Abstract
Background
Impaired insight into illness (clinical insight) in schizophrenia has negative effects on treatment adherence and clinical outcomes. Schizophrenia is described as a disorder of disrupted brain connectivity. In line with this concept, resting state networks (RSNs) appear differentially affected in persons with schizophrenia. Therefore, impaired clinical, or the related construct of cognitive insight (which posits that impaired clinical insight is a function of metacognitive deficits), may reflect alterations in RSN functional connectivity (fc). Based on our previous research, which showed that impaired insight into illness was associated with increased left hemisphere volume relative to right, we hypothesized that impaired clinical insight would be associated with increased connectivity in the DMN with specific left hemisphere brain regions.
Methods
Resting state MRI scans were acquired for participants with schizophrenia or schizoaffective disorder (n = 20). Seed-to-voxel and ROI-to-ROI fc analyses were performed using the CONN-fMRI fc toolbox v13 for established RSNs. Clinical and cognitive insight were measured with the Schedule for the Assessment of Insight—Expanded Version and Beck Cognitive Insight Scale, respectively, and included as the regressors in fc analyses.
Results
As hypothesized, impaired clinical insight was associated with increased connectivity in the default mode network (DMN) with the left angular gyrus, and also in the self-referential network (SRN) with the left insula. Cognitive insight was associated with increased connectivity in the dorsal attention network (DAN) with the right inferior frontal cortex (IFC) and left anterior cingulate cortex (ACC).
Conclusion
Increased connectivity in DMN and SRN with the left angular gyrus and insula, respectively, may represent neural correlates of impaired clinical insight in schizophrenia spectrum disorders, and is consistent with the literature attributing impaired insight to left hemisphere dominance. Increased connectivity in the DAN with the IFC and ACC in relation to cognitive insight may facilitate enhanced mental flexibility in this sample.
Keywords: Anosognosia, Insight, Cognitive insight, Illness awareness, Resting state networks, Default mode network, Functional MRI
1. Introduction
Failure to recognize that one has a mental illness is a core feature of schizophrenia spectrum disorders. Kraeplin in his original writings described individuals with schizophrenia (i.e. dementia praecox) as “completely unaware of the gravity of their illness” (Kraepelin, 1919). Originally viewed as a dichotomous construct, contemporarily, insight into illness (clinical insight) is recognized as a multidimensional construct that exists on a continuum (David, 1990), consisting of four core domains: awareness of having a serious mental illness; awareness and attribution of symptoms to the illness; acceptance of the need for treatment; and awareness of the social, occupational or other negative consequences (i.e. hospitalization, legal issues, etc.) of the illness (David, 1990; Orfei et al., 2008).
The related, but distinct construct of cognitive insight posits that impaired clinical insight may be a function of global mental inflexibility or meta-cognitive deficits (Markova et al., 2003; Spalletta et al., 2014). Metacognition is regarded as a higher-order cognitive function consisting of awareness or knowledge of one's mental or cognitive processes, i.e. one's ability for self-awareness and self-monitoring (Markova et al., 2003; Spalletta et al., 2014). According to Beck et al. (2004), in relation to clinical insight, cognitive insight represents the overarching ability to evaluate and correct distorted beliefs and misperceptions as measured by reduced self-reflectiveness and rigid self-certainty (Beck et al., 2004). Where assessments of clinical insight are based on patients' verbal admissions and may reflect repetition of previously heard information, cognitive insight reflects the ability to integrate new information into one's own thought processes and refute erroneous convictions.
Although individuals with schizophrenia may have impaired self-monitoring abilities and mental inflexibility in relation to their mental illness, symptoms and need for treatment, it does not necessarily hold, however, that they would be inflexible with regard to other beliefs, e.g. how best to perform certain tasks, political opinions, and details while recounting a story. Moreover, awareness in one domain of clinical insight (e.g. awareness of need for treatment) does not preclude awareness in another domain (e.g. correct symptom attribution) (Bota et al., 2006). Indeed, the literature suggests that there is only a modest association between clinical and cognitive insight (Pedrelli et al., 2004; Warman et al., 2007; Greenberger and Serper, 2010; Nair et al., 2014).
Schizophrenia is described as a ‘dysconnection syndrome’ consisting of aberrant neural connectivity between brain regions (Bullmore et al., 1997; Stephan et al., 2009). To test this hypothesis, a number of studies have explored whether there exist alterations of the resting state networks, which consist of temporally related brain regions of functional significance (Fox and Raichle, 2007). Well recognized resting state networks (i.e. distinct neural networks that emerge when one is not engaged in any particular task) include the default mode network (DMN; posterior cingulate), dorsal attention network (DAN; parietal–frontal), executive control network (ECN; dorsolateral prefrontal), salience network (frontal-insular/anterior cingulate), and self-referential network (SRN; medial prefrontal) (Damoiseaux et al., 2006; De Luca et al., 2006; van den Heuvel and Hulshoff Pol, 2010; Woodward et al., 2011).
The DMN, the most well known of the resting state networks, is task independent, deactivating during goal directed activity (Raichle et al., 2001). It is thought to reflect spontaneous, stimulus independent, internal mentation, self-referential or semantic processing—”a stream of consciousness” (McKiernan et al., 2006; Buckner et al., 2008). In schizophrenia, DMN hyperactivity or a failure of DMN suppression during task-related activity is thought to lead to excessive self-referential processing, contributing to impaired reality testing and delusional thinking (Buckner et al., 2008; Menon et al., 2011; Anticevic et al., 2012; Kindler et al., 2013). Results of functional magnetic resonance imaging (fMRI) studies comparing patients to healthy controls, however, are mixed (Orliac et al., 2013) with studies showing increased connectivity (Rotarska-Jagiela et al., 2010), decreased connectivity (Bluhm et al., 2007; Vercammen et al., 2010) or both (Garrity et al., 2007; Skudlarski et al., 2010; Manoliu et al., 2014) in the DMN. Other studies have investigated the influence of symptomatology on resting state networks, including positive (Rotarska-Jagiela et al., 2010; Vercammen et al., 2010), negative (Mingoia et al., 2012) and cognitive symptoms (Camchong et al., 2011). We are not aware of any studies that have investigated resting state connectivity in relation to cognitive insight, and we are aware of only one study that has examined the relationship between impaired clinical insight into illness and resting state DMN connectivity (Liemburg et al., 2012). The results of the few studies that have explored the functional neural correlates of self-evaluation in schizophrenia using an fMRI task design are inconclusive (Murphy et al., 2010; Holt et al., 2011; Bedford et al., 2012), but suggest that the default mode network, cortical midline structures, and other regions implicated in self-awareness, including the insula and inferior parietal lobes, may play an influential role (Raij et al., 2012; van der Meer et al., 2013). Intriguingly, self-certainty, a subdimension of the Beck Cognitive Insight Scale (Beck et al., 2004) representing mental inflexibility, is linked with lower performance on verbal memory and executive function tasks (Lepage et al., 2008; Cooke et al., 2010; Orfei et al., 2010) and with structural changes in related brain regions, specifically greater FA values in the right fornix and volumetric reductions in the hippocampus (Buchy et al., 2010, 2012).
As such, we aimed to explore the DMN and other resting state network connectivity in relation to clinical and cognitive insight. We hypothesized that impaired clinical insight and cognitive insight in schizophrenia would have differing effects on resting state connectivity. Based on our previous research, which showed that impaired insight into illness was associated with increased left hemisphere volume relative to right (Gerretsen et al., 2013), we hypothesized that impaired clinical insight would be associated with increased connectivity in the DMN in left hemisphere brain regions, namely the left parietal, medial prefrontal cortex (mPFC), dorsolateral prefrontal cortex (DLPFC), insula, and anterior temporal lobe. Exploratory analyses were performed between clinical and cognitive insight and the other resting state networks.
2. Method
2.1. Participants
Participants (n = 20) with diagnoses of schizophrenia or schizoaffective disorder and varying degrees of clinical insight were recruited from the Schizophrenia Program at the Centre for Addiction & Mental Health (CAMH). Written informed consent was obtained after full explanation of the study procedures and risks. Capacity to consent was confirmed for all participants with the MacArthur Test of Competence (MacCAT) (Appelbaum and Grisso, 1995). An assessment of psychiatric disorders was performed using the MINI-Plus structured interview (Sheehan et al., 1998). Inclusion criteria for patients were as follows: (i) age 18–65; (ii) fluency in English; (iii) DSM-IV diagnosis of schizophrenia or schizoaffective disorder; (iv) voluntary status; (v) able to undergo fMRI of approximately 50 min duration; and (vi) right handed. Handedness was assessed using the Edinburgh Handedness Inventory (Dragovic, 2004). Exclusion criteria included: (i) serious, unstable medical illness or any concomitant major medical or neurological illness; (ii) acute suicidal and/or homicidal ideation; (iii) DSM-IV substance dependence (except caffeine and nicotine) within 3 months; (iv) current major depressive or manic episode; (v) metal implants, cardiac pacemaker, claustrophobia or other limitations to participating in the MRI component of the study; (vi) illegal psychoactive drug use in the last two weeks; (vii) severe head injury resulting in a loss of consciousness over 30 min; and, (viii) Scale for the Assessment of Positive Symptoms (SAPS) formal thought disorder rating of ≥3. Urine toxicology screens were done as part of the initial assessment. The study was approved by the Research Ethics Board of CAMH.
2.2. Study measures
Clinical insight was measured using the Schedule for the Assessment of Insight—Expanded version (SAI-E) (Kemp and David, 1997). The SAI-E is an 11 item clinician-rated measure with a maximum score of 28 that was designed to assess the four core domains of clinical insight; namely, awareness of having a serious mental illness; awareness and attribution of symptoms to the illness; acceptance of the need for treatment; and awareness of negative consequences attributable to the disorder (i.e. social and occupational dysfunction, etc.). Cognitive insight was evaluated with the Beck Cognitive Insight Scale (BCIS) (Beck et al., 2004), which was developed to evaluate patients' self-reflectiveness and their overconfidence in their interpretations of their experiences. The BCIS is a 15-item self-report questionnaire with a 9-item self-reflectiveness subscale and a 6-item self-certainty subscale. A composite score can be obtained by subtracting the self-certainty sub-scale score from the self-reflectiveness subscale score. The SAPS and the Scale for the Assessment of Negative Symptoms (SANS) were used to assess symptoms of schizophrenia (Andreasen et al., 1995). The Wide Range Achievement Test (WRAT-3) reading subtest was used to measure Premorbid IQ (Wilkenson and Jastak, 1993).
2.3. Statistical analyses
Statistical analyses of clinical, demographic and behavioral variables were carried out with PASW software (Released 2009. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc). Means and standard deviations were calculated for the demographic and clinical data. Bivariate Pearson correlations were performed between clinical insight (SAI-E) scores and relevant demographic and clinical variables. The significance level for tests was established at p ≤ 0.05.
2.4. MRI data acquisition
MRI scans were acquired with a GE Signa 1.5 T scanner (General Electric, Waukesha, WI) equipped with standard head coils. A 5 minute (299 s) resting-state echo-planar imaging (EPI) scan (36 axial slices; slice thickness = 4.4 mm; TR = 2300 ms; TE = 40 ms; matrix 64 × 64; FOV 20 cm; 130 volumes) was acquired for each participant. Participants were instructed to rest quietly with their eyes open and to remain awake during the scan. High resolution IR-Prepped 3D FSPGR T1-weighted anatomical images (120 contiguous axial 1.1 mm thick slices) were also acquired (TR = 12 ms; TE = 5.4 ms; flip angle 20°; matrix 256 × 256; FOV 20 cm).
2.5. Image pre-processing
The data were pre-processed and analyzed using SPM8 (The Wellcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm8). All functional images were slice-timing corrected and realigned to the first volume using a six-parameter rigid body transformation. The mean image generated was spatially normalized into standard stereotactic space, using the Montreal Neurological Institute (MNI) echo planar imaging (EPI) template. Computed transformation parameters were applied to all functional images, interpolated to isotropic voxels of 2 mm3 and the resulting images were smoothed using an 8-mm full-width half-maximum (FWHM), isotropic Gaussian kernel.
2.6. Functional connectivity analyses
Functional connectivity analyses were carried out using the CONN-fMRI Functional Connectivity toolbox v13 (Whitfield-Gabrieli and Nieto-Castanon, 2012) (http://www.nitrc.org/projects/conn). Using the default preprocessing parameters, the possible confounding effects of head motion artifacts, and white matter and CSF BOLD signal were defined and addressed (Whitfield-Gabrieli and Nieto-Castanon, 2012). BOLD signal noise from the white matter and CSF was characterized with the principal component-based noise-correction ‘CompCor’ method utilized in this toolbox (Murphy et al., 2009). Band-pass filtering was performed with a frequency window of 0.01 to 0.1 Hz.
Seed-to-voxel and ROI-to-ROI functional connectivity maps were created for each participant. The mean BOLD time series was computed across all voxels within each ROI. Bivariate-regression analyses were used to determine the linear association of the BOLD time series between each pair of sources. Each scan was Hanning weighted (Whitfield-Gabrieli and Nieto-Castanon, 2012).
Predefined regions-of-interest (a spherical ROI with MNI coordinates and radius 10 mm) from the CONN-fMRI Functional Connectivity toolbox were chosen based on prior studies as seeds to create connectivity maps of the default mode (DMN, posterior cingulate cortex/precuneus, −6, −52, 40), dorsal attention (DAN, left and right inferior parietal lobes, BA 40, −50, −40, 30; 51, −40, 39), executive control (ECN, left and right DLPFC, BA 46, −46, 36, 16; 49, 37, 15), salience (left and right frontoinsular cortex, BA 47, −34, 24, 11; 36, 24, −11), and self-referential networks (SRNs, medial prefrontal cortex, 0, 48, −4) (Damoiseaux et al., 2006; De Luca et al., 2006; van den Heuvel and Hulshoff Pol, 2010; Woodward et al., 2011).
To explore the effect of impaired insight on resting state functional connectivity we first performed second level analyses for the entire sample to create seed-to-voxel connectivity maps and ROI-to-ROI connectivity matrices, and then, included participants' insight into illness scores (i.e. SAI-E scores) as the principal regressor. Due to the strong correlation between impaired insight into illness and positive symptom severity, the SAPS total score was included as a covariate. For seed-to-voxel analyses a peak voxel threshold of p ≤ 0.001 and a cluster extent threshold of p ≤ 0.05 were set for bidirectional explorations of connectivity (i.e. positive and negative associations). Results of exploratory analyses were considered significant if clusters survived FWE correction p ≤ 0.05. For ROI-to-ROI analyses a threshold of p ≤ 0.001 was used for bidirectional explorations of connectivity (i.e. positive and negative associations). Results of exploratory analyses were considered significant if they survived correction for multiple comparisons (FDR ≤ 0.05).
3. Results
3.1. Demographic & clinical data
The demographic and clinical data of the sample (n = 20) are presented in Table 1. Participants had a wide range of clinical and cognitive insight scores. Although participants scored within the absent-to-mild range of positive symptom severity, SAI-E scores were inversely correlated with SAPS total average scores (r = −0.68, p < 0.001) suggesting a strong association between the presence of active psychotic symptoms and impaired insight into illness.
Table 1.
Relationship between clinical and cognitive insight and participant characteristics.
| SAI-E | p-Value | Self-reflectiveness | p-Value | Self-certainty | p-Value | Composite | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| N | 20 | ||||||||
| Male:female | 12:8 | ||||||||
| Schizophrenia:schizoaffective | 12:8 | ||||||||
| Mean (SD) | r | r | r | r | |||||
| Age, range | 43.0 (12.1), 18–65 | −0.35 | 0.129 | −0.23 | 0.335 | 0.17 | 0.472 | −0.19 | 0.444 |
| Age of illness onset | 24.0 (6.9) | −0.26 | 0.270 | −0.45 | 0.047* | 0.52 | 0.019* | −0.54 | 0.015* |
| Duration (years) of illness | 18.2 (11.9) | −0.23 | 0.368 | −0.03 | 0.906 | −0.12 | 0.603 | 0.10 | 0.701 |
| Antipsychotic CPZ equivalents | 357.9 (208.6) | −0.14 | 0.567 | −0.35 | 0.135 | 0.45 | 0.045* | −0.44 | 0.053 |
| Test score | |||||||||
| IQ (WRAT-III) | 109.2 (7.4) | 0.28 | 0.240 | 0.35 | 0.135 | −0.06 | 0.807 | 0.26 | 0.279 |
| Degree of right handednessa | 87.3 (17.8) | −0.25 | 0.336 | 0.07 | 0.786 | 0.11 | 0.681 | −0.01 | 0.974 |
| Global illness awareness | |||||||||
| SAI-E score (max = 28), range | 19.1 (8.1), 7–28 | – | – | 0.58 | 0.007* | −0.23 | 0.341 | 0.48 | 0.034* |
| Psychotic/positive symptoms | |||||||||
| SAPS total average score (max = 5) | 0.7 (0.7) | −0.68 | <0.001** | −0.48 | 0.032* | 0.28 | 0.237 | −0.44 | 0.053 |
| Negative symptoms | |||||||||
| SANS total average score (max = 5) | 1.1 (0.6) | 0.25 | 0.290 | 0.16 | 0.510 | 0.17 | 0.471 | 0.02 | 0.944 |
| Cognitive insight | |||||||||
| Self-reflectiveness | 15.4 (4.6) | 0.58 | 0.007* | – | – | −0.60 | 0.006 | −0.92 | <0.001** |
| Self-certainty | 6.4 (3.5) | −0.23 | 0.341 | −0.60 | 0.006* | – | – | −0.86 | <0.001** |
| Composite score | 9.0 (7.3) | 0.48 | 0.034* | 0.92 | <0.001** | −0.86 | <0.001** | – | – |
SCZ, schizophrenia; SCZ-AFF, schizoaffective disorder; IQ, intelligence quotient; WRAT-III, Wide Range Achievement Test, 3rd Edition; SAI-E, Schedule for the Assessment of Insight—Extended Version; SAPS, Scale for the Assessment of Positive Symptoms; SANS, Scale for the Assessment of Negative Symptoms.
Edinburgh Handedness Inventory.
p ≤ 0.05.
p ≤ 0.001, after Bonferroni correction for multiple comparisons.
There was a strong positive correlation between SAI-E scores and BCIS self-reflectiveness (r = 0.58, p = 0.007) and the BCIS composite score (r = 0.48, p = 0.028). BCIS self-reflectiveness was negatively associated with positive symptom severity (r = −0.48, p = 0.032) and, as one would expect, negatively correlated with BCIS self-certainty (r = −0.60, p = 0.006). These associations, however, were not significant after Bonferroni correction for multiple comparisons.
Age of illness onset was negatively correlated with BCIS self-reflectiveness (r = −0.45, p = 0.047) and BCIS composite score (r = −0.54, p = 0.015), and positively correlated with BCIS self-certainty (r = 0.52, p = 0.019), indicating an association between earlier age of onset and lower cognitive insight. However, these associations were also not significant after Bonferroni correction for multiple comparisons.
3.2. Seed-to-voxel analysis
The group connectivity maps for each resting state network and their relationship to clinical insight are presented in Table 2, Fig. 1 and Supplementary Fig. 1. Impaired insight was associated with greater connectivity in the DMN with the left angular gyrus and left posterior cingulate cortex. Analyses of connectivity in other resting state networks showed that impaired clinical insight was associated with greater connectivity in the following: self-referential network with the left superior temporal gyrus/insula and right primary auditory cortex; right ECN and right salience network with the cerebellum; and right salience network with the left temporal parietal junction. Only the cluster showing the association between impaired insight and greater connectivity in the DMN with the left angular gyrus survived FWE correction (p < 0.05).
Table 2.
Resting state networks for impaired clinical insight in schizophrenia spectrum disorders—seed-to-voxel analysis.
| Network | Brain region | Voxels per cluster | Cluster maxima
|
Minimum T value | p value uncorrected | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Default mode network | L angular gyrus (BA 39) | 190 | −42 | −72 | 36 | 3.97 | <0.001* |
| L posterior cingulate cortex (BA 30) | 72 | −14 | −56 | 18 | 3.97 | <0.001 | |
| Self-referential network | L sup. temporal gyrus/insula (BA 22/13) | 102 | −48 | −06 | 04 | 3.97 | <0.001 |
| R primary auditory cortex (BA 41) | 55 | 46 | −36 | 12 | 3.97 | <0.001 | |
| L dorsal attention network | Nil | ||||||
| R dorsal attention network | Nil | ||||||
| L executive control network | Nil | ||||||
| R executive control network | L cerebellum | 113 | −10 | −32 | −42 | 3.97 | <0.001 |
| L salience network | Nil | ||||||
| R salience network | L cerebellum | 59 | −16 | −30 | −46 | 3.97 | <0.001 |
| R cerebellum | 50 | 26 | −40 | −42 | 3.97 | <0.001 | |
| L angular gyrus (BA 39) | 48 | −42 | −58 | 16 | 3.97 | <0.001 | |
BA, Brodmann area.
Cluster FWE ≤ 0.05.
Fig. 1.
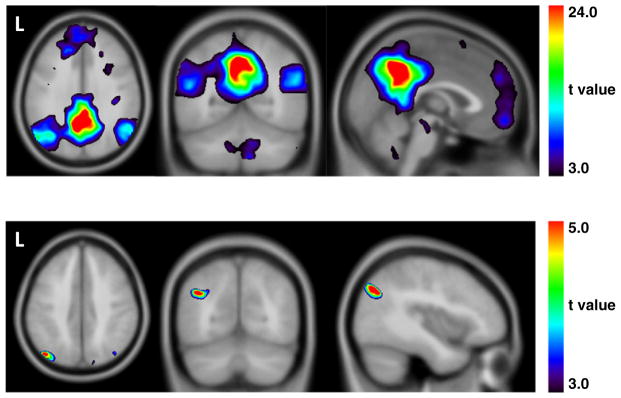
Top row The default mode network (PCC as seed) for participants with schizophrenia spectrum disorders. Bottom row. The default mode network (PCC as seed) for impaired insight in participants with schizophrenia spectrum disorders. Peak voxel in the left angular gyrus (−42, −72, 36).
The group connectivity maps for each resting state network and their relationship to cognitive insight are presented in Table 3, Supplementary Tables 1 and 2, and Supplementary Figs. 2 and 3. No component of cognitive insight was associated with altered connectivity in the DMN. In other resting state networks, the only area of altered connectivity that survived FWE correction (p < 0.05) was the cluster showing the association between greater self-certainty and reduced connectivity in the left inferior frontal cortex pars opercularis in the right DAN (Table 3).
Table 3.
Resting state networks for BCIS self-certainty in schizophrenia spectrum disorders—seed-to-voxel analysis.
| Network | Brain region | Voxels per cluster | Cluster maxima
|
Minimum T value | p value uncorrected | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Default mode network | Nil | ||||||
| Self-referential network | ↓L cerebellum | 78 | −30 | −50 | −22 | 3.92 | <0.001 |
| L dorsal attention network | ↓L & R secondary visual cortex (BA 18) | 128 | 0 | −98 | −10 | 3.92 | <0.001 |
| L premotor cortex (BA 6) | 100 | −16 | −4 | 56 | 3.92 | <0.001 | |
| ↓L inf. temporal gyrus/temporopolar area (BA 20/38) | 84 | −44 | 0 | −40 | 3.92 | <0.001 | |
| R dorsal attention network | ↓L IFC pars opercularis (BA 44) | 143 | −48 | 16 | 4 | 3.92 | <0.001* |
| ↓R cerebellum | 124 | 24 | −86 | −26 | 3.92 | <0.001 | |
| L primary motor cortex (BA 4) | 70 | −34 | −18 | 46 | 3.92 | <0.001 | |
| L executive control network | ↓ L cerebellum | 110 | −20 | −48 | −48 | 3.92 | <0.001 |
| R executive control network | ↓R inf. temporal gyrus (BA 20) | 63 | 46 | −4 | −48 | 3.92 | <0.001 |
| ↓R premotor cortex/dorsal frontal cortex (BA 6/8) | 53 | 16 | 30 | 56 | 3.92 | <0.001 | |
| L salience network | Nil | ||||||
| R salience network | Nil | ||||||
BCIS, Beck Cognitive Insight Scale; BA, Brodmann area; IFC, inferior frontal cortex; ↓, reduced connectivity.
Cluster FWE ≤ 0.05.
3.3. ROI-to-ROI analysis
The results of the ROI-to-ROI analyses for each resting state network and their relationship to clinical insight are consistent with the findings of the seed-to-voxel analyses. Results are presented in Table 4. Impaired clinical insight was associated with greater connectivity in the DMN with the left angular gyrus and in the salience network with the left insula. The latter finding survived correction for multiple comparisons (FDR = 0.035).
Table 4.
Resting state networks for impaired clinical insight in schizophrenia spectrum disorders—Seed ROI-to-ROI analysis.
| Network | Brain region | Cluster maxima
|
T value | p value FDR corr. | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Default mode network | L Angular gyrus (BA 39) | −46 | −70 | 36 | 4.11 | 0.076 |
| Self-referential network | L insular cortex (BA 13) | −38 | −7 | 9 | 4.47 | 0.035* |
| L dorsal attention network | Nil | |||||
| R dorsal attention network | Nil | |||||
| L executive control network | Nil | |||||
| R executive control network | ||||||
| L salience network | Nil | |||||
| R salience network | Nil | |||||
BA, Brodmann area.
FDR ≤ 0.05.
The results of the ROI-to-ROI analyses for each resting state network in relation to cognitive insight are presented in Supplementary Tables 3, 4 and 5. Self-reflectiveness was associated with greater connectivity in the left DAN with the left ACC (Supplementary Table 3); however, these findings failed to reach significance (FDR = 0.064). Self-certainty was associated with reduced connectivity in the right DAN with the inferior frontal cortex pars triangularis, which is consistent with the results from the seed-to-voxel analysis (Supplementary Table 4). BCIS composite score was associated with greater connectivity in the left DAN with the ACC, and in the right DAN with the right inferior frontal cortex pars triangularis and opercularis. BCIS composite score was associated with reduced connectivity in the right ECN with the left subcentral area and anterior entorhinal cortex (Supplementary Table 5); however, these findings failed to reach significance (FDR = 0.083).
4. Discussion
The neural correlates of clinical and cognitive insight remain largely unknown. Here we present results from our investigation of the relationships of impaired clinical and cognitive insight with resting state functional connectivity in patients with schizophrenia. As expected, clinical insight and cognitive insight were associated with differences in resting state connectivity. Our main result showed that impaired clinical insight was associated with greater connectivity in the DMN with the left angular gyrus and left posterior cingulate cortex. Our study was predicated on the theory that impaired clinical insight stems from inter-hemispheric imbalance. Lesion models suggest that impaired clinical insight can occur with right hemisphere brain lesions secondary to stroke, traumatic brain injury and dementia (Orfei et al., 2008). Impaired clinical insight in these contexts is thought to arise from left dominant brain hemisphere activity and serves as a model for understanding impaired insight in other neuropsychiatric disorders, such as schizophrenia (Ramachandran, 1995; Ramachandran et al., 2007; Shad et al., 2007). Volume-based analyses using a region of interest approach support this model of impaired clinical insight, reporting an association between reduced right hemisphere brain volume and impaired insight into illness (Flashman et al., 2001; Shad et al., 2004, 2006, 2007). Results from studies that have used a whole brain, voxel-wise approach, however, are inconsistent (Ha et al., 2004; Bassitt et al., 2007; Cooke et al., 2008; Morgan et al., 2010; Berge et al., 2011). A recent analysis of hemispheric asymmetry by our group found relatively reduced right hemisphere (or relatively increased left hemisphere) volume, specifically within the angular gyrus, mPFC, DLPFC, insula, and anterior temporal lobe, in relation to impaired clinical insight in schizophrenia (Gerretsen et al., 2013). The results of the one previous study we are aware of that investigated the relationship of impaired clinical insight with resting state connectivity in schizophrenia, however, do not support the model of interhemispheric imbalance. Contrary to our findings, participants with impaired clinical insight (>2 on PANSS item G12) had reduced connectivity in the ACC and precuneus in the DMN when compared to those with good insight (1 or 2 on PANSS item G12), which they suggest represents reduced self-referential processing (Liemburg et al., 2012). They did not find a significant cluster when comparing “poor” versus “good” insight groups. Despite the differing results, both the present study and the study by Liemburg and colleagues converge in linking aberrant DMN functional connectivity with deficits in clinical insight in patients with schizophrenia.
Only recently has research focused on the neural correlates of cognitive or metacognitive insight in schizophrenia. In one study exploring the neural correlates of cognitive insight, increased brain volume was positively related to BCIS self-reflectiveness in the right ventrolateral PFC (Orfei et al., 2013). No significant results emerged for the relationship between volume and BCIS self-certainty or composite scores. In a more recent study by the same group investigating the brain regions associated with metacognitive insight, lower metacognitive insight was associated with reduced volume in the left ventrolateral PFC, right dorsolateral PFC, right insula, and premotor areas and putamen bilaterally (Spalletta et al., 2014). Metacognitive insight was measured with the Insight Scale, which is based on a wider concept of clinical insight as a form of ‘self-knowledge’ (Markova et al., 2003). Taken together, results of these studies suggest that the prefrontal cortex features prominently in self-monitoring or in self-reflection.
By comparison, our exploratory analyses of resting state functional connectivity implicate the DAN in cognitive insight. In the left DAN, greater cognitive insight (i.e. higher BCIS self-reflectiveness and composite scores) was associated with increased connectivity with the left ACC. In the right DAN, greater cognitive insight (i.e. higher BCIS composite and lower BCIS self-certainty scores) correlated with increased connectivity with the inferior frontal cortex (BA 44 and BA 45), primarily the left pars opercularis (BA 44).
The DAN and the ventral attention system, together, represent two distinct anticorrelated networks (Fox et al., 2006) that flexibly interact to mediate conscious awareness (van den Heuvel and Hulshoff Pol, 2010). In general, the DAN is activated during ‘top-down’ goal-directed activity, while the ventral network attends to ‘bottom-up’ unexpected, salient stimuli serving a re-orienting response (Vossel et al., 2014). Increased functional connectivity in the DAN with the ACC and IFC in association with cognitive insight may represent enhanced mental flexibility in individuals with schizophrenia spectrum disorders. The ACC is implicated in conflict and performance monitoring (MacDonald et al., 2000; Kerns et al., 2004), while the IFC may function as an interface between the dorsal and ventral attention systems in response to discrepant information (Vossel et al., 2014).
It appears that resting state functional connectivity may be a facilitator or marker of a symptom or function's intensity whereby increased connectivity maintains integrated functional systems in either an active adaptive or maladaptive state. In support of this, studies of long-term motor learning have shown that motor skills' training increases resting-state activity in motor areas (Xiong et al., 2009; Ma et al., 2011). In the same way, DAN functional connectivity with the lateral PFC may facilitate meta-cognitive abilities. Conversely, persistent psychotic symptom misattribution and illness denial may enhance DMN connectivity with left hemisphere regions (i.e. angular gyrus, insula, etc.) in a maladaptive manner. However, it remains unclear the degree these functions/symptoms or brain networks are mutable. One recent study demonstrated alterations of resting state connectivity in the frontoparietal and default mode networks after 6 weeks of working memory training (Jolles et al., 2013). In another study, antipsychotics were shown to increase synchronous regional brain function in the resting state at 6 weeks relative to pretreatment, which was correlated with clinical improvement (Lui et al., 2010).
In summary, the results of our study revealed that impaired clinical insight and cognitive insight had differing effects on resting state connectivity (or conversely, that alterations in resting state connectivity had differing effects on clinical and cognitive insight): (i) impaired clinical insight was associated with increased connectivity in the DMN with the left angular gyrus and PCC, and increased connectivity in the salience network with the left insula; and (ii) cognitive insight was associated with increased connectivity in the DAN with the ACC and lateral PFC.
These results are limited by two main factors. First, by utilizing a seed method to assess resting state functional connectivity the analysis is limited to the functional connections of each seed region. The seeds selected, however, are well known hubs of the resting state networks we explored (e.g. the PCC was the seed region for the DMN). Moreover, seed-based and model free methods, such as independent component analyses, tend to produce overlapping results (van den Heuvel and Hulshoff Pol, 2010). Second, our sample population was purposely restricted to stable patients with schizophrenia spectrum disorders. This was done intentionally to better control for illness severity, which is highly associated with clinical insight depending on stage of illness (Heinrichs et al., 1985; Mintz et al., 2003; De Hert et al., 2009; Parellada et al., 2011; Gerretsen et al., 2013). Nevertheless, there was still a strong association between impaired insight into illness and illness severity, which was statistically controlled for in our analyses. Another possible limitation of this study was the lack of a healthy control comparison group. In the original design of this study, however, we felt that healthy controls would not be a valid comparison group, primarily as they do not have a schizophrenia spectrum disorder, and thus, do not have a neuropsychiatric illness or psychotic symptoms for which they can have insight impairment. We believe that the only true valid comparison is between participants with schizophrenia with impaired or intact insight using functional MRI.
In conclusion, the results of this study provide functional imaging evidence in support of the interhemispheric imbalance theory of impaired clinical insight in schizophrenia, whereby impaired clinical insight arises from left dominant brain hemisphere activity. The results of this study build on our previous structural imaging study (Gerretsen et al., 2013) and structural imaging studies that used an ROI approach (Flashman et al., 2001; Shad et al., 2004, 2006, 2007). Future research is required to replicate these findings and to determine if they are generalizable to other neuropsychiatric disorders that may exhibit impaired clinical or cognitive insight. Noninvasive techniques, including transcranial magnetic, direct current or caloric vestibular stimulation—shown to induce spatial hemineglect in healthy controls, transiently reverse anosognosia in right hemisphere damaged stroke patients, and improve clinical insight in schizophrenia spectrum and bipolar disorders (Cappa et al., 1987; Oliveri et al., 2001; Miller and Ngo, 2007; Sparing et al., 2009; Kortte and Hillis, 2011; Levine et al., 2012), can be used to further investigate the theory of interhemispheric imbalance of clinical insight in schizophrenia spectrum disorders.
Supplementary Material
Acknowledgments
Role of funding sources
The research was partially supported by Ontario Mental Health Foundation (OMHF) grant (AG), National Institute of Health RO1MH084886-01A2 (AG and DM), the Clinician Scientist Program, Department of Psychiatry, University of Toronto (PG), and Ontario Mental Health Foundation fellowship awards (PG).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Wanna Mar, research coordinator; Kathryn Kalahani, research student; Zhe Feng, research student; Min Tae Matt Park, research student.
Footnotes
Contributors
All authors contributed to the preparation and review of the manuscript, and have approved the manuscript for submission.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.schres.2014.10.015.
Conflicts of interest
P.G. has received fellowship awards from the Ontario Mental Health Foundation and the Centre for Addiction and Mental Health.
M.M. receives research support from the Ontario Mental Health Foundation, the Brain and Behaviour Research Foundation and the Canadian Institutes of Health Research.
D.M. receives research support from the National Institute of Health and the Canadian Institutes of Health Research. He has received investigator-initiated research support from Pfizer Canada over the past three years.
G.F. reports no competing interests.
G.R. has received research support from the Canadian Diabetes Association, the Canadian Institutes of Health Research, Medicure, Neurocrine Biosciences, Novartis, Research Hospital Fund—Canada Foundation for Innovation, and the Schizophrenia Society of Ontario and has served as a consultant or speaker for Novartis, Laboratorios Farmacéuticos Rovi, Synchroneuron, and Roche.
B.G.P. receives research support from the National Institute of Health and the Canadian Institutes of Health Research. Within the past 5 years, he has been a member of the advisory board of Lundbeck Canada (final meeting was May 2009) and Forest Laboratories (final meeting was March 2008). He has served one time as a consultant for Wyeth (October 2008) and Takeda (July 2007). He was also a faculty member of the Lundbeck International Neuroscience Foundation (LINF) (final meeting was April 2010).
A.G. receives grant support from the National Institute of Health, Canadian Institute of Health Research, Ontario Mental Health Foundation, NARSAD, CONACyT, and ICyTDF.
References
- Andreasen NC, Arndt S, Miller D, Flaum M, Nopoulos P. Correlational studies of the Scale for the Assessment of Negative Symptoms and the Scale for the Assessment of Positive Symptoms: an overview and update. Psychopathology. 1995;28 (1):7–17. doi: 10.1159/000284894. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, Niciu MJ, Morgan PT, Surti TS, Bloch MH, Ramani R, Smith MA, Wang XJ, Krystal JH, Corlett PR. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci U S A. 2012;109(41):16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum PS, Grisso T. The Macarthur Treatment Competence Study. 1. Mental-illness and competence to consent to treatment. Law Hum Behav. 1995;19(2):105–126. doi: 10.1007/BF01499321. [DOI] [PubMed] [Google Scholar]
- Bassitt DP, Neto MR, de Castro CC, Busatto GF. Insight and regional brain volumes in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2007;257(1):58–62. doi: 10.1007/s00406-006-0685-z. [DOI] [PubMed] [Google Scholar]
- Beck AT, Baruch E, Balter JM, Steer RA, Warman DM. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2–3):319–329. doi: 10.1016/S0920-9964(03)00189-0. [DOI] [PubMed] [Google Scholar]
- Bedford NJ, Surguladze S, Giampietro V, Brammer MJ, David AS. Self-evaluation in schizophrenia: an fMRI study with implications for the understanding of insight. BMC Psychiatr. 2012;12:106. doi: 10.1186/1471-244X-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge D, Carmona S, Rovira M, Bulbena A, Salgado P, Vilarroya O. Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand. 2011;123(6):431–439. doi: 10.1111/j.1600-0447.2010.01635.x. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33(4):1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota RG, Munro JS, Ricci WF, Bota DA. The dynamics of insight in the prodrome of schizophrenia. CNS Spectr. 2006;11(5):355–362. doi: 10.1017/s1092852900014486. [DOI] [PubMed] [Google Scholar]
- Buchy L, Czechowska Y, Chochol C, Malla A, Joober R, Pruessner J, Lepage M. Toward a model of cognitive insight in first-episode psychosis: verbal memory and hippocampal structure. Schizophr Bull. 2010;36(5):1040–1049. doi: 10.1093/schbul/sbp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchy L, Luck D, Czechowska Y, Malla A, Joober R, Lepage M. Diffusion tensor imaging tractography of the fornix and belief confidence in first-episode psychosis. Schizophr Res. 2012;137(1–3):80–84. doi: 10.1016/j.schres.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Frangou S, Murray RM. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28(2–3):143–156. doi: 10.1016/s0920-9964(97)00114-x. [DOI] [PubMed] [Google Scholar]
- Camchong J, MacDonald AW, 3rd, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37(3):640–650. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappa S, Sterzi R, Vallar G, Bisiach E. Remission of hemineglect and anosognosia during vestibular stimulation. Neuropsychologia. 1987;25:775–782. doi: 10.1016/0028-3932(87)90115-1. [DOI] [PubMed] [Google Scholar]
- Cooke MA, Fannon D, Kuipers E, Peters E, Williams SC, Kumari V. Neurological basis of poor insight in psychosis: a voxel-based MRI study. Schizophr Res. 2008;103(1–3):40–51. doi: 10.1016/j.schres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke MA, Peters ER, Fannon D, Aasen I, Kuipers E, Kumari V. Cognitive insight in psychosis: the relationship between self-certainty and self-reflection dimensions and neuropsychological measures. Psychiatry Res. 2010;178(2):284–289. doi: 10.1016/j.psychres.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David AS. Insight and psychosis. Br J Psychiatry. 1990;156 (6):798–808. doi: 10.1192/bjp.156.6.798. [DOI] [PubMed] [Google Scholar]
- De Hert MA, Simon V, Vidovic D, Franic T, Wampers M, Peuskens J, van Winkel R. Evaluation of the association between insight and symptoms in a large sample of patients with schizophrenia. Eur Psychiatry. 2009;24 (8):507–512. doi: 10.1016/j.eurpsy.2009.04.004. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage. 2006;29 (4):1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Dragovic M. Towards an improved measure of the Edinburgh Handedness Inventory: a one-factor congeneric measurement model using confirmatory factor analysis. Laterality. 2004;9 (4):411–419. doi: 10.1080/13576500342000248. [DOI] [PubMed] [Google Scholar]
- Flashman LA, McAllister TW, Johnson SC, Rick JH, Green RL, Saykin AJ. Specific frontal lobe subregions correlated with unawareness of illness in schizophrenia: a preliminary study. J Neuropsychiatry Clin Neurosci. 2001;13(2):255–257. doi: 10.1176/jnp.13.2.255. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164 (3):450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Gerretsen P, Chakravarty MM, Mamo D, Menon M, Pollock BG, Rajji TK, Graff-Guerrero A. Frontotemporoparietal asymmetry and lack of illness awareness in schizophrenia. Hum Brain Mapp. 2013;34(5):1035–1043. doi: 10.1002/hbm.21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger C, Serper MR. Examination of clinical and cognitive insight in acute schizophrenia patients. J Nerv Ment Dis. 2010;198(7):465–469. doi: 10.1097/NMD.0b013e3181e4f35d. [DOI] [PubMed] [Google Scholar]
- Ha TH, Youn T, Ha KS, Rho KS, Lee JM, Kim IY, Kim SI, Kwon JS. Gray matter abnormalities in paranoid schizophrenia and their clinical correlations. Psychiatry Res. 2004;132(3):251–260. doi: 10.1016/j.pscychresns.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Heinrichs DW, Cohen BP, Carpenter WT., Jr Early insight and the management of schizophrenic decompensation. J Nerv Ment Dis. 1985;173(3):133–138. doi: 10.1097/00005053-198503000-00001. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Cassidy BS, Andrews-Hanna JR, Lee SM, Coombs G, Goff DC, Gabrieli JD, Moran JM. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol Psychiatry. 2011;69 (5):415–423. doi: 10.1016/j.biopsych.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles DD, van Buchem MA, Crone EA, Rombouts SA. Functional brain connectivity at rest changes after working memory training. Hum Brain Mapp. 2013;34(2):396–406. doi: 10.1002/hbm.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp R, David AS. Insight and compliance. In: Blackwell B, editor. Treatment compliance and the therapeutic alliance. Gordon and Breach Publishing Group; Newark, NJ: 1997. pp. 61–84. [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303 (5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kindler J, Jann K, Homan P, Hauf M, Walther S, Strik W, Dierks T, Hubl D. Static and dynamic characteristics of cerebral blood flow during the resting state in schizophrenia. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt180. http://dx.doi.org/10.1093/schbul/sbt180 (2013 Dec 18 Epub ahead of print) [DOI] [PMC free article] [PubMed]
- Kortte KB, Hillis AE. Recent trends in rehabilitation interventions for visual neglect and anosognosia for hemiplegia following right hemisphere stroke. Future Neurol. 2011;6(1):33–43. doi: 10.2217/fnl.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Dementia praecox. Edinburgh; 1919. [Google Scholar]
- Lepage M, Buchy L, Bodnar M, Bertrand MC, Joober R, Malla A. Cognitive insight and verbal memory in first episode of psychosis. Eur Psychiatry. 2008;23 (5):368–374. doi: 10.1016/j.eurpsy.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Levine J, Toder D, Geller V, Kraus M, Gauchman T, Puterman M, Grisaru N. Beneficial effects of caloric vestibular stimulation on denial of illness and manic delusions in schizoaffective disorder: a case report. Brain Stimul. 2012;5(3):267–273. doi: 10.1016/j.brs.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Liemburg EJ, van der Meer L, Swart M, Curcic-Blake B, Bruggeman R, Knegtering H, Aleman A. Reduced connectivity in the self-processing network of schizophrenia patients with poor insight. PLoS One. 2012;7 (8):e42707. doi: 10.1371/journal.pone.0042707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, Yue Q, Huang X, Chan RC, Collier DA, Meda SA, Pearlson G, Mechelli A, Sweeney JA, Gong Q. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67 (8):783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- Ma L, Narayana S, Robin DA, Fox PT, Xiong J. Changes occur in resting state network of motor system during 4 weeks of motor skill learning. NeuroImage. 2011;58 (1):226–233. doi: 10.1016/j.neuroimage.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288 (5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Manoliu A, Riedl V, Zherdin A, Muhlau M, Schwerthoffer D, Scherr M, Peters H, Zimmer C, Forstl H, Bauml J, Wohlschlager AM, Sorg C. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull. 2014;40(2):428–437. doi: 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markova IS, Roberts KH, Gallagher C, Boos H, McKenna PJ, Berrios GE. Assessment of insight in psychosis: a re-standardization of a new scale. Psychiatry Res. 2003;119(1–2):81–88. doi: 10.1016/s0165-1781(03)00101-x. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, D'Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. NeuroImage. 2006;29 (4):1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon M, Schmitz TW, Anderson AK, Graff A, Korostil M, Mamo D, Gerretsen P, Addington J, Remington G, Kapur S. Exploring the neural correlates of delusions of reference. Biol Psychiatry. 2011;70 (12):1127–1133. doi: 10.1016/j.biopsych.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Miller S, Ngo TT. Studies of caloric vestibular stimulation: implications for the cognitive neurosciences, the clinical neurosciences and neurophilosophy. Acta Neuropsychiatr. 2007;19:183–203. doi: 10.1111/j.1601-5215.2007.00208.x. [DOI] [PubMed] [Google Scholar]
- Mingoia G, Wagner G, Langbein K, Maitra R, Smesny S, Dietzek M, Burmeister HP, Reichenbach JR, Schlosser RG, Gaser C, Sauer H, Nenadic I. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr Res. 2012;138(2–3):143–149. doi: 10.1016/j.schres.2012.01.036. [DOI] [PubMed] [Google Scholar]
- Mintz AR, Dobson KS, Romney DM. Insight in schizophrenia: a meta-analysis. Schizophr Res. 2003;61(1):75–88. doi: 10.1016/s0920-9964(02)00316-x. [DOI] [PubMed] [Google Scholar]
- Morgan KD, Dazzan P, Morgan C, Lappin J, Hutchinson G, Suckling J, Fearon P, Jones PB, Leff J, Murray RM, David AS. Insight, grey matter and cognitive function in first-onset psychosis. Br J Psychiatry J Ment Sci. 2010;197(2):141–148. doi: 10.1192/bjp.bp.109.070888. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44 (3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ER, Brent BK, Benton M, Pruitt P, Diwadkar V, Rajarethinam RP, Keshavan MS. Differential processing of metacognitive evaluation and the neural circuitry of the self and others in schizophrenia: a pilot study. Schizophr Res. 2010;116(2–3):252–258. doi: 10.1016/j.schres.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Nair A, Palmer EC, Aleman A, David AS. Relationship between cognition, clinical and cognitive insight in psychotic disorders: a review and meta-analysis. Schizophr Res. 2014;152(1):191–200. doi: 10.1016/j.schres.2013.11.033. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Bisiach E, Brighina F, Piazza A, La Bua V, Buffa D, Fierro B. rTMS of the unaffected hemisphere transiently reduces contralesional visuospatial hemineglect. Neurology. 2001;57 (7):1338–1340. doi: 10.1212/wnl.57.7.1338. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Robinson RG, Bria P, Caltagirone C, Spalletta G. Unawareness of illness in neuropsychiatric disorders: phenomenological certainty versus etiopathogenic vagueness. Neuroscientist Rev J Neurobiol Neurol Psychiatry. 2008;14 (2):203–222. doi: 10.1177/1073858407309995. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Spoletini I, Banfi G, Caltagirone C, Spalletta G. Neuropsychological correlates of cognitive insight in schizophrenia. Psychiatry Res. 2010;178(1):51–56. doi: 10.1016/j.psychres.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Piras F, Macci E, Caltagirone C, Spalletta G. The neuroanatomical correlates of cognitive insight in schizophrenia. Soc Cogn Affect Neurosci. 2013;8(4):418–423. doi: 10.1093/scan/nss016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orliac F, Naveau M, Joliot M, Delcroix N, Razafimandimby A, Brazo P, Dollfus S, Delamillieure P. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr Res. 2013;148(1–3):74–80. doi: 10.1016/j.schres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Parellada M, Boada L, Fraguas D, Reig S, Castro-Fornieles J, Moreno D, Gonzalez-Pinto A, Otero S, Rapado-Castro M, Graell M, Baeza I, Arango C. Trait and state attributes of insight in first episodes of early-onset schizophrenia and other psychoses: a 2-year longitudinal study. Schizophr Bull. 2011;37(1):38–51. doi: 10.1093/schbul/sbq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrelli P, McQuaid JR, Granholm E, Patterson TL, McClure F, Beck AT, Jeste DV. Measuring cognitive insight in middle-aged and older patients with psychotic disorders. Schizophr Res. 2004;71(2–3):297–305. doi: 10.1016/j.schres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raij TT, Riekki TJ, Hari R. Association of poor insight in schizophrenia with structure and function of cortical midline structures and frontopolar cortex. Schizophr Res. 2012;139(1–3):27–32. doi: 10.1016/j.schres.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS. Anosognosia in parietal lobe syndrome. Conscious Cogn. 1995;4(1):22–51. doi: 10.1006/ccog.1995.1002. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, McGeoch PD, Williams L. Can vestibular caloric stimulation be used to treat Dejerine–Roussy Syndrome? Med. Hypotheses. 2007;69 (3):486–488. doi: 10.1016/j.mehy.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117(1):21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Shad MU, Muddasani S, Prasad K, Sweeney JA, Keshavan MS. Insight and prefrontal cortex in first-episode schizophrenia. NeuroImage. 2004;22 (3):1315–1320. doi: 10.1016/j.neuroimage.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Shad MU, Tamminga CA, Cullum M, Haas GL, Keshavan MS. Insight and frontal cortical function in schizophrenia: a review. Schizophr Res. 2006;86(1–3):54–70. doi: 10.1016/j.schres.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Shad MU, Keshavan MS, Tamminga CA, Cullum CM, David A. Neurobiological underpinnings of insight deficits in schizophrenia. Int Rev Psychiatry. 2007;19 (4):437–446. doi: 10.1080/09540260701486324. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 (Suppl 20):22–33. (quiz 34–57.) [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68 (1):61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalletta G, Piras F, Piras F, Caltagirone C, Orfei MD. The structural neuroanatomy of metacognitive insight in schizophrenia and its psychopathological and neuropsy-chological correlates. Hum Brain Mapp. 2014;35(9):4729–4740. doi: 10.1002/hbm.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparing R, Thimm M, Hesse MD, Kust J, Karbe H, Fink GR. Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain. 2009;132 (Pt 11):3011–3020. doi: 10.1093/brain/awp154. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35(3):509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- van der Meer L, de Vos AE, Stiekema AP, Pijnenborg GH, van Tol MJ, Nolen WA, David AS, Aleman A. Insight in schizophrenia: involvement of self-reflection networks? Schizophr. Bull. 2013;39(6):1288–1295. doi: 10.1093/schbul/sbs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen A, Knegtering H, den Boer JA, Liemburg EJ, Aleman A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biol Psychiatry. 2010;67 (10):912–918. doi: 10.1016/j.biopsych.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist Rev J Neurobiol Neurol Psychiatry. 2014;20 (2):150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warman DM, Lysaker PH, Martin JM. Cognitive insight and psychotic disorder: the impact of active delusions. Schizophr Res. 2007;90(1–3):325–333. doi: 10.1016/j.schres.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wilkenson GS, Jastak J. Wide Range Achievement Test—Third Edition (WRAT-3) Wide Range, Inc; Wilmington, DE: 1993. [Google Scholar]
- Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130(1–3):86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Ma L, Wang B, Narayana S, Duff EP, Egan GF, Fox PT. Long-term motor training induced changes in regional cerebral blood flow in both task and resting states. NeuroImage. 2009;45 (1):75–82. doi: 10.1016/j.neuroimage.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


