Abstract
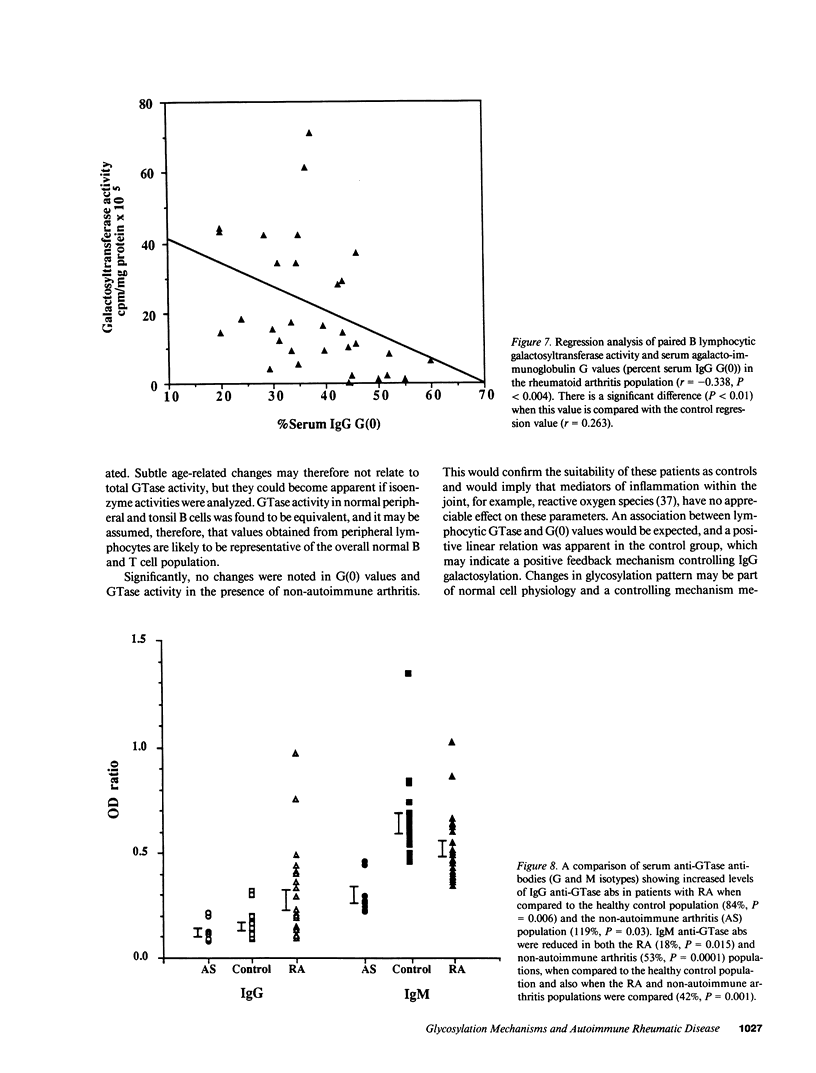
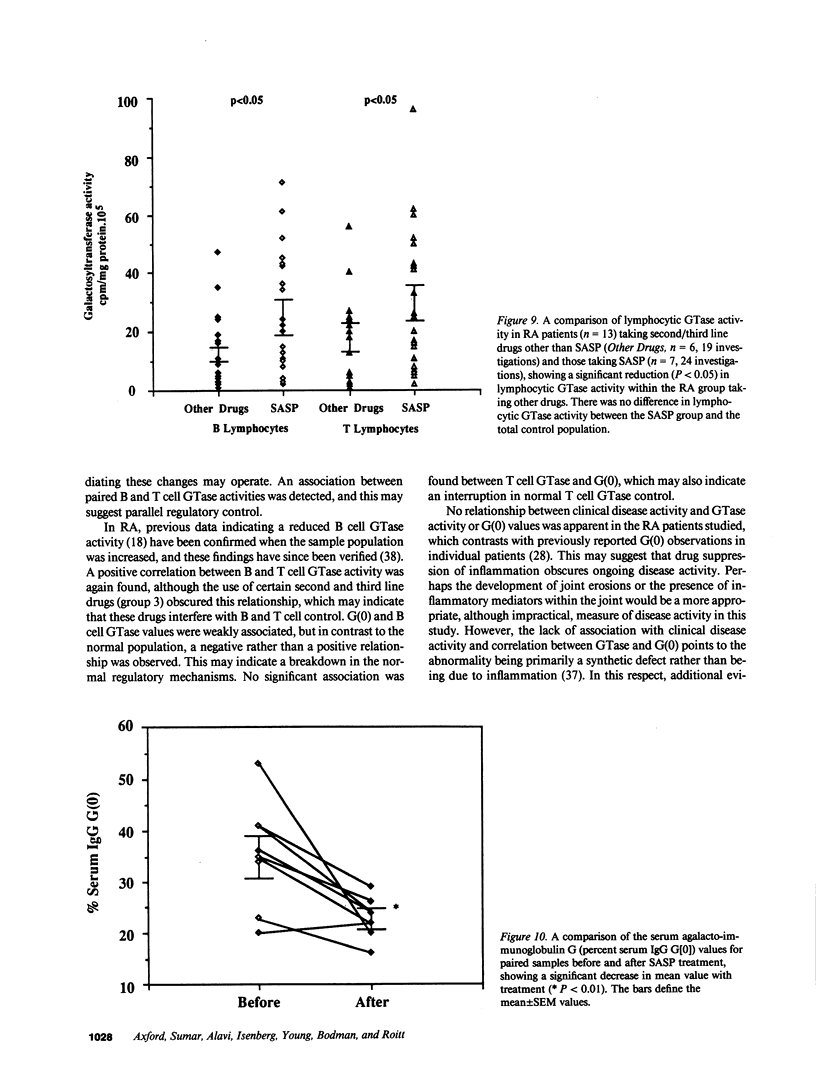
To investigate potential mechanisms controlling protein glycosylation we have studied the interrelationship between lymphocytic galactosyltransferase (GTase) activity and serum agalactosylated immunoglobulin G levels (G(0)) in healthy individuals and patients with rheumatoid arthritis and non-autoimmune arthritis. In RA there was reduced GTase activity and increased G(0). A positive linear correlation between B and T cell GTase was found in all individuals. The relationship between GTase and G(0) was found to be positive and linear in the control population and negative and linear in the RA population. Sulphasalazine therapy maintained normal levels of GTase and caused a reduction in G(0) in the RA population. IgG anti-GTase antibodies (abs) were significantly increased in the RA population, whereas IgM anti-GTase abs were significantly decreased in both the RA and the non-autoimmune arthritis groups. These data describe a defect in RA lymphocytic GTase, with associated abnormal G(0) changes, which is corrected by sulphasalazine. A possible regulatory mechanism controlling galactosylation in normal cells is suggested, in which there is parallel control of B and T cell GTase. IgM anti-GTase abs may be integrated into this normal regulatory process. This is disrupted in RA, where the positive feedback between GTase and G(0) is lost and there is an associated increase in IgG anti-GTase abs, which may result from isotype switching as IgM anti-GTase abs are reduced. We suggest that these mechanisms are of relevance to the pathogenesis of RA, and that their manipulation may form part of a novel therapeutic approach.
Full text
PDF
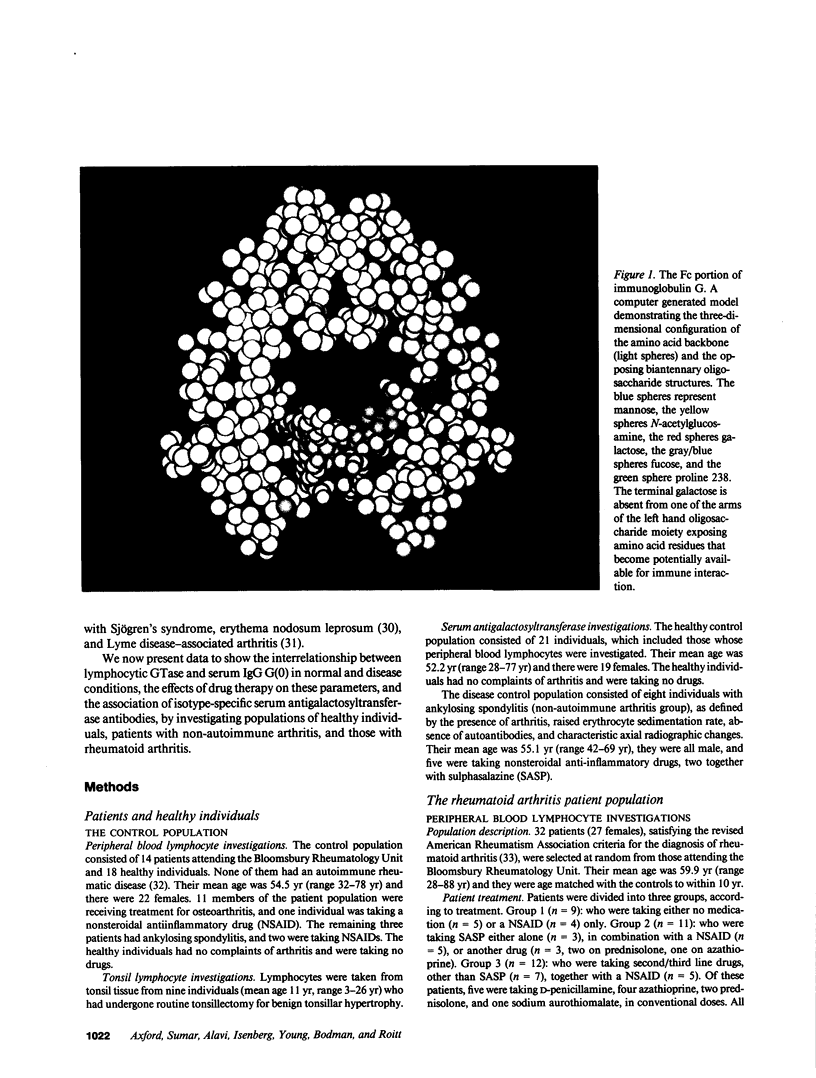


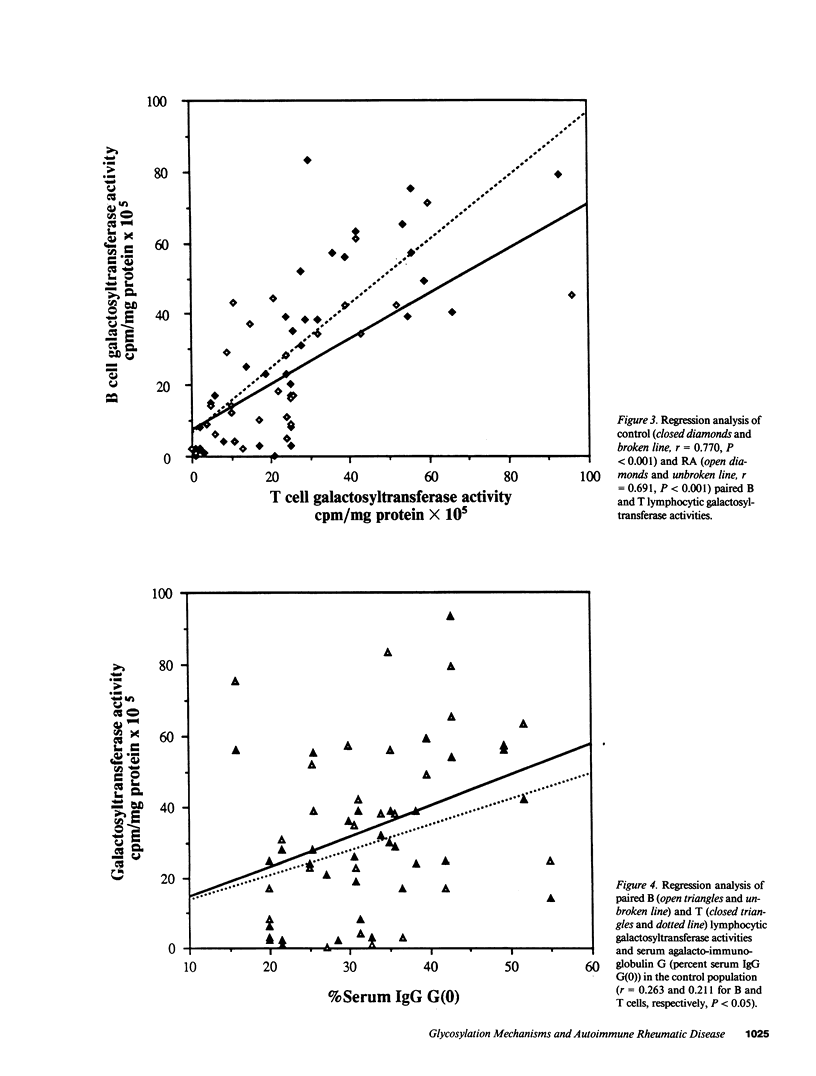
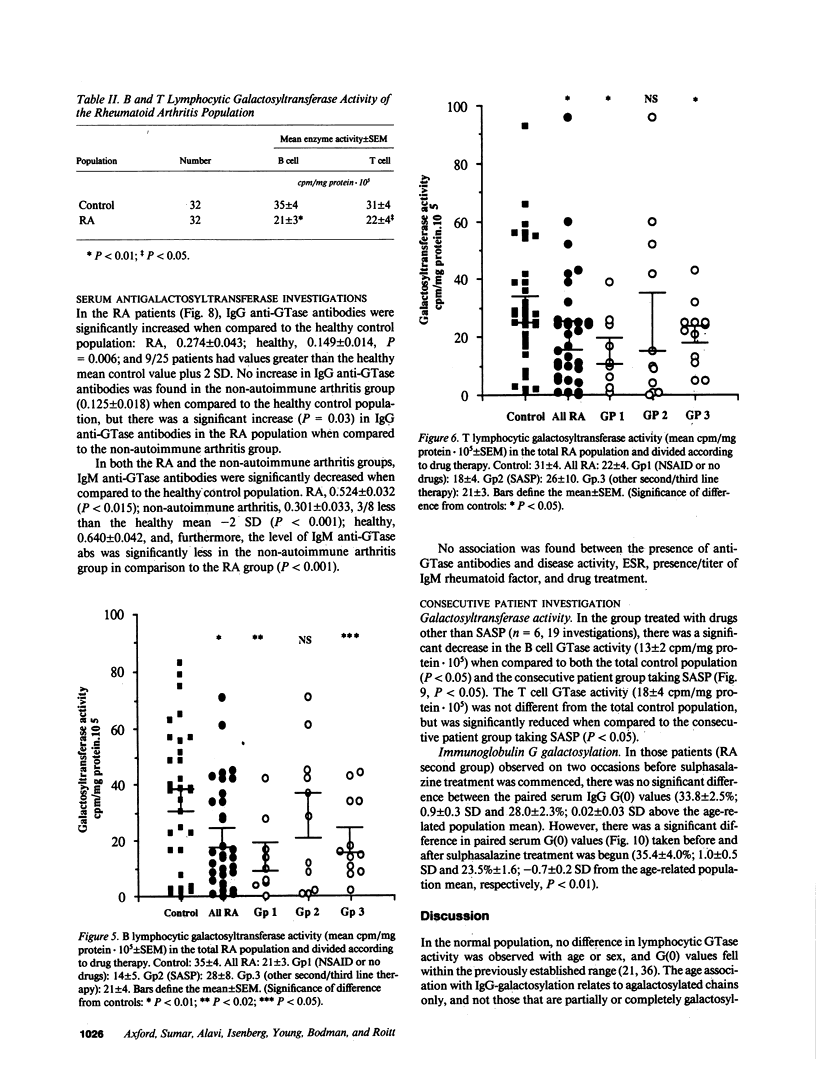



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Axford J. S., Mackenzie L., Lydyard P. M., Hay F. C., Isenberg D. A., Roitt I. M. Reduced B-cell galactosyltransferase activity in rheumatoid arthritis. Lancet. 1987 Dec 26;2(8574):1486–1488. doi: 10.1016/s0140-6736(87)92621-3. [DOI] [PubMed] [Google Scholar]
- Bax D. E., Amos R. S. Sulphasalazine: a safe, effective agent for prolonged control of rheumatoid arthritis. A comparison with sodium aurothiomalate. Ann Rheum Dis. 1985 Mar;44(3):194–198. doi: 10.1136/ard.44.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayna E. M., Runyan R. B., Scully N. F., Reichner J., Lopez L. C., Shur B. D. Cell surface galactosyltransferase as a recognition molecule during development. Mol Cell Biochem. 1986 Nov-Dec;72(1-2):141–151. doi: 10.1007/BF00230641. [DOI] [PubMed] [Google Scholar]
- Bayna E. M., Shaper J. H., Shur B. D. Temporally specific involvement of cell surface beta-1,4 galactosyltransferase during mouse embryo morula compaction. Cell. 1988 Apr 8;53(1):145–157. doi: 10.1016/0092-8674(88)90496-5. [DOI] [PubMed] [Google Scholar]
- Berger E. G., Thurnher M., Müller U. Galactosyltransferase and sialyltransferase are located in different subcellular compartments in HeLa cells. Exp Cell Res. 1987 Nov;173(1):267–273. doi: 10.1016/0014-4827(87)90352-1. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M., Chatterjee S. K., Barlow J. J. Uridine 5'-diphosphate-galactose:glycoprotein galactosyltransferase activity in the ovarian cancer patient. Cancer Res. 1976 Jun;36(6):2096–2101. [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Riberdy J., Ang S. L., Seidman J. G., Devlin P., Krangel M. S. Two forms of the T-cell receptor gamma protein found on peripheral blood cytotoxic T lymphocytes. Nature. 1987 Feb 19;325(6106):689–694. doi: 10.1038/325689a0. [DOI] [PubMed] [Google Scholar]
- Delves P. J., Lund T., Axford J. S., Alavi-Sadrieh A., Lydyard P. M., MacKenzie L., Smith M. D., Kidd V. J. Polymorphism and expression of the galactosyltransferase-associated protein kinase gene in normal individuals and galactosylation-defective rheumatoid arthritis patients. Arthritis Rheum. 1990 Nov;33(11):1655–1664. doi: 10.1002/art.1780331108. [DOI] [PubMed] [Google Scholar]
- Filley E., Andreoli A., Steele J., Waters M., Wagner D., Nelson D., Tung K., Rademacher T., Dwek R., Rook G. A. A transient rise in agalactosyl IgG correlating with free interleukin 2 receptors, during episodes of erythema nodosum leprosum. Clin Exp Immunol. 1989 Jun;76(3):343–347. [PMC free article] [PubMed] [Google Scholar]
- Gribben J. G., Devereux S., Thomas N. S., Keim M., Jones H. M., Goldstone A. H., Linch D. C. Development of antibodies to unprotected glycosylation sites on recombinant human GM-CSF. Lancet. 1990 Feb 24;335(8687):434–437. doi: 10.1016/0140-6736(90)90665-r. [DOI] [PubMed] [Google Scholar]
- Heyman B., Nose M., Weigle W. O. Carbohydrate chains on IgG2b: a requirement for efficient feedback immunosuppression. J Immunol. 1985 Jun;134(6):4018–4023. [PubMed] [Google Scholar]
- Isenberg D. A., Martin P., Hajirousou V., Todd-Pokropek A., Goldstone A. H., Snaith M. L. Haematological reassessment of rheumatoid arthritis using an automated method. Br J Rheumatol. 1986 May;25(2):152–157. doi: 10.1093/rheumatology/25.2.152. [DOI] [PubMed] [Google Scholar]
- Leatherbarrow R. J., Rademacher T. W., Dwek R. A., Woof J. M., Clark A., Burton D. R., Richardson N., Feinstein A. Effector functions of a monoclonal aglycosylated mouse IgG2a: binding and activation of complement component C1 and interaction with human monocyte Fc receptor. Mol Immunol. 1985 Apr;22(4):407–415. doi: 10.1016/0161-5890(85)90125-7. [DOI] [PubMed] [Google Scholar]
- Lund J., Tanaka T., Takahashi N., Sarmay G., Arata Y., Jefferis R. A protein structural change in aglycosylated IgG3 correlates with loss of huFc gamma R1 and huFc gamma R111 binding and/or activation. Mol Immunol. 1990 Nov;27(11):1145–1153. doi: 10.1016/0161-5890(90)90103-7. [DOI] [PubMed] [Google Scholar]
- Lunec J., Blake D. R., McCleary S. J., Brailsford S., Bacon P. A. Self-perpetuating mechanisms of immunoglobulin G aggregation in rheumatoid inflammation. J Clin Invest. 1985 Dec;76(6):2084–2090. doi: 10.1172/JCI112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire E. J., Kerlin R., Cebra J. J., Roth S. A human milk galactosyltransferase is specific for secreted, but not plasma, IgA. J Immunol. 1989 Nov 1;143(9):2933–2938. [PubMed] [Google Scholar]
- Nardella F. A., Teller D. C., Mannik M. Studies on the antigenic determinants in the self-association of IgG rheumatoid factor. J Exp Med. 1981 Jul 1;154(1):112–125. doi: 10.1084/jem.154.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newkirk M. M., Lemmo A., Rauch J. Importance of the IgG isotype, not the state of glycosylation, in determining human rheumatoid factor binding. Arthritis Rheum. 1990 Jun;33(6):800–809. doi: 10.1002/art.1780330606. [DOI] [PubMed] [Google Scholar]
- Nicholas N. S., Panayi G. S. Rheumatoid arthritis and pregnancy. Clin Exp Rheumatol. 1988 Apr-Jun;6(2):179–182. [PubMed] [Google Scholar]
- Nose M., Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6632–6636. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Néel D., Merlu B., Turpin E., Rabourdin-Combe C., Mach B., Goussault Y., Charron D. J. Characterization of N-linked oligosaccharides of an HLA-DR molecule expressed in different cell lines. Biochem J. 1987 Jun 1;244(2):433–442. doi: 10.1042/bj2440433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Roitt I. M., Isenberg D. A., Dwek R. A., Ansell B. M., Rademacher T. W. Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet. 1988 Apr 30;1(8592):966–969. doi: 10.1016/s0140-6736(88)91781-3. [DOI] [PubMed] [Google Scholar]
- Parekh R., Isenberg D., Rook G., Roitt I., Dwek R., Rademacher T. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J Autoimmun. 1989 Apr;2(2):101–114. doi: 10.1016/0896-8411(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Parekh R., Roitt I., Isenberg D., Dwek R., Rademacher T. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J Exp Med. 1988 May 1;167(5):1731–1736. doi: 10.1084/jem.167.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekelharing J. M., Hepp E., Kamerling J. P., Gerwig G. J., Leijnse B. Alterations in carbohydrate composition of serum IgG from patients with rheumatoid arthritis and from pregnant women. Ann Rheum Dis. 1988 Feb;47(2):91–95. doi: 10.1136/ard.47.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinals R. S., Kaplan S. B., Lawson J. G., Hepburn B. Sulfasalazine in rheumatoid arthritis. A double-blind, placebo-controlled trial. Arthritis Rheum. 1986 Dec;29(12):1427–1434. doi: 10.1002/art.1780291202. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Weiser M. M., Isselbacher K. J., Cohen A. M. A cancer-associated galactosyltransferase isoenzyme. N Engl J Med. 1978 Sep 28;299(13):703–705. doi: 10.1056/NEJM197809282991306. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Weiser M. M., Westwood J. C., Gammon M. Cancer-associated serum galactosyltransferase activity. Demonstration in an animal model system. J Biol Chem. 1977 Mar 10;252(5):1807–1813. [PubMed] [Google Scholar]
- Pullar T., Hunter J. A., Capell H. A. Sulphasalazine in the treatment of rheumatoid arthritis: relationship of dose and serum levels to efficacy. Br J Rheumatol. 1985 Aug;24(3):269–276. doi: 10.1093/rheumatology/24.3.269. [DOI] [PubMed] [Google Scholar]
- Quentin E., Gladen A., Rodén L., Kresse H. A genetic defect in the biosynthesis of dermatan sulfate proteoglycan: galactosyltransferase I deficiency in fibroblasts from a patient with a progeroid syndrome. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1342–1346. doi: 10.1073/pnas.87.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A., Isenberg D., Rook G., Axford J. S., Roitt I. The role of IgG glycoforms in the pathogenesis of rheumatoid arthritis. Springer Semin Immunopathol. 1988;10(2-3):231–249. doi: 10.1007/BF01857227. [DOI] [PubMed] [Google Scholar]
- Russo R. N., Shaper N. L., Shaper J. H. Bovine beta 1----4-galactosyltransferase: two sets of mRNA transcripts encode two forms of the protein with different amino-terminal domains. In vitro translation experiments demonstrate that both the short and the long forms of the enzyme are type II membrane-bound glycoproteins. J Biol Chem. 1990 Feb 25;265(6):3324–3331. [PubMed] [Google Scholar]
- Shaper N. L., Shaper J. H., Bertness V., Chang H., Kirsch I. R., Hollis G. F. The human galactosyltransferase gene is on chromosome 9 at band p13. Somat Cell Mol Genet. 1986 Nov;12(6):633–636. doi: 10.1007/BF01671948. [DOI] [PubMed] [Google Scholar]
- Sheldon P. HLA-B27 related arthritis, sulphasalazine and rheumatoid arthritis. Br J Rheumatol. 1987 Oct;26(5):321–324. doi: 10.1093/rheumatology/26.5.321. [DOI] [PubMed] [Google Scholar]
- Sheldon P., Webb C., Grindulis K. A. Effect of sulphasalazine and its metabolites on mitogen induced transformation of lymphocytes--clues to its clinical action? Br J Rheumatol. 1988 Oct;27(5):344–349. doi: 10.1093/rheumatology/27.5.344. [DOI] [PubMed] [Google Scholar]
- Shur B. D., Hall N. G. Sperm surface galactosyltransferase activities during in vitro capacitation. J Cell Biol. 1982 Nov;95(2 Pt 1):567–573. doi: 10.1083/jcb.95.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shur B. D., Roth S. Cell surface glycosyltransferases. Biochim Biophys Acta. 1975 Dec 29;415(4):473–512. doi: 10.1016/0304-4157(75)90007-6. [DOI] [PubMed] [Google Scholar]
- Singer L., Crozier D., Moscarello M. A. The levels of galactosyltransferase activity in sera from normal children and patients with cystic fibrosis. Clin Biochem. 1974 Jun;7(2):146–149. doi: 10.1016/s0009-9120(74)91334-4. [DOI] [PubMed] [Google Scholar]
- Situnayake R. D., McConkey B. Resin-coated 5-aminosalicylic acid (Asacol) in rheumatoid arthritis. Br J Rheumatol. 1985 May;24(2):226–227. doi: 10.1093/rheumatology/24.2.226. [DOI] [PubMed] [Google Scholar]
- Sumar N., Bodman K. B., Rademacher T. W., Dwek R. A., Williams P., Parekh R. B., Edge J., Rook G. A., Isenberg D. A., Hay F. C. Analysis of glycosylation changes in IgG using lectins. J Immunol Methods. 1990 Jul 20;131(1):127–136. doi: 10.1016/0022-1759(90)90242-n. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N., Endo T., Matsuta K., Yoshinoya S., Aikawa T., Kosuge E., Takeuchi F., Miyamoto T., Kobata A. Effects of galactose depletion from oligosaccharide chains on immunological activities of human IgG. J Rheumatol. 1989 Mar;16(3):285–290. [PubMed] [Google Scholar]
- Yamaguchi Y., Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature. 1988 Nov 17;336(6196):244–246. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]
- Young A., Sumar N., Bodman K., Goyal S., Sinclair H., Roitt I., Isenberg D. Agalactosyl IgG: an aid to differential diagnosis in early synovitis. Arthritis Rheum. 1991 Nov;34(11):1425–1429. doi: 10.1002/art.1780341113. [DOI] [PubMed] [Google Scholar]