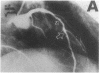
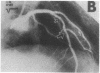
Abstract
Endothelial dysfunction has been implicated as a cause of coronary vasospasm in patients with variant angina. This study aimed to determine if endothelium-dependent vasodilation evoked with substance P (SP) was altered at the spastic site where vasospasm was induced by acetylcholine (ACH) in patients with variant angina. It has been shown that SP evokes endothelium-dependent vasodilation with no direct effect on vascular smooth muscle in excised human coronary arteries. SP and ACH were infused into the coronary arteries in nine patients with variant angina in whom coronary arteriograms showed normal or mild atherosclerotic lesions. The vasomotor responses of coronary arteries were assessed by quantitative arteriography. ACH at a high dose (100 micrograms/min) provoked coronary vasospasm associated with anginal attack in all patients. In contrast, SP at graded doses (13.5, 40, and 135 ng/min) caused the dose-dependent and comparable increases in the coronary diameter at the spastic and control sites. ACH at a low dose (10 micrograms/min) also caused comparable vasodilation at the spastic and control sites in patients with normal coronary arteries. Coronary vasodilating responses to SP were comparable in patients with variant angina and those with atypical chest pain. The results indicate that endothelium-dependent vasodilation evoked with SP and ACH at the low dose was present at the vasospastic site in patients with variant angina. These findings suggest that the ACH-induced coronary vasospasm in patients with variant angina results from hyperreactivity of vascular smooth muscle to ACH but not from endothelial dysfunction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassenge E., Busse R. Endothelial modulation of coronary tone. Prog Cardiovasc Dis. 1988 Mar-Apr;30(5):349–380. doi: 10.1016/0033-0620(88)90003-5. [DOI] [PubMed] [Google Scholar]
- Bossaller C., Habib G. B., Yamamoto H., Williams C., Wells S., Henry P. D. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5'-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J Clin Invest. 1987 Jan;79(1):170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester A. H., O'Neil G. S., Moncada S., Tadjkarimi S., Yacoub M. H. Low basal and stimulated release of nitric oxide in atherosclerotic epicardial coronary arteries. Lancet. 1990 Oct 13;336(8720):897–900. doi: 10.1016/0140-6736(90)92269-n. [DOI] [PubMed] [Google Scholar]
- Crossman D. C., Larkin S. W., Fuller R. W., Davies G. J., Maseri A. Substance P dilates epicardial coronary arteries and increases coronary blood flow in humans. Circulation. 1989 Sep;80(3):475–484. doi: 10.1161/01.cir.80.3.475. [DOI] [PubMed] [Google Scholar]
- Egashira K., Shimokawa H., Tomoike H., Nakamura M. Prostanoids, coronary circulation and coronary artery spasm in open chest dogs and miniature pigs. Jpn Circ J. 1987 Apr;51(4):459–461. doi: 10.1253/jcj.51.459. [DOI] [PubMed] [Google Scholar]
- Egashira K., Tomoike H., Yamamoto Y., Yamada A., Hayashi Y., Nakamura M. Histamine-induced coronary spasm in regions of intimal thickening in miniature pigs: roles of serum cholesterol and spontaneous or induced intimal thickening. Circulation. 1986 Oct;74(4):826–837. doi: 10.1161/01.cir.74.4.826. [DOI] [PubMed] [Google Scholar]
- Freiman P. C., Mitchell G. G., Heistad D. D., Armstrong M. L., Harrison D. G. Atherosclerosis impairs endothelium-dependent vascular relaxation to acetylcholine and thrombin in primates. Circ Res. 1986 Jun;58(6):783–789. doi: 10.1161/01.res.58.6.783. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mügge A., Alheid U., Haverich A., Frölich J. C. Selective attenuation of endothelium-mediated vasodilation in atherosclerotic human coronary arteries. Circ Res. 1988 Feb;62(2):185–190. doi: 10.1161/01.res.62.2.185. [DOI] [PubMed] [Google Scholar]
- Heistad D. D., Armstrong M. L., Marcus M. L., Piegors D. J., Mark A. L. Augmented responses to vasoconstrictor stimuli in hypercholesterolemic and atherosclerotic monkeys. Circ Res. 1984 Jun;54(6):711–718. doi: 10.1161/01.res.54.6.711. [DOI] [PubMed] [Google Scholar]
- Hillis L. D., Braunwald E. Coronary-artery spasm. N Engl J Med. 1978 Sep 28;299(13):695–702. doi: 10.1056/NEJM197809282991305. [DOI] [PubMed] [Google Scholar]
- Kalsner S. Cholinergic mechanisms in human coronary artery preparations: implications of species differences. J Physiol. 1985 Jan;358:509–526. doi: 10.1113/jphysiol.1985.sp015564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaski J. C., Crea F., Meran D., Rodriguez L., Araujo L., Chierchia S., Davies G., Maseri A. Local coronary supersensitivity to diverse vasoconstrictive stimuli in patients with variant angina. Circulation. 1986 Dec;74(6):1255–1265. doi: 10.1161/01.cir.74.6.1255. [DOI] [PubMed] [Google Scholar]
- Ludmer P. L., Selwyn A. P., Shook T. L., Wayne R. R., Mudge G. H., Alexander R. W., Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986 Oct 23;315(17):1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Richard V., Tschudi M., Yang Z. H., Boulanger C. Endothelial control of vascular tone in large and small coronary arteries. J Am Coll Cardiol. 1990 Mar 1;15(3):519–527. doi: 10.1016/0735-1097(90)90619-z. [DOI] [PubMed] [Google Scholar]
- Maseri A., Davies G., Hackett D., Kaski J. C. Coronary artery spasm and vasoconstriction. The case for a distinction. Circulation. 1990 Jun;81(6):1983–1991. doi: 10.1161/01.cir.81.6.1983. [DOI] [PubMed] [Google Scholar]
- Nakamura M. Myocardial ischemia. Jpn Circ J. 1985 Jan;49(1):1–12. doi: 10.1253/jcj.49.1. [DOI] [PubMed] [Google Scholar]
- Nichols A. B., Gabrieli C. F., Fenoglio J. J., Jr, Esser P. D. Quantification of relative coronary arterial stenosis by cinevideodensitometric analysis of coronary arteriograms. Circulation. 1984 Mar;69(3):512–522. doi: 10.1161/01.cir.69.3.512. [DOI] [PubMed] [Google Scholar]
- Shepherd J. T., Vanhoutte P. M. Spasm of the coronary arteries: causes and consequences (the scientist's viewpoint). Mayo Clin Proc. 1985 Jan;60(1):33–46. doi: 10.1016/s0025-6196(12)65280-x. [DOI] [PubMed] [Google Scholar]
- Toda N., Okamura T. Endothelium-dependent and -independent responses to vasoactive substances of isolated human coronary arteries. Am J Physiol. 1989 Sep;257(3 Pt 2):H988–H995. doi: 10.1152/ajpheart.1989.257.3.H988. [DOI] [PubMed] [Google Scholar]
- Verbeuren T. J., Jordaens F. H., Zonnekeyn L. L., Van Hove C. E., Coene M. C., Herman A. G. Effect of hypercholesterolemia on vascular reactivity in the rabbit. I. Endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986 Apr;58(4):552–564. doi: 10.1161/01.res.58.4.552. [DOI] [PubMed] [Google Scholar]
- Vita J. A., Treasure C. B., Nabel E. G., McLenachan J. M., Fish R. D., Yeung A. C., Vekshtein V. I., Selwyn A. P., Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990 Feb;81(2):491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- Waters D. D., Szlachcic J., Bonan R., Miller D. D., Dauwe F., Theroux P. Comparative sensitivity of exercise, cold pressor and ergonovine testing in provoking attacks of variant angina in patients with active disease. Circulation. 1983 Feb;67(2):310–315. doi: 10.1161/01.cir.67.2.310. [DOI] [PubMed] [Google Scholar]
- Werns S. W., Walton J. A., Hsia H. H., Nabel E. G., Sanz M. L., Pitt B. Evidence of endothelial dysfunction in angiographically normal coronary arteries of patients with coronary artery disease. Circulation. 1989 Feb;79(2):287–291. doi: 10.1161/01.cir.79.2.287. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Tomoike H., Egashira K., Kobayashi T., Kawasaki T., Nakamura M. Pathogenesis of coronary artery spasm in miniature swine with regional intimal thickening after balloon denudation. Circ Res. 1987 Jan;60(1):113–121. doi: 10.1161/01.res.60.1.113. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Tomoike H., Egashira K., Nakamura M. Attenuation of endothelium-related relaxation and enhanced responsiveness of vascular smooth muscle to histamine in spastic coronary arterial segments from miniature pigs. Circ Res. 1987 Dec;61(6):772–778. doi: 10.1161/01.res.61.6.772. [DOI] [PubMed] [Google Scholar]
- Yasue H., Horio Y., Nakamura N., Fujii H., Imoto N., Sonoda R., Kugiyama K., Obata K., Morikami Y., Kimura T. Induction of coronary artery spasm by acetylcholine in patients with variant angina: possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation. 1986 Nov;74(5):955–963. doi: 10.1161/01.cir.74.5.955. [DOI] [PubMed] [Google Scholar]
- Zeiher A. M., Drexler H., Wollschläger H., Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991 Feb;83(2):391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]