Summary
Bone is a regenerative tissue with an innate ability to self-remodel in response to environmental stimuli and the need to repair damage. Rodent models of fracture healing, and in particular genetic mouse models, can be used to study the contributions of specific molecular switches to skeletal repair, as well as to recreate and exacerbate biological development and repair mechanisms in postnatal skeletons. Here, we describe methodology for producing fully stabilized, single-cortex defects in mouse femurs to study mechanisms of intramembranous bone regeneration.
Keywords: Intramembranous bone formation, Fracture healing, Stabilized defect repair, Mouse model
1. Introduction
The skeleton is a classic example of regenerative biology, as bone possesses an inherent ability to remodel its structure and composition in response to a need to repair damage, modify architecture, or modulate calcium needs within the body. Repair of skeletal fractures occurs when progenitor cells from the periosteum, intact bone tissue, or bone marrow differentiate into chondrocytes that form cartilage and osteoblasts that form bone (1), where the tissue phenotype developed depends on the mechanical strain environment encountered by cells in the repair site (2–4). In particular, intramembranous bone, which forms without the need for a preceding cartilage template, is generated within a perfectly stabilized fracture (5). This result can be achieved with careful external fixation of a transverse femoral or tibial defect (4,6), which can be used to promote immediate or prolonged healing, the latter of which is applied in the process of distraction osteogenesis (7).
Rigid, externally stabilized fractures, while scientifically useful and most relevant to clinical situations, can be technically challenging to produce. An alternative approach for studying intramembranous bone regeneration mechanisms within the appendicular skeleton is to surgically induce a small void in one bone cortex, leaving the remainder of the bone to rigidly stabilize the defect. This approach allows the researcher to functionally investigate healing processes involving pure bone formation. This method gives insight into bone repair mechanisms, and can also be useful for exacerbating or magnifying developmental skeletal changes that only occur over long time periods. For example, Axin2−/− mice develop high bone mass in the appendicular skeleton due to increased Wnt signaling, but this developmental effect is only apparent at older ages (6 to 12 months of age) (8). However, Axin2−/− mice rapidly heal single-cortex femoral defects at a faster rate than wildtype littermates, at least as early as 2 to 3 months of age (9), indicating that the molecular pathway is active in younger mice and is responsive to stresses that require repair.
2. Materials
2.1. Surgical Tools and Materials
Hair clippers or depilatory cream (e.g., Nair® hair remover lotion)
Recirculating water warming pad and pump
Povidone-iodone antiseptic solution (10% topical solution)
Stainless steel micro-burr drill bit (Fine Science Tools #19008-07, 0.7 mm diameter)
Drill: Variable speed rotary tool (e.g., Dremel) and appropriately sized collet, or suitable alternative
Scalpel (#11 or #15)
Dissecting forceps and blunt probe
Sutures (Vicryl 7-0 diameter) or tissue adhesive
Isoflurane vaporizer and anesthesia system
Sterile 0.9% saline for injection
Buprenorphine (Buprenex for injection: 0.3 mg/mL stock concentration, diluted to 0.02 mg/mL in sterile saline for injection)
Acetaminophen (liquid suspension, 160 mg in 5 mL stock concentration, diluted to 1 mg/mL in drinking water)
2.2. Analysis Tools and Materials
X-ray system
MicroCT system
10% neutral buffered formalin
Graded ethanol (EtOH) solutions, 70–100%
-
EDTA solution for decalcification
2.0L dH2O
140 mL NH3OH (add additional 40 mL later for 180 mL total)
-
280 g Ethylenediaminetetraacetic acid (EDTA)
In a chemical fume hood, slowly dissolve 280g EDTA + 140 mL NH3OH in 2.0 L dH2O with constant stirring
Slowly add remaining NH3OH to bring pH up to 7.1 (approximately 40 mL, but do not go over pH 7.1)
Store EDTA solution at room temperature
Paraffin tissue embedding medium
Xylenes, histological grade
Vacuum oven
Microtome
-
Safranin O / Fast green staining reagents
-
Weigert’s working solution: 1:1 combination of “ solution A” + “solution B”, diluted 1:1 with H2O prior to use
Solution A: FeCl stock = 0.25 g Ferric Cl + 15 mL H2O + 0.17 mL concentrated HCl
Solution B: Hematoxylin stock = 0.17 g hematoxylin + 1.5 mL 100% EtOH, diluted in an additional 13.5 mL 95% EtOH
0.001% Fast green solution (FCF C.I. 42053): 0.25 g fast green + 250 mL H2O
0.1% Safranin O solution (C.I. 50240): 0.1 g safranin O + 100 mL H2O
1% acetic acid solution: 1 mL glacial acetic acid + 99 mL H2O, mixed fresh
-
-
VonKossa silver nitrate / MacNeal’s Tetrachrome staining reagents
Silver nitrate solution: 5 g silver nitrate + 100 mL distilled water. Filter before use.
Sodium carbonate-formaldehyde solution: 5 g sodium carbonate, 25 mL formaldehyde, 75 mL distilled water.
-
Farmer’s diminisher: 20 g sodium thiosulfate, 210 mL distilled water. Dissolve sodium thiosulfate in 210 mL distilled water first, then add 1 gram potassium ferricyanide.
This solution is stable for 45 min once potassium ferricyanide is added. Mix a fresh batch for each use.
MacNeal’s tetrachrome: 2 g MacNeal’s tetrachrome powder + 100 mL distilled water. Combine and bring briefly to a boil. Remove from heat and stir, at least overnight. Filter before use
3. Methods
3.1. Prepare tools and animal(s) for surgery
Twenty-four hours prior to surgery, begin administration of acetaminophen in the drinking water (1 mg/mL final concentration) for ad-libitum consumption (see Note 1).
Sterilize all surgical tools in an autoclave prior to use, and sanitize between animals with a hot bead sterilizer (preferred method) or another acceptable means.
One hour prior to surgery, inject animal with buprenorphine (0.1 mg/kg body mass, subcutaneous injection) to provide analgesia.
Prepare one hind leg for aseptic surgery by removing hair from the lateral surface of the thigh (shave hair or apply depilatory cream and wash/rinse thoroughly) (11).
Induce anesthesia in an isoflurane vaporizer chamber with 3 to 5% isoflurane, 3.0 LPM oxygen delivery rate
Transfer mouse to a nose cone and reduce isoflurane administration to 1 to 2% concentration for anesthesia maintenance.
Place mouse on a recirculating water warming pad to maintain a constant body temperature of 36 to 38 deg C while under anesthesia. Monitor respiration at all times to ensure lack of respiratory distress.
Swab skin surface with povidone-iodine antiseptic solution to sanitize immediately prior to surgery.
3.2. Surgical procedures
Create an incision through the skin (but not the underlying muscle) on the lateral surface of the thigh, centered over top of the femur extending roughly along the length of the femoral diaphysis with a #11 or #15 scalpel blade.
Expose the femur via blunt dissection with a probe and/or forceps, without transecting muscle tissues.
Visually identify anatomical landmarks on the femur including the greater trochanter and the knee joint capsule, and estimate the mid-point between identified landmarks on the anterior surface of the bone. Grip the bone with dissecting forceps immediately above and below the intended defect location, using forceps to retract soft tissues.
-
Create a single-cortex drilled defect in the anterior aspect of the bone using a 0.7 mm diameter burr drill bit and a rotary drill speed of approximately 10,000 rpm
Irrigate the wound with sterile saline to avoid thermal necrosis and rinse the newly created defect to dislodge any bone fragments
Do not continue the defect into or through the opposite cortical bone wall
Immediately after defect creation, obtain an x-ray of the operated leg to ensure proper defect location and the lack of full transverse fracture
Suture the skin incision with 7-0 diameter sutures, or close incision with tissue adhesive
Transfer the animal to a dry, clean cage and ensure recovery from anesthesia (see Note 2).
Administer 0.1 mg/kg buprenorphine subcutaneously at 12- and 24-hours after surgery to ensure adequate analgesia, and check wound closure periodically to ensure proper healing.
3.3. Longitudinal monitoring of healing via x-ray
Defect healing can be monitored via radiography after surgery. Typical time points include 7, 14, and 21 days after surgery (Figure 1).
Induce anesthesia with isoflurane prior to x-ray as described in Section 3.1.
Ensure consistent animal positioning to permit longitudinal evaluation of bone defect radiopacity over time. This can be easily done with a radiolucent template to guide animal placement.
Perform other procedures (e.g., fluorochrome labeling) as necessary (see Note 3).
Figure 1.
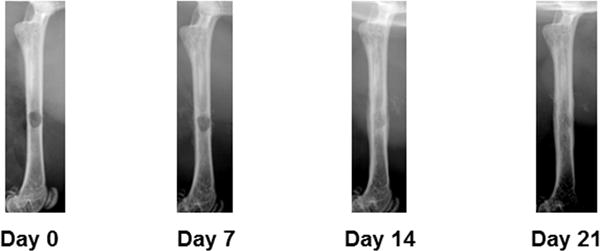
Longitudinal x-ray monitoring of defect repair in a wildtype mouse. Note the increasing radiopacity of the defect over time.
3.4. Tissue harvest and storage
-
Sacrifice mouse by carbon dioxide inhalation at postoperative time point of choice. Suggested time points include:
Postoperative day 7: immunohistochemistry, signaling analysis of early healing mechanisms.
Postoperative day 14 or 21: histology, quantification of bone architecture at mid-healing stages via microCT.
Postoperative day 28: histology, bone architecture at endpoints of healing via microCT. Wildtype mice regularly demonstrate complete healing by this time point (5), and thus this can be a useful time point for comparison of healing in transgenic or mutant mouse strains to wildtype littermates.
Remove the operated femur, keeping full bone anatomy intact. Carefully remove overlying soft tissues, but do not disturb tissue makeup in or around the healing defect (see Note 4).
Fix the operated femur in 10% neutral buffered formalin for 24 hours
Transfer to 70% ethanol for long-term storage.
3.5. Immunohistochemistry (decalcified bone)
Remove femoral epiphyses and decalcify femoral diaphysis for at least 7 days in EDTA solution (confirm complete decalcification by x-ray).
-
Dehydrate decalcified tissue through graded ethanols and infiltrate with xylenes and molten paraffin as described below. Note time, temperature, pressure indicated for each step.
70% EtOH, overnight, room temperature and pressure (RTP)
95% EtOH, 1.5 hrs, 60 deg C, 15mmHg vacuum
95% EtOH, 1 hour, RTP
100% EtOH, 1.5 hrs, 60 deg C, 15mmHg vacuum
100% EtOH, 1 hr, RTP
100% EtOH, 1 hr, RTP
100% EtOH, 1 hr, RTP
Xylenes, 1 hour, 60 deg C, 15mmHg vacuum
Xylenes, 1 hr, RTP
Xylenes, 1 hr, RTP
50% Xylenes/50% molten paraffin, 2 hrs, 60 deg C, 15mmHg vacuum
Molten paraffin, 2 hrs, 60 deg C, 15mmHg vacuum
Embed decalcified bone segments in paraffin for longitudinal sectioning; note tissue orientation and location of defect prior to embedding.
Obtain longitudinal thin (8 micron) sections through the defect with a microtome
Perform immunohistochemical staining with antibodies of choice according to established protocols (9).
3.6. MicroCT analysis of defect bone architecture (undecalcified bone)
Scan the mid-diaphysis of each femur, centered about the defect, in 70% ethanol with a microCT system at 5 to 10 μm voxel size. Recommended settings are energy = 70 kVp and integration time = 300 ms.
Analyze the architecture of the regenerated bone spicules within the defect region (hereafter referred to as “trabecular bone”) using the manufacturer’s software. Parameters of interest may include bone volume fraction (Tb.BV/TV, %), trabecular number (Tb.N, mm−1), trabecular thickness (Tb.Th, mm), and trabecular separation (Tb.Sp, mm). Report all variables and relevant microCT scan settings according to established guidelines (12).
Generate transverse and longitudinal image views from the center of the defect with the manufacturer’s software for quantification of defect diameter using image analysis software (Figure 2).
Figure 2.
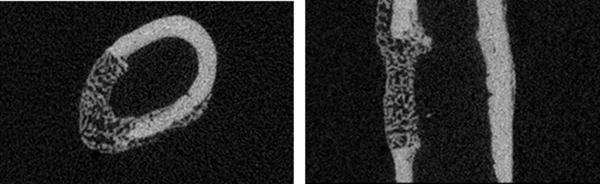
Transverse and longitudinal images through the center of the defect in a wildtype mouse (postoperative day 14). Images reproduced from (9), with permission.
3.7. Histological analysis of tissue morphology (decalcified or undecalcified bone)
Prepare and embed samples via decalcified paraffin embedding (Section 3.5) or undecalcified plastic embedding methodology (9,13,14) as desired.
-
Obtain thin (5 to 8 μm) longitudinal bone sections through the center of the healing defect and prepare histologically. Suggested tissue-specific stains include:
-
Safranin O/fast green (paraffin embedding) to confirm a lack of cartilage formation
Deparaffinize and hydrate sections to water
Add 20μl Weigert’s working solution to each section and stain for 30 seconds to 7 minutes (depending on age of reagents)
Wash sections with water for 10 minutes; change water as necessary
Add 20μl fast green solution to each section and stain for 3 minutes
Immerse sections in 1% acetic acid for 10–15 seconds
Wash sections with water for 1 minute
Add 20μl Safranin O solution to each section and stain for 5 minutes
Dehydrate and clear sections with graded ethanols and xylenes, then mount in resinous medium.
-
b. VonKossa/MacNeal’s tetrachrome (plastic embedding, undecalcified bone) to highlight osteoblast and mineralized bone surfaces
Deplastify sections and hydrate to water.
Stain in silver nitrate solution for 10 minutes in the dark.
Rinse in distilled water x 3 for 1 minute each
Stain in sodium carbonate-formaldehyde solution for 2 minutes
Rinse in distilled water x 2 for 1 minute each
Add potassium ferricyanide to Farmer’s diminisher solution. Stain in Farmer’s diminisher for 30 seconds (time is critical).
Wash in running tap water for 20 minutes
Rinse in distilled water for 1 minute
Stain in MacNeal’s tetrachrome solution for 10 – 15 minutes
Rinse in distilled water x 3 for 1 minute each
Dehydrate in one change each of 70% EtOH, 95% EtOH and 100% EtOH; blot between changes of alcohol
Clear in 2 changes of xylenes.
Coverslip with xylenes-based mounting medium.
Unstained sections (plastic embedding, undecalcified bone) for visualization of fluorochrome labels, if administered (see Note 3).
-
-
Quantify osteoblastic histomorphometric indices across the entire defect region via image analysis software (15). Suggested indices include:
Osteoblast surface/bone surface (Ob.S/BS, %)
Osteoblast number per bone area (N.Ob/B.Ar #/mm2)
Osteoblast number per tissue area (N.Ob/T.Ar #/mm2)
Acknowledgments
The NIH (R01 DE020194, T32 AR056950, F32 AR60140) and the Mayo Clinic Center for Regenerative Medicine supported this work. The authors thank Keith Condon (Indiana University School of Medicine) for the VonKossa/MacNeal’s tetrachrome staining protocol.
Footnotes
It can be helpful to replace standard water bottles with ones featuring a long spout, to promote easy access to water without the necessity to rear on hind legs.
The most common complication observed from this procedure is the creation of a full femoral fracture. The reported rate of this complication is approximately 4% (i.e., 1 out of 24 animals (5). If this occurs, mice should be removed from the study and humanely euthanized. For mice with relatively weak skeletons, reduce cage housing density of the animals to limit the risk of femoral fracture from routine activity.
Fluorochrome labeling can be performed prior to tissue harvest to highlight sites of new bone formation. A suggested dosage and administration schedule for calcein has been previously described as 10 mg/kg body mass, injected 10 days after surgery and 4 days prior to animal sacrifice (5).
It may be advantageous to harvest and save the contralateral femur, for an intact comparison, or other bones for simultaneous analysis of developmental or systemic phenotypes.
References
- 1.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24(2):274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan EF, Salisbury Palomares KT, Gleason RE, Bellin DL, Chien KB, Unnikrishnan GU, Leong PL. Correlations between local strains and tissue phenotypes in an experimental model of skeletal healing. Journal of biomechanics. 2010;43(12):2418–2424. doi: 10.1016/j.jbiomech.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le AX, Miclau T, Hu D, Helms JA. Molecular aspects of healing in stabilized and non-stabilized fractures. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2001;19(1):78–84. doi: 10.1016/S0736-0266(00)00006-1. [DOI] [PubMed] [Google Scholar]
- 4.Cullinane DM, Fredrick A, Eisenberg SR, Pacicca D, Elman MV, Lee C, Salisbury K, Gerstenfeld LC, Einhorn TA. Induction of a neoarthrosis by precisely controlled motion in an experimental mid-femoral defect. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2002;20(3):579–586. doi: 10.1016/S0736-0266(01)00131-0. [DOI] [PubMed] [Google Scholar]
- 5.Monfoulet L, Rabier B, Chassande O, Fricain JC. Drilled hole defects in mouse femur as models of intramembranous cortical and cancellous bone regeneration. Calcified tissue international. 2010;86(1):72–81. doi: 10.1007/s00223-009-9314-y. [DOI] [PubMed] [Google Scholar]
- 6.Yu YY, Bahney C, Hu D, Marcucio RS, Miclau T., 3rd Creating rigidly stabilized fractures for assessing intramembranous ossification, distraction osteogenesis, or healing of critical sized defects. J Vis Exp. 2012;(62) doi: 10.3791/3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isefuku S, Joyner CJ, Simpson AH. A murine model of distraction osteogenesis. Bone. 2000;27(5):661–665. doi: 10.1016/s8756-3282(00)00385-9. [DOI] [PubMed] [Google Scholar]
- 8.Yan Y, Tang D, Chen M, Huang J, Xie R, Jonason JH, Tan X, Hou W, Reynolds D, Hsu W, Harris SE, Puzas JE, Awad H, O’Keefe RJ, Boyce BF, Chen D. Axin2 controls bone remodeling through the beta-catenin-BMP signaling pathway in adult mice. J Cell Sci. 2009;122(Pt 19):3566–3578. doi: 10.1242/jcs.051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGee-Lawrence ME, Ryan ZC, Carpio LR, Kakar S, Westendorf JJ, Kumar R. Sclerostin deficient mice rapidly heal bone defects by activating beta-catenin and increasing intramembranous ossification. Biochemical and biophysical research communications. 2013;441(4):886–890. doi: 10.1016/j.bbrc.2013.10.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenk RK, Olah AJ, Herrmann W. Preparation of calcified tissue for light microscopy. In: Dickson GR, editor. Methods of Calcified Tissue Preparation. Elsevier; New York: 1984. pp. 1–56. [Google Scholar]
- 11.Cunliffe-Beamer TL. Applying principles of aseptic surgery to rodents. Animal Welfare Information Center Newsletter. 1993;4(2):3–6. [Google Scholar]
- 12.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 13.An YH, Moreira PL, Kang QK, Gruber HE. Principles of Embedding and Common Protocols. In: An YH, Martin KL, editors. Handbook of Histology Methods for Bone and Cartilage. Humana Press Inc; Totowa, NJ: 2003. pp. 185–206. [Google Scholar]
- 14.Ries WL. Techniques for Sectioning Undecalcified Bone Tissue Using Microtomes. In: An YH, Martin KL, editors. Handbook of Histology Methods for Bone and Cartilage. Humana Press Inc; Totowa, NJ: 2003. pp. 221–232. [Google Scholar]
- 15.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28(1):2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]


