Abstract
Purpose of review
Right ventricular failure (RVF) is associated with significant morbidity and mortality. There is an increasing interest in proper assessment of right ventricle (RV) function as well as understanding mechanisms behind RVF.
Recent findings
Within this manuscript, we discuss the metabolic changes that occur in the RV that occur in response to RVF- in particular, a shift towards glycolysis and increased glutaminolysis. We will detail the advances made in non-invasive imaging in assessing the function of the RV and review the methods to assess right ventricle-pulmonary artery coupling. We lastly investigate the role of new treatment options in the failing RV, such as β-blocker therapy.
Summary
RVF is a complicated entity. Although some inferences on RV function and treatment can be made from LV, the RV has unique features, anatomically, metabolically and embryologically, which requires dedicated RV-directed research.
Keywords: Right Ventricle, Right Heart Failure, Ventricular-Vascular Coupling, Warburg effect, glycolysis, Randle's cycle
Introduction
For many years, the right heart and its potential role in disease had been largely ignored. Observations from patients with Fontan circulation who could live for periods of time without heart failure symptoms contributed to the belief that the right heart was essentially nothing more than a passive conduit. These patients in fact did do well, until the systemic ventricle began to fail or pulmonary hypertension (PH) developed – both situations in which RV afterload is increased. It is now evident that the function of the right heart is directly related to outcomes in many disease states including pulmonary arterial hypertension (PAH) [1,2], heart failure with reduced [3,4] and preserved [5,6] ejection fraction, and heart failure after implantation of left ventricular assist devices (LVAD).[7] The International Right Heart Foundation Working Group recently proposed a comprehensive definition of right heart failure: “ a clinical syndrome due to an alteration of structure and/or function of the right heart circulatory system that leads to suboptimal delivery of blood flow (high or low) to the pulmonary circulation and/or elevated venous pressures—at rest or with exercise.”[8] Although the RV is a very important part of the right heart system (and the main focus of this review), failure of any component of the circulation from the systemic veins up to the pulmonary capillaries can result in right heart failure symptoms.
The Normal Right Ventricle
The normal RV is a thin-walled crescent shaped structure that is composed of the RV free wall, interventricular septum, and RV outflow tract. Although it is a distinct embryologic structure from the left ventricle (LV), the interventricular septum has shared fibers with the LV. [9] As a consequence of these shared fibers, the LV accounts for as much as 30-50% of RV contractile function. [10] The septum itself, via its efficient longitudinal/twisting contraction, contributes most to overall RV function as compared with the transverse motion of the RV free wall.[9] In fact, it has been is estimated that longitudinal shortening accounts for almost 80% of RV function in normal physiologic states.[11]
Etiology of Right Ventricular Failure
The etiologies of RV failure (RVF) are many and varied but LV failure remains the leading cause (Table 1). The same factors that lead to left-sided dysfunction can also impact the RV, causing intrinsic RV dysfunction. RV contractility may further be reduced because the RV depends on the LV for a substantial portion of its function as noted above.
Table 1.
Etiology of Right Ventricular Failure
| Increased afterload | Pulmonary Hypertension, Group 1-5. Increased left ventricular end-diastolic pressure. Mitral valve disease. Hypoxic pulmonary vasoconstriction. Pulmonary thrombo-embolus, acute or chronic. Pulmonary embolus (septic, amniotic, fat, air, injectate, other). Pulmonary valve stenosis. Right ventricular outflow tract obstruction. Vaso-occlusive sickle cell crisis. Mechanical ventilation. |
| Decreased preload | Hypovolemia. Systemic vasodilatory shock (anaphylaxis, extensive burn injury, sepsis, other). Tamponade. Constrictive pericarditis. Superior vena cava syndrome. Tricuspid stenosis. |
| Right ventricular myocardial abnormality | Right ventricular infarction. Infiltrative and restrictive cardiomyopathy. Arrhythmogenic right ventricular dysplasia. Cardiomyopathy, in particular left ventricular systolic dysfunction. Right ventricular ischemia in setting of right ventricular pressure overload. Microvascular diseases and capillary rarefaction. |
The RV normally ejects blood into a low afterload, highly compliant arterial circuit. When afterload is increased, especially acutely, a marked reduction in RV function can occur.[12] Elevated left-sided filling pressures not only increase pulmonary pressure passively but also lower vascular compliance, thereby augmenting pulsatile RV afterload.[13] Over time, LV failure may also precipitate pulmonary arterial vasoconstriction and/or remodeling further elevating afterload. In some cases of chronic or slowly progressive elevations in afterload, the RV is able to compensate relatively well, as is the case with Eisenmenger's syndrome.[14] In others, it is not. Continued efforts are underway to diagnose and predict the development of RVF secondary to increased left-sided filling pressures, most particularly as it complicates implantation of LVADs. With increased understanding of cardiomyocyte dysfunction in PH and RVF, it is worth reviewing the advances made in the past year on molecular signaling in the failing RV.
Molecular Changes in the Right Ventricle
There is increasing appreciation and acceptance of the metabolic changes that develop in the failing RV[15], and has been particularly well studied in PAH-associated RVF. In RVF, a metabolic shift from mitochondrial oxidative phosphorylation to cytoplasmic glycolysis has been identified as the RV undergoes hypertrophy, as evidenced by increased uptake of Fluorodeoxyglucose-Positron emission tomography (FDG- PET) scan[16] and by direct measurement of metabolism in an RV working heart model.[17] This increase in glycolysis is accompanied by a decrease in fatty acid oxidation, referred to as the Warburg effect, a phenomenon that has traditionally been used in discussing metabolism of cancer cells[18], and is mediated by hyperpolarized mitochondrial membrane potential. In PAH, the accompanying increase in RV afterload creates an ischemic RV, due at least in part to compromised right coronary artery (RCA) blood flow.[19] Furthermore, there is observed capillary rarefaction in the RV of patients with PAH, most particularly with scleroderma-associated PAH.[17]
In the setting of an ischemic myocardium, mitochondria-dependent apoptosis is suppressed and there is decreased production of mitochondria-derived reactive oxygen species (mROS), potentially to minimize stress in a hypertrophying myocardium.[20] While the RV remains compensated, there is an increase in Hypoxia-Inducible Factor 1α(HIF1α), which may promote angiogenesis to counteract for the increased O2 demands of a hypertrophied RV. When the RV becomes decompensated, there is an increase in mROS, a decrease in HIF1α and a decrease in glucose oxidation. In this setting, patients with PAH enter into a syndrome of pronounced RVF, especially as the decrease in HIF1α is accompanied by decreased angiogenesis and exacerbation of ischemia in the RV.[20]
Additive to the cancer phenotype is the recent observation that there is an increase in glutaminolysis within the RV of patients and animals with PAH.[21] Glutaminolysis is the metabolism of glutamine, which in cancer allows for rapid cell growth without apoptosis.[22] In RVH, capillary rarefaction and RV ischemia activate the proto-oncogene cMyc, which increases glutamine uptake and in turn increases production of α-ketoglutarate (α-KG). α-KG then enters Krebs cycle producing malate. This Krebs cycle-derived malate then generates cytosolic pyruvate, which in turn is converted by lactate dehydrogenase A to lactate. In the setting of increased glutaminolysis, this is accompanied by inhibition of glucose oxidation. This maladaptive pathway has the potential to be therapeutically manipulated by inhibitors of glutaminolysis, which would then optimize cellular metabolism in the failing RV by increasing glucose oxidation.
Peroxisome proliferator-activated receptor-gamma coactivator 1alpha (PGC-1α) governs a large numbers of genes that regulate mitochondrial function[23] and has been shown to be downregulated in the failing RV.[24] This decreased expression of RV PGC-1α results in a loss of mitochondrial protein and oxidative capacity. In fact, not only is this decrease in PGC-1α associated with decreased mitochondrial number, but also the mitochondria become hyperpolarized, abnormally shaped and have a reduced ADP-stimulated (state 3) rate for complex I.[24] These structural and functional abnormalities of the RV mitochondria provide credence to the theory of mitochondrially-mediated metabolic abnormalities in the failing RV and offer therapeutic potential in the form of metabolic modulators such as the pyruvate dehydrogenase kinase inhibitor, dichloroacetate (DCA), which is currently the study of clinical trials in PAH.[25]
There is ongoing debate and controversy regarding the sequence of events in the RV, namely the timely of mitochondrial dysfunction as it relates to the development of RVF[26], but it is apparent that the glycolytic shift that occurs in the RV is a maladaptive response and can ultimately contribute to the “burning out” of the RV.[26] Furthermore, the loss of the angiogenic program contributes to the loss of capillaries which contributes to ischemia and the loss of mitochondrial hyperpolarization facilitates myocardial apoptosis, which can potentially thin out the RV and contribute to RV decompensation and failure.[20]
Assessment of Right Ventricular Function
Non-invasive Measures
Accurate and comprehensive assessment of RV function remains a challenge. The RV can be technically difficult to image and the afterload dependence of the RV can also lead to misleading interpretations of intrinsic RV function. Noninvasively, echocardiography and magnetic resonance imaging (MRI) are the most often used clinical tools. Because the majority of RV function occurs via longitudinal shortening, tricuspid annular systolic plane excursion (TAPSE) is an attractive candidate to assess RV function. TAPSE has been shown to correlate with outcomes in multiple cohorts of patients with PAH[2,27] and LV failure.[4-6] However, other data suggests changes in TAPSE may not be reliable marker of disease progression[28] and may also not represent global RV function in congenital heart disease[29] or after cardiac surgery.[30] After cardiac surgery, longitudinal shortening is depressed – from acquired septal dysfunction[31] and/or loss of pericardial constraint[32] - and the RV becomes more dependent on transverse shortening by the RV free wall.[30] Despite the decline in longitudinal function, RV fraction area change (RVFAC) and RV ejection fraction (RVEF) may be preserved, especially if afterload is low.[30,33] Although RVEF is difficult to interpret by conventional echocardiography, RVFAC and TAPSE correlated well with MRI derived RVEF in a recent study of patients with PH[34].
Strain imaging is increasingly being utilized to assess RV function. Strain is likely a composite measure of RV loading and dysfunction and as such abnormal strain patterns likely develop from microvascular ischemia (Table 1) as well as myocardial disarray from distension of the RV. Sachdev studied RV longitudinal strain in 80 patients with PAH and discovered that worse RV strain was associated with disease progression, higher diuretic use, and mortality.[35] Although risk scores have traditionally focused on clinical parameters[36] to predict RVF after LVAD implantation, more recent efforts have concentrated on using echocardiographic strain assessments of the RV to risk stratify patients. In this setting, RV strain patterns have been shown to add incremental benefit on top of clinical risk scores.[37] Free-wall RV longitudinal strain also best predicts clinical outcomes in patients referred for heart transplantation, when compared with N-terminal pro-BNP, global strain patterns, left ventricular ejection fraction and other echocardiographic parameters.[38] Additionally, in Group 1, 3 and 4 PH, RV longitudinal peak systolic strain as assessed and measured by speckle-tracking echocardiography can predict survival even when adjusted for invasive hemodynamics and RV strain provides incremental prognostic value over conventional echocardiographic and clinical variables.[39] Serial assessments and changes in strain rates in response to treatment may also have prognostic value[40], although reliance solely on longitudinal strain may not account for changes in the contractile patterns late in disease or after surgery.[41] Freed recently demonstrated RV longitudinal strain measured by echocardiogram correlated with MRI-derived RVEF but only correlated moderately with MRI-derived strain.[42] This later finding suggests strain measures may not be interchangeable among imaging modalities. Routine assessment of RV strain in patients with risk factors and/or diseases that can predispose to RV failure may become a necessary skill to develop in echocardiography laboratories over the next few years.
Cardiac MRI has become the gold standard for noninvasive assessment of RV function and is then most accurate method for determining RV mass, volume, and EF.[43] Van de Veerdonk and colleagues found a change in RVEF, assessed by MRI, was the variable most predictive of outcome in patients receiving PAH-specific therapy.[1] In another study by the same group, increases in RV size and declining RVEF predicted clinical worsening in patients with idiopathic PAH.[44] Investigators from the multicenter EURO-MR study have shown on-treatment changes in RV (especially RVEF) and LV parameters predict improvement in functional capacity.[45] As in PAH, lower RVEF portends a worse outcome in left heart failure.[3,46]
Invasive Hemodynamics
Hemodynamic variables obtain via right heart catheterization may aid in assessing right heart function. Elevated right atrial pressure (RAP) and low cardiac index are associated with worse survival in historical and more modern cohorts of PAH.[47,48] However, one must remember than factors other than RV dysfunction may contribute to elevations in RAP including pericardial constraint .[49] Elevation in pulmonary vascular resistance (PVR) (>3 Wood units) is associated with poor prognosis in left heart disease [50,51] and PAH.[48] More recently, pulmonary vascular compliance (estimated as stroke volume/pulmonary pulse pressure) has shown prognostic value in PAH [52] as well as left heart failure.[53-55] In left heart failure, compliance appears more predictive than PVR, because it incorporates the effect of pulsatile loading caused by elevated left side filling pressures.[13,53] Despite their prognostic value, both PVR and compliance are measures of afterload and do not directly reflect RV function.
Measures of Right Ventricular-Pulmonary Arterial Coupling
The gold standard for assessment of ventricular function and the effect of afterload is through the relation of ventricular pressure and volume. This type of analysis has been extensively performed in the LV, but the same principles also hold true for the RV. [56,57] By varying preload or afterload, a family of PV loops can be created, and the maximal ratio of pressure to volume (P/[V-Vo], where Vo is the volume-intercept) can be determined for each loop. As opposed to the LV, the normal RV pressure-volume (PV) loop is triangular in shape (Figure 1A) with pressure declining throughout ejection - indicative of the low impedance vascular system. Therefore, this maximal ratio of pressure and volume may not occur near endsystole, making PV assessment more difficult. However, the shape of the RV PV loop changes to more of a square shape (similar to the LV) in mild PH (Figure 1B) or even a trapezoidal shape in more severe PH (Figure 1C), with pressure continuing to rise throughout ejection[58]. In the latter two cases, the maximal ratio of pressure and volume will occur near end-systole as it does in the LV. The end-systolic pressure-volume relationship (ESPVR) can then be determined by connecting the end-systolic pressure (ESP) points of each PV loop, the slope of which is the endsystolic elastance (Ees) - a relatively load-independent measure of contractility. By comparison, TAPSE, RVFAC, and RVEF are all load-dependent, and therefore do not directly measure intrinsic RV contractility. Afterload may be estimated via the PV loop as the effective arterial elastance (Ea) – ESP divided by the stroke volume (SV). This ‘lumped’ parameter of afterload takes into account both resistive and pulsatile components and more completely describes total RV afterload than PVR alone. The ratio of these two elastances (Ees/Ea) can then be used to illustrate ventricular-vascular coupling. Measures of coupling are particularly attractive because they may help identify sub-clinical right heart failure. Impaired RV-PA coupling in systemic sclerosis-associated PAH compared with idiopathic PAH was recently shown via this technique when other imaging and hemodynamic variables failed to discriminate between the two groups.[58]
Figure 1.
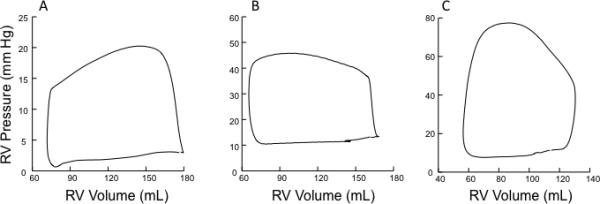
Examples of human right ventricular pressure-volume loops A) under normal loading conditions B) in mild pulmonary hypertension C) in more severe pulmonary hypertension. For B) and C), pressure rises throughout ejection and peaks near end-systole.
Although certainly feasible, these types of invasive measures require specialized equipment and significant expertise, and are not practical for routine clinical use. Therefore, less invasive ways to measure RV-PA coupling are currently being sought. One of these approaches uses a single, steady-state PV loop to estimate Ees, negating the need for load variation and multi-beat measures (so-called ‘single beat’ methods). First proposed by Brimioulle for the RV[59], one technique involves fitting a sine wave to the isovolumic portions of the RV pressure tracing to determine Pmax (Figure 2A), the theoretical maximal pressure the RV could generate if ejecting into infinite load. Thus, a second point (Pmax, end-diastolic volume) along the ESPVR is determined and Ees can be calculated (Figure 2B). Although good correlation was found between estimated and actual Pmax in normotensive dogs, the technique was not sensitive to inotropic induced changes in contractility[60] and was not predictive of outcome in a recent human study.[61] A second, even simpler estimation of Ees, assumes the volume intercept of the ESPVR is zero (Vo=0). In this case, Ees could be estimated as the ratio of ESP/end-systolic volume (ESV), and the ratio of elastances as SV/ESV[62]. Recently, a study by Vanderpool and colleagues, found SV/ESV was a predictor of survival in 50 patients with PAH[61]. The Vo=0 is approach does have limitations however as it appear to understimate Ees[63]. Additionally, Vo can be highly variable depending on the contractile state of the RV[58], and therefore it is unlikely one can assume Vo=0. It is also important to remember that RVEF is directly related to Ees/Ea only if it is assumed that Vo=0[64], and therefore is not a direct measure RV-PA coupling.
Figure 2.
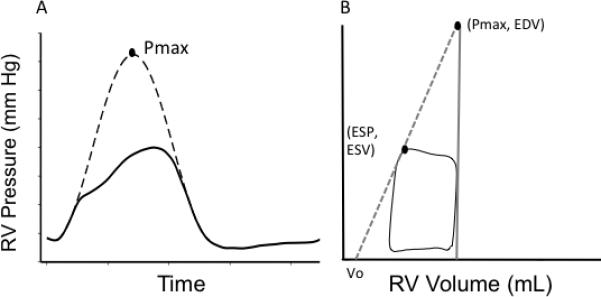
Single-beat method of calculating end-systolic elastance (Ees): A) Sine wave is fit to the isovolumetric portions of a high-fidelity RV pressure tracing to determine Pmax. B) End-systolic elastance (slope of the grey dotted line) can be determined by the two points (Pmax, end-diastolic volume) and (end-systolic pressure, end-systolic volume).
Given the limitations with direct measures of coupling, yet its importance in our understanding of the RV, other indirect measures of coupling have been explored. Combining noninvasive measures of contractile function and afterload into single parameters has shown prognostic promise in left heart failure[4] and predicted RV failure after LVAD implantation.[65] Using a pig model of PAH, Guihaire and colleagues recently showed that RV contractile reserve - measured by change in SV index, dP/dt max and Ees - were strongly related to resting RV-PA coupling.[66] Sharma reported RV contractile reserve limitation in 18 PAH patients, including those with “normal” resting function. In this group, lack of RV reserve correlated linearly with exercise capacity.[67] The idea that RV reserve may be predictive of resting coupling is intriguing because RV reserve (or lack thereof) has been predictive of prognosis in several recent PAH studies.[68,69] The relationship of RV reserve to resting coupling and its use as a clinical tool warrants further investigation.
Management of Right Ventricular Failure
The optimum management of RV failure remains elusive, especially when it comes to the choice of inotropic agent to support the decompensated RV. In a recent study of PAH providers, there was considerable disagreement as to the first line inotrope in patients with PAH-related RVF with no standardized practice or globally accepted guidelines currently available for RVF.[70] Animal models of PAH provide some insight to support claims that inotropy in RVF (secondary to PAH) is best achieved with dobutamine due to improved coupling to adenylyl cyclase.[71] The potency of inotropes in the RV Langendorff model (in descending order) is: dobutamine = isoproterenol > dopamine > phenylephrine. Additionally, the superiority of dobutamine over dopamine may reflect the fact that dopamine relies heavily on Dopamine-1 receptor signaling, which is impaired in RV hypertrophy.[71]
Admission to an intensive care unit for RVF and consequent inotropic support has an inpatient mortality rate of over 40%.[72] In this setting, and with the absence of standardized protocols, there has been some interest in developing mechanical circulatory support targeting the RV.[73] To this end, the RECOVER Right Study is ongoing[74] in patients who developed RVF within 48 hours after implantation of a LVAD, or patients in post-cardiotomy shock or patients with post- acute myocardial infarction (AMI) shock. The results of this study will provide insight into the ability to unload the RV.
Although there is downregulation and desensitization of adrenergic (β1- and α1-adrenoreceptors) receptors in RVF, there is increasing interest in the feasibility of the use of β-blocker in chronic RVF (likely not acute RVF, akin to the practice in left ventricular systolic dysfunction). Carvedilol can reverse established RVF in two different rat models of PAH and the improvement in RV function is associated with improved capillary density and reduced hypertrophy of the myocardium.[75] Additionally, bisoprolol has been shown to delay progression toward RVF in animal model of PAH, and can, at least in part, preserve RV systolic and diastolic function.[76] Of note, genes encoding proteins involved in the mitochondrial dysfunction pathway are upregulated in PAH animals treated with carvedilol and protein ubiquitination pathways and genes encoding proteins involved in cardiac hypertrophy are downregulated in the RV by carvedilol.[77] When formally studied in humans with PAH, β- blockers are found to at least be safe. However, although no adverse events have been identified[78], no therapeutic clinical benefit have yet been observed either[79], so this will remain an area of investigation over the coming years as our efforts to modulate RV metabolism continue.
Conclusion
The diagnosis and treatment of the RVF continues to be challenging. As our understanding of molecular mechanisms evolve, we should be able to offer more therapeutic options of this final common pathway. However, whether the therapeutic targets will be on the neuroendocrine system, or metabolic modulators remains to be seen and will be dependent on insight offered by cellular studies as well as through intricate hemodynamics.
Key points.
- Comprehensive assessment of RV function is difficult as it requires consideration of both RV contractility and afterload (RV-PA coupling).
- RV contractile reserve is prognostic in pulmonary hypertension and may identify patients with uncoupling at rest.
- RV failure has a very poor prognosis and no standardized treatment options are available.
- RV failure is accompanied by increased glycolysis, decreased glucose oxidation and increased glutaminolysis- and these abnormalities in metabolism are mediated through mitochondrial dysfunction.
Acknowledgements
None.
Financial support and sponsorship: RJT is supported by funding from the National Heart, Lung, and Blood Institute (grants 1R01HL114910-03 and L30 HL110304).
Footnotes
Conflicts of interest: None.
References
- 1.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive Right Ventricular Dysfunction in Patients With Pulmonary Arterial Hypertension Responding to Therapy. J Am Coll Cardiol. 2011;58:2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 2.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid Annular Displacement Predicts Survival in Pulmonary Hypertension. Am J Respir Crit Care Med. 2006;174:1034–1041. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 3.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- *4.Guazzi M, Bandera F, Pelissero G, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. American Journal of Physiology - Heart and Circulatory Physiology. 2013;305:H1373–H1381. doi: 10.1152/ajpheart.00157.2013. [Two hundred ninety-three HF patients with reduced (HFrEF, n = 247) or with preserved left ventricular (LV) ejection fraction (HFpEF, n = 46) underwent echo-Doppler studies and were tracked for adverse events. The TAPSE to PASP ratio is shifted downward in nonsurvivors and was useful prognotically as a measure of RV contractile function.] [DOI] [PubMed] [Google Scholar]
- *5.Melenovsky V, Hwang S, Lin G, et al. Right heart dysfunction in heart failure with preserved ejection fraction. European Heart Journal. 2014 doi: 10.1093/eurheartj/ehu193. [Heart failure and preserved ejection fraction patients (n = 96) and controls (n = 46) were referred for right heart catheterization, echocardiographic assessment, and follow-up. RV failure was common in HFpEF and was caused by both RV contractile impairment and afterload mismatch from pulmonary hypertension.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *6.Mohammed SF, Hussain I, Abou Ezzeddine OF, et al. Right Ventricular Function in Heart Failure with Preserved Ejection Fraction: A Community Based Study. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.113.008461. [562 patients with HFpEF from Olmsted County, Minnesota underwent echocardiography at HF diagnosis and followed up for cause specific mortality and HF hospitalization. RV failure was found to be common in HFpEF patients, and was predictive of poorer outcomes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: A 10,000-patient database. The Journal of Heart and Lung Transplantation. 2014;33:555–564. doi: 10.1016/j.healun.2014.04.010. [Report from the INTERMACS registry of LVAD implantations. One of several analysis shows right ventricular failure after implant portends a worse prognosis.] [DOI] [PubMed] [Google Scholar]
- *8.Mehra MR, Park MH, Landzberg MJ, et al. Right heart failure: toward a common language. Pulmonary Circulation. 2013;3:963–967. doi: 10.1086/674750. [Consensus statement from the International Right Heart Foundation Working Group includes a comprehensive definition of right heart failure.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckberg GD, the RESTORE Group The ventricular septum: the lion of right ventricular function, and its impact on right ventricular restoration. European Journal of Cardio-Thoracic Surgery. 2006;29:S272–S278. doi: 10.1016/j.ejcts.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Damiano RJ, La Follette P, Cox JL, et al. Significant left ventricular contribution to right ventricular systolic function. American Journal of Physiology - Heart and Circulatory Physiology. 1991;261:H1514–H1524. doi: 10.1152/ajpheart.1991.261.5.H1514. [DOI] [PubMed] [Google Scholar]
- 11.Brown SB, Raina A, Katz D, et al. Longitudinal shortening accounts for the majority of right ventricular contraction and improves after pulmonary vasodilator therapy in normal subjects and patients with pulmonary arterial hypertension. Chest. 2011;140:27–33. doi: 10.1378/chest.10-1136. [DOI] [PubMed] [Google Scholar]
- 12.Abel FL, Waldhausen JA. Effects of alterations in pulmonary vascular resistance on right ventricular function. J Thorac Cardiovasc Surg. 1967;54:886–894. [PubMed] [Google Scholar]
- 13.Tedford RJ, Hassoun PM, Mathai SC, et al. Pulmonary Capillary Wedge Pressure Augments Right Ventricular Pulsatile Loading / Clinical Perspective. Circulation. 2012;125:289–297. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *14.Gomez-Arroyo J, Santos-Martinez L, Aranda A, et al. Differences in Right Ventricular Remodeling Secondary to Pressure Overload in Patients with Pulmonary Hypertension. Am J Respir Crit Care Med. 2014;189:603–606. doi: 10.1164/rccm.201309-1711LE. [Post-mortem study of RV and LV structures showing more RV hypertrophy in those with PAH due to Eisenmenger's syndrome compared with idiopathic PAH.] [DOI] [PubMed] [Google Scholar]
- *15.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circulation research. 2014;115:148–64. doi: 10.1161/CIRCRESAHA.115.301130. [This is a comprehensive review article on the metabolic changes that occur in the RV and pulmonary arteries of patients with PAH.] [DOI] [PubMed] [Google Scholar]
- 16.Oikawa M, Kagaya Y, Otani H, et al. Increased [18F]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. Journal of the American College of Cardiology. 2005;45:1849–55. doi: 10.1016/j.jacc.2005.02.065. [DOI] [PubMed] [Google Scholar]
- *17.Archer SL, Fang YH, Ryan JJ, Piao L. Metabolism and bioenergetics in the right ventricle and pulmonary vasculature in pulmonary hypertension. Pulmonary circulation. 2013;3:144–52. doi: 10.4103/2045-8932.109960. [In this review, the authors provide an overview of glycolysis and fatty acid oxidation in the RV and pulmonary vasculature in PAH.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. The Journal of general physiology. 1927;8:519–30. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogaard HJ, Natarajan R, Henderson SC, et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–60. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- *20.Sutendra G, Dromparis P, Paulin R, et al. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. Journal of molecular medicine. 2013;91:1315–27. doi: 10.1007/s00109-013-1059-4. [The transition from compensated right ventricular hypertrophy (RVH) to decompensated RVH is associated with a decrease in HIF-1α and mROS.] [DOI] [PubMed] [Google Scholar]
- *21.Piao L, Fang YH, Parikh K, et al. Cardiac glutaminolysis: a maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. Journal of molecular medicine. 2013;91:1185–97. doi: 10.1007/s00109-013-1064-7. [Glutaminolysis has been identified in the proliferation of cancer cells and was identified in this article as also being present in the RV myocytes of RVH in PAH.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dang CV. Glutaminolysis: supplying carbon or nitrogen or both for cancer cells? Cell cycle. 2010;9:3884–6. doi: 10.4161/cc.9.19.13302. [DOI] [PubMed] [Google Scholar]
- *23.Ryan JJ, Marsboom G, Fang YH, et al. PGC1alpha-mediated mitofusin-2 deficiency in female rats and humans with pulmonary arterial hypertension. American journal of respiratory and critical care medicine. 2013;187:865–78. doi: 10.1164/rccm.201209-1687OC. [The metabolic and mitochondrial changes that are found in the pulmonary artery smooth muscle cells are caused at least in part by PGC1α-mediated downregulation of mitofusin-2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Gomez-Arroyo J, Mizuno S, Szczepanek K, et al. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circulation Heart failure. 2013;6:136–44. doi: 10.1161/CIRCHEARTFAILURE.111.966127. [RV failure demonstrates a gene expression profile compatible with impaired fatty acid metabolism and significant mitochondrial dysfunction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. http://clinicaltrials.gov/show/NCT01083524.
- 26.Rich S, Pogoriler J, Husain AN, et al. Long-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertension. Chest. 2010;138:1234–9. doi: 10.1378/chest.09-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathai SC, Sibley CT, Forfia PR, et al. Tricuspid Annular Plane Systolic Excursion Is a Robust Outcome Measure in Systemic Sclerosis-associated Pulmonary Arterial Hypertension. The Journal of Rheumatology. 2011;38:2410–2418. doi: 10.3899/jrheum.110512. [DOI] [PubMed] [Google Scholar]
- 28.Mauritz G, Kind T, Marcus JT, et al. Progressive changes in right ventricular geometric shortening and long-term survival in pulmonary arterial hypertension. CHEST Journal. 2012;141:935–943. doi: 10.1378/chest.10-3277. [DOI] [PubMed] [Google Scholar]
- *29.Mercer-Rosa L, Parnell A, Forfia PR, et al. Tricuspid Annular Plane Systolic Excursion in the Assessment of Right Ventricular Function in Children and Adolescents after Repair of Tetralogy of Fallot. Journal of the American Society of Echocardiography. 2013;26:1322–1329. doi: 10.1016/j.echo.2013.06.022. [In a study of 125 patients with Tetralogy of Fallot, TAPSE was not associated with RV ejection fraction or exercise performance.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- **30.Raina A, Vaidya A, Gertz ZM, et al. Marked changes in right ventricular contractile pattern after cardiothoracic surgery: Implications for post-surgical assessment of right ventricular function. The Journal of Heart and Lung Transplantation. 2013;32:777–783. doi: 10.1016/j.healun.2013.05.004. [In those undergoing cardiac bypass or orthotopic heart transplantation, there was relative loss of longitudinal shortening and gain in transverse shortening despite normal global RV function. Longitudinal shortening was better preserved in lung transplantation patients in whom the pericardium remained intact.] [DOI] [PubMed] [Google Scholar]
- 31.Lehmann KG, Lee FA, McKenzie WB, et al. Onset of altered interventricular septal motion during cardiac surgery. Assessment by continuous intraoperative transesophageal echocardiography. Circulation. 1990;82:1325–1334. doi: 10.1161/01.cir.82.4.1325. [DOI] [PubMed] [Google Scholar]
- 32.Unsworth B, Casula RP, Kyriacou AA, et al. The right ventricular annular velocity reduction caused by coronary artery bypass graft surgery occurs at the moment of pericardial incision. Am Heart J. 2009;159:314–322. doi: 10.1016/j.ahj.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamborini G, Muratori M, Brusoni D, et al. Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. European Journal of Echocardiography. 2009;10:630–634. doi: 10.1093/ejechocard/jep015. [DOI] [PubMed] [Google Scholar]
- *34.Shiran H, Zamanian RT, McConnell MV, et al. Relationship between Echocardiographic and Magnetic Resonance Derived Measures of Right Ventricular Size and Function in Patients with Pulmonary Hypertension. Journal of the American Society of Echocardiography. 2014;27:405–412. doi: 10.1016/j.echo.2013.12.011. [In 40 patients with pulmonary hypertension undergoing both echocardiography and MRI, estimates of RV volume (by RV end-diastolic area) and function (by RV fractional area change and tricuspid annular plane systolic excursion) offered good approximations of MRI derived RV size and function.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sachdev A, Villarraga HR, Frantz RP, et al. RIght ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest. 2011;139:1299–1309. doi: 10.1378/chest.10-2015. [DOI] [PubMed] [Google Scholar]
- 36.Drakos SG, Janicki L, Horne BD, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. The American journal of cardiology. 2010;105:1030–5. doi: 10.1016/j.amjcard.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 37.Grant AD, Smedira NG, Starling RC, Marwick TH. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. Journal of the American College of Cardiology. 2012;60:521–8. doi: 10.1016/j.jacc.2012.02.073. [DOI] [PubMed] [Google Scholar]
- *38.Cameli M, Righini FM, Lisi M, et al. Comparison of right versus left ventricular strain analysis as a predictor of outcome in patients with systolic heart failure referred for heart transplantation. The American journal of cardiology. 2013;112:1778–84. doi: 10.1016/j.amjcard.2013.07.046. [Ninety-eight patients with advanced systolic heart failure referred for cardiac transplantation and underwent echocardiography strain analysis. RV longitudinal strain was found to be a stronger predictor of outcome than LV longitudinal strain and other conventional parameters.] [DOI] [PubMed] [Google Scholar]
- **39.Fine NM, Chen L, Bastiansen PM, et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circulation Cardiovascular imaging. 2013;6:711–21. doi: 10.1161/CIRCIMAGING.113.000640. [In 575 patients with pulmonary hypertension who underwent echocardiography, assessment of RV free-wall systolic strain was found to be feasible and a powerful predictor of the clinical outcomes.] [DOI] [PubMed] [Google Scholar]
- *40.Hardegree EL, Sachdev A, Villarraga HR, et al. Role of Serial Quantitative Assessment of Right Ventricular Function by Strain in Pulmonary Arterial Hypertension. Am J Cardiol. 2013;111:143–148. doi: 10.1016/j.amjcard.2012.08.061. [RV longitudinal systolic function was assessed in 50 patients with PAH before and after the initiation of therapy. After adjusting for age, functional class, and RV strain at baseline, patients with ≥ 5% improvement in RV free wall systolic strain had a greater than seven-fold lower mortality risk at 4 years.] [DOI] [PubMed] [Google Scholar]
- *41.Marston N, Brown JP, Olson N, et al. Right Ventricular Strain before and after Pulmonary Thromboendarterectomy in Patients with Chronic Thromboembolic Pulmonary Hypertension. Echocardiography. 2014 doi: 10.1111/echo.12812. [Thirty consecutive patients with chronic thromboembolic pulmonary hypertension referred for pulmonary endarterctomy (PTE) where studied with pre- and post-PTE strain imaging. RV basal strain worsened following PTE leading the authors to conclude that RV basal strain cannot be used as a surrogate marker for surgical success early after PTE.] [DOI] [PubMed] [Google Scholar]
- *42.Freed BH, Tsang W, Bhave NM, et al. Right Ventricular Strain in Pulmonary Arterial Hypertension: A 2D Echocardiography and Cardiac Magnetic Resonance Study. Echocardiography. 2014 doi: 10.1111/echo.12662. [Thirty patients with PAH underwent strain echo and cardiac MRI within a 2 hour period. Even though RV longitudinal strain by echo provided a good alternative for MRI RVEF, there was only moderate agreement in strain measurements between echo and MRI.] [DOI] [PubMed] [Google Scholar]
- *43.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right Heart Adaptation to Pulmonary Arterial Hypertension: Physiology and Pathobiology. J Am Coll Cardiol. 2013;62:D22–D33. doi: 10.1016/j.jacc.2013.10.027. [One of several documents summarizing recommendations from the 5th World Symposium on Pulmonary Hypertension in Nice, France.] [DOI] [PubMed] [Google Scholar]
- *44.van de Veerdonk MC, Marcus JT, Westerhof N, et al. Signs of right ventricular deterioration in clinically stable patients with pulmonary arterial hypertension. Chest. 2014 doi: 10.1378/chest.14-0701. [This study included 22 patients with IPAH who had MRI at baseline and 1.5, 3.5, 6.5 and, when still alive, ten years of follow-up. Late-progressive patients demonstrated a gradually increased RV end-diastolic volume and RV end-systolic volume and a declined RVEF whereas long-term stable patients did not show any RV changes.] [DOI] [PubMed] [Google Scholar]
- *45.Peacock AJ, Crawley S, McLure L, et al. Changes in Right Ventricular Function Measured by Cardiac Magnetic Resonance Imaging in Patients Receiving Pulmonary Arterial Hypertension–Targeted Therapy: The EURO-MR Study. Circulation: Cardiovascular Imaging. 2014;7:107–114. doi: 10.1161/CIRCIMAGING.113.000629. [Multi-center study of 91 patients with PAH who underwent clinical and cardiac MRI assessments at baseline and after 12 months of disease-targeted therapy. Significant improvements were achieved in RV and LVEF, RV stroke volume index, and left ventricular end-diastolic volume index. Increases in 6-minute walk distance were noted, and correlated with change in RV ejection fraction and left ventricular end-diastolic volume.] [DOI] [PubMed] [Google Scholar]
- 46.Meyer P, Filippatos GS, Ahmed MI, et al. Effects of Right Ventricular Ejection Fraction on Outcomes in Chronic Systolic Heart Failure. Circulation. 2010;121:252–258. doi: 10.1161/CIRCULATIONAHA.109.887570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in Patients with Primary Pulmonary Hypertension. Annals of Internal Medicine. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 48.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting Survival in Pulmonary Arterial Hypertension: Insights From the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 49.Tyberg JV, Taichman GC, Smith ER, et al. The relationship between pericardial pressure and right atrial pressure: an intraoperative study. Circulation. 1986;73:428–432. doi: 10.1161/01.cir.73.3.428. [DOI] [PubMed] [Google Scholar]
- *50.Miller WL, Grill DE, Borlaug BA. Clinical Features, Hemodynamics, and Outcomes of Pulmonary Hypertension Due to Chronic Heart Failure With Reduced Ejection FractionPulmonary Hypertension and Heart Failure. JACC: Heart Failure. 2013;1:290–299. doi: 10.1016/j.jchf.2013.05.001. [Ambulatory patients with heart failure and reduced ejection fraction (HFrEF) who underwent right heart catheterization for clinical reasons were reviewed. Pulmonary hypertension, and in particular those with elevated pulmonary vascular resistance and low pulmonary arterial compliance, was associated with worse prognosis.] [DOI] [PubMed] [Google Scholar]
- *51.Tampakakis E, Leary PJ, Selby VN, et al. The Diastolic Pulmonary Gradient Does Not Predict Survival in Patients With Pulmonary Hypertension Due to Left Heart Disease. JACC: Heart Failure. 2014 doi: 10.1016/j.jchf.2014.07.010. [In a study of predominately heart failure with reduced ejection fraction, elevated pulmonary vascular resistance and transpulmonary gradient was associated with worse survival, although an elevated diastolic pulmonary gradient was not.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahapatra S, Nishimura RA, Sorajja P, et al. Relationship of Pulmonary Arterial Capacitance and Mortality in Idiopathic Pulmonary Arterial Hypertension. J Am Coll Cardiol. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 53.Dupont M, Mullens W, Skouri HN, et al. Prognostic Role of Pulmonary Arterial Capacitance in Advanced Heart Failure. Circulation: Heart Failure. 2012;5:778–785. doi: 10.1161/CIRCHEARTFAILURE.112.968511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *54.Pellegrini P, Rossi A, Pasotti M, et al. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. CHEST Journal. 2013 doi: 10.1378/chest.13-1510. [Pulmonary arterial compliance was a strong prognostic factor in patients with heart failure with reduced ejection fraction, even when pulmonary vascular resistance was normal.] [DOI] [PubMed] [Google Scholar]
- *55.Dragu R, Rispler S, Habib M, et al. Pulmonary arterial capacitance in patients with heart failure and reactive pulmonary hypertension. European Journal of Heart Failure. 2014 doi: 10.1002/ejhf.192. [In another study of heart failure patients, development of ‘reactive’ pulmonary hypertension was associated with a marked reduction in compliance. Pulmonary arterial compliance was a strong independent haemodynamic marker of mortality.] [DOI] [PubMed] [Google Scholar]
- 56.Maughan WL, Shoukas AA, Sagawa K, Weisfeldt ML. Instantaneous pressure-volume relationship of the canine right ventricle. Circulation Research. 1979;44:309–315. doi: 10.1161/01.res.44.3.309. [DOI] [PubMed] [Google Scholar]
- 57.Bishop A, White P, Oldershaw P, et al. Clinical application of the conductance catheter technique in the adult human right ventricle. Int J Cardiol. 1997;58:211–221. doi: 10.1016/s0167-5273(96)02880-x. [DOI] [PubMed] [Google Scholar]
- *58.Tedford RJ, Mudd JO, Girgis RE, et al. Right Ventricular Dysfunction in Systemic Sclerosis Associated Pulmonary Arterial Hypertension. Circulation: Heart Failure. 2013;6:953–963. doi: 10.1161/CIRCHEARTFAILURE.112.000008. [Using pressure-volume relations, right ventricular-pulmonary arterial coupling was impaired in systemic sclerosis associated PAH compared with IPAH and systemic sclerosis patients without PH.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brimioulle S, Wauthy P, Ewalenko P, et al. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. American Journal of Physiology - Heart and Circulatory Physiology. 2003;284:H1625–H1630. doi: 10.1152/ajpheart.01023.2002. [DOI] [PubMed] [Google Scholar]
- 60.Lambermont B, Segers P, Ghuysen A, et al. Comparison between single-beat and multiple- beat methods for estimation of right ventricular contractility. Crit Care Med. 2004;32:1886–1890. doi: 10.1097/01.ccm.0000139607.38497.8a. [DOI] [PubMed] [Google Scholar]
- **61.Vanderpool RR, Pinsky MR, Naeije R, et al. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart. 2014 doi: 10.1136/heartjnl-2014-306142. [In a study of 50 patients with PH, stroke volume/end-systolic volume was the strongest hemodynamic predictor of survival. RV-PA coupling assessed via the single-beat approach was not associated with survival.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanz J, García-Alvarez A, Fernández-Friera L, et al. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart. 2012;98:238–243. doi: 10.1136/heartjnl-2011-300462. [DOI] [PubMed] [Google Scholar]
- 63.Trip P, Kind T, van de Veerdonk MC, et al. Accurate assessment of load-independent right ventricular systolic function in patients with pulmonary hypertension. The Journal of Heart and Lung Transplantation. 2013;32:50–55. doi: 10.1016/j.healun.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 64.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. American Journal of Physiology - Heart and Circulatory Physiology. 1983;245:H773–H780. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- *65.Dandel M, Potapov E, Krabatsch T, et al. Load Dependency of Right Ventricular Performance Is a Major Factor to be Considered in Decision Making Before Ventricular Assist Device Implantation. Circulation. 2013;128:S14–S23. doi: 10.1161/CIRCULATIONAHA.112.000335. [Novel echo parameters indexing contractility (strain) to afterload and afterload to RV size/volume best predicted RV failure after ventricular assist device implantation.] [DOI] [PubMed] [Google Scholar]
- **66.Guihaire JH, F., Noly P, Boulate D, et al. Right Ventricular Reserve in a Piglet Model of Chronic Pulmonary Hypertension. European Respiratory Journal. 2014 doi: 10.1183/09031936.00081314. [Using an animal model of PAH, the authors show resting RV-PA uncoupling is associated with impaired RV contractile reserve.] [DOI] [PubMed] [Google Scholar]
- *67.Sharma T, Lau EMT, Choudhary P, et al. Dobutamine stress for evaluation of right ventricular reserve in pulmonary arterial hypertension. European Respiratory Journal. 2014 doi: 10.1183/09031936.00089914. [Sixteen PAH patients and 18 age-matched controls underwent low-dose dobutamine stress echocardiography. Those with PAH had impaired RV contractile reserve, even those with normal resting function.] [DOI] [PubMed] [Google Scholar]
- **68.Grünig E, Tiede H, Enyimayew EO, et al. Assessment and Prognostic Relevance of Right Ventricular Contractile Reserve in Patients With Severe Pulmonary Hypertension. Circulation. 2013;128:2005–2015. doi: 10.1161/CIRCULATIONAHA.113.001573. [This is a prospective study of 124 patients with IPAH or inoperable chronic thromboembolic pulmonary hypertension who underwent stress echocardiography. Those patients unable to augment echo-estimated pulmonary artery systolic pressure during exercise by 30mmHg were also with worse prognosis. When coupled with low maximal oxygen consumption, this parameter was even more predictive of a worse prognosis.] [DOI] [PubMed] [Google Scholar]
- *69.Blumberg FC, Arzt M, Lange T, et al. Impact of right ventricular reserve on exercise capacity and survival in patients with pulmonary hypertension. European Journal of Heart Failure. 2013;15:771–775. doi: 10.1093/eurjhf/hft044. [In a study of 36 PH patients, exercise cardiac index correlated linearly with maximal oxygen consumption; both factors predicted survival.] [DOI] [PubMed] [Google Scholar]
- *70.Ryan JJ, Butrous G, Maron BA. The Heterogeneity of Clinical Practice Patterns among an International Cohort of Pulmonary Arterial Hypertension Experts. Pulmonary circulation. 2014 doi: 10.1086/677357. [This study of 105 experts from 25 countries demonstrated important variability in the approach to diagnosis, treatment, and long-term management of PAH.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piao L, Fang YH, Parikh KS, et al. GRK2-mediated inhibition of adrenergic and dopaminergic signaling in right ventricular hypertrophy: therapeutic implications in pulmonary hypertension. Circulation. 2012;126:2859–69. doi: 10.1161/CIRCULATIONAHA.112.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campo A, Mathai SC, Le Pavec J, et al. Outcomes of hospitalization for right heart failure in pulmonary arterial hypertension. European Respiratory Journal. 2011 doi: 10.1183/09031936.00148310. [DOI] [PubMed] [Google Scholar]
- 73.Kar B, Basra SS, Shah NR, Loyalka P. Percutaneous circulatory support in cardiogenic shock: interventional bridge to recovery. Circulation. 2012;125:1809–17. doi: 10.1161/CIRCULATIONAHA.111.040220. [DOI] [PubMed] [Google Scholar]
- 74. http://clinicaltrials.gov/show/NCT01777607.
- 75.Bogaard HJ, Natarajan R, Mizuno S, et al. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. American journal of respiratory and critical care medicine. 2010;182:652–60. doi: 10.1164/rccm.201003-0335OC. [DOI] [PubMed] [Google Scholar]
- 76.de Man FS, Handoko ML, van Ballegoij JJ, et al. Bisoprolol delays progression towards right heart failure in experimental pulmonary hypertension. Circulation Heart failure. 2012;5:97–105. doi: 10.1161/CIRCHEARTFAILURE.111.964494. [DOI] [PubMed] [Google Scholar]
- *77.Drake JI, Gomez-Arroyo J, Dumur CI, et al. Chronic carvedilol treatment partially reverses the right ventricular failure transcriptional profile in experimental pulmonary hypertension. Physiological genomics. 2013;45:449–61. doi: 10.1152/physiolgenomics.00166.2012. [In a rat model of PAH, carvedilol downregulated RV genes encoding proteins in the cardiac hypertrophy and protein ubiquitination pathways, whereas carvedilol upregulated genes encoding proteins in the mitochondrial dysfunction pathway.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *78.Grinnan D, Bogaard HJ, Grizzard J, et al. Treatment of group I pulmonary arterial hypertension with carvedilol is safe. American journal of respiratory and critical care medicine. 2014;189:1562–4. doi: 10.1164/rccm.201311-2025LE. [In 6 patients with PAH, beta-blocker therapy was noted to be safe and feasible on top of pulmonary vasodilator therapy.] [DOI] [PubMed] [Google Scholar]
- 79.Thenappan T, Roy SS, Duval S, et al. beta-Blocker Therapy Is Not Associated With Adverse Outcomes in Patients With Pulmonary Arterial Hypertension: A Propensity Score Analysis. Circulation Heart failure. 2014;7:903–10. doi: 10.1161/CIRCHEARTFAILURE.114.001429. [In patients referred to and being followed in a PAH center, there was no observed adverse outcomes in patients being treated with β-blockers.] [DOI] [PubMed] [Google Scholar]


