Abstract
The mechanism of action of prolactin (PRL), a lactogenic and immunoregulatory hormone, has remained undetermined despite its critical role in development. This study identifies a DNA-binding factor induced by PRL that appears to mediate a signal from the cell surface receptor to specific gene expression in the nucleus. PRL stimulates the proliferation of Nb2 T-lymphoma cells and activates transcription of the interferon-regulatory factor 1 (IRF-1) gene. Within minutes of PRL stimulation, a PRL-induced factor (PRLIF) is activated and binds to a target site in the promoter of the IRF-1 gene. The PRLIF-binding site contains an inverted GAAA repeat that is also functional in the hormone-responsive beta-casein gene. The PRL-receptor complex signals tyrosine phosphorylation of JAK2, a nonreceptor tyrosine kinase, which may lead to activation of PRLIF. T-cell proliferation and transcriptional activation of the IRF-1 gene is also induced by the cytokine interleukin 2 (IL-2). This report demonstrates the rapid activation of an IL-2 nuclear-activated factor that recognizes the same GAAA inverted repeat in the IRF-1 promoter. PRLIF and IL-2 nuclear-activated factor are newly identified factors that appear to serve fundamental roles in the signal transduction pathways of PRL and IL-2, respectively, leading to the transcriptional regulation of responsive genes.
Full text
PDF
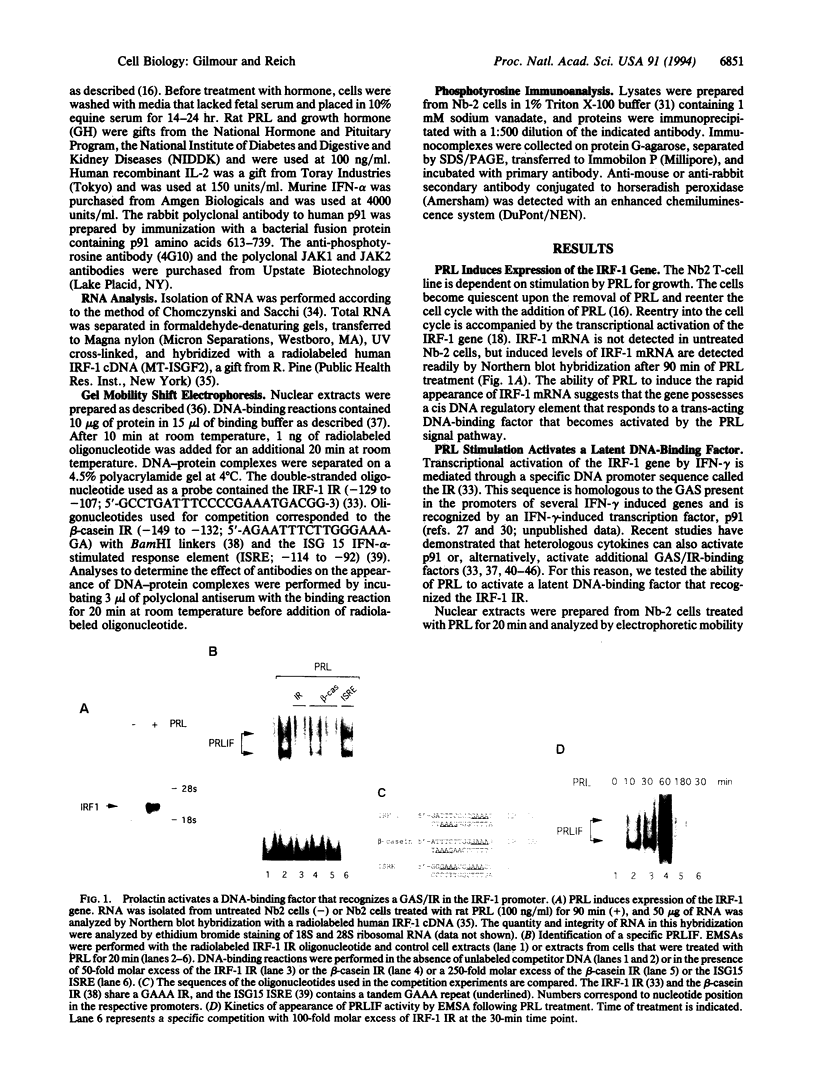



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argetsinger L. S., Campbell G. S., Yang X., Witthuhn B. A., Silvennoinen O., Ihle J. N., Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993 Jul 30;74(2):237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- Ball R. K., Friis R. R., Schoenenberger C. A., Doppler W., Groner B. Prolactin regulation of beta-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J. 1988 Jul;7(7):2089–2095. doi: 10.1002/j.1460-2075.1988.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayat-Sarmadi M., Houdebine L. M. Effect of various protein kinase inhibitors on the induction of milk protein gene expression by prolactin. Mol Cell Endocrinol. 1993 Mar;92(1):127–134. doi: 10.1016/0303-7207(93)90083-v. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. Haemopoietic receptors and helical cytokines. Immunol Today. 1990 Oct;11(10):350–354. doi: 10.1016/0167-5699(90)90139-z. [DOI] [PubMed] [Google Scholar]
- Bonni A., Frank D. A., Schindler C., Greenberg M. E. Characterization of a pathway for ciliary neurotrophic factor signaling to the nucleus. Science. 1993 Dec 3;262(5139):1575–1579. doi: 10.1126/science.7504325. [DOI] [PubMed] [Google Scholar]
- Boutin J. M., Jolicoeur C., Okamura H., Gagnon J., Edery M., Shirota M., Banville D., Dusanter-Fourt I., Djiane J., Kelly P. A. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988 Apr 8;53(1):69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G., Carbajo P., Rillema J. A. Possible role of tyrosine kinases in the prolactin stimulation of cell division in Nb2 node lymphoma cells. Horm Metab Res. 1993 May;25(5):256–258. doi: 10.1055/s-2007-1002091. [DOI] [PubMed] [Google Scholar]
- Fu X. Y., Zhang J. J. Transcription factor p91 interacts with the epidermal growth factor receptor and mediates activation of the c-fos gene promoter. Cell. 1993 Sep 24;74(6):1135–1145. doi: 10.1016/0092-8674(93)90734-8. [DOI] [PubMed] [Google Scholar]
- Fujita T., Reis L. F., Watanabe N., Kimura Y., Taniguchi T., Vilcek J. Induction of the transcription factor IRF-1 and interferon-beta mRNAs by cytokines and activators of second-messenger pathways. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9936–9940. doi: 10.1073/pnas.86.24.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gala R. R. Prolactin and growth hormone in the regulation of the immune system. Proc Soc Exp Biol Med. 1991 Oct;198(1):513–527. doi: 10.3181/00379727-198-43286b. [DOI] [PubMed] [Google Scholar]
- Gout P. W., Beer C. T., Noble R. L. Prolactin-stimulated growth of cell cultures established from malignant Nb rat lymphomas. Cancer Res. 1980 Jul;40(7):2433–2436. [PubMed] [Google Scholar]
- Gutch M. J., Daly C., Reich N. C. Tyrosine phosphorylation is required for activation of an alpha interferon-stimulated transcription factor. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11411–11415. doi: 10.1073/pnas.89.23.11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H., Kitagawa M., Tanaka N., Yamamoto H., Harada K., Ishihara M., Taniguchi T. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science. 1993 Feb 12;259(5097):971–974. doi: 10.1126/science.8438157. [DOI] [PubMed] [Google Scholar]
- Hartmann D. P., Holaday J. W., Bernton E. W. Inhibition of lymphocyte proliferation by antibodies to prolactin. FASEB J. 1989 Aug;3(10):2194–2202. doi: 10.1096/fasebj.3.10.2787766. [DOI] [PubMed] [Google Scholar]
- Kanai A., Nonomura N., Yoshimura M., Oka T. DNA-binding proteins and their cis-acting sites controlling hormonal induction of a mouse beta-casein::CAT fusion protein in mammary epithelial cells. Gene. 1993 Apr 30;126(2):195–201. [PubMed] [Google Scholar]
- Kelly P. A., Djiane J., Postel-Vinay M. C., Edery M. The prolactin/growth hormone receptor family. Endocr Rev. 1991 Aug;12(3):235–251. doi: 10.1210/edrv-12-3-235. [DOI] [PubMed] [Google Scholar]
- Kondo M., Takeshita T., Ishii N., Nakamura M., Watanabe S., Arai K., Sugamura K. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993 Dec 17;262(5141):1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- Kotanides H., Reich N. C. Requirement of tyrosine phosphorylation for rapid activation of a DNA binding factor by IL-4. Science. 1993 Nov 19;262(5137):1265–1267. doi: 10.1126/science.7694370. [DOI] [PubMed] [Google Scholar]
- Larner A. C., David M., Feldman G. M., Igarashi K., Hackett R. H., Webb D. S., Sweitzer S. M., Petricoin E. F., 3rd, Finbloom D. S. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993 Sep 24;261(5129):1730–1733. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Decker T., Strehlow I., Darnell J. E. Overlapping elements in the guanylate-binding protein gene promoter mediate transcriptional induction by alpha and gamma interferons. Mol Cell Biol. 1991 Jan;11(1):182–191. doi: 10.1128/mcb.11.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütticken C., Wegenka U. M., Yuan J., Buschmann J., Schindler C., Ziemiecki A., Harpur A. G., Wilks A. F., Yasukawa K., Taga T. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994 Jan 7;263(5143):89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Kimura T., Kitagawa M., Pfeffer K., Kawakami T., Watanabe N., Kündig T. M., Amakawa R., Kishihara K., Wakeham A. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell. 1993 Oct 8;75(1):83–97. [PubMed] [Google Scholar]
- Minami Y., Kono T., Miyazaki T., Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- Miyamoto M., Fujita T., Kimura Y., Maruyama M., Harada H., Sudo Y., Miyata T., Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988 Sep 9;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Montgomery D. W., Zukoski C. F., Shah G. N., Buckley A. R., Pacholczyk T., Russell D. H. Concanavalin A-stimulated murine splenocytes produce a factor with prolactin-like bioactivity and immunoreactivity. Biochem Biophys Res Commun. 1987 Jun 15;145(2):692–698. doi: 10.1016/0006-291x(87)91020-5. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Mastro A. M., Hymer W. C. Prolactin induction of interleukin-2 receptors on rat splenic lymphocytes. Endocrinology. 1990 Jan;126(1):88–94. doi: 10.1210/endo-126-1-88. [DOI] [PubMed] [Google Scholar]
- Müller M., Briscoe J., Laxton C., Guschin D., Ziemiecki A., Silvennoinen O., Harpur A. G., Barbieri G., Witthuhn B. A., Schindler C. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993 Nov 11;366(6451):129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- Noguchi M., Nakamura Y., Russell S. M., Ziegler S. F., Tsang M., Cao X., Leonard W. J. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993 Dec 17;262(5141):1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- Pellegrini I., Lebrun J. J., Ali S., Kelly P. A. Expression of prolactin and its receptor in human lymphoid cells. Mol Endocrinol. 1992 Jul;6(7):1023–1031. doi: 10.1210/mend.6.7.1508218. [DOI] [PubMed] [Google Scholar]
- Pellegrini S., Schindler C. Early events in signalling by interferons. Trends Biochem Sci. 1993 Sep;18(9):338–342. doi: 10.1016/0968-0004(93)90070-4. [DOI] [PubMed] [Google Scholar]
- Pine R. Constitutive expression of an ISGF2/IRF1 transgene leads to interferon-independent activation of interferon-inducible genes and resistance to virus infection. J Virol. 1992 Jul;66(7):4470–4478. doi: 10.1128/jvi.66.7.4470-4478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine R., Decker T., Kessler D. S., Levy D. E., Darnell J. E., Jr Purification and cloning of interferon-stimulated gene factor 2 (ISGF2): ISGF2 (IRF-1) can bind to the promoters of both beta interferon- and interferon-stimulated genes but is not a primary transcriptional activator of either. Mol Cell Biol. 1990 Jun;10(6):2448–2457. doi: 10.1128/mcb.10.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillema J. A., Campbell G. S., Lawson D. M., Carter-Su C. Evidence for a rapid stimulation of tyrosine kinase activity by prolactin in Nb2 rat lymphoma cells. Endocrinology. 1992 Aug;131(2):973–975. doi: 10.1210/endo.131.2.1639035. [DOI] [PubMed] [Google Scholar]
- Ruff-Jamison S., Chen K., Cohen S. Induction by EGF and interferon-gamma of tyrosine phosphorylated DNA binding proteins in mouse liver nuclei. Science. 1993 Sep 24;261(5129):1733–1736. doi: 10.1126/science.8378774. [DOI] [PubMed] [Google Scholar]
- Rui H., Djeu J. Y., Evans G. A., Kelly P. A., Farrar W. L. Prolactin receptor triggering. Evidence for rapid tyrosine kinase activation. J Biol Chem. 1992 Nov 25;267(33):24076–24081. [PubMed] [Google Scholar]
- Rui H., Kirken R. A., Farrar W. L. Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol Chem. 1994 Feb 18;269(7):5364–5368. [PubMed] [Google Scholar]
- Sadowski H. B., Shuai K., Darnell J. E., Jr, Gilman M. Z. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science. 1993 Sep 24;261(5129):1739–1744. doi: 10.1126/science.8397445. [DOI] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Schmitt-Ney M., Doppler W., Ball R. K., Groner B. Beta-casein gene promoter activity is regulated by the hormone-mediated relief of transcriptional repression and a mammary-gland-specific nuclear factor. Mol Cell Biol. 1991 Jul;11(7):3745–3755. doi: 10.1128/mcb.11.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz L. A., Stevens A. M., Hrachovy J. A., Yu-Lee L. Y. Interferon regulatory factor-1 is inducible by prolactin, interleukin-2 and concanavalin A in T cells. Mol Cell Endocrinol. 1992 Jul;86(1-2):103–110. doi: 10.1016/0303-7207(92)90180-e. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Sakal E., Cohen R., Cohen-Chapnik N., Gertler A. Hydroxyphenyl acetate derivatives inhibit protein tyrosine kinase activity and proliferation in Nb2 rat lymphoma cells and insulin-induced lipogenesis in rat adipocytes. Mol Cell Endocrinol. 1991 Sep;80(1-3):183–192. doi: 10.1016/0303-7207(91)90155-l. [DOI] [PubMed] [Google Scholar]
- Shuai K., Schindler C., Prezioso V. R., Darnell J. E., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992 Dec 11;258(5089):1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Shuai K., Ziemiecki A., Wilks A. F., Harpur A. G., Sadowski H. B., Gilman M. Z., Darnell J. E. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993 Dec 9;366(6455):580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Schindler C., Schlessinger J., Levy D. E. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science. 1993 Sep 24;261(5129):1736–1739. doi: 10.1126/science.8378775. [DOI] [PubMed] [Google Scholar]
- Sims S. H., Cha Y., Romine M. F., Gao P. Q., Gottlieb K., Deisseroth A. B. A novel interferon-inducible domain: structural and functional analysis of the human interferon regulatory factor 1 gene promoter. Mol Cell Biol. 1993 Jan;13(1):690–702. doi: 10.1128/mcb.13.1.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skwarło-Sońta K. Prolactin as an immunoregulatory hormone in mammals and birds. Immunol Lett. 1992 Jul;33(2):105–121. doi: 10.1016/0165-2478(92)90034-l. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Kawakami T., Taniguchi T. Recognition DNA sequences of interferon regulatory factor 1 (IRF-1) and IRF-2, regulators of cell growth and the interferon system. Mol Cell Biol. 1993 Aug;13(8):4531–4538. doi: 10.1128/mcb.13.8.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watling D., Guschin D., Müller M., Silvennoinen O., Witthuhn B. A., Quelle F. W., Rogers N. C., Schindler C., Stark G. R., Ihle J. N. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature. 1993 Nov 11;366(6451):166–170. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- Wilks A. F., Harpur A. G., Kurban R. R., Ralph S. J., Zürcher G., Ziemiecki A. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol. 1991 Apr;11(4):2057–2065. doi: 10.1128/mcb.11.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willman C. L., Sever C. E., Pallavicini M. G., Harada H., Tanaka N., Slovak M. L., Yamamoto H., Harada K., Meeker T. C., List A. F. Deletion of IRF-1, mapping to chromosome 5q31.1, in human leukemia and preleukemic myelodysplasia. Science. 1993 Feb 12;259(5097):968–971. doi: 10.1126/science.8438156. [DOI] [PubMed] [Google Scholar]
- Yu-Lee L. Y., Hrachovy J. A., Stevens A. M., Schwarz L. A. Interferon-regulatory factor 1 is an immediate-early gene under transcriptional regulation by prolactin in Nb2 T cells. Mol Cell Biol. 1990 Jun;10(6):3087–3094. doi: 10.1128/mcb.10.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]