Abstract
Purpose
To evaluate whether ejaculated human spermatozoa undergo complete apoptosis or necrosis during experimental semen bacterial infection in vitro.
Methods
Apoptotic markers, including mitochondrial transmembrane potential (ΔΨm), phosphatidylserine (PS) externalization, and DNA fragmentation, have been detected simultaneously in ejaculated human sperm after their incubation with a known pathogenic (Escherichia coli), as well as with conditionally pathogenic bacterial strains (Staphylococcus haemolyticus, Bacteroides ureolyticus) and/or leukocytes. The ΔΨm and translocation of PS was evaluated using the JC-1 and Annexin V binding tests, respectively. A modified TUNEL assay with additional staining for sperm viability was used to detect the DNA fragmentation level.
Results
The exposure of ejaculated spermatozoa to bacterial strains was associated with a simultaneous decrease in the percentage of sperm with normal ΔΨm and an increase in the proportion of Annexin V-positive sperm. Additionally, in the presence of S. haemolyticus, B. ureolyticus and/or leukocytes, a significant increase in the percentage of live TUNEL-positive (apoptotic) as well as dead TUNEL-positive (necrotic) sperm cells was also observed.
Conclusions
The cellular death observed in spermatozoa in the presence of inflammatory mediators may be due to both apoptosis and necrosis. Here, we demonstrate for the first time that direct contact of conditionally pathogenic bacteria with ejaculated human sperm may play an even greater role in the promotion of apoptosis than in case of some pathogenic bacterial strains. These findings suggest that significant bacteriospermia and leukocytospermia may be direct causes of subfertility or additional negative factors worsening the prognosis of fertility in natural and assisted procreation.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-015-0462-x) contains supplementary material, which is available to authorized users.
Keywords: Semen bacterial infection, Sperm apoptosis, Sperm Necrosis, Modified TUNEL assay
Introduction
The significance of urogenital bacterial infections for sperm quality has been discussed in recent years. Clinicians are not in full agreement about the effects of leukocytospermia and bacteriospermia on sperm function, especially in the context of the contamination or colonization of semen samples by ‘nonpathogenic’ bacteria, and also regarding the impact of increased levels of bacteria and leukocytes in semen samples for the outcome of assisted reproductive techniques (ARTs) [1–4]. The problem is complicated by the fact that, in subfertile/infertile men, the infectious process is often clinically silent. There is growing evidence that some bacterial species contribute to the deterioration of the sperm quality, directly reducing sperm viability and motility, as well as changing sperm morphology and indirectly influencing sperm quality through oxidative stress, immune or autoimmune reactions, the induction of apoptosis or necrosis, and secretory dysfunction of the male accessory glands [5–8]. However, the most critical mechanism leading to the sperm death pathway occurring in ejaculated spermatozoa during genitourinary tract inflammation/infection is related to apoptosis.
In somatic cells, the molecular mechanism of apoptosis induction by bacteria is well-known; it depends on the type of strain and takes place through classical intrinsic and extrinsic apoptotic pathways [9]. However, current research offers no clear consensus on the relationship between bacteriospermia and human sperm apoptosis. Among the various bacterial strains, well-established causative pathogens such as Escherichia coli and Chlamydia trachomatis and their soluble factors are most often presented as the inducers of apoptosis in human spermatozoa. This is confirmed by numerous experimental studies revealing the induction of early apoptotic markers by these bacteria, as reflected by phosphatidylserine (PS) translocation, mitochondrial membrane potential (ΔΨm), sperm viability, and motility [5,10–16]. However, only one report has focused on the culminating effect of apoptotic cascade namely, DNA fragmentation [13].
The premise that bacteria and leukocytes contribute to complete apoptosis in ejaculated human sperm has not been completely demonstrated. Recently, the detrimental effect of different types of bacterial strains (E. coli O75:HNT, Staphylococcus haemolyticus, and Bacteroides ureolyticus) and/or leukocytes on sperm motility, lipid sperm membrane peroxidation, and sperm membrane architecture was investigated by our group [17]. Here, we examine markers typically considered to be signs of apoptosis, such as sperm PS externalization, ΔΨm, and DNA fragmentation subsequent upon the incubation of spermatozoa with the selected infectious mediators. This study is the first of its kind, in which an expression of early and late apoptotic markers is simultaneously demonstrated in ejaculated sperm during experimental conditionally pathogenic bacteria infection in vitro.
Materials and methods
Semen sample collection and preparation
The studied population consisted of 30 healthy normozoospermic volunteers, between 20 and 35 years of age, recruited at an Andrology Outpatient Clinic in Poznan, Poland. This study was approved by the Local Bioethical Committee of Medical University of Poznan, Poland. All the selected volunteers were asymptomatic for urogenital tract inflammation and varicocoele. Semen specimens were obtained through masturbation after 3–5 days of sexual abstinence. Following liquefaction (30 min at room temperature), the specimens were subjected to routine semen analysis in accordance with the guidelines published by the World Health Organization [18]. The data recorded included semen volume, pH, sperm count, motility, morphology, and viability. The number of peroxidase-positive leukocytes in the ejaculates was evaluated using the Endtz test [19]. Additionally, each semen sample was tested for the presence of antisperm antibodies using the direct immunobead test (Irvine Scientific, Santa Ana, CA, USA). All tested samples were subjected to microbiological analysis and examined for the presence of aerobes, anaerobes, and atypical bacteria using an automated ATB Expression computer system (Bio Merieux SA, Marcy-L’Étoile, France). Semen samples with normal sperm parameters and without antisperm antibodies and signs of bacterial infection (peroxidase-positive leukocytes <0.2 × 106/ml and negative culture for bacteria) were selected for the study. The characteristics of the selected semen samples are presented in Supplementary Table 1.
Spermatozoa from the collected semen samples were separated from seminal plasma by centrifugation at 600 g for 8 min. The sperm pellets were washed in warm phosphate buffer saline (PBS), pH 7.4 (Biomed, Lublin, Poland), and adjusted to a final concentration of 4 × 107 sperm per ml of PBS.
Blood sample collection and preparation
Peripheral blood leukocytes were obtained from fresh heparinized venous blood samples of healthy donors attending the Regional Blood Centre, Poznan, Poland. The density-gradient centrifugation technique (Histopaque-1077 g/cm3, Sigma, St. Louis, MO, USA) was used for peripheral blood leukocyte isolation (400 g, 20 min, room temperature), as previously described [20]. After washing twice with Hank’s balanced salt solution (HBSS; Biomed, Lublin, Poland), the cell pellets were checked for viability via trypan blue staining. The sperm pellets were finally resuspended to a concentration of 1 × 107/mL in PBS.
Bacteria collection and preparation
The Escherichia coli, Staphylococcus haemolyticus, and Bacteroides ureolyticus strains were isolated from the semen samples of infertile patients attending the Outpatient Andrology Clinic/Unit of Microbiology of Poznan Hospital Medical College, with active infection in the urogenital tract (leukocytospermia and significant bacteriospermia >1 × 104 CFU/mL of semen for all bacterial strains). The isolates were identified according to conventional techniques. Fresh subcultures were prepared by streaking thawed stock cultures on Columbia agar containing 5 % sheep blood cells and incubated at 37 °C under aerobic or anaerobic systems, respectively. On the day of the experiment, bacterial working suspensions containing approximately 3 × 106 CFU/mL were prepared in sterile 0.85 % saline. After incubation, the stock bacterial suspensions were reseeded on Columbia agar in order to verify bacteria viability. All the bacterial strains were alive during the experiments.
E. coli serotyping
The O (lipopolysaccharide) and H (flagellar) antigens of E. coli were determined using specific monovalent rabbit antisera, as has been previously described [21].
Sperm incubation conditions
Sample mixtures containing 2 × 107 spermatozoa in PBS were incubated with bacterial strains or leukocytes by means of an orbital shaker with a speed of 200 rpm for 2 h at 37 °C. The bacterial strains were added at a concentration of 1 × 105 CFU/mL and the leukocytes at a concentration of 1 × 106 cells/mL of coincubated mixtures. Untreated sperm were used as a negative control. Following incubation, the spermatozoa were washed in warm PBS at 600 g for 5 min; the sperm pellets were immediately subjected to analysis of their mitochondrial membrane potential (ΔΨm), phosphatidylserine (PS) externalization, and DNA fragmentation.
Determination of ΔΨm
Mitochondrial membrane potential was determined using JC-1 lipophilic cationic dye (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbocyanine iodide; Molecular Probes, Inc., Eugene, OR, USA). Sperm suspensions (2 × 106/mL) were stained with JC-1 (for a final concentration in the sperm suspension of 10 μg/mL) for 20 min at 37 °C in CO2 atmosphere, in the dark. After incubation, sperm smears were evaluated under a fluorescent microscope (Olympus BX41, Tokyo, Japan). A triple emission filter (DAPI/FITC/TexasRed) was applied to simultaneously view the green JC-1 monomers (Ex/Em 490/529 nm) and the orange JC-1 aggregates (Ex/Em 514/590 nm). The means of the duplicate counts of 200 spermatozoa at 400 × magnification were recorded as previously described [22].
Determination of PS externalization
The Annexin V-FITC Apoptosis Detection Kit (Beckman Coulter, Fullerton, CA, USA) was used according to the manufacturer’s instructions in order to detect PS translocation. The sperm suspensions (2 × 106/mL) were washed once in PBS supplemented with Ca2+ and were then double-stained with Annexin V-FITC and propidium iodide (PI). Following 15 min incubation on ice in the dark, cells were diluted with 1 × binding buffer. Flow cytometry analysis was conducted for 15 min. Based on green and red fluorescence, cells were divided into four sperm populations: viable sperm (negative for both dyes), apoptotic sperm (positive for Annexin V only), early necrotic sperm (positive for both dyes), and dead sperm (positive for PI only). The results were expressed as the percentage of apoptotic Annexin V-FITC positive sperm.
Evaluation of DNA fragmentation
The level of DNA fragmentation was measured using a modified TUNEL assay [23]. Briefly, sperm cells were incubated with LIVE/DEAD Fixable Dead Cell Far Red Stain (Molecular Probes) for 30 min at 37 °C. After triple washing with PBS, the sperm were exposed to 2 mM dithiothreitol (DTT) for 45 min at room temperature. The cells were then washed in PBS and fixed with 2 % paraformaldehyde for the TUNEL assay. TUNEL analysis was performed with a FlowTacs Apoptosis Detection Kit (Trevigen, Inc., Gaithersburg, MD, USA) according to manufacturer’s instructions, as previously described [24]. Background fluorescence was assessed in comparison to both the negative control (sperm exposed to the reaction mixture without TdT and Far Red Stain) and the positive control (sperm pretreated with DNase I) controls. Based on cell viability, the spermatozoa were divided into live (FL3-negative) and dead (FL3-positive) populations. The percentage of TUNEL-positive sperm cells among the live and dead cells was determined separately. This was expressed as the percentage of live TUNEL-positive cells (effect of apoptosis) and dead TUNEL-positive cells (effect of necrosis), respectively.
Flow cytometry analysis
Flow cytometry analysis of sperm samples was performed using a Beckman Coulter flow cytometer (Cell LabQuanta SC MPL, Beckman Coulter, Fullerton, CA, USA) equipped with a 488 nm argon-ion laser. For each analysis, at least 10,000 events, at a rate of 200–250 events/s were collected and analyzed using Cell LabQuanta SC MPL Analysis software (Beckman Coulter). The sperm population was gated on the basis of measurements of Electronic Volume (EV, parameter depends on cell size) and Side Scatter (SS, parameter depends on cellular granules). The green (480–550) and red (590–670) fluorescences were detected using the FL1 and FL3 channels, respectively. The fluorescent data were obtained at a fixed gain setting in logarithmic (FL1, FL3) mode. The fluorescence readings were repeated twice with distinct samples.
Statistical analysis
All statistical calculations were performed using the STATISTICA software package, version 10.0 (StatSoft, Tulsa, OK, USA). The distribution of the results was checked using the Shapiro–Wilk test. A nonparametric analysis of variances (using the Kruskal–Wallis test), followed by Dunn’s multiple comparison test were applied to assess the effects of the bacteria. Additionally, the nonparametric Mann–Whitney U-test was used to compare the results obtained without leukocytes and following leukocyte incubation applied to selected bacterial strains. All the data were expressed as medians ± average deviations (AD). P < .05 was considered significant, P < .01 very significant, and P < .001 most significant.
Results
Effect of different bacteria on ΔΨm
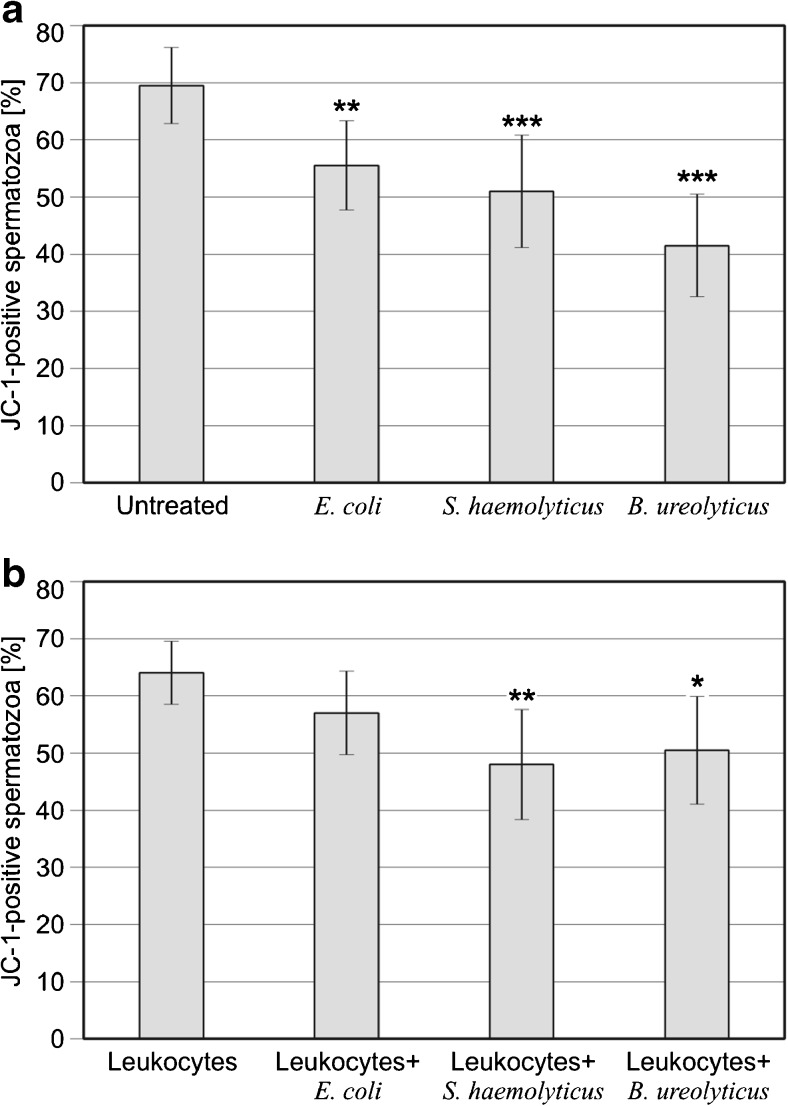
The results for ΔΨm in spermatozoa incubated with bacteria, leukocytes, or both are presented in Fig. 1. The application of every bacterial strain significantly decreased the percentage of sperm with normal ΔΨm, and this effect was highly significant for S. haemolyticus and B. ureolyticus (P < .01 for E. coli; P < .001 for S. haemolyticus and B. ureolyticus; panel A). The incubation of ejaculated sperm with S. haemolyticus or B. ureolyticus, both used together with leukocytes, in comparison to sperm incubated with leukocytes alone, also significantly decreased the proportion of sperm with normal ΔΨm (P < .01 and P < .05, for S. haemolyticus and B. ureolyticus, respectively; panel B).
Fig. 1.
Influence of selected bacterial strains on sperm mitochondrial membrane potential (a) without leukocytes and (b) after incubation with leukocytes. The results are expressed as medians ± average deviations. **P < .01; ***P < .001; calculated using the Kruskal–Wallis test, compared with respective controls; n = 30
Effect of different bacteria on PS externalization
The results for PS externalization in spermatozoa following their incubation with bacteria, leukocytes, or both are shown in Fig. 2. The incubation of sperm with bacteria was associated with an increase in the proportion of Annexin V-positive cells over untreated cells. However, it was only in the case of S. haemolyticus and anaerobic bacteria that this effect was statistically significant (P < .05; panel A). A significant increase in the percentage of sperm with PS externalization, as compared with sperm incubated with leukocytes alone, was observed only with respect to B. ureolyticus used together with leukocytes (P < .05; panel B).
Fig. 2.
Influence of selected bacterial strains on sperm phosphatidylserine externalization (a) without leukocytes and (b) after incubation with leukocytes. The results are expressed as medians ± average deviations. *P < .05; calculated using the Kruskal–Wallis test, compared with respective controls; n = 30
Effect of different bacteria on DNA fragmentation
The results for DNA fragmentation in spermatozoa treated with bacteria, leukocytes, or both are summarized in Fig. 3. The presence of S. haemolyticus and B. ureolyticus, compared with the control sperm, was associated with a significant increase in the percentage of live TUNEL-positive (P < .001) and dead TUNEL-positive spermatozoa (P < .01 for S. haemolyticus and P < .001 for B. ureolyticus; panel A). In general, the addition of leukocytes to the coincubated mixtures resulted in an elevated proportion of both live and dead TUNEL-positive sperm cells. A significant increase in live TUNEL-positive cells, as compared to sperm incubated with leukocytes alone, was observed for S. haemolyticus and B. ureolyticus, both used together with leukocytes, (P < .05 and P < .01, respectively). The incubation of sperm with anaerobic bacteria and leukocytes also significantly increased the percentage of dead TUNEL-positive cells, as compared to sperm treated with leukocytes alone (P < .01; panel B).
Fig. 3.
Influence of selected bacterial strains on sperm DNA fragmentation (a) without leukocytes and (b) after incubation with leukocytes. The results are expressed as medians ± average deviations. *P < .05; **P < .01; ***P < .001; calculated using the Kruskal–Wallis test, compared with respective controls; n = 30
Effect of leukocytes on ΔΨm, PS externalization, and DNA fragmentation
The Mann–Whitney test discriminated the leukocyte effect on ΔΨm, PS externalization, and DNA fragmentation in spermatozoa, depending on the bacterial strain used in the study (Table 1). As for ΔΨm and PS externalization, there were no statistical differences in sperm incubated with leukocytes and sperm incubated without leukocytes. The presence of leukocytes had a statistically significant influence on the percentage of both live and dead TUNEL-positive sperm cells, irrespective of whether they were used alone or together with bacteria.
Table 1.
Comparison of the percentage of JC-1 positive, Annexin V-positive/PI-negative and TUNEL-positive cells in both sperm incubated without leukocytes or after leukocyte incubation applied to selected bacterial strain
| No bacteria vs leukocytes | E. coli vs E. coli + leukocytes | S. haemolyticus vs S. haemolyticus + Leukocytes | B. ureolyticus vs B. ureolyticus + Leukocytes | |||||
|---|---|---|---|---|---|---|---|---|
| Z | P | Z | P | Z | P | Z | P | |
| JC-1-positive sperm (%) | 1.704 | NS | −0.811 | NS | −0.825 | NS | −1.866 | NS |
| AnV+/PI- sperm (%) | −1.349 | NS | −0.571 | NS | −0.584 | NS | −1.524 | NS |
| Live TUNEL-positive sperm (%) | −5.669 | .000000 | −5.551 | .000000 | −4.401 | .000011 | −2.253 | .024226 |
| Dead TUNEL-positive sperm (%) | −5.105 | .000000 | −3.298 | .000974 | −2.758 | .005815 | −2.664 | .007719 |
Abbreviations: Z Mann–Whitney test values, NS Non statistically significant
Discussion
This study provides evidence for the proapoptotic effect of bacterial infections of semen under in vitro conditions. Bacteria-induced PS translocation from the inner to the outer sperm membrane seems to be a natural consequence of sperm membrane lipid architecture loss, as has been previously showed using the merocyanine 540 (M540) test [17]. There are reports in which lipid disorders have been revealed simultaneously in M540-positive and Annexin V-positive sperm [25,26]. Moreover, those authors agreed that, in human spermatozoa, such molecular changes in membranes are characteristic of damaged, but not capacitated cells, and might represent true apoptotic events in sperm. Taking our recent and current findings into consideration, it can be assumed that a loss of phospholipid asymmetry is one of the mechanisms by which bacteria interfere with ejaculated spermatozoa, with subsequent induction of their death pathways.
The importance of well-functioning mitochondria for sperm fertilizing potential is repeatedly mentioned in the literature [27–33]. The existence of proapoptotic mechanisms associated with these organelles of the male gamete, similar to those found in somatic cells, has also been strongly suggested by others [33]. Moreover, according to recent reports, mitochondria, through their generation of reactive oxygen species (ROS), are an integral part of the intrinsic cascade of apoptosis in spermatozoa [34]. The results obtained here showed that bacterial agents, in addition to changes in sperm membranes, contribute to decreased ΔΨm. The results are consistent with our earlier morphological study, in which the use of a triple combination of fluorochromes (SYBR-14, PI, and JC-1) allowed us to see that the loss of sperm viability and ΔΨm changes may occur simultaneously after their incubation with bacteria [16]. Moreover, the presence of both early apoptotic markers, as evidenced by PS externalization, and depolarized mitochondria is a strong indicator of the initiation of apoptotic events in male germ cells as a consequence of the direct contact of spermatozoa with bacteria and their toxins.
Although an early apoptosis-induced phenotype in ejaculated sperm in the presence of some pathogens or their toxins has been reported by others, the presence of apoptosis in sperm nuclei has not been extensively studied. However, some groups have demonstrated an increase in human sperm DNA fragmentation due to incubation with C. trachomatis (13) and Candida albicans [35] using conventional TUNEL assay. In this study, we have applied a modified TUNEL assay, with staining for live sperm, which allowed the separate assessment of DNA fragmentation in live (net apoptotic effect) and dead (net necrotic effect) sperm as recently proposed by Aitken’s group [23]. Interestingly, the treatment of ejaculated human spermatozoa with conditionally pathogenic bacterial agents significantly increased DNA damage in both live and dead TUNEL-positive sperm populations. However, the high percentage of nonviable TUNEL-positive cells (necrosis), as compared to live TUNEL-positive sperm (apoptosis) was observed. Not surprisingly, the strongest effect was visible in the presence of anaerobic strains whose unique cytotoxic properties have also recently been showed [17]. The use of a combination of the TUNEL assay and sperm viability was able to dispel doubts about the nature of the DNA breaks observed during experimental semen bacterial infection in vitro. Our findings have provided evidence for the simultaneous induction of complete apoptosis and necrosis in ejaculated human sperm as a result of direct contact with bacteria or their toxins. These molecular observations are also consistent with other results that reveal the increased presence of sperm with altered morphology typical of apoptosis and necrosis in patients with urogenital bacterial infections [36] and bacterial contamination of semen [2]. However, in an animal model of uropathogenic E. coli (UPEC) epididymo-orchitis, necrosis has been recently demonstrated to be the main death pathway in infected rat testes [37].
Escherichia coli is a very frequent agent of urogenital infections and has the best documented direct negative impact on sperm quality. We know, however, that the pathogenicity of different serotypes of E. coli is heterogeneous in their effects on ejaculated spermatozoa [38]. In turn, coagulase-negative Staphylococci are commonly isolated from the semen samples of both fertile and infertile men, but often appear to be neutral for germ cell physiology [39]. Anaerobic microorganisms can be found in the ejaculate of the majority of infertile patients, although this depends on the extent of the routine microbial screening of the semen [40,41]. The use of bacteria clearly differing in metabolism and pathogenicity seems to be interesting especially in the context of the results here obtained. In our study, the ejaculated human spermatozoa had greater apoptotic and necrotic changes following treatment with B. ureolyticus and S. haemolyticus than with uropathogenic E. coli O75:HNT. The mechanism of pathogenesis with the S. haemolyticus strain is still poorly understood, although the depolarization of mitochondria and the induction of caspase-dependent apoptosis in macrophages after treatment with S. haemolyticus strains has recently been found [42]. Our present results suggest a similar pathogenicity of S. haemolyticus for ejaculated human sperm. These results are supported by the strong adhesion of these bacterial strains to the surface of the sperm head, midpiece, and principal piece, as has been recently documented [16]. In turn, the link between B. ureolyticus and male infertility was discussed long ago in the literature [43]. Interestingly, our earlier in vitro experiments indicated that free radical species mediate the cytotoxic effect of these bacteria on spermatozoa as a consequence of lipid peroxidative damage to the membranes [20]. According to some authors, these changes may lead to the induction of apoptosis in spermatozoa, characterized by enhanced mitochondrial ROS generation, PS exposure, and oxidative DNA damage [44]. In this context, the results observed suggest a similar mechanism of inducing sperm death pathways in the presence of some bacterial strains. Here, we demonstrate for the first time that the direct contact of conditionally pathogenic bacteria and their toxins with ejaculated human sperm may play a role in promoting apoptosis, even to a greater extent than known pathogenic bacterial strains.
In analyzing the influence of the semen bacterial infection on the apoptosis of ejaculated spermatozoa, the effect of leukocytes should also be taken into account. The increased expression of early or late apoptotic biomarkers in spermatozoa in response to seminal leukocytes has already been demonstrated [30,45–47]. However, a direct causal relationship between DNA damage in human spermatozoa and leukocyte infiltration has not always been confirmed [48]. In our study, a comparison of the expression of apoptotic markers in both sperm incubated with and without leukocytes has shown that the leukocyte effect was significant only in the TUNEL assay. Most probably, the increase in DNA fragmentation in live and dead spermatozoa that was shown in the presence of leukocytes was mainly due to the free radical attacks generated by the leukocytes. This is in agreement with previous studies, in which the increased expression of apoptotic markers was correlated with leukocyte concentration and reactive oxygen species (ROS) production in the ejaculate [30,46]. Interestingly, in another recent study, the level of 8-hydroxy-2′-deoxyguanosine (8-OHdG)—the main marker of oxidative DNA stress—and the caspase activity were clearly associated with DNA fragmentation in live sperm only, suggesting the role of oxidative stress in generating sperm DNA fragmentation by inducing an apoptotic mechanism [49]. Additionally, the apoptotic and necrotic effects of leukocytes on sperm observed in our study depended on the bacterial strain used, being greatest in the case of B. ureolyticus. Taking into account the notion that apoptosis could be signaled by ROS and that the infectious process is inevitably connected with the oxidative stress, the hypothesis of at least partial oxidative-damaged DNA during leukocytospermia and bacteriospermia is highly feasible.
The data obtained in this study indicate, for the first time, that coincubation of ejaculated human spermatozoa with inflammatory mediators results in complete apoptosis. However, DNA damage observed in human spermatozoa during bacterial infection could be due in part to the induction of apoptosis and necrosis. Since DNA damage induced in live sperm cells is clinically relevant, our results may have major implications for the prevention, diagnosis, and treatment of bacterial infections of semen, as suggested by others [34]. Here, we demonstrate for the first time that the induction of sperm apoptosis and necrosis could contribute to the mechanisms of pathogenesis through which various bacteria kill sperm cells. Moreover, apoptotic and necrotic changes in sperm membranes, mitochondria, and DNA could be more severe in the presence of bacteria that so far have been considered ‘nonpathogenic’, compared to some known pathogenic bacterial strains. These findings support our view that each semen culture that is positive for bacteria should be treated with care, especially in patients with subfertility/infertility and qualified for ARTs treatment. It cannot be excluded that the molecular changes observed in this study could be critical for semen processing procedures. Future studies should address this topic in order to provide new recommendations for the more effective preparation of spermatozoa for ARTs, including semen processing methods for the selection of nonapoptotic sperm cells and seminal bacteria removal [48,50].
We are aware of the limitations of in vitro assessment of the influence of bacteria and leukocytes on purified spermatozoa, and some bias may arise between in vitro models and in vivo semen bacterial infection. However, in our in vitro experiments, we employed bacterial and leukocyte concentrations that reflected real and pathognomonic in vivo bacterial infections of semen. Our future research will be focused on the verification of the present results revealed in vitro in bacteriospermia and leukocytospermia in in situ conditions.
Electronic supplementary material
(DOCX 16 kb)
Acknowledgments
The authors wish to thank Anna Czernikiewicz, M.Sc. for blood sample processing. Tomasz Kolanowski is a scholarship recipient of EU 8.2.2 OP- Innovative Economy.
Grant support
This work was financed by Ministry of Science and Higher Education grant No. NN 407283539 and National Centre for Research and Development grant No. NR 13006606.
Ethical statement
This study was approved by the Local Bioethical Committee of Medical University of Poznan, Poland. Informed consent was obtained from all the participants included to the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Capsule Ejaculated human spermatozoa undergo complete apoptosis and necrosis during semen bacterial infection; it is possible to induce sperm apoptosis/necrosis in the presence of conditionally pathogenic bacterial strains.
References
- 1.Cottell E, Harrison RF, McCaffrey M, Walsh T, Mallon E, Barry-Kinsella C. Are seminal fluid microorganisms of significance or merely contaminants? Fertil Steril. 2000;74:465–70. doi: 10.1016/S0015-0282(00)00709-3. [DOI] [PubMed] [Google Scholar]
- 2.Moretti E, Capitani S, Figura N, Pammolli A, Federico MG, Giannerini V, et al. The presence of bacteria species in semen and sperm quality. J Assist Reprod Genet. 2009;26:47–56. doi: 10.1007/s10815-008-9283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barraud-Lange V, Pont JC, Pocate K, Kunstmann JM, Chalas-Boissonas C, Ducot B, et al. Seminal leukocytes and clinical outcomes with donor sperm insemination. Fertil Steril. 2011;96:1320–4. doi: 10.1016/j.fertnstert.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Cavagna M, Oliveira JB, Petersen CG, Mauri AL, Silva LF, Massaro FC, et al. The influence of leukocytospermia on the outcomes of assisted reproductive technology. Reprod Biol Endocrinol. 2012;10:44. doi: 10.1186/1477-7827-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villegas J, Schulz M, Soto L, Sanchez R. Bacteria induce expression of apoptosis in human spermatozoa. Apoptosis. 2005;10:105–10. doi: 10.1007/s10495-005-6065-8. [DOI] [PubMed] [Google Scholar]
- 6.Allam JP, Fronhoffs F, Fathy A, Novak N, Oltermann I, Bieber T, et al. High percentage of apoptotic spermatozoa in ejaculates from men with chronic genital tract inflammation. Andrologia. 2008;40:329–34. doi: 10.1111/j.1439-0272.2008.00864.x. [DOI] [PubMed] [Google Scholar]
- 7.Domes T, Lo KC, Grober ED, Mullen JB, Mazzulli T, Jarvi K. The incidence and effect of bacteriospermia and elevated seminal leukocytes on semen parameters. Fertil Steril. 2012;97:1050–5. doi: 10.1016/j.fertnstert.2012.01.124. [DOI] [PubMed] [Google Scholar]
- 8.La Vignera S, Condorelli R, D’Agata R, Vicari E, Calogero AE. Semen alterations and flow-citometry evaluation in patients with male accessory gland infections. J Endocrinol Investig. 2012;35:219–23. doi: 10.3275/7924. [DOI] [PubMed] [Google Scholar]
- 9.Grassmé H, Jendrossek V, Gulbins E. Molecular mechanisms of bacteria induced apoptosis. Apoptosis. 2001;6:441–5. doi: 10.1023/A:1012485506972. [DOI] [PubMed] [Google Scholar]
- 10.Diemer T, Weidner W, Michelmann HW, Schiefer HG, Rovan E, Mayer F. Influence of Escherichia coli on motility parameters of human spermatozoa in vitro. Int J Androl. 1996;19:271–7. doi: 10.1111/j.1365-2605.1996.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 11.Köhn FM, Erdmann I, Oeda T, el Mulla KF, Schiefer HG, Schill WB. Influence of urogenital infections on sperm functions. Andrologia. 1998;30:73–80. doi: 10.1111/j.1439-0272.1998.tb02829.x. [DOI] [PubMed] [Google Scholar]
- 12.Eley A, Hosseinzadeh S, Hakimi H, Geary I, Pacey AA. Apoptosis of ejaculated human sperm is induced by co-incubation with Chlamydia trachomatis lipopolysaccharide. Hum Reprod. 2005;20:2601–7. doi: 10.1093/humrep/dei082. [DOI] [PubMed] [Google Scholar]
- 13.Satta A, Stivala A, Garozzo A, Morello A, Perdichizzi A, Vicari E, et al. Experimental Chlamydia trachomatis infection causes apoptosis in human sperm. Hum Reprod. 2006;21:134–7. doi: 10.1093/humrep/dei269. [DOI] [PubMed] [Google Scholar]
- 14.Berktas M, Aydin S, Yilmaz Y, Cecen K, Bozkurt H. Sperm motility changes after coincubation with various uropathogenic microorganisms: an in vitro experimental study. Int Urol Nephrol. 2008;40:383–9. doi: 10.1007/s11255-007-9289-4. [DOI] [PubMed] [Google Scholar]
- 15.Schulz M, Sánchez R, Soto L, Risopatrón J, Villegas J. Effect of Escherichia coli and its soluble factors on mitochondrial membrane potential, phosphatidylserine translocation, viability, and motility of human spermatozoa. Fertil Steril. 2010;94:619–23. doi: 10.1016/j.fertnstert.2009.01.140. [DOI] [PubMed] [Google Scholar]
- 16.Fraczek M, Piasecka M, Gaczarzewicz D, Szumala-Kakol A, Kazienko A, Lenart S, et al. Membrane stability and mitochondrial activity of human-ejaculated spermatozoa during in vitro experimental infection with Escherichia coli, Staphylococcus haemolyticus and Bacteroides ureolyticus. Andrologia. 2012;44:315–29. doi: 10.1111/j.1439-0272.2012.01283.x. [DOI] [PubMed] [Google Scholar]
- 17.Fraczek M, Wiland E, Piasecka M, Boksa M, Gaczarzewicz D, Szumala-Kakol A, et al. Fertilizing potential of ejaculated human spermatozoa during in vitro semen bacterial infection. Fertil Steril. 2014;102:711–9. doi: 10.1016/j.fertnstert.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5. Geneva: WHO Press; 2010. [Google Scholar]
- 19.Endtz AW. A rapid staining method for differentiating granulocytes from germinal cells in Papanicolau-stained semen. Acta Cytol. 1974;18:2–7. [PubMed] [Google Scholar]
- 20.Fraczek M, Szumala-Kakol A, Jedrzejczak P, Kamieniczna M, Kurpisz M. Bacteria trigger oxygen radical release and sperm lipid peroxidation in in vitro model of semen inflammation. Fertil Steril. 2007;88:1076–85. doi: 10.1016/j.fertnstert.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Beutin L, Miko A, Krause G, Pries K, Haby S, Steege K, et al. Identification of human-pathogenic strains of Shiga toxin-producing Escherichia coli from food by a combination of serotyping and molecular typing of Shiga toxin genes. Appl Environ Microbiol. 2007;73:4769–75. doi: 10.1128/AEM.00873-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piasecka M, Kawiak J. Sperm mitochondria of patients with normal sperm motility and with asthenozoospermia: morphological and functional study. Folia Histochem Cytobiol. 2003;41:125–39. [PubMed] [Google Scholar]
- 23.Mitchell LA, De Iuliis GN, Aitken RJ. The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: development of an improved methodology. Int J Androl. 2011;34:2–13. doi: 10.1111/j.1365-2605.2009.01042.x. [DOI] [PubMed] [Google Scholar]
- 24.Fraczek M, Szumala-Kakol A, Dworacki G, Sanocka D, Kurpisz M. In vitro reconstruction of inflammatory reaction in human semen: effect on sperm DNA fragmentation. J Reprod Immunol. 2013;100:76–85. doi: 10.1016/j.jri.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Muratori M, Porazzi I, Luconi M, Marchiani S, Forti G, Baldi E. AnnexinV binding and merocyanine staining fail to detect human sperm capacitation. J Androl. 2004;25:797–810. doi: 10.1002/j.1939-4640.2004.tb02858.x. [DOI] [PubMed] [Google Scholar]
- 26.Martin G, Sabido O, Durand P, Levy R. Phosphatidylserine externalization in human sperm induced by calcium ionophore A23187: relationship with apoptosis, membrane scrambling and the acrosome reaction. Hum Reprod. 2005;20:3459–68. doi: 10.1093/humrep/dei245. [DOI] [PubMed] [Google Scholar]
- 27.Marchetti P, Ballot C, Jouy N, Thomas P, Marchetti C. Influence of mitochondrial membrane potential of spermatozoa on in vitro fertilisation outcome. Andrologia. 2012;44:136–41. doi: 10.1111/j.1439-0272.2010.01117.x. [DOI] [PubMed] [Google Scholar]
- 28.Marchetti C, Jouy N, Leroy-Martin B, Defossez A, Formstecher P, Marchetti P. Comparison of four fluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility. Hum Reprod. 2004;19:2267–76. doi: 10.1093/humrep/deh416. [DOI] [PubMed] [Google Scholar]
- 29.Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum Reprod. 2002;17:1257–65. doi: 10.1093/humrep/17.5.1257. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Sharma RK, Gupta A, George V, Thomas AJ, Falcone T, et al. Alterations in mitochondria membrane potential and oxidative stress in infertile men: a prospective observational study. Fertil Steril. 2003;80:844–50. doi: 10.1016/S0015-0282(03)00983-X. [DOI] [PubMed] [Google Scholar]
- 31.Barroso G, Taylor S, Morshedi M, Manzur F, Gaviño F, Oehninger S. Mitochondrial membrane potential integrity and plasma membrane translocation of phosphatidylserine as early apoptotic markers: a comparison of two different sperm subpopulations. Fertil Steril. 2006;85:149–54. doi: 10.1016/j.fertnstert.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 32.Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, Aitken RJ. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab. 2008;93:3199–207. doi: 10.1210/jc.2007-2616. [DOI] [PubMed] [Google Scholar]
- 33.Espinoza JA, Schulz MA, Sánchez R, Villegas JV. Integrity of mitochondrial membrane potential reflects human sperm quality. Andrologia. 2009;41:51–4. doi: 10.1111/j.1439-0272.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- 34.Aitken RJ, Baker MA. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int J Dev Biol. 2013;57:265–72. doi: 10.1387/ijdb.130146ja. [DOI] [PubMed] [Google Scholar]
- 35.Burrello N, Salmeri M, Perdichizzi A, Bellanca S, Pettinato G, D’Agata R, et al. Candida albicans experimental infection: effects on human sperm motility, mitochondrial membrane potential and apoptosis. Reprod Biomed Online. 2009;18:496–501. doi: 10.1016/S1472-6483(10)60125-3. [DOI] [PubMed] [Google Scholar]
- 36.Collodel G, Baccetti B, Capitani S, Moretti E. Necrosis in human spermatozoa. I. Ultrastructural features and FISH study in semen from patients with uro-genital infections. J Submicrosc Cytol Pathol. 2005;37:67–73. [PubMed] [Google Scholar]
- 37.Lu Y, Bhushan S, Tchatalbachev S, Marconi M, Bergmann M, Weidner W, et al. Necrosis is the dominant cell death pathway in uropathogenic Escherichia coli elicited epididymo-orchitis and is responsible for damage of rat testis. PLoS One. 2013;8:e52919. doi: 10.1371/journal.pone.0052919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boguen R, Uribe P, Treulen F, Villegas JV. Distinct isolates of uropathogenic Escherichia coli differentially affect human sperm parameters in vitro. Andrologia. 2013;46:943–7. doi: 10.1111/and.12167. [DOI] [PubMed] [Google Scholar]
- 39.Rodin DM, Larone D, Goldstein M. Relationship between semen cultures, leukospermia, and semen analysis in men undergoing fertility evaluation. Fertil Steril. 2003;79:1555–8. doi: 10.1016/S0015-0282(03)00340-6. [DOI] [PubMed] [Google Scholar]
- 40.Stovall DW, Bailey LE, Talbert LM. The role of aerobic and anaerobic semen cultures in asymptomatic couples undergoing in vitro fertilization: effects on fertilization and pregnancy rates. Fertil Steril. 1993;59:197–201. doi: 10.1016/s0015-0282(16)55639-8. [DOI] [PubMed] [Google Scholar]
- 41.Eggert-Kruse W, Probst S, Rohr G, Aufenanger J, Runnebaum B. Screening for subclinical inflammation in ejaculates. Fertil Steril. 1995;64:1012–22. [PubMed] [Google Scholar]
- 42.Krzymińska S, Szczuka E, Kaznowski A. Staphylococcus haemolyticus strains target mitochondria and induce caspase-dependent apoptosis of macrophages. Antonie Van Leeuwenhoek. 2012;102:611–20. doi: 10.1007/s10482-012-9756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balmelli T, Stamm J, Dolina-Giudici M, Peduzzi R, Piffaretti-Yanez A, Balerna M. Bacteroides ureolyticus in men consulting for infertility. Andrologia. 1994;26:35–8. doi: 10.1111/j.1439-0272.1994.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 44.Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl. 2011;13:36–42. doi: 10.1038/aja.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villegas J, Schulz M, Soto L, Iglesias T, Miska W, Sánchez R. Influence of reactive oxygen species produced by activated leukocytes at the level of apoptosis in mature human spermatozoa. Fertil Steril. 2005;83:808–10. doi: 10.1016/j.fertnstert.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 46.Zorn B, Ihan A, Kopitar AN, Kolbezen M, Sesek-Briski A, Meden-Vrtovec H. Changes in sperm apoptotic markers as related to seminal leukocytes and elastase. Reprod Biomed Online. 2010;21:84–92. doi: 10.1016/j.rbmo.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 47.Mupfiga C, Fisher D, Kruger T, Henkel R. The relationship between seminal leukocytes, oxidative status in the ejaculate, and apoptotic markers in human spermatozoa. Syst Biol Reprod Med. 2013;59:304–11. doi: 10.3109/19396368.2013.821540. [DOI] [PubMed] [Google Scholar]
- 48.Said T, Agarwal A, Grunewald S, Rasch M, Baumann T, Kriegel C, et al. Selection of nonapoptotic spermatozoa as a new tool for enhancing assisted reproduction outcomes: an in vitro model. Biol Reprod. 2006;74:530–7. doi: 10.1095/biolreprod.105.046607. [DOI] [PubMed] [Google Scholar]
- 49.Muratori, M, Tamburrino L, Marchiani S, Azzari C, Forti G, Baldi E. Investigation of the origin of sperm DNA fragmentation: the role of apoptosis, immaturity and oxidative stress. Proceedings of the 8th Congress of the European Academy of Andrology; 2014 October 15–17; Barcelona, Spain. American Society of Andrology and European Academy of Andrology.
- 50.Fourie J, Loskutoff N, Huyser C. Elimination of bacteria from human semen during sperm preparation using density gradient centrifugation with a novel tube insert. Andrologia. 2012;44:513–7. doi: 10.1111/j.1439-0272.2011.01217.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 16 kb)