Abstract
BACKGROUND
Brachytherapy has been shown to be an efficacious and cost-effective treatment among patients with localized prostate cancer. In this study, the authors examined trends in brachytherapy use for localized prostate cancer using a large national cancer registry.
METHODS
In the National Cancer Data Base (NCDB), a total of 1,547,941 patients with localized prostate cancer were identified from 1998 through 2010. Excluding patients with lymph node-positive or metastatic disease, the authors examined primary treatment trends focusing on the use of brachytherapy over time. Patients with available data (2004–2009) were stratified by National Comprehensive Cancer Network risk criteria. Controlling for year of diagnosis and demographic, clinical, and pathologic characteristics, multivariate analyses were performed examining the association between patient characteristics and receipt of brachytherapy.
RESULTS
In the study cohort, brachytherapy use reached a peak of 16.7% in 2002, and then steadily declined to a low of 8% in 2010. Of the 719,789 patients with available data for risk stratification, 41.1%, 35.3%, and 23.6%, respectively, met low, intermediate, and high National Comprehensive Cancer Network risk criteria. After adjustment, patients of increasing age and those with Medicare insurance were more likely to receive brachytherapy. In contrast, patients with intermediate-risk or high-risk disease, Medicaid insurance, increasing comorbidity count, and increasing year of diagnosis were less likely to receive brachytherapy.
CONCLUSIONS
For patients with localized prostate cancer who are treated at National Cancer Data Base institutions, there has been a steady decline in brachytherapy use since 2003. For low-risk patients, the declining use of brachytherapy monotherapy compared with more costly emerging therapies has significant health policy implications.
Keywords: brachytherapy, prostatic neoplasms, use, radiotherapy, clinical practice patterns, prostatectomy
INTRODUCTION
First performed in the 1960s, brachytherapy has been shown to be a clinically and cost-effective treatment strategy in the management of localized prostate cancer.1 Although tumor registry studies using the National Cancer Data Base (NCDB) and Surveillance, Epidemiology, and End Results (SEER) data have documented increasing use trends after the adoption of an ultrasound-guided transperineal approach first described in 1983, the performance of brachytherapy has lagged behind other treatment options such as surgery, external beam radiotherapy, and intensity-modulated radiotherapy (IMRT).2–4
Primary treatment decision-making for patients presenting with localized prostate cancer has grown increasingly complex in recent years with the rapid introduction of new technologies including robotic surgery, IMRT, and proton therapy. As comparative effectiveness and cost-effectiveness research becomes increasingly emphasized under contemporary health care reform, the management of patients with prostate cancer has been highlighted as a prominent example of how the adoption of novel technologies has outpaced the evidence base defining the risks and benefits when compared with conventional treatments.5–7
The objective of the current study was to use the NCDB registry to examine recent trends in brachytherapy use for the treatment of patients with localized prostate cancer in comparison with alternative surgical and radiotherapy treatment modalities.
MATERIALS AND METHODS
The NCDB, a joint program of the American College of Surgeons Commission on Cancer (CoC) and the American Cancer Society, is a nationwide oncology outcomes database containing information regarding patterns of cancer care and treatment outcomes. The NCDB has been collecting data on newly diagnosed cancers since 1985 and now includes information regarding >29 million cancers from >1500 hospitals with CoC-accredited cancer programs in the United States and Puerto Rico.8 Approximately 70% of new cancer cases in the United States each year are diagnosed and treated at such hospitals and reported to the NCDB. The data collection methods for this database have been previously described.8
The NCDB was queried for all patients diagnosed with localized prostate cancer from 1998 through 2010. Patients with lymph node-positive or metastatic disease were excluded from this analysis. Due to limitations regarding the completeness of clinical staging data, a sub-cohort of patients diagnosed from 2004 through 2009 was identified due to the availability of data regarding clinical T classification, prostate-specific antigen, and Gleason score to facilitate stratification by National Comprehensive Cancer Network (NCCN) risk grouping criteria.9 Information was not available for the percentage of involved biopsy cores, thereby preventing further stratification of the intermediate-risk group into favorable and unfavorable.10 Patients missing all 3 risk stratification variables (4364 patients; 0.6%) were excluded from the analysis. If data were missing for a given risk grouping, it was assumed that the unknown variable would not increase the risk category of the individual.
Patient treatments were categorized by initial treatment received into surgery, all radiotherapy, hormone therapy, or no treatment. The radiotherapy group was then further stratified as brachytherapy (monotherapy), brachytherapy as a boost, and other radiotherapy (including 3-dimensional conformal radiotherapy, IMRT, and radiotherapy not otherwise specified). The NCDB does contain categories for low-dose rate brachytherapy or high-dose rate brachytherapy, but due to concerns regarding data fidelity these patients were excluded from the current analyses.
Covariates available for severity adjustment include race, Hispanic ethnicity, age, insurance, urban/rural status, median income, education, Charlson/Deyo comorbidity score, facility type, and geographic region. For comorbidity assessment, the Charlson/Deyo score is truncated to 0 (no comorbid conditions reported), 1, or 2 (> 1 comorbid conditions reported), and is available for all cases diagnosed after 2003. Each facility that reports cases to the NCDB is classified by 1 of 4 types: community cancer program, comprehensive community cancer program, academic/research program (includes National Cancer Institute-designated comprehensive cancer centers), or other. These classifications are made by the CoC over a 3-year period based on facility type, services provided, and cases accessioned.
Using the full analytic cohort (1998–2010), we compared trends in primary treatment modality performance over time. Then, in a NCCN risk-stratified cohort (2004–2009), we compared demographic, clinical, and pathologic characteristics between patients treated with brachytherapy (standard or boost) versus alternative treatment methods using chi-square tests. We then tested the associations between available covariates and receipt of brachytherapy using multivariable logistic regressions adjusting for risk group, age, race, Hispanic ethnicity, insurance type, median income, education, urban setting, Charlson/Deyo score, year of diagnosis, and geographic region. Logistic regression analysis was also performed for each risk group separately (using the above covariates) and an interaction model was applied to assess differences attributed to year by risk group. To account for within-facility correlation, we used generalized estimating equations with robust standard errors to estimate regression parameters.11
RESULTS
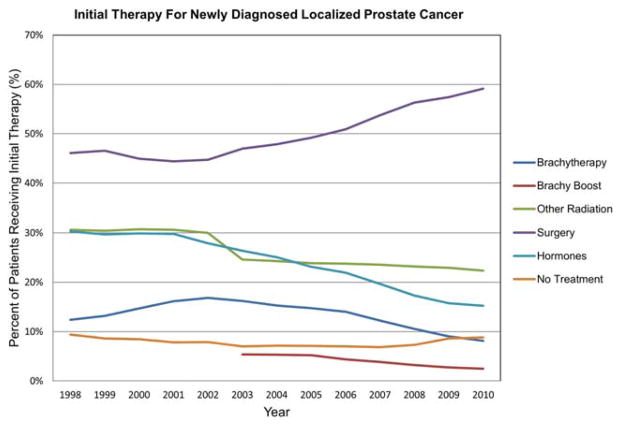
In the NCDB, a total of 1,547,941 patients with localized prostate cancer were identified from 1998 through 2010. Overall, 13.4% of patients were treated with brachytherapy, with an additional 2.6% treated with brachytherapy boost, compared with 49.8% treated with surgery, 26.3% with nonbrachytherapy radiotherapy, 24.1% who received hormone therapy, and 7.8% who received no treatment. The percentage of patients treated with brachytherapy as monotherapy changed significantly over this time period, rising to a peak of 16.9% of patients with localized prostate cancer in 2002 and then persistently declining to a low of 8.2% in 2010 (P <.001) (Fig. 1). In the NCDB, brachytherapy as a boost was not counted separately from other radiotherapy until 2003. Since then, it has steadily declined from 5.4% of total cases to 2.5% in 2010 (P <.001). Although performance rates of alternative radiotherapy modalities remained consistent, rates of surgery markedly increased from 46.1% in 1998 to 59.1% in 2010 (P <.001).
Figure 1.
Initial therapy for patients with newly diagnosed localized prostate cancer is shown as a percentage. Brachy indicates brachytherapy.
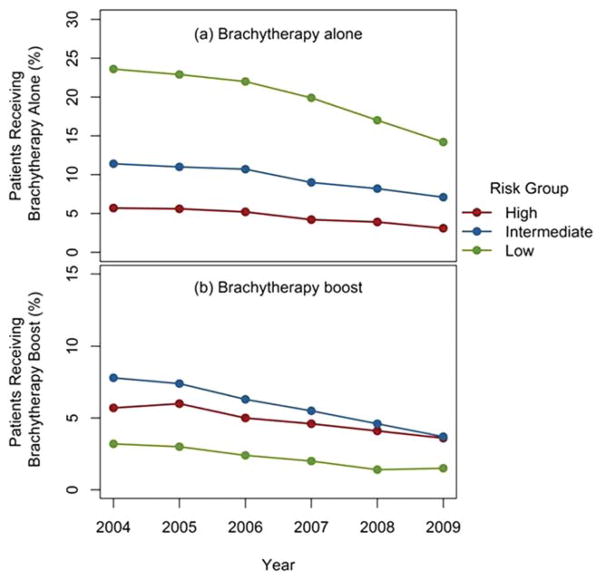
Of the 719,789 patients with available clinical staging information to facilitate NCCN risk stratification, 41.1% were stratified as low risk, 35.3% were stratified as intermediate risk, and 23.6% were stratified as high risk. Of these patients, 67.8% had complete data available, 26.7% were missing 1 of 3 variables, and 5.5% were missing 2 of 3 variables. For patients receiving brachytherapy alone, low-risk patients exhibited the largest decrease of 9.4% (23.6% in 2004 to 14.2% in 2009), followed by intermediate-risk patients (−4.3%) and high-risk patients (−2.6%) (Fig. 2a). Intermediate-risk patients had the greatest decrease of 4.1% for patients receiving a brachytherapy boost (7.8% in 2004 to 3.7% in 2009), followed by high-risk patients (−2.1%) and low-risk patients (−1.7%) (Fig. 2b). For low-risk patients, the use of non-brachytherapy radiotherapy remained relatively constant from 2004 through 2009 (17.9%–18.5%; P = .04), whereas the rates of surgery (46.6%–53.8%; P <.001) and patients not receiving treatment (8.4%–12.2%; P <.001) both increased.
Figure 2.
(a) Percentage of patients treated with brachytherapy alone by year from 2004 through 2009 is shown stratified by National Comprehensive Cancer Network risk grouping. (b) Percentage of patients treated with brachytherapy boost by year from 2004 through 2009 is shown stratified by National Comprehensive Cancer Network risk grouping.
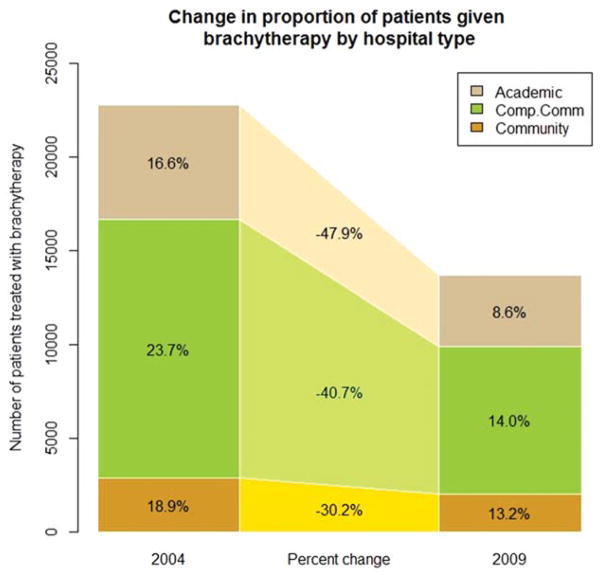
When comparing patients treated with brachytherapy with those who were not, significant differences were noted with regard to age, race, ethnicity, Charlson/Deyo score, NCCN risk group, payor group, urban/rural status, geographic region, facility type, median income, and education (all P values <.01) (Table 1). The Southeast region had the highest percentage of patients treated with brachytherapy (22.1%), whereas the West region had the lowest (11.6%). The percentage change in brachytherapy by facility type between 2004 and 2009 was most dramatic for the academic/research programs (47.9% decrease) compared with comprehensive community, community, and unknown facility types (Fig. 3).
TABLE 1.
Patient Characteristics and Univariate Chi-Square Analysis for Predictors of Receiving Prostate Brachytherapy
| Characteristic | Patients Not Receiving Brachytherapy | Patients Receiving Brachytherapy | Chi-Square P |
|---|---|---|---|
| Risk group | <.0001 | ||
| Low | 77.8% | 22.2% | |
| Intermediate | 84.7% | 15.3% | |
| High | 90.6% | 9.4% | |
| Race | <.0001 | ||
| White | 83.2% | 16.8% | |
| African American | 82.8% | 17.2% | |
| Ethnicity | <.0001 | ||
| Hispanic | 87.5% | 12.5% | |
| Non-Hispanic | 82.9% | 15.3% | |
| Age, y | <.0001 | ||
| <50 | 92.5% | 7.5% | |
| 51–60 | 87.5% | 12.5% | |
| 61–70 | 82.2% | 17.8% | |
| ≥71 | 80.5% | 19.5% | |
| Charlson/Deyo comorbidity index | <.0001 | ||
| 0 | 82.8% | 17.2% | |
| 1 | 85.3% | 14.7% | |
| 2 | 88.4% | 11.6% | |
| Facility type | <.0001 | ||
| Community cancer program | 82.7% | 17.3% | |
| Comprehensive community cancer program | 80.5% | 19.5% | |
| Academic/research program | 87.2% | 12.8% | |
| Other | 86.6% | 13.4% | |
| Insurance | <.0001 | ||
| Private/HMO | 85.2% | 14.8% | |
| Medicaid | 88.1% | 11.9% | |
| Medicare | 80.4% | 19.6% | |
| None/other/unknown | 85.7% | 14.3% | |
| Urban setting | <.0001 | ||
| Large metropolitan | 82.9% | 17.1% | |
| Small metropolitan | 84.2% | 15.8% | |
| Suburban | 81.4% | 18.6% | |
| Rural | 84.0% | 16.0% | |
| Median income quartile | .0012 | ||
| <$30,000 | 83.2% | 16.8% | |
| $30,000–$35,000 | 83.0% | 17.0% | |
| $35,000–$45,999 | 83.3% | 16.7% | |
| >$46,000 | 83.4% | 16.6% | |
| % in region without high school diploma | <.0001 | ||
| >29% | 83.5% | 16.5% | |
| 20%–28.9% | 82.5% | 17.5% | |
| 14%–19.9% | 82.8% | 17.2% | |
| <14% | 83.9% | 16.1% | |
| Region | <.0001 | ||
| Northeast (CT, MA, ME, NH, RI, and VT) | 85.4% | 14.6% | |
| Atlantic (NJ, NY, and PA) | 81.3% | 18.7% | |
| Southeast (DC, DE, FL, GA, MD, NC, SC, VA, and WV) | 77.9% | 22.1% | |
| Great Lakes (IL, IN, MI, OH, and WI) | 83.9% | 16.1% | |
| South (AL, KY, MS, and TN) | 84.7% | 15.3% | |
| Midwest (IA, KS, MN, MO, ND, NE, and SD) | 87.0% | 13.0% | |
| West (AR, LA, OK, and TX) | 88.4% | 11.6% | |
| Mountain (AZ, CO, ID, MT, NM, NV, UT, and WY) | 86.1% | 13.9% | |
| Pacific (AK, CA, HI, OR, and WA) | 85.9% | 14.1% |
Abbreviation: HMO, health maintenance organization.
Figure 3.
Receipt of prostate brachytherapy is shown by type of facility. Comp. Comm. indicates comprehensive community cancer program.
After adjustment, patients were less likely to be treated with brachytherapy if they were Hispanic (odds ratio [OR], 0.89; 95% confidence interval [95% CI], 0.84–0.94), met intermediate (OR, 0.67; 95% CI, 0.65–0.69) or high (OR, 0.40; 95% CI, 0.38–0.42) NCCN risk criteria, had elevated Charlson/Deyo scores (1: OR, 0.84 [95% CI, 0.80–0.88] or ≥2: OR, 0.64 [95% CI, 0.60–0.69]), had Medicaid insurance (OR, 0.88; 95% CI, 0.81–0.95), or received care in a non-rural setting (large metropolitan area: OR, 0.87 [95% CI, 0.79–0.96]; small metropolitan area: OR, 0.80 [95% CI, 0.75–0.85], and suburban: OR, 0.92 [95% CI, 0.87–0.97]) (Table 2). Patients were more likely to receive brachytherapy with each increase in age category (51 years–60 years: OR, 1.65 [95% CI, 1.57–1.74], 61 years–70 years: OR, 2.28 [95% CI, 2.13–2.44], and ≥ 71 years: OR, 2.30 [95% CI, 2.10–2.51]) and if covered by Medicare (OR, 1.10; 95% CI, 1.06–1.15). With each incremental increase in year of diagnosis, the odds of being treated with brachytherapy as primary treatment decreased by 10% from 2004 through 2009 (OR, 0.90; 95% CI, 0.88–0.91). Results from the logistic regression analysis stratified by NCCN risk group demonstrated a similar effect per year across risk groups for low-risk (OR, 0.90; 95% CI, 0.88–0.91), intermediate-risk (OR, 0.91; 95% CI, 0.89–0.92), and high-risk (OR, 0.91; 95% CI, 0.89–0.93) patients. The interaction model revealed no significant difference in the odds of being treated with brachytherapy by year when comparing different risk groups (low to intermediate risk: P = .15; and low to high risk: P = .07).
DISCUSSION
Prior studies examining rates of brachytherapy use for the treatment of patients with localized prostate cancer demonstrated the increased adoption of this modality through the early 2000s.3,4 Using SEER data, Jani et al4 demonstrated a steady increase in the use of radiotherapy overall from 1973 to 2004, with a dramatic rise in the use of brachytherapy from 1994 to 2004. Using NCDB data, Mettlin et al reported an increase in cases treated with brachytherapy from 1.4% to 3.0% (of all prostate cancer cases including metastatic disease) from 1992 through1996.3 Confirming an observation recently made by Mahmood et al12 using SEER data, our contemporary study examining treatment trends for all localized prostate cancer cases reported to the NCDB from 1998 through 2010 revealed that brachytherapy use increased to a peak in 2002 (16.7%) followed by a precipitous decline to 8.0% in 2010.12
When comparing trends between the previously mentioned studies, careful attention must be paid to the number of patients and inclusion criteria impacting the denominator for direct comparison of results. Mahmood et al found that brachytherapy use decreased from 44.5% in 2005 to 38% in 2009 among all patients treated with radiotherapy.12 To make a direct comparison, when we limited the current study data to only patients treated with radiotherapy, we observed a similar decrease from 45.5% to 34% over the same time period. Although there is inherent value to validating trends in use with different tumor registry data sources, the results of the current analyses demonstrated that patients in the mid- to late 2000s were more likely to be treated with surgery, which markedly increased, rather than other radiotherapy modalities, whose rates remained relatively constant over the same time period. The findings of the current study also demonstrated that patients receiving brachytherapy compared with alternative treatment types were younger, hade minimal comorbidity, and were more likely to have low-risk disease.
There are multiple possible explanations for the decline in brachytherapy use for the treatment of patients with localized prostate cancer demonstrated in the current study. Significant trends in the adoption of new more costly technologies in the management of localized prostate cancer have been demonstrated and have attracted significant attention from the media as well as policymakers.7 The change in clinical practice with the greatest impact is likely the substantial increase in the number of prostatectomies performed, as indicated in the data from the current study as well as in several other articles.13–15 To the best of our knowledge, the first report of a robot-assisted laparoscopic prostatectomy was published in 2001, and despite the absence of level I evidence supporting a benefit over conventional open prostatectomy, this technique has been widely adopted across the United States.16 The current study data demonstrated that surgery accounted for approximately 44% of treatment among patients with localized prostate cancer before the introduction of robotic prostatectomy in the early 2000s, and has since risen to 60% of patients in 2010.
The landscape of managing patients with clinically localized prostate cancer has dramatically changed over the past decade. With growing concerns regarding the overtreatment of patients with low-risk disease, overuse of new technologies, and little forthcoming level 1 data to influence practice, the challenge to urologists and radiation oncologists is now not only how best to treat but who to treat with the least negative impact on quality of life.7,17,18 For men with low-volume low-risk disease wishing to defer the potential side effects of primary treatment, active surveillance has become an increasingly used management strategy.19
Prostate brachytherapy has been demonstrated to be as effective as surgery or external beam radiotherapy for based on primary treatment by Wilson et al reached a similar conclusion, finding costs to be lowest for brachytherapy ($35,143), second only to watchful waiting.23 Because of the documented efficacy, acceptable toxicity profile, and relative cost efficiency in the treatment of patients with low-risk disease, we find it surprising and concerning that rates of brachytherapy use are declining compared with those of other primary treatment options.9,24,25
For men with unfavorable intermediate-risk or high-risk prostate cancer, brachytherapy may be incorporated into their treatment as a boost, but is not recommended as monotherapy.9 Unfortunately, we were unable to parse the patients in the intermediate-risk group into favorable or unfavorable categories due to the lack of information regarding the percentage of positive biopsy cores in the database. Results from the current study have demonstrated a small decline in the already limited use of brachytherapy monotherapy for men with intermediate-risk (11.4% in 2004 to 7.1% in 2009) and high-risk (5.7% in 2004 to 3.1% in 2009) disease, which would be appropriate with current NCCN recommendations.9 However, a large decrease in brachytherapy monotherapy for patients with low-risk disease was noted from 2004 through 2009 (23.6% vs 14.2%;, P <.001). Although a component of this decline may be attributed to the appropriate use of active surveillance (percentage of patients receiving no treatment increased from 8.4% to 12.2%; P <.001), it is also likely due to the increased rates of use of nonbrachytherapy radiotherapy and surgery also noted over the same time period (63.3% to 71.4%; P <.001).
The limitations of the current study include concerns generic to secondary analyses using tumor registry data, most notably selection biases, incomplete data, and coding errors. For patients with missing information regarding NCCN risk stratification, the assumption that the missing information would not change their risk stratification introduces a threat to inference. Furthermore, brachytherapy dose and whether the treatment was given with a low-dose rate or high-dose rate technique were not coded consistently enough to facilitate inclusion in the current analysis. Perhaps most important, the usefulness of the current study findings is restricted to hospitals reporting cases to the NCDB, which should not be interpreted as generalizable to community practices in the United States. Hospitals reporting to the NCDB are all CoC-designated cancer programs and have been shown to be larger, more frequently located in urban locations, and to have more cancer-related services available when compared with non–CoC-approved hospitals.26 NCDB data are hospital-based and therefore will be missing patients who are referred directly for outpatient external beam radiotherapy or brachytherapy performed at nonhospital sites. This could lead to concerns that NCDB data may be missing a higher percentage of patients treated with radio-therapy than more generalizable data such as the SEER registry. Nonetheless, the findings of the current study are closely aligned with recently reported SEER trend data that alleviate these concerns.12
Conclusions
In hospitals reporting to the NCDB, there has been a consistent decline in the use of brachytherapy for the treatment of patients with localized prostate cancer since 2002. Under contemporary health care reform, there is renewed interest in providing high-quality and cost-effective care.27 Prior studies have consistently demonstrated that brachytherapy is among the most cost-effective treatment options for patients with localized prostate cancer.1,28,29 Identifying barriers to the use of brachytherapy may be an attractive alternative to emerging technologies for patients with low-risk and intermediate-risk prostate cancer who are poor candidates for or who are not interested in active surveillance.
Acknowledgments
FUNDING SUPPORT
Supported by National Cancer Institute grants P30-CA006927 and K07-CA163616.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
The data used in the current study were derived from a deidentified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigators. The interpretation and reporting of these data are the sole responsibility of the authors.
References
- 1.Hayes JH, Ollendorf DA, Pearson SD, et al. Observation versus initial treatment for men with localized, low-risk prostate cancer: a cost-effectiveness analysis. Ann Intern Med. 2013;158:853–860. doi: 10.7326/0003-4819-158-12-201306180-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holm HH, Juul N, Pedersen JF, Hansen H, Stroyer I. Transperineal 125iodine seed implantation in prostatic cancer guided by transrectal ultrasonography. J Urol. 1983;130:283–286. doi: 10.1016/s0022-5347(17)51108-8. [DOI] [PubMed] [Google Scholar]
- 3.Mettlin CJ, Murphy GP, McDonald CJ, Menck HR. The National Cancer Data base Report on increased use of brachytherapy for the treatment of patients with prostate carcinoma in the U.S. Cancer. 1999;86:1877–1882. [PubMed] [Google Scholar]
- 4.Jani AB, Johnstone PA, Liauw SL, Master VA, Rossi PJ. Prostate cancer modality time trend analyses from 1973 to 2004: a Surveillance, Epidemiology, and End Results registry analysis. Am J Clin Oncol. 2010;33:168–172. doi: 10.1097/COC.0b013e3181a44ebe. [DOI] [PubMed] [Google Scholar]
- 5.Iglehart JK. Prioritizing comparative-effectiveness research–IOM recommendations. N Engl J Med. 2009;361:325–328. doi: 10.1056/NEJMp0904133. [DOI] [PubMed] [Google Scholar]
- 6.Yu JB, Soulos PR, Herrin J, et al. Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. J Natl Cancer Inst. 2013;105:25–32. doi: 10.1093/jnci/djs463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs BL, Zhang Y, Schroeck FR, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA. 2013;309:2587–2595. doi: 10.1001/jama.2013.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. [Accessed on March 20, 2014];Prostate Cancer V1. 2014 http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 10.Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64:895–902. doi: 10.1016/j.eururo.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Liang KY, Zeger SL. Longitudinal data-analysis using generalized linear-models. Biometrika. 1986;73:13–22. [Google Scholar]
- 12.Mahmood U, Pugh T, Frank S, et al. Declining use of brachytherapy for the treatment of prostate cancer. Brachytherapy. 2014;13:157–162. doi: 10.1016/j.brachy.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Stitzenberg KB, Wong YN, Nielsen ME, Egleston BL, Uzzo RG. Trends in radical prostatectomy: centralization, robotics, and access to urologic cancer care. Cancer. 2012;118:54–62. doi: 10.1002/cncr.26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowrance WT, Eastham JA, Savage C, et al. Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. J Urol. 2012;187:2087–2092. doi: 10.1016/j.juro.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbash GI, Glied SA. New technology and health care costs–the case of robot-assisted surgery. N Engl J Med. 2010;363:701–704. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 16.Abbou CC, Hoznek A, Salomon L, et al. Laparoscopic radical prostatectomy with a remote controlled robot. J Urol. 2001;165:1964–1966. doi: 10.1097/00005392-200106000-00027. [DOI] [PubMed] [Google Scholar]
- 17.Addressing the evidence gap-randomized controlled trials for the surgical management of localized genitourinary tract malignancies. Urol Oncol. 2013;31:393–397. [PubMed] [Google Scholar]
- 18.Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–445. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeb S, Berglund A, Stattin P. Population based study of use and determinants of active surveillance and watchful waiting for low and intermediate risk prostate cancer. J Urol. 2013;190:1742–1749. doi: 10.1016/j.juro.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 20.Grimm P, Billiet I, Bostwick D, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012;109(suppl 1):22–29. doi: 10.1111/j.1464-410X.2011.10827.x. [DOI] [PubMed] [Google Scholar]
- 21.Sylvester JE, Grimm PD, Wong J, Galbreath RW, Merrick G, Blasko JC. Fifteen-year biochemical relapse-free survival, cause-specific survival, and overall survival following I(125) prostate brachytherapy in clinically localized prostate cancer: Seattle experience. Int J Radiat Oncol Biol Phys. 2011;81:376–381. doi: 10.1016/j.ijrobp.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 22.Taira AV, Merrick GS, Butler WM, et al. Long-term outcome for clinically localized prostate cancer treated with permanent interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2011;79:1336–1342. doi: 10.1016/j.ijrobp.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Wilson LS, Tesoro R, Elkin EP, et al. Cumulative cost pattern comparison of prostate cancer treatments. Cancer. 2007;109:518–527. doi: 10.1002/cncr.22433. [DOI] [PubMed] [Google Scholar]
- 24.Cooperberg MR, Ramakrishna NR, Duff SB, et al. Primary treatments for clinically localised prostate cancer: a comprehensive lifetime cost-utility analysis. BJU Int. 2013;111:437–450. doi: 10.1111/j.1464-410X.2012.11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY. Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27:4177–4181. doi: 10.1200/JCO.2008.21.7018. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum L. The whole ball game–overcoming the blind spots in health care reform. N Engl J Med. 2013;368:959–962. doi: 10.1056/NEJMms1301576. [DOI] [PubMed] [Google Scholar]
- 28.Shah C, Lanni TB, Jr, Ghilezan MI, et al. Brachytherapy provides comparable outcomes and improved cost-effectiveness in the treatment of low/intermediate prostate cancer. Brachytherapy. 2012;11:441–445. doi: 10.1016/j.brachy.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Keegan KA, Dall’Era MA, Durbin-Johnson B, Evans CP. Active surveillance for prostate cancer compared with immediate treatment: an economic analysis. Cancer. 2012;118:3512–3518. doi: 10.1002/cncr.26688. [DOI] [PMC free article] [PubMed] [Google Scholar]