. 2.
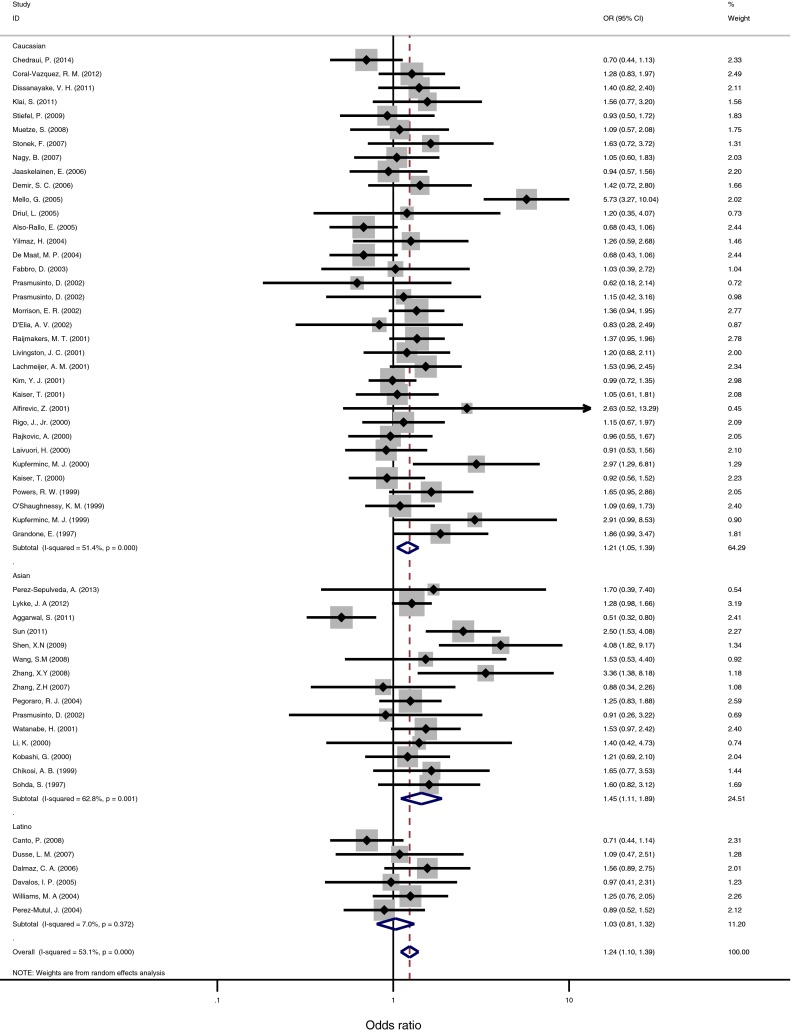
Forest plot of ORs of PE for T-carriers allele (TT + CT) when compared to the CC genotype. The squares and horizontal lines correspond to the study-specific OR and 95 % CI. The area of the squares reflects the study-specific weight. The diamond represents the pooled OR and 95 % CI
Publication bias
Funnel plot and Egger’s test were performed to quantitatively evaluate the publication bias of literatures on PE. The results of Egger’s test provided statistical evidence for funnel plot symmetry (P = 0.819) in overall results, suggesting the absence of publication bias.
Discussion
In this study, we investigated that MTHFR C677T polymorphism is positively related to PE risk. The frequency of MTHFR C677T was 1.371 times higher in case patients than in control patients. The association appeared to be stronger in Caucasian and Asian patients. However, the correlation was not found between MTHFR A1298C and PE. We speculated that MTHFR C677T might be an early marker for PE diagnosis.
The mechanism of PE is still relatively unclear. Some maternal metabolic disorders like diabetes and chronic hypertension probably contribute to the aberrant endothelial function observed in preeclampsia. On the other hand, some clinical studies have documented a familial tendency toward development of preeclampsia, suggesting a genetic factors could predispose women to develop it [47]. Hyperhomocysteinemia has also been described as a risk factor for PE and a promoter of endothelial dysfunction in preeclampsia [10, 48].
The meta-analysis examined the MTHFR gene polymorphisms C677T and A1298C and their relationship to the risk of PE. The frequency of T-carriers genotypes was found significantly higher among the women with PE than the control groups indicated that MTHFR C677T polymorphism would be expected to play a major role to bring about PE. Some studies reported significantly increased prevalence of MTHFR C677T among cases [22–24, 49]. In contrast, other studies reported an insignificant association between the MTHFR C677T and PE [19, 20, 25, 32, 36, 39, 50]. For MTHFR A1298C polymorphism, much more contradictory reports have been presented. The differences in ethnicity may be a major reason for the controversy.
Three meta-analysis summarizing studies on association between the MTHFR C677T polymorphism and the risk of PE until August 2012 have been performed [51–53], however, their meta-analysis did not perform analyses on association between the MTHFR A1298C and the risk of PE. Six studies with 1103 /988 cases /controls for MTHFR A1298C were chosen. We found that the previous meta-analysis did not include at least 5 studies in the last updated meta-analysis [4, 36, 41, 54, 55]. In addition, two new studies have been published since July 2014 [11, 56]. We excluded two studies in the previous meta-analyses after evaluating the articles.
The summary OR from our meta-analyses evidenced the importance of the ethnical origin when approaching the issue, as we have observed a correlation between MTHFR C677T and preeclampsia in Caucasian (p = 0.015) and Asian (p =0.001 ). When the MTHFR A1298C was considered, we failed to find any association with the risk for preeclampsia.
The C677T polymorphism in MTHFR gene was associated with elevated plasma homocysteine level, increased risk of arterial stiffness [57]. Women with elevated total homocysteine concentrations showed a significant association with cellular fibronectin concentration,a marker of endothelial dysfunction [10]. Cellular fibronectin (cFN), an isoform of fibronectin synthesized locally by endothelial cells in response to tissue injury, has been reported in several studies to be elevated in women with preeclampsia [58, 59]. It is also possible that the association of the MTHFR 677TT genotype with PE, independent of hyperhomocysteinemia, was due to interference with red blood cell folate metabolism.
There are limitations that are present in this analysis, which mainly relate to the lack of other risk factors between the subjects in the available studies. In these cases, few investigators reported results from subgroup analysis for other risk factors, such as maternal age, dietary parameters, and behavioral factors and so on. Therefore, the association in these factors could not be assessed. There is a need for larger and wider case–control studies to explore the role of other factors that are likely to cause PE.
This is a meta-analysis with sufficient individual data to stratify results by ethnicity. In the stratified analysis, individuals with the TT genotype in the recessive model had increased risk of PE (OR = 0.282, 95 % CI: 1.048-1.567, p=0.015) in Caucasian subjects and in Asian subjects (OR = 2.078, 95 % CI: 1.368-3.157, p=0.001). In conclusion, the overall result of the present meta-analysis demonstrated that the MTHFR T677T genotype had increased risk of PE. Our finding, showing MTHFR 677TT polymorphism is associated with PE,may provide a clue toward a better understanding of the correlation between MTHFR 677 TT genotype and the pathogenesis of PE in human.
Footnotes
Capsule This meta-analysis showed that MTHFR C677T genotype had increased risk of preeclampsia.
Contributor Information
Ying Luo, Email: yingluo@kmust.edu.cn.
Wenru Tang, Phone: +86-871-65920753, Email: twr@sina.com.
References
- 1.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol, 2000. 183(1): p. S1-S22. [PubMed]
- 2.Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol. 1992;99(7):547–53. doi: 10.1111/j.1471-0528.1992.tb13818.x. [DOI] [PubMed] [Google Scholar]
- 3.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet, 2002. 77(1): p. 67–75. [PubMed]
- 4.Kupferminc MJ, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med. 1999;340(1):9–13. doi: 10.1056/NEJM199901073400102. [DOI] [PubMed] [Google Scholar]
- 5.Sohda S, et al. Methylenetetrahydrofolate reductase polymorphism and pre-eclampsia. J Med Genet. 1997;34(6):525–6. doi: 10.1136/jmg.34.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grandone E, et al. Factor V Leiden, C > T MTHFR polymorphism and genetic susceptibility to preeclampsia. Thromb Haemost. 1997;77(6):1052–4. [PubMed] [Google Scholar]
- 7.Steegers-Theunissen RP, et al. Hyperhomocysteinaemia and recurrent spontaneous abortion or abruptio placentae. Lancet. 1992;339(8801):1122–3. doi: 10.1016/0140-6736(92)90725-i. [DOI] [PubMed] [Google Scholar]
- 8.Stamler JS, Slivka A. Biological chemistry of thiols in the vasculature and in vascular-related disease. Nutr Rev. 1996;54(1 Pt 1):1–30. doi: 10.1111/j.1753-4887.1996.tb03770.x. [DOI] [PubMed] [Google Scholar]
- 9.Rajkovic A, Catalano PM, Malinow MR. Elevated homocyst(e)ine levels with preeclampsia. Obstet Gynecol. 1997;90(2):168–71. doi: 10.1016/S0029-7844(97)00223-8. [DOI] [PubMed] [Google Scholar]
- 10.Powers RW, et al. Plasma homocysteine concentration is increased in preeclampsia and is associated with evidence of endothelial activation. Am J Obstet Gynecol. 1998;179(6 Pt 1):1605–11. doi: 10.1016/s0002-9378(98)70033-x. [DOI] [PubMed] [Google Scholar]
- 11.Chedraui P, et al. Polymorphisms of the methylenetetrahydrofolate reductase gene (C677T and A1298C) in nulliparous women complicated with preeclampsia. Gynecol Endocrinol. 2014;30(5):392–6. doi: 10.3109/09513590.2014.895807. [DOI] [PubMed] [Google Scholar]
- 12.Mislanova C, et al. Placental markers of folate-related metabolism in preeclampsia. Reproduction. 2011;142(3):467–76. doi: 10.1530/REP-10-0484. [DOI] [PubMed] [Google Scholar]
- 13.Lachmeijer AM, et al. Mutations in the gene for methylenetetrahydrofolate reductase, homocysteine levels, and vitamin status in women with a history of preeclampsia. Am J Obstet Gynecol. 2001;184(3):394–402. doi: 10.1067/mob.2001.109393. [DOI] [PubMed] [Google Scholar]
- 14.Egan KM, et al. Genetic polymorphisms in GSTM1, GSTP1, and GSTT1 and the risk for breast cancer: results from the Shanghai Breast Cancer Study and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2004;13(2):197–204. doi: 10.1158/1055-9965.epi-03-0294. [DOI] [PubMed] [Google Scholar]
- 15.Waterman M, et al. Distinct and overlapping genetic loci in Crohn’s disease and ulcerative colitis: correlations with pathogenesis. Inflamm Bowel Dis. 2011;17(9):1936–42. doi: 10.1002/ibd.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179(5):1359–75. doi: 10.1016/s0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 17.Sun, N.J., Study on the relationship between N5,10-methylenetetrahydrofolate reductase gene and endothelial nitric oxide synthase gene polymorphisms and preeclampsia and eclampsia in the Han nationality women of Guangdong Prog Obstet Gynecol, 2011. 20(3).
- 18.Canto P, et al. Methylenetetrahydrofolate reductase C677T and glutathione S-transferase P1 A313G are associated with a reduced risk of preeclampsia in Maya-Mestizo women. Hypertens Res. 2008;31(5):1015–9. doi: 10.1291/hypres.31.1015. [DOI] [PubMed] [Google Scholar]
- 19.Dalmaz CA, et al. Relationship between polymorphisms in thrombophilic genes and preeclampsia in a Brazilian population. Blood Cells Mol Dis. 2006;37(2):107–10. doi: 10.1016/j.bcmd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Davalos IP, et al. Methylenetetrahydrofolate reductase C677T polymorphism and Factor V Leiden variant in Mexican women with preeclampsia/eclampsia. Blood Cells Mol Dis. 2005;35(1):66–9. doi: 10.1016/j.bcmd.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Swanton A, et al. IVF outcome in women with PCOS, PCO and normal ovarian morphology. Eur J Obstet Gynecol Reprod Biol. 2010;149(1):68–71. doi: 10.1016/j.ejogrb.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Prasmusinto D, et al. The methylenetetrahydrofolate reductase 677 C–>T polymorphism and preeclampsia in two populations. Obstet Gynecol. 2002;99(6):1085–92. doi: 10.1016/s0029-7844(02)01997-x. [DOI] [PubMed] [Google Scholar]
- 23.Kobashi G, et al. Absence of association between a common mutation in the methylenetetrahydrofolate reductase gene and preeclampsia in Japanese women. Am J Med Genet. 2000;93(2):122–5. doi: 10.1002/1096-8628(20000717)93:2<122::aid-ajmg8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 24.Sunkara SK, et al. The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: a systematic review and meta-analysis. Hum Reprod. 2010;25(2):418–29. doi: 10.1093/humrep/dep396. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Mishra VV. Review: toxicants in reproductive fluid and in vitro fertilization (IVF) outcome. Toxicol Ind Health. 2010;26(8):505–11. doi: 10.1177/0748233710373081. [DOI] [PubMed] [Google Scholar]
- 26.Also-Rallo E, et al. Polymorphisms of genes involved in homocysteine metabolism in preeclampsia and in uncomplicated pregnancies. Eur J Obstet Gynecol Reprod Biol. 2005;120(1):45–52. doi: 10.1016/j.ejogrb.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Veleva Z, et al. Elective single embryo transfer with cryopreservation improves the outcome and diminishes the costs of IVF/ICSI. Hum Reprod. 2009;24(7):1632–9. doi: 10.1093/humrep/dep042. [DOI] [PubMed] [Google Scholar]
- 28.Perin PM, et al. Impact of short-term preconceptional exposure to particulate air pollution on treatment outcome in couples undergoing in vitro fertilization and embryo transfer (IVF/ET) J Assist Reprod Genet. 2010;27(7):371–82. doi: 10.1007/s10815-010-9419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison ER, et al. Prothrombotic genotypes are not associated with pre-eclampsia and gestational hypertension: results from a large population-based study and systematic review. Thromb Haemost. 2002;87(5):779–85. [PubMed] [Google Scholar]
- 30.Raijmakers MT, et al. Hyperhomocysteinaemia: a risk factor for preeclampsia? Eur J Obstet Gynecol Reprod Biol. 2001;95(2):226–8. doi: 10.1016/s0301-2115(00)00497-8. [DOI] [PubMed] [Google Scholar]
- 31.Kim YJ, et al. Genetic susceptibility to preeclampsia: roles of cytosineto-thymine substitution at nucleotide 677 of the gene for methylenetetrahydrofolate reductase, 68-base pair insertion at nucleotide 844 of the gene for cystathionine beta-synthase, and factor V Leiden mutation. Am J Obstet Gynecol. 2001;184(6):1211–7. doi: 10.1067/mob.2001.110411. [DOI] [PubMed] [Google Scholar]
- 32.Mann JS, et al. Endogenous gonadotropin flare following microdose leuprolide (MDL) stimulation protocol does not correlate with in vitro fertilization (IVF) outcome. Fertil Steril. 2010;94(6):2427–9. doi: 10.1016/j.fertnstert.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Rigo J, Jr, et al. Maternal and neonatal outcome of preeclamptic pregnancies: the potential roles of factor V Leiden mutation and 5,10 methylenetetrahydrofolate reductase. Hypertens Pregnancy. 2000;19(2):163–72. doi: 10.1081/prg-100100132. [DOI] [PubMed] [Google Scholar]
- 34.Check JH, et al. A prospective comparison of in vitro fertilization (IVF) outcome following controlled ovarian hyperstimulation (COH) regimens using follitropin alpha exclusively or with the addition of low dose human chorionic gonadotropin (hCG) and ganirelix. Clin Exp Obstet Gynecol. 2009;36(4):217–8. [PubMed] [Google Scholar]
- 35.O’Shaughnessy KM, et al. Factor V Leiden and thermolabile methylenetetrahydrofolate reductase gene variants in an East Anglian preeclampsia cohort. Hypertension. 1999;33(6):1338–41. doi: 10.1161/01.hyp.33.6.1338. [DOI] [PubMed] [Google Scholar]
- 36.Pegoraro RJ, et al. Methylenetetrahydrofolate reductase gene polymorphisms in black South Africans and the association with preeclampsia. Acta Obstet Gynecol Scand. 2004;83(5):449–54. doi: 10.1111/j.0001-6349.2004.0355.x. [DOI] [PubMed] [Google Scholar]
- 37.Livingston JC, et al. Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185(1):153–7. doi: 10.1067/mob.2001.114691. [DOI] [PubMed] [Google Scholar]
- 38.Farhi J, et al. Effect of coasting on IVF cycle characteristics and outcome in short vs. long GnRH agonist protocols. Gynecol Endocrinol. 2010;26(3):187–92. doi: 10.3109/09513590903015601. [DOI] [PubMed] [Google Scholar]
- 39.Dickerson EH, et al. Insulin resistance and free androgen index correlate with the outcome of controlled ovarian hyperstimulation in non-PCOS women undergoing IVF. Hum Reprod. 2010;25(2):504–9. doi: 10.1093/humrep/dep393. [DOI] [PubMed] [Google Scholar]
- 40.Lauszus FF, Gron PL, Klebe JG. Association of polymorphism of methylene-tetrahydro-folate-reductase with urinary albumin excretion rate in type 1 diabetes mellitus but not with preeclampsia, retinopathy, and preterm delivery. Acta Obstet Gynecol Scand. 2001;80(9):803–6. doi: 10.1034/j.1600-0412.2001.080009803.x. [DOI] [PubMed] [Google Scholar]
- 41.de Maat MP, et al. Preeclampsia and its interaction with common variants in thrombophilia genes. J Thromb Haemost. 2004;2(9):1588–93. doi: 10.1111/j.1538-7836.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 42.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48. [PubMed] [Google Scholar]
- 43.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 44.Egger M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klai S, et al. Association of MTHFR A1298C polymorphism (but not of MTHFR C677T) with elevated homocysteine levels and placental vasculopathies. Blood Coagul Fibrinolysis. 2011;22(5):374–8. doi: 10.1097/MBC.0b013e328344f80f. [DOI] [PubMed] [Google Scholar]
- 46.Zusterzeel PL, et al. Methylenetetrahydrofolate reductase polymorphisms in preeclampsia and the HELLP syndrome. Hypertens Pregnancy. 2000;19(3):299–307. doi: 10.1081/prg-100101991. [DOI] [PubMed] [Google Scholar]
- 47.Myatt L, Miodovnik M. Prediction of preeclampsia. Semin Perinatol. 1999;23(1):45–57. doi: 10.1016/s0146-0005(99)80059-7. [DOI] [PubMed] [Google Scholar]
- 48.Lopez-Quesada E, Vilaseca MA, Lailla JM. Plasma total homocysteine in uncomplicated pregnancy and in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2003;108(1):45–9. doi: 10.1016/s0301-2115(02)00367-6. [DOI] [PubMed] [Google Scholar]
- 49.Okohue JE, et al. The effect of endometrial thickness on in vitro fertilization (IVF)-embryo transfer/intracytoplasmic sperm injection (ICSI) outcome. Afr J Reprod Health. 2009;13(1):113–21. [PubMed] [Google Scholar]
- 50.Li, M., et al., [Association of the clinical characteristics and the IVF-ET outcome in infertile women with polycystic ovarian syndrome of different subtypes]. Nan Fang Yi Ke Da Xue Xue Bao, 2009. 29(2): p. 224–7. [PubMed]
- 51.Wang XM, Wu HY, Qiu XJ. Methylenetetrahydrofolate reductase (MTHFR) gene C677T polymorphism and risk of preeclampsia: an updated meta-analysis based on 51 studies. Arch Med Res. 2013;44(3):159–68. doi: 10.1016/j.arcmed.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Xia XP, Chang WW, Cao YX. Meta-analysis of the methylenetetrahydrofolate reductase C677T polymorphism and susceptibility to pre-eclampsia. Hypertens Res. 2012;35(12):1129–34. doi: 10.1038/hr.2012.117. [DOI] [PubMed] [Google Scholar]
- 53.Kosmas IP, Tatsioni A, Ioannidis JP. Association of C677T polymorphism in the methylenetetrahydrofolate reductase gene with hypertension in pregnancy and pre-eclampsia: a meta-analysis. J Hypertens. 2004;22(9):1655–62. doi: 10.1097/00004872-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Kaiser T, Brennecke SP, Moses EK. C677T methylenetetrahydrofolate reductase polymorphism is not a risk factor for pre-eclampsia/eclampsia among Australian women. Hum Hered. 2001;51(1–2):20–2. doi: 10.1159/000022954. [DOI] [PubMed] [Google Scholar]
- 55.NJ, S., Study on the relationship between N5,10-methylenetetrahydrofolate reductase gene and endothelial nitric oxide synthase gene polymorphisms and preeclampsia and eclampsia in the Han nationality women of Guangdong. Prog Obstet Gynecol, 2011. 20: p. 3.
- 56.Coral-Vazquez RM, et al. Analysis of polymorphisms and haplotypes in genes associated with vascular tone, hypertension and oxidative stress in Mexican-Mestizo women with severe preeclampsia. Clin Biochem. 2013;46(7–8):627–32. doi: 10.1016/j.clinbiochem.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 57.Teles JS, et al. Association of FCRL3 C-169T promoter single-nucleotide polymorphism with idiopathic infertility and infertility-related endometriosis. J Reprod Immunol. 2011;89(2):212–5. doi: 10.1016/j.jri.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Chavarria ME, et al. Maternal plasma cellular fibronectin concentrations in normal and preeclamptic pregnancies: a longitudinal study for early prediction of preeclampsia. Am J Obstet Gynecol. 2002;187(3):595–601. doi: 10.1067/mob.2002.123281. [DOI] [PubMed] [Google Scholar]
- 59.Powers RW, et al. Homocysteine and cellular fibronectin are increased in preeclampsia, not transient hypertension of pregnancy. Hypertens Pregnancy. 2001;20(1):69–77. doi: 10.1081/PRG-100104173. [DOI] [PubMed] [Google Scholar]
- 60.Lykke JA, et al. Thrombophilias and adverse pregnancy outcomes: results from the Danish National Birth Cohort. J Thromb Haemost. 2012;10(7):1320–5. doi: 10.1111/j.1538-7836.2012.04773.x. [DOI] [PubMed] [Google Scholar]
- 61.Dissanayake VH, et al. Candidate gene study of genetic thrombophilic polymorphisms in pre-eclampsia and recurrent pregnancy loss in Sinhalese women. J Obstet Gynaecol Res. 2012;38(9):1168–76. doi: 10.1111/j.1447-0756.2012.01846.x. [DOI] [PubMed] [Google Scholar]
- 62.Aggarwal S, et al. Preeclampsia in North Indian women: the contribution of genetic polymorphisms. J Obstet Gynaecol Res. 2011;37(10):1335–41. doi: 10.1111/j.1447-0756.2010.01523.x. [DOI] [PubMed] [Google Scholar]
- 63.Stiefel P, et al. Genotype of the CYBA promoter -930A/G, polymorphism C677T of the MTHFR and APOE genotype in patients with hypertensive disorders of pregnancy: an observational study. Med Clin (Barc) 2009;133(17):657–61. doi: 10.1016/j.medcli.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 64.Alfirevic Z, et al. Postnatal screening for thrombophilia in women with severe pregnancy complications. Obstet Gynecol. 2001;97(5 Pt 1):753–9. doi: 10.1016/s0029-7844(01)01190-5. [DOI] [PubMed] [Google Scholar]
- 65.Muetze S, et al. Maternal factor V Leiden mutation is associated with HELLP syndrome in Caucasian women. Acta Obstet Gynecol Scand. 2008;87(6):635–42. doi: 10.1080/00016340802112740. [DOI] [PubMed] [Google Scholar]
- 66.Stonek F, et al. Methylenetetrahydrofolate reductase C677T polymorphism and pregnancy complications. Obstet Gynecol. 2007;110(2 Pt 1):363–8. doi: 10.1097/01.AOG.0000270122.13198.6f. [DOI] [PubMed] [Google Scholar]
- 67.Nagy B, Hupuczi P, Papp Z. High frequency of methylenetetrahydrofolate reductase 677TT genotype in Hungarian HELLP syndrome patients determined by quantitative real-time PCR. J Hum Hypertens. 2007;21(2):154–8. doi: 10.1038/sj.jhh.1002122. [DOI] [PubMed] [Google Scholar]
- 68.Dusse LM, et al. Inherited thrombophilias and pre-eclampsia in Brazilian women. Eur J Obstet Gynecol Reprod Biol. 2007;134(1):20–3. doi: 10.1016/j.ejogrb.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 69.Jaaskelainen E, et al. MTHFR C677T polymorphism is not associated with placental abruption or preeclampsia in Finnish women. Hypertens Pregnancy. 2006;25(2):73–80. doi: 10.1080/10641950600745137. [DOI] [PubMed] [Google Scholar]
- 70.Demir SC, et al. The relationship between pregnancy induced hypertension and congenital thrombophilia. Saudi Med J. 2006;27(8):1161–6. [PubMed] [Google Scholar]
- 71.Mello G, et al. Thrombophilia is significantly associated with severe preeclampsia: results of a large-scale, case-controlled study. Hypertension. 2005;46(6):1270–4. doi: 10.1161/01.HYP.0000188979.74172.4d. [DOI] [PubMed] [Google Scholar]
- 72.Driul L, et al. Genetic thrombophilias and uterine artery Doppler velocimetry and preeclampsia. Int J Gynaecol Obstet. 2005;88(3):265–70. doi: 10.1016/j.ijgo.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 73.Yilmaz H, et al. Association of pre-eclampsia with hyperhomocysteinaemia and methylenetetrahydrofolate reductase gene C677T polymorphism in a Turkish population. Aust N Z J Obstet Gynaecol. 2004;44(5):423–7. doi: 10.1111/j.1479-828X.2004.00283.x. [DOI] [PubMed] [Google Scholar]
- 74.Williams MA, et al. Methylenetetrahydrofolate reductase 677 C–>T polymorphism and plasma folate in relation to pre-eclampsia risk among Peruvian women. J Matern Fetal Neonatal Med. 2004;15(5):337–44. doi: 10.1080/14767050410001680037. [DOI] [PubMed] [Google Scholar]
- 75.Perez-Mutul J, et al. A mutation in the 5,10-methylenetetrahydrofolate reductase gene is not associated with preeclampsia in women of southeast Mexico. Arch Med Res. 2004;35(3):231–4. doi: 10.1016/j.arcmed.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 76.Fabbro D, et al. Association between plasminogen activator inhibitor 1 gene polymorphisms and preeclampsia. Gynecol Obstet Invest. 2003;56(1):17–22. doi: 10.1159/000072326. [DOI] [PubMed] [Google Scholar]
- 77.D’Elia AV, et al. Frequency of factor V, prothrombin and methylenetetrahydrofolate reductase gene variants in preeclampsia. Gynecol Obstet Invest. 2002;53(2):84–7. doi: 10.1159/000052998. [DOI] [PubMed] [Google Scholar]
- 78.Watanabe H, et al. Evidence for an association of the R485K polymorphism in the coagulation factor V gene with severe preeclampsia from screening 35 polymorphisms in 27 candidate genes. Thromb Haemost. 2001;86(6):1594–5. [PubMed] [Google Scholar]
- 79.Rajkovic A, et al. Methylenetetrahydrofolate reductase 677 C –>T polymorphism, plasma folate, vitamin B(12) concentrations, and risk of preeclampsia among black African women from Zimbabwe. Mol Genet Metab. 2000;69(1):33–9. doi: 10.1006/mgme.1999.2952. [DOI] [PubMed] [Google Scholar]
- 80.Li, K., et al., [The common C677T polymorphism in the methylenetetrahydrofolate reductase gene is associated with neural tube defects and preeclampsia]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi, 2000. 17(2): p. 76–8. [PubMed]
- 81.Laivuori H, et al. 677 C–>T polymorphism of the methylenetetrahydrofolate reductase gene and preeclampsia. Obstet Gynecol. 2000;96(2):277–80. doi: 10.1016/s0029-7844(00)00896-6. [DOI] [PubMed] [Google Scholar]
- 82.Kupferminc MJ, et al. Severe preeclampsia and high frequency of genetic thrombophilic mutations. Obstet Gynecol. 2000;96(1):45–9. doi: 10.1016/s0029-7844(00)00861-9. [DOI] [PubMed] [Google Scholar]
- 83.Kaiser T, Brennecke SP, Moses EK. Methylenetetrahydrofolate reductase polymorphisms are not a risk factor for pre-eclampsia/eclampsia in Australian women. Gynecol Obstet Invest. 2000;50(2):100–2. doi: 10.1159/000010291. [DOI] [PubMed] [Google Scholar]
- 84.Powers RW, et al. Methylenetetrahydrofolate reductase polymorphism, folate, and susceptibility to preeclampsia. J Soc Gynecol Investig. 1999;6(2):74–9. doi: 10.1016/s1071-5576(98)00052-5. [DOI] [PubMed] [Google Scholar]
- 85.Chikosi AB, et al. 5,10 methylenetetrahydrofolate reductase polymorphism in black South African women with pre-eclampsia. Br J Obstet Gynaecol. 1999;106(11):1219–20. doi: 10.1111/j.1471-0528.1999.tb08152.x. [DOI] [PubMed] [Google Scholar]