Abstract
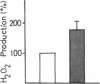
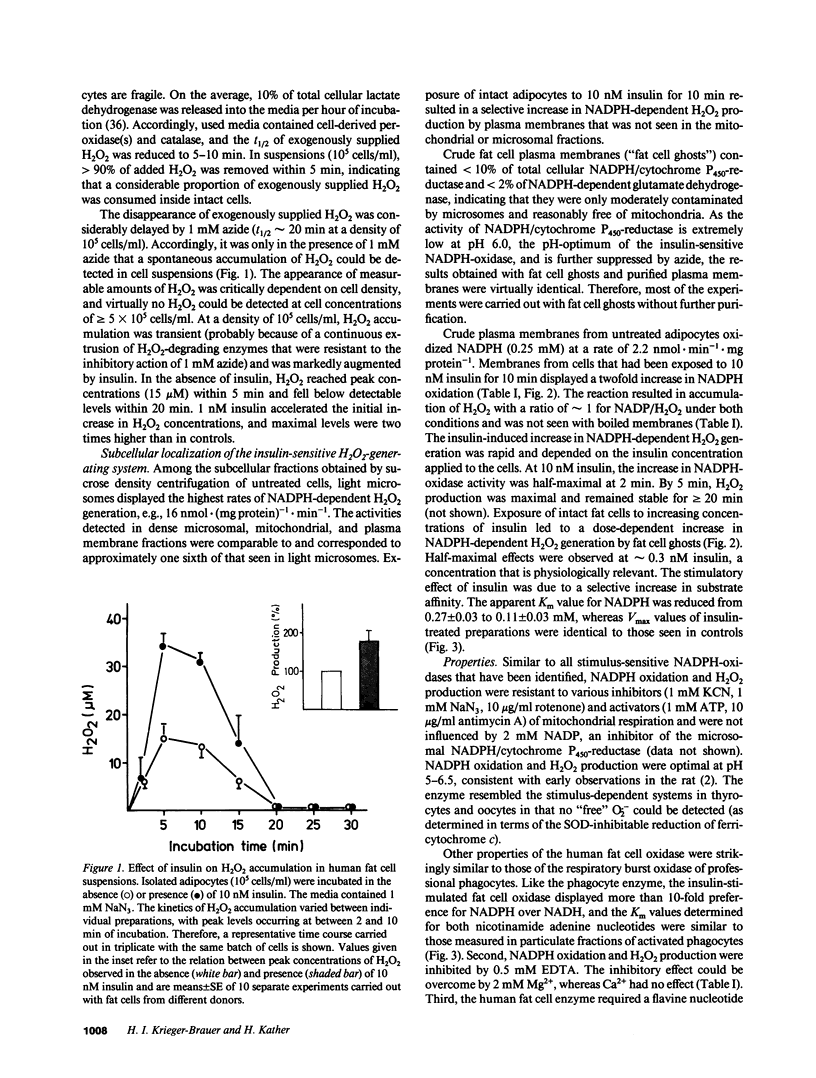
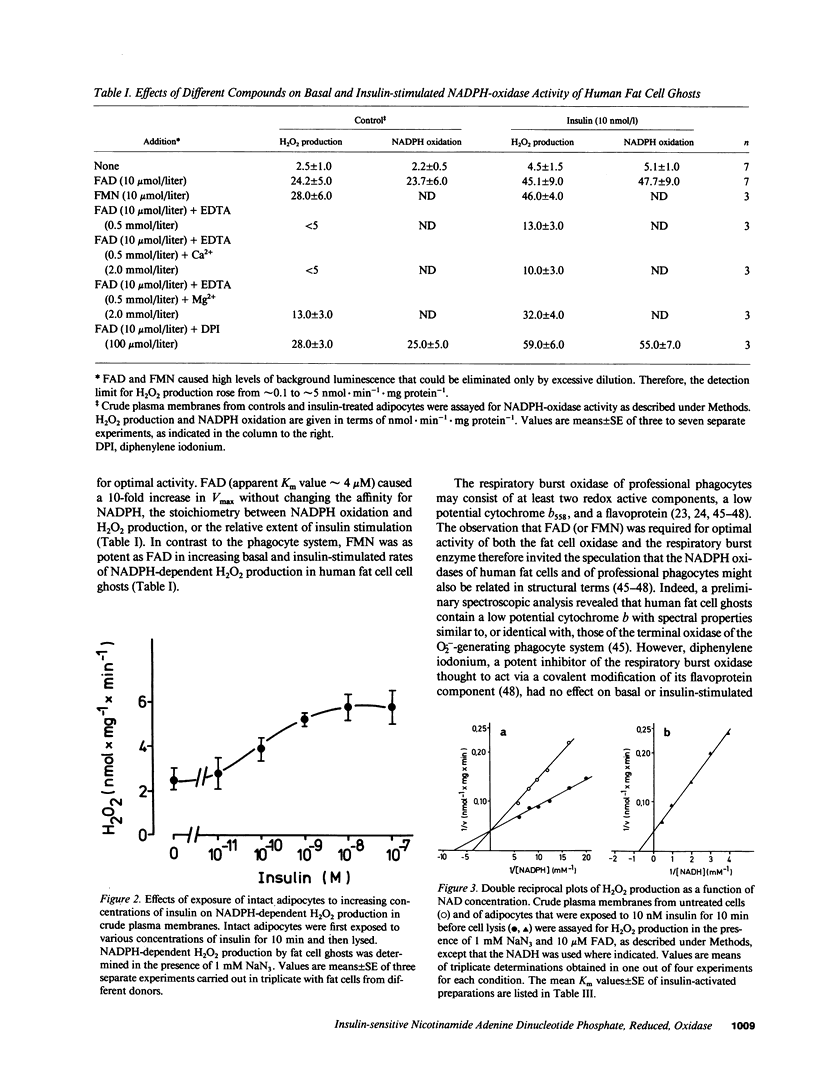
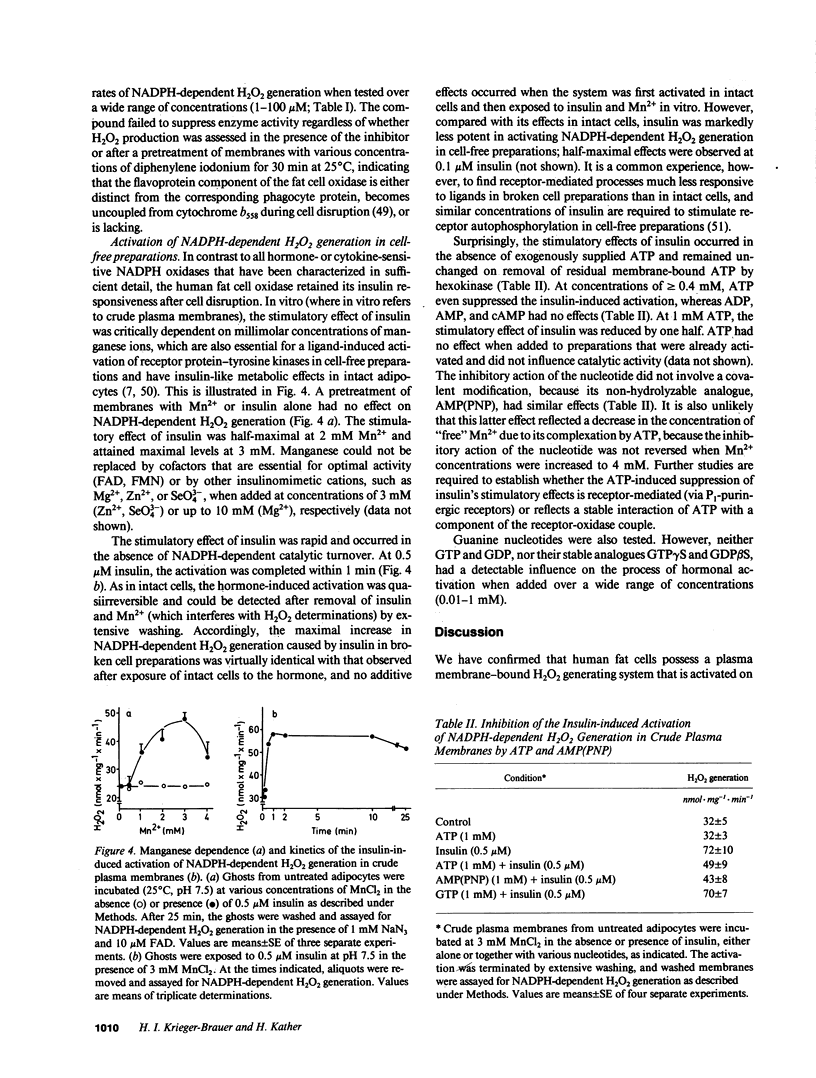
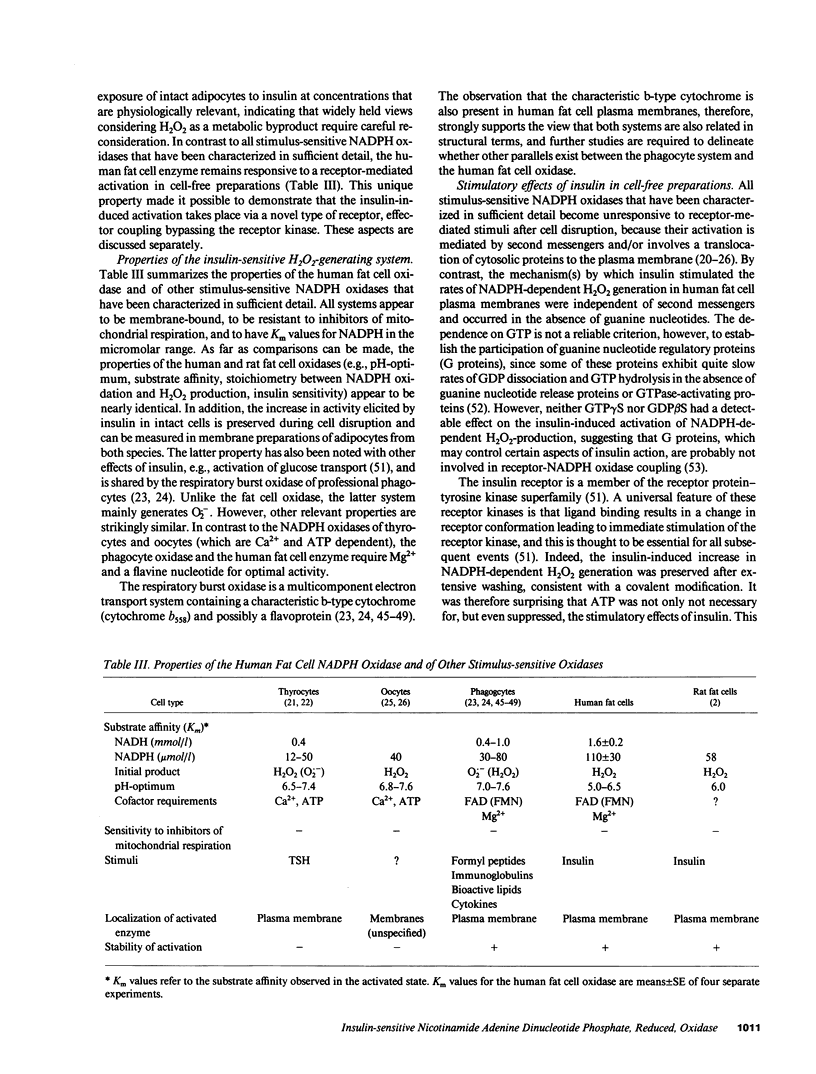
Insulin caused a transient increase in H2O2 accumulation in human fat cell suspensions that was observed only in the presence of an inhibitor of catalase and heme-containing peroxidases, such as azide, and reached peak levels of 30 microM within 5 min. The cells contained a plasma membrane-bound NADPH oxidase, producing 1 mol H2O2/mol of NADPH oxidation, that was activated on exposure of intact cells to insulin at contrations that are physiologically relevant (0.1-10 nM). The hormone effect was rapid and was due to a selective increase in substrate affinity. The enzyme was magnesium dependent, required a flavine nucleotide for optimal activity, and was most active at pH 5.0-6.5. In contrast to all other hormone- or cytokine-sensitive NADPH oxidases that have been characterized in sufficient detail, the human fat cell oxidase retained its hormone responsiveness after cell disruption, and only Mn2+, but no ATP, was required for a ligand-induced activation in crude plasma membranes. The results demonstrate that insulin utilizes tyrosine kinase-independent pathways for receptor signaling and strongly support the view that H2O2 contributes to the intracellular propagation of the insulin signal.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Becker J., Mezger V., Courgeon A. M., Best-Belpomme M. Hydrogen peroxide activates immediate binding of a Drosophila factor to DNA heat-shock regulatory element in vivo and in vitro. Eur J Biochem. 1990 May 20;189(3):553–558. doi: 10.1111/j.1432-1033.1990.tb15522.x. [DOI] [PubMed] [Google Scholar]
- Bellavite P., Corso F., Dusi S., Grzeskowiak M., Della-Bianca V., Rossi F. Activation of NADPH-dependent superoxide production in plasma membrane extracts of pig neutrophils by phosphatidic acid. J Biol Chem. 1988 Jun 15;263(17):8210–8214. [PubMed] [Google Scholar]
- Bolinder J., Lindblad A., Engfeldt P., Arner P. Studies of acute effects of insulin-like growth factors I and II in human fat cells. J Clin Endocrinol Metab. 1987 Oct;65(4):732–737. doi: 10.1210/jcem-65-4-732. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Henderson L., Jones O. T., Delpiano M. A., Hentschel J., Acker H. Involvement of an NAD(P)H oxidase as a pO2 sensor protein in the rat carotid body. Biochem J. 1990 Dec 15;272(3):743–747. doi: 10.1042/bj2720743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R. Inhibitors of the leukocyte superoxide generating oxidase: mechanisms of action and methods for their elucidation. Free Radic Biol Med. 1990;8(1):71–93. doi: 10.1016/0891-5849(90)90147-b. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T., Harper A. M., Segal A. W. Oxidation-reduction properties of the cytochrome b found in the plasma-membrane fraction of human neutrophils. A possible oxidase in the respiratory burst. Biochem J. 1981 Feb 15;194(2):599–606. doi: 10.1042/bj1940599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deme D., Virion A., Hammou N. A., Pommier J. NADPH-dependent generation of H2O2 in a thyroid particulate fraction requires Ca2+. FEBS Lett. 1985 Jul 1;186(1):107–110. doi: 10.1016/0014-5793(85)81349-1. [DOI] [PubMed] [Google Scholar]
- DiGirolamo M., Edén S., Enberg G., Isaksson O., Lönnroth P., Hall K., Smith U. Specific binding of human growth hormone but not insulin-like growth factors by human adipocytes. FEBS Lett. 1986 Sep 1;205(1):15–19. doi: 10.1016/0014-5793(86)80856-0. [DOI] [PubMed] [Google Scholar]
- Ekholm R. Biosynthesis of thyroid hormones. Int Rev Cytol. 1990;120:243–288. doi: 10.1016/s0074-7696(08)61602-2. [DOI] [PubMed] [Google Scholar]
- Gabig T. G., Babior B. M. The O2(-) -forming oxidase responsible for the respiratory burst in human neutrophils. Properties of the solubilized enzyme. J Biol Chem. 1979 Sep 25;254(18):9070–9074. [PubMed] [Google Scholar]
- Garrett I. R., Boyce B. F., Oreffo R. O., Bonewald L., Poser J., Mundy G. R. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990 Mar;85(3):632–639. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk W. K. The pathway mediating insulin's effects on pyruvate dehydrogenase bypasses the insulin receptor tyrosine kinase. J Biol Chem. 1991 May 15;266(14):8814–8819. [PubMed] [Google Scholar]
- Hayes G. R., Lockwood D. H. Role of insulin receptor phosphorylation in the insulinomimetic effects of hydrogen peroxide. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8115–8119. doi: 10.1073/pnas.84.22.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffetz D., Bushkin I., Dror R., Zick Y. The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. J Biol Chem. 1990 Feb 15;265(5):2896–2902. [PubMed] [Google Scholar]
- Heinecke J. W., Meier K. E., Lorenzen J. A., Shapiro B. M. A specific requirement for protein kinase C in activation of the respiratory burst oxidase of fertilization. J Biol Chem. 1990 May 15;265(14):7717–7720. [PubMed] [Google Scholar]
- Heinecke J. W., Shapiro B. M. Respiratory burst oxidase of fertilization. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1259–1263. doi: 10.1073/pnas.86.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbel R. E. Insulin-like growth factors I and II. Eur J Biochem. 1990 Jul 5;190(3):445–462. doi: 10.1111/j.1432-1033.1990.tb15595.x. [DOI] [PubMed] [Google Scholar]
- Hurst J. K., Barrette W. C., Jr Leukocytic oxygen activation and microbicidal oxidative toxins. Crit Rev Biochem Mol Biol. 1989;24(4):271–328. doi: 10.3109/10409238909082555. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol. 1991 Feb 15;41(4):485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- Kather H. Purine accumulation in human fat cell suspensions. Evidence that human adipocytes release inosine and hypoxanthine rather than adenosine. J Biol Chem. 1988 Jun 25;263(18):8803–8809. [PubMed] [Google Scholar]
- Kather H., Wieland E., Waas W. Chemiluminescent determination of adenosine, inosine, and hypoxanthine/xanthine. Anal Biochem. 1987 May 15;163(1):45–51. doi: 10.1016/0003-2697(87)90091-1. [DOI] [PubMed] [Google Scholar]
- Kuthan H., Ullrich V., Estabrook R. W. A quantitative test for superoxide radicals produced in biological systems. Biochem J. 1982 Jun 1;203(3):551–558. doi: 10.1042/bj2030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laporte F., Doussiere J., Vignais P. V. Respiratory burst of rabbit peritoneal neutrophils. Transition from an NADPH diaphorase activity to an .O2(-)-generating oxidase activity. Eur J Biochem. 1990 Nov 26;194(1):301–308. doi: 10.1111/j.1432-1033.1990.tb19457.x. [DOI] [PubMed] [Google Scholar]
- Luttrell L., Kilgour E., Larner J., Romero G. A pertussis toxin-sensitive G-protein mediates some aspects of insulin action in BC3H-1 murine myocytes. J Biol Chem. 1990 Oct 5;265(28):16873–16879. [PubMed] [Google Scholar]
- Malter J. S., Hong Y. A redox switch and phosphorylation are involved in the post-translational up-regulation of the adenosine-uridine binding factor by phorbol ester and ionophore. J Biol Chem. 1991 Feb 15;266(5):3167–3171. [PubMed] [Google Scholar]
- Maly F. E., Cross A. R., Jones O. T., Wolf-Vorbeck G., Walker C., Dahinden C. A., De Weck A. L. The superoxide generating system of B cell lines. Structural homology with the phagocytic oxidase and triggering via surface Ig. J Immunol. 1988 Apr 1;140(7):2334–2339. [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol. 1986 Nov 15;137(10):3295–3298. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McKeel D. W., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. J Cell Biol. 1970 Feb;44(2):417–432. doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier B., Radeke H. H., Selle S., Younes M., Sies H., Resch K., Habermehl G. G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem J. 1989 Oct 15;263(2):539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller D. E., Benecke H., Flier J. S. Biologic activities of naturally occurring human insulin receptor mutations. Evidence that metabolic effects of insulin can be mediated by a kinase-deficient insulin receptor mutant. J Biol Chem. 1991 Jun 15;266(17):10995–11001. [PubMed] [Google Scholar]
- Muchmore D. B., Little S. A., de Haën C. Counterregulatory control of intracellular hydrogen peroxide production by insulin and lipolytic hormones in isolated rat epididymal fat cells: a role of free fatty acids. Biochemistry. 1982 Aug 3;21(16):3886–3892. doi: 10.1021/bi00259a025. [DOI] [PubMed] [Google Scholar]
- Mukherjee S. P., Lynn W. S. Reduced nicotinamide adenine dinucleotide phosphate oxidase in adipocyte plasma membrane and its activation by insulin. Possible role in the hormone's effects on adenylate cyclase and the hexose monophosphate shunt. Arch Biochem Biophys. 1977 Nov;184(1):69–76. doi: 10.1016/0003-9861(77)90327-7. [DOI] [PubMed] [Google Scholar]
- Mukherjee S. P., Mukherjee C. Stimulation of pyruvate dehydrogenase activity in adipocytes by oxytocin: evidence for mediation of the insulin-like effect by endogenous hydrogen peroxide independent of glucose transport. Arch Biochem Biophys. 1982 Mar;214(1):211–222. doi: 10.1016/0003-9861(82)90024-8. [DOI] [PubMed] [Google Scholar]
- Murrell G. A., Francis M. J., Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990 Feb 1;265(3):659–665. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo T. T., Lenhoff H. M. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal Biochem. 1980 Jul 1;105(2):389–397. doi: 10.1016/0003-2697(80)90475-3. [DOI] [PubMed] [Google Scholar]
- Paetzke-Brunner I., Wieland O. H. Activation of the pyruvate dehydrogenase complex in isolated fat cell mitochondria by hydrogen peroxide and t-butyl hydroperoxide. FEBS Lett. 1980 Dec 15;122(1):29–32. doi: 10.1016/0014-5793(80)80394-2. [DOI] [PubMed] [Google Scholar]
- Pollard H. B., Stopak S. S., Pazoles C. J., Creutz C. E. A simple and novel method for radiometric analysis of glucose utilization by adrenal chromaffin cells. Anal Biochem. 1981 Jan 15;110(2):424–430. doi: 10.1016/0003-2697(81)90214-1. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Ramasarma T. Generation of H2O in biomembranes. Biochim Biophys Acta. 1982 Aug 11;694(1):69–93. doi: 10.1016/0304-4157(82)90014-4. [DOI] [PubMed] [Google Scholar]
- Rossi F. The O2- -forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim Biophys Acta. 1986 Nov 4;853(1):65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Ames B. N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990 Apr 13;248(4952):189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Regulation of ferritin and transferrin receptor mRNAs. J Biol Chem. 1990 Mar 25;265(9):4771–4774. [PubMed] [Google Scholar]
- Thomas G., Ramwell P. Induction of vascular relaxation by hydroperoxides. Biochem Biophys Res Commun. 1986 Aug 29;139(1):102–108. doi: 10.1016/s0006-291x(86)80085-7. [DOI] [PubMed] [Google Scholar]
- Toledano M. B., Leonard W. J. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Robinson F. W., Smith M. M., Kono T. Effects of divalent cations on the regulation of insulin-sensitive glucose transport and cAMP phosphodiesterase in adipocytes. Insulin-like effects of divalent cations. J Biol Chem. 1984 Aug 10;259(15):9520–9525. [PubMed] [Google Scholar]
- Wente S. R., Villalba M., Schramm V. L., Rosen O. M. Mn2(+)-binding properties of a recombinant protein-tyrosine kinase derived from the human insulin receptor. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2805–2809. doi: 10.1073/pnas.87.7.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K. T., Khalaf N., Czech M. P. Insulin stimulates the tyrosine phosphorylation of a Mr = 160,000 glycoprotein in rat adipocyte plasma membranes. J Biol Chem. 1987 Jun 5;262(16):7865–7873. [PubMed] [Google Scholar]
- Zick Y. The insulin receptor: structure and function. Crit Rev Biochem Mol Biol. 1989;24(3):217–269. doi: 10.3109/10409238909082554. [DOI] [PubMed] [Google Scholar]
- Ziegler D. M. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]
- Zoccarato F., Deana R., Cavallini L., Alexandre A. Generation of hydrogen peroxide by cerebral-cortex synaptosomes. Stimulation by ionomycin and plasma-membrane depolarization. Eur J Biochem. 1989 Mar 15;180(2):473–478. doi: 10.1111/j.1432-1033.1989.tb14670.x. [DOI] [PubMed] [Google Scholar]