Abstract
Vitamin D receptor (VDR) expression and action in non-human skeletal muscle have recently been reported in several studies, yet data on the activity and expression of VDR in human muscle cells are scarce. We conducted a series of studies to examine the (1) effect of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) on VDR gene expression in human primary myoblasts, (2) effect of 16-week supplementation with vitamin D3 on intramuscular VDR gene expression in older women, and (3) association between serum 25-hydroxyvitamin D (25OHD) and intramuscular VDR protein concentration in older adults. Human primary myoblasts were treated with increasing concentrations of 1,25(OH)2D3 for 18 h. A dose-dependent treatment effect was noted with 1 nmol/L of 1,25OH2D3 increasing intramuscular VDR mRNA expression (mean fold change ± SD 1.36 ± 0.33; P = 0.05). Muscle biopsies were obtained at baseline and 16 weeks after vitamin D3 supplementation (4,000 IU/day) in older adults. Intramuscular VDR mRNA was significantly different from placebo after 16 weeks of vitamin D3 (1.2 ± 0.99; −3.2 ± 1.7, respectively; P = 0.04). Serum 25OHD and intramuscular VDR protein expression were examined by immunoblot. 25OHD was associated with intramuscular VDR protein concentration (R = 0.67; P = 0.0028). In summary, our study found VDR gene expression increases following treatment with 1,25OH2D3 in human myoblasts. 25OHD is associated with VDR protein and 16 weeks of supplementation with vitamin D3 resulted in a persistent increase in VDR gene expression of vitamin D3 in muscle tissue biopsies. These findings suggest treatment with vitamin D compounds results in sustained increases in VDR in human skeletal muscle.
Keywords: Vitamin D, Skeletal muscle, Vitamin D receptor, Human, Primary myoblasts, Aging
Introduction
Several observational studies suggest a positive association between serum 25-hydroxyvitamin D (25OHD) concentration and muscle strength and physical function in older adults [1–3]. However, the specific mechanism of action by which vitamin D acts on skeletal muscle has been debated. One mechanism proposed is by way of a vitamin D effect on calcium and phosphate [4]. An increasing number of studies in both non-human and human skeletal muscle cells report that the actions of vitamin D are also mediated by the VDR located within skeletal muscle cells [5–10]. These studies have used a variety of VDR-specific antibodies (Santa Cruz D6 [8–10], Santa Cruz 333C6a [8], Affinity BioReagents 9A7 [7], Perseus Proteomics VDR NR 1|1 [8], among others) to locate VDR in animal muscle cell lines or human muscle biopsies using immunoblotting and/or immunohistochemical techniques. Additionally, gene expression studies have been conducted in murine muscle cell lines [9, 10]. Nevertheless, not all studies have confirmed these findings in human skeletal muscle [11].
Factors that regulate expression of VDR in skeletal muscle have not been extensively studied in human skeletal muscle. One previous paper found that VDR concentration is inversely associated with age in humans [12]. An age association was also noted in mice [10]. Additionally in animal models, it has been shown that both 25OHD and 1,25OH2D3 increase VDR protein content in murine C2C12 myoblasts [9, 13] and murine primary myotubes [10]. To date, however, few data are available on the effect of 1,25OH2D3 or its parent compounds on VDR expression and activity in human muscle.
We conducted three separate studies to examine the relationships between vitamin D and VDR gene expression and protein concentration in human skeletal muscle. First, we investigated whether 1,25OH2D3 had a direct effect on intramuscular VDR, by measuring changes in the gene expression of VDR and its target gene, cytochrome P450 family 24 subfamily A polypeptide 1 (CYP24A1), in human primary myoblasts following 18 h of 1,25OH2D3 treatment. Second, we examined the effect of 16 weeks of vitamin D3 supplementation on VDR gene expression in skeletal muscle tissue biopsies of older mobility-limited women. Within this second study, we evaluated whether changes in intramuscular VDR occurred in non-nuclear areas of skeletal muscle cross-sections. Third, we assessed the relationship between baseline serum concentrations of 25OHD and baseline intramuscular VDR gene and protein concentration in skeletal muscle in older mobility-limited adults.
Materials and Methods
Subjects
Cell Culture
Primary myoblasts were established from three healthy young adults: age 19–30 years, BMI 20.3–23.4 kg/m2, 1 female, and 2 male. This portion of our study was approved by the Ethics Committee of the Karolinska Institutet (Stockholm, Sweden).
Human Muscle Biopsies
Muscle biopsy data were derived from two prior clinical studies. The first was a vitamin D3 supplementation study which included 20 women aged 65 years and over with moderately low baseline 25OHD and mobility limitations as determined by the short physical performance battery (SPPB) [14]. These women were participating in a randomized controlled intervention study examining the effects of vitamin D3 versus placebo on skeletal muscle morphology and VDR protein concentration [15]. Mean age (±SD) was 78.5 ± 4.79 years, mean BMI was 27.0 ± 5.5 kg/m2, a mean SPPB score was 7.9 ± 1.55, and mean baseline 25OHD concentration was 18 ± 3.86 ng/mL. Subjects underwent a muscle biopsy before and after 16 weeks of 4,000 IU per day of oral vitamin D3 supplementation or matching placebo. This study protocol and inclusion/exclusion criteria have been described in detail elsewhere [15].
The second was a study which included 20 older adults (male = 8, female = 12) who were also mobility-limited as determined by the short physical performance battery (SPPB) [14]. These subjects had been selected for a larger randomized study for which inclusion and exclusion criteria are presented elsewhere [16]. Mean age was 77.9 ± 4.05 years, mean BMI was 27.06 ± 2.94 kg/m2, and mean SPPB was 8.65 ± 1.23. Baseline (pre-intervention) serum samples and muscle biopsies were obtained for the purposes of our analyses. Both of the above studies were approved by the Institutional Review Board of the Tufts University Health Sciences Campus (Boston, MA).
Human Myoblast Cell Culture
Cell Line Preparation
Muscle biopsies were obtained from the vastus lateralis of three healthy young subjects via the percutaneous needle biopsy technique and were kept as individual cultures throughout the experiment. Visible fat and connective tissue were removed, and then put in a 15 mL tube containing PBS + 1 % AbAm (antibiotic/antimycotic, Sigma Aldrich) and was stored at 4 °C overnight. The tissue was washed twice in serum-free DMEM medium (Dulbecco’s modified eagle medium:nutrient mixture F-12 (DMEM/F-12), Life Technologies) and transferred to a small sterile beaker with 0.25 % trypsin, final volume 5 mL. The beaker was put on a magnetic stirrer in an incubator, 37 °C, low speed for 20–30 min. The beaker stood for about 5 min inside the sterile hood, then supernatant was transferred to a sterile tube. Trypsin was stopped by adding DMEM medium with FCS and centrifuged at 300×g for 10 min. The supernatant was removed and myoblasts were re-suspended in medium containing 20 % FCS and 1 % AbAm. Cell suspension was pre-plated for 20–30 min in a Petri dish. Cell solution was transferred to a 75-mL culture flask. Isolated human myoblasts were cultured in DMEM-F12 with 20 % FBS and 1 % ABAM (proliferation media) at 37 °C, 5 % CO2. Culture dishes were precoated with collagen I (Gibco® Collagen I, Bovine 5 mg/mL Invitrogen) diluted to a final concentration of 50 µg/mL in 0.02 M acetic acid according to the manufacturer’s manual. Medium was switched every third day. Myoblasts were split into new flasks when reaching approximately 60–70 % confluency. To control for cell culture population purity (i.e., whether or not the isolated population were predominantly of myogenic origin), cells were immunocytochemically analyzed for the myogenic marker, desmin. A sample of cells was trypsinized in 0.25 % trypsin–EDTA for 5 min and spun down onto a cover glass for subsequent immunocytochemistry. The cells were then fixated in 4 % para-formaldehyde for 8 min. Following fixation, cells were washed 3 × 5 min in 1× PBS (hereafter referred to simply as washing step) prior to a 30-min block in 4 % bovine serum albumin (BSA, Invitrogen). Mouse-derived monoclonal antibody against human desmin (DAKO D33) diluted 1:200 in 1× PBS containing 1 % BSA and 0.1 % Triton-X was used for the 60-min primary antibody incubation. Following another washing step, the rat anti-mouse IgG monoclonal antibody (ALEXA Fluor 568) diluted 1:1,000 in 1× PBS was used for the 30-min secondary antibody incubation. Following the subsequent washing step, the cells were mounted in Vectashield and 4′,6-diamidino-2-phenylindole (DAPI) before quantification of the percentage of desmin positive cells. We calculated the percentage of desmin-positive nuclei by counting the total number of nuclei in three fields of 10× magnification and the number of desmin-positive nuclei in the same fields. A minimum of 100 cells was counted for each individual experimental set. All isolated cell populations with fewer than 85 % desmin-positive cells were rejected for further analysis. Cells from passage two were utilized for analysis. Untreated myoblasts were controls. Each of the myoblasts were then treated in triplicate for 18 h with one of three doses of 1,25OH2D3 (1 pmol/L, 10 pmol/L, and 1 nmol/L).
mRNA Extraction
Total RNA was prepared by the Trizol method (Invitrogen) according to the manufacturer’s protocol and quantified spectrophotometrically by absorbance at 260 nm. One microgram of total RNA was reverse transcribed by Superscript reverse transcriptase (Life Technologies) using random hexamer primers (Roche Diagnostics) in a total volume of 20 µL.
Real-Time qPCR
VDR: quantitative real-time PCR was performed utilizing a commercially available reaction mixture (SsoAdvanced SYBR Green Supermix; Bio-Rad Laboratories, Hercules, CA) on a CFX96 Real-Time System (Bio-Rad Laboratories, Hercules, CA). cDNA of VDR (QT01010170) were measured using commercially available primer mixtures (Quantitect Primer Assays: Qiagen). 5 µL aliquots of cDNA together with 20 µL iScript (12.5 µL of iTaQ Supermix, 2.5 µL of primer and 5 µL of nuclease-free H2O) were assayed in duplicate on a 96-well heat-sealed PCR plate (Bio-Rad Laboratories, Hercules, CA). Changes in target gene expression were calculated relative to values from glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (QT00199633). Efficiencies of each primer set were assessed using a standard curve, and analyzed using 0.0025–25 ng of control cDNA. CYP24A1: detection of mRNA for CYP24A1 (Hs00167999_m1, Perkin-Elmer Applied Biosystems) was performed on an ABIPRISM7700 sequence detector (Perkin-Elmer Applied Biosystems) with changes in target gene expression calculated relative to values of GAPDH (402869, Perkin-Elmer Applied Biosystems). Data are presented as fold change from untreated controls.
Human Skeletal Muscle Biopsy
Muscle Biopsies
Baseline and 16-week follow-up human muscle biopsies were obtained from the vastus lateralis at the level of the mid-thigh under local anesthesia (1 % Lidocaine). At baseline and following vitamin D3 supplementation, the specimens were split and either flash frozen in liquid nitrogen or mounted in a vinyl cryomold (Tissue-Tek, USA) and secured using a viscous mounting medium (O.C.T., Tissue-Tek, USA) and then frozen in an isopentane/liquid nitrogen slurry. Samples were stored in liquid nitrogen until analysis.
mRNA Preparation
Vastus lateralis muscle (~50 mg) was homogenized by bead milling with zirconium oxide beads in 1 mL PureZOL RNA Isolation Reagent (Bio-Rad Laboratories, Hercules, CA). RNA extraction was completed utilizing Aurum Total RNA Fatty and Fibrous Tissue RNA Extraction Kit (Bio-Rad Laboratories, Hercules, CA). cDNA conversion was performed utilizing a commercially available reaction mixture (iScript Reverse Transcription SuperMix for RT-qPCR Bio-Rad Laboratories, Hercules, CA) on a T100 Thermal Cycler (Bio-Rad Laboratories, Hercules, CA). Real-time qPCR was performed as outlined above.
Western Blotting
Immunoblotting was utilized to examine protein concentration of VDR in human skeletal muscle. Vastus lateralis muscle was prepared as previously described [17]. Membranes were incubated overnight at 4 °C with primary antibodies specific for VDR (1:1,000 in 5 % bovine serum albumin and TBS-Tween; VDR NR 1|1 Perseus Proteomics Tokyo, Japan via R&D Systems, Minneapolis, MN). Membranes were rinsed three times for 10 min in TBS-Tween and incubated at room temperature with secondary goat-anti-mouse IgG2Aa HRP conjugate antibody (1:2,000 in 5 % nonfat dry milk and TBS-Tween; Invitrogen, Frederick, MD). Membranes were rinsed three times for 10 min in TBS-Tween and the immunoreactive proteins were detected with Supersignal Chemiluminescent Substrate (Thermo Scientific, Rockford, IL) and quantified by optical density (Image Lab 3.0.1; Bio-Rad Laboratories, Hercules, CA). Changes in protein expression were calculated relative to values of constitutive control GAPDH (14C10, Cell Signaling Technologies, Beverly, MA). Data are presented in arbitrary units and represent concentration as determined by optical density.
Immunohistochemistry
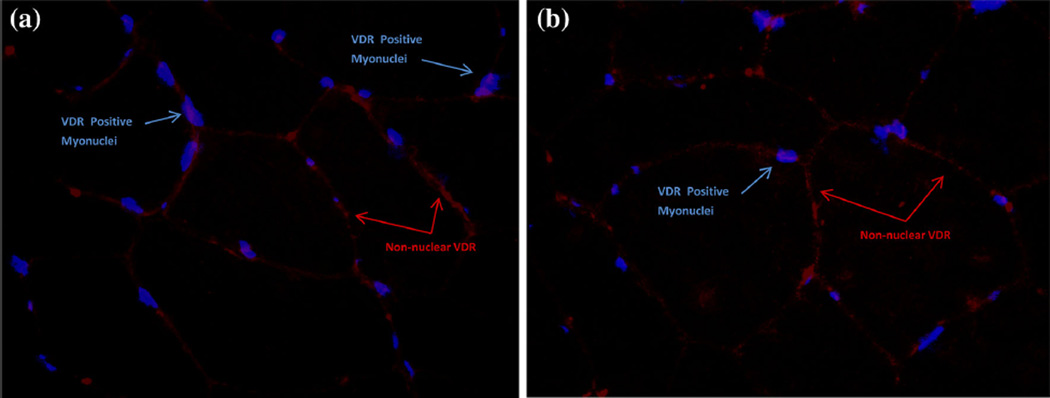
Immunohistochemistry and fluorescent microscopy methods were described in detail previously [8]. Digital images of immunofluorescent marker staining of VDR, DAPI, and laminin in a subgroup of the supplementation study (vitamin D3 n = 6; placebo n = 5) were further analyzed by OracleBio (Glasgow, Scotland, UK) to determine concentration of non-intramyonuclear-associated VDR protein signal. A software algorithm was utilized to detect total VDR signal area and VDR-positive myonuclei across individual field images of skeletal muscle. The VDR and DAPI layer images were overlaid for detection of positive nuclei. VDR signal was aggregated across the field, then VDR signal colocalized with myonuclei was subtracted from total VDR signal to determine the non-nuclear fraction. The non-nuclear signal was normalized to image area so that individual time points (pre- and post-) could be compared. The non-nuclear signal from three images was averaged for each time point per subject.
Biochemical Measurements
Fasting morning blood samples were drawn from human subjects at baseline and 16-week follow-up. Serum 25OHD concentration was analyzed utilizing a commercially available assay kit (Diasorin, LIAISON® 25OH Vitamin D Total Assay). Vitamin D deficiency was defined as 25OHD serum concentrations below 12 ng/mL, insufficiency as 12–19 ng/mL, and sufficiency ≥20 ng/mL [17].
Statistical Analysis
All statistical analyses were performed using JMP statistical software (v. 10.0, SAS Institute Inc., Cary, NC) except for CYP24A1 data which were analyzed with STATISTICA statistical software (version 10 StatSoft Inc.). Statistically significant differences were evaluated between 1,25OH2D3 concentrations by the use of one-way ANOVA. Tukey’s post hoc test was used to locate differences in mean values. Associations between linear variables were examined with Pearson correlation analysis. Formal hypothesis testing within and across groups was completed with two-sided t tests. All variables were examined for normal distribution, and were log-transformed before analysis as needed to better approximate a normal distribution. Cook’s distance was applied to all data points to identify outliers and observations. A Cook’s distance greater than three times the mean Cook’s distance for that variable was determined as an outlier. Data are presented as mean ± SD and were determined to be statistically significant at an α value of ≤0.05.
As studies examining VDR protein in human skeletal muscle via western blot are lacking in the literature, we estimated statistical power a priori to detect between-group differences based on a 95 % confidence interval with 80 % power from previously available immunohistochemical studies in humans [12, 15]. From these data, we estimated a between-group difference of 1.5-fold in VDR expression and our calculations required a within-group sample size of n = 4 to give an 80 % chance of rejecting the two-sided null hypothesis of no difference between the group means at the 0.05 level of significance.
Results
Human Primary Myoblasts
VDR mRNA Fold Change
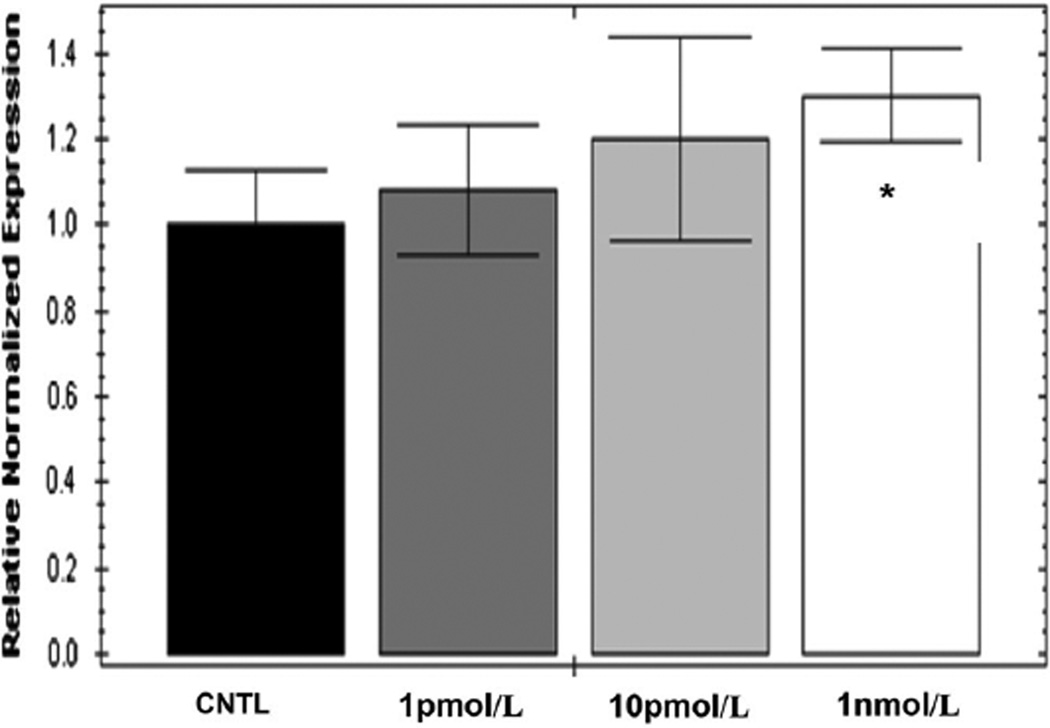
After being treated for 18 h with three concentrations of 1,25OH2D3 (1 pmol/L, 10 pmol/L, and 1 nmol/L), myoblast VDR mRNA expression increased in a dose-dependent manner (Fig. 1). The mean fold change ± SD in VDR mRNA between untreated control and after 18 h of 1 nmol/L 1,25OH2D3 was 1.36 ± 0.33 (P = 0.05).
Fig. 1.
Human primary myoblast VDR mRNA fold change. After being treated for 18 h with three concentrations of 1,25(OH)2D3 (1 pmol/L, 10 pmol/L, and 1 nmol/L), fold change of VDR mRNA was significantly increased between control and 1 nmol/L (1.36 ± 0.33) in human primary myoblasts established from three healthy young adults (P = 0.05). Data presented as (mean ± SD) and analyzed via ANOVA and Tukey’s post hoc test. Statistical significance versus control group, *P ≤ 0.05
CYP24A1 mRNA Fold Change
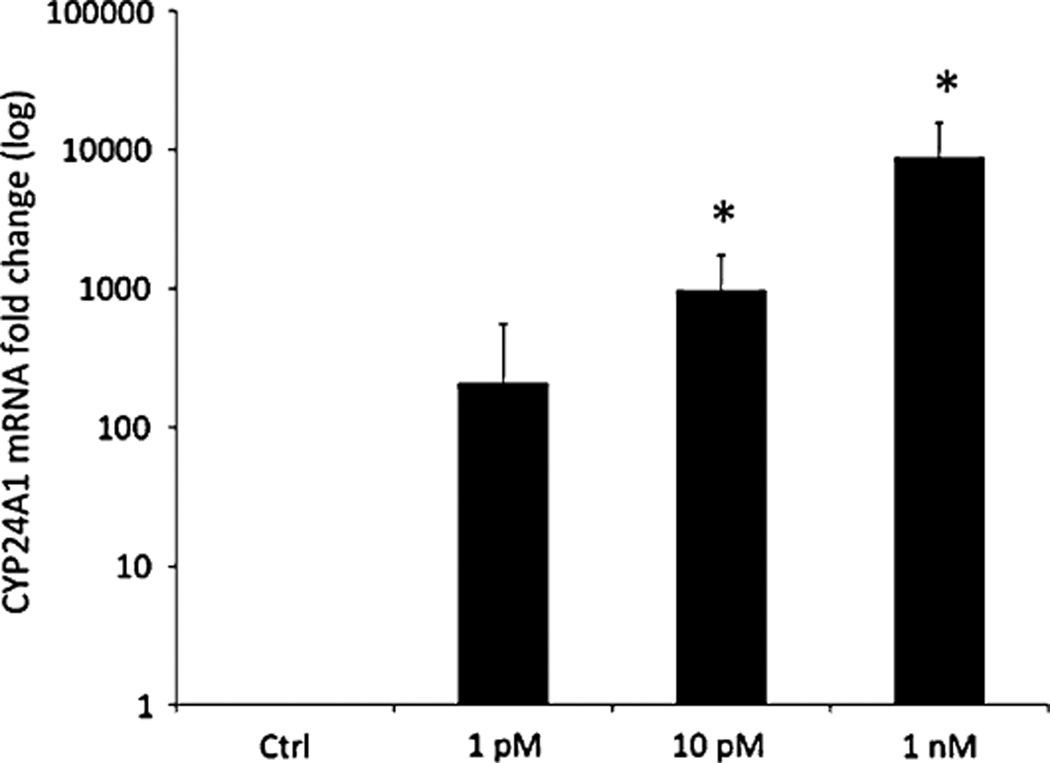
In human primary cell myoblasts, there were also dose-dependent increases in mean fold change in CYP24A1 mRNA expression following 18 h of 1,25OH2D3 (203.6 ± 349.5 for 1 pmol/L; 951.5 ± 784.1 for 10 pmol/L; 8,633.9 ± 6,957.5 for 1 nmol/L; P < 0.05 between control and 10 pmol/L and 1 nmol/L, Fig. 2). CYP24A1 mRNA concentration was expressed in very low to non-detectable concentration in myoblasts in the basal state, as made apparent by high CT values (CT value range 33–36).
Fig. 2.
Gene expression of the 24-hydroxylase (CYP24A1). Human primary cell myoblasts were stimulated with 1 pmol/L, 10 pmol/L, and 1 nmol/L of 1,25(OH)2D3 for 18 h. Data presented as mean ± SD fold change relative to controls (1 pmol/L 203.6 ± 349.5; 10 pmol/L 951.5 ± 784.1; 1 nmol/L 8,633.9 ± 6,957.5). Statistical significance versus control group, *P ≤ 0.05
Human Muscle Biopsies
VDR mRNA Expression in Human Skeletal Muscle Biopsies Following Vitamin D3 Supplementation
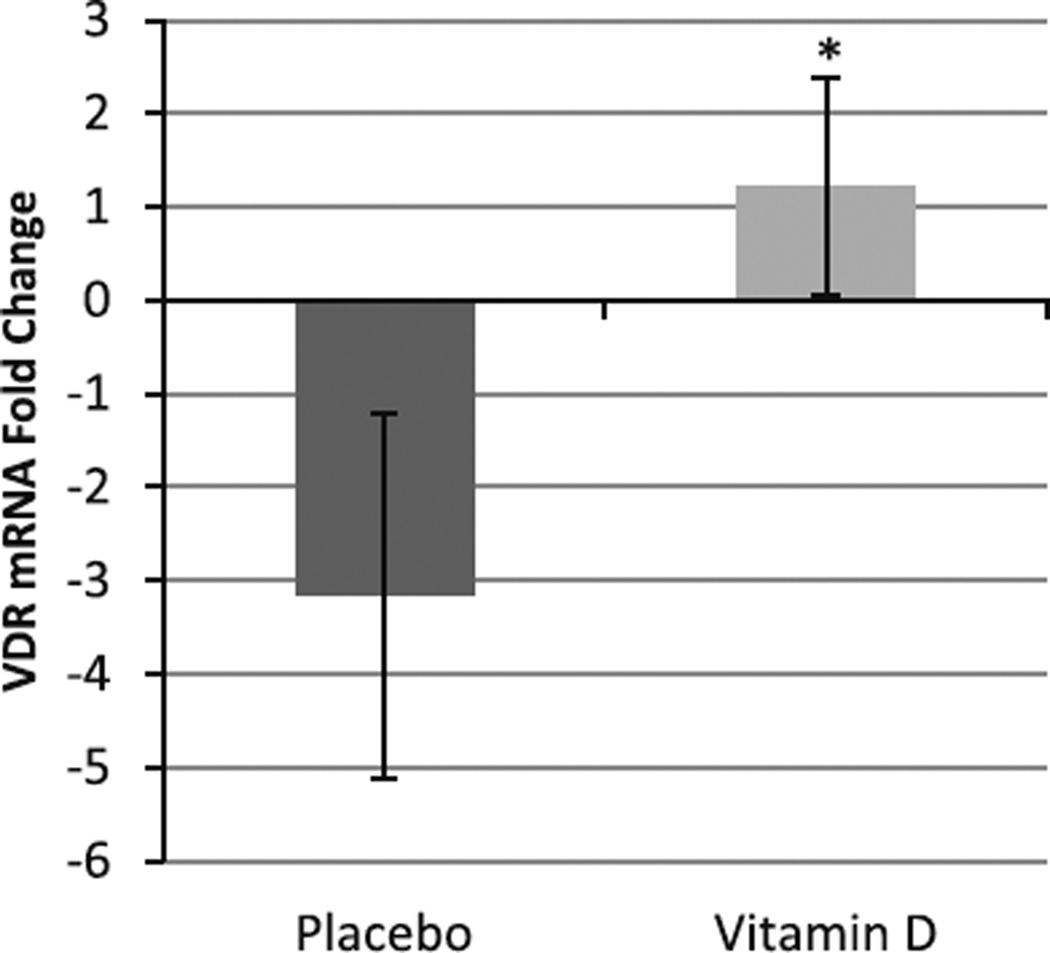
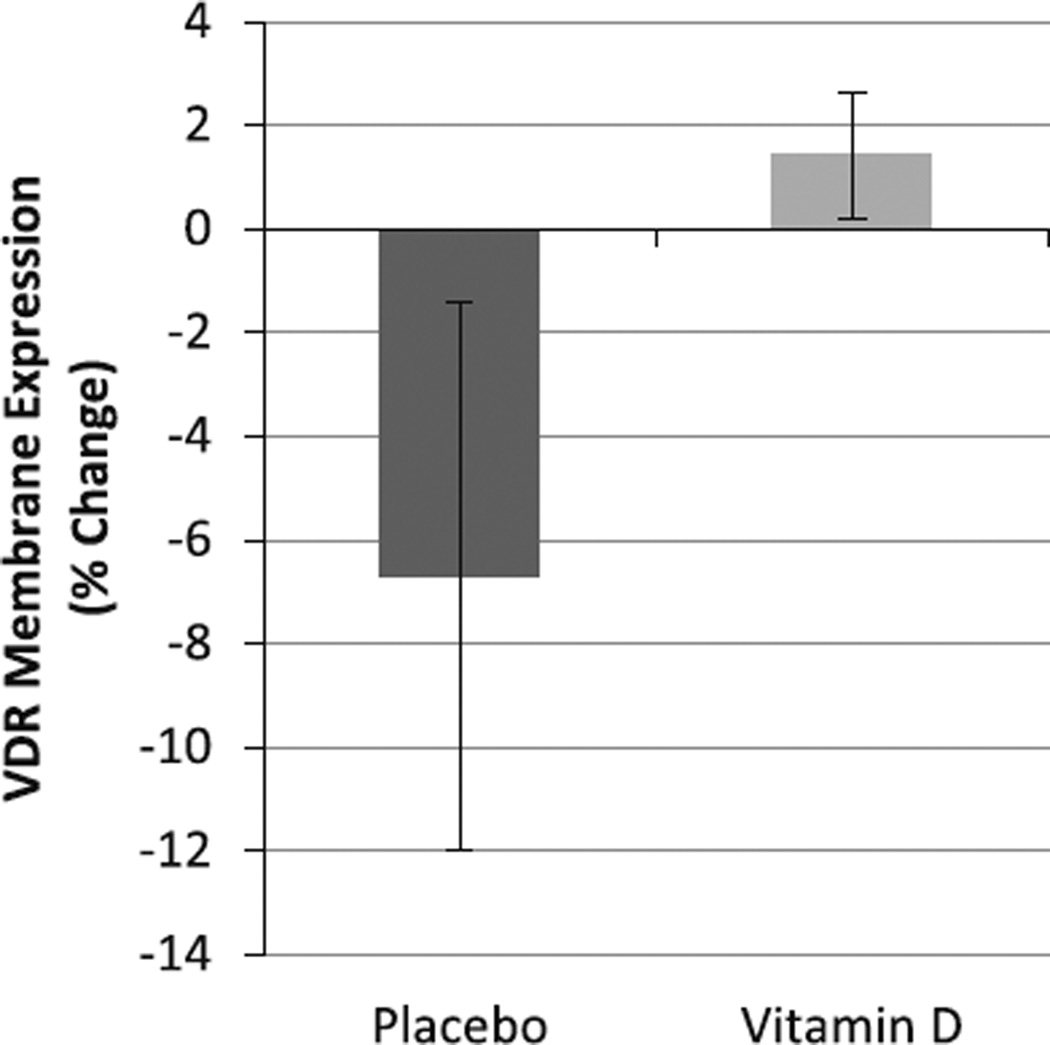
Baseline and follow-up mean serum 25OHD concentrations in the older women in this study have previously been reported [15]. After 16 weeks, fold change in muscle VDR mRNA expression was 1.2 ± 0.99-fold in the vitamin D3-supplemented women (n = 9) versus −3.2 ± 1.7-fold in the placebo-treated women (n = 10; P = 0.04; Fig. 3). Of note, one placebo subject whose sample demonstrated a VDR mRNA expression fold change of 69.55 (vs an average of −3.2) was determined to be an outlier and was excluded from analysis. An analysis of the human muscle cross-sections after vitamin D3 supplementation in older women found that the mean percent change of total nonnuclear VDR area was 1.42 ± 1.21 % for the vitamin D3-supplemented group (n = 9) and −6.7 ± 5.26 % for the placebo group (n = 10; P = 0.2, Figs. 4, 5a, b).
Fig. 3.
Human VDR mRNA fold change. Mean (±SD) fold change of VDR mRNA was increased (1.2 ± 2.6 times; n = 9) in the vitamin D3 supplementation group compared to a decrease in the placebo group (−3.2 ± 4.6 times; n = 11) in older mobility-limited women (P = 0.04) as demonstrated with PCR and analyzed by Student’s t test. Statistical significance between groups, *P ≤ 0.05
Fig. 4.
Location of VDR in human skeletal muscle after 16 weeks of 4,000 IU/day vitamin D3 supplementation. The vitamin D3 supplemented group (n = 9) increased skeletal muscle plasma membrane VDR protein by 1.42 % (±1.21) while the placebo group (n = 11) decreased by −6.7 % (±5.26) (P = 0.2) as demonstrated by immunohistochemistry and determined by Student’s t test. Statistical significance between groups, *P ≤ 0.05
Fig. 5.
Immunofluorescent images of VDR localization (nuclear vs plasma membrane). Representative image of VDR and DAPI detection in skeletal muscle cross-sections of a single subject at baseline (a) and after 16 weeks of vitamin D3 supplementation (b). A software algorithm was utilized to colocalize the total VDR signal area (red) and myonuclei (blue DAPI) across individual field images of skeletal muscle (Color figure online)
Serum 25OHD Concentration and Intramuscular VDR
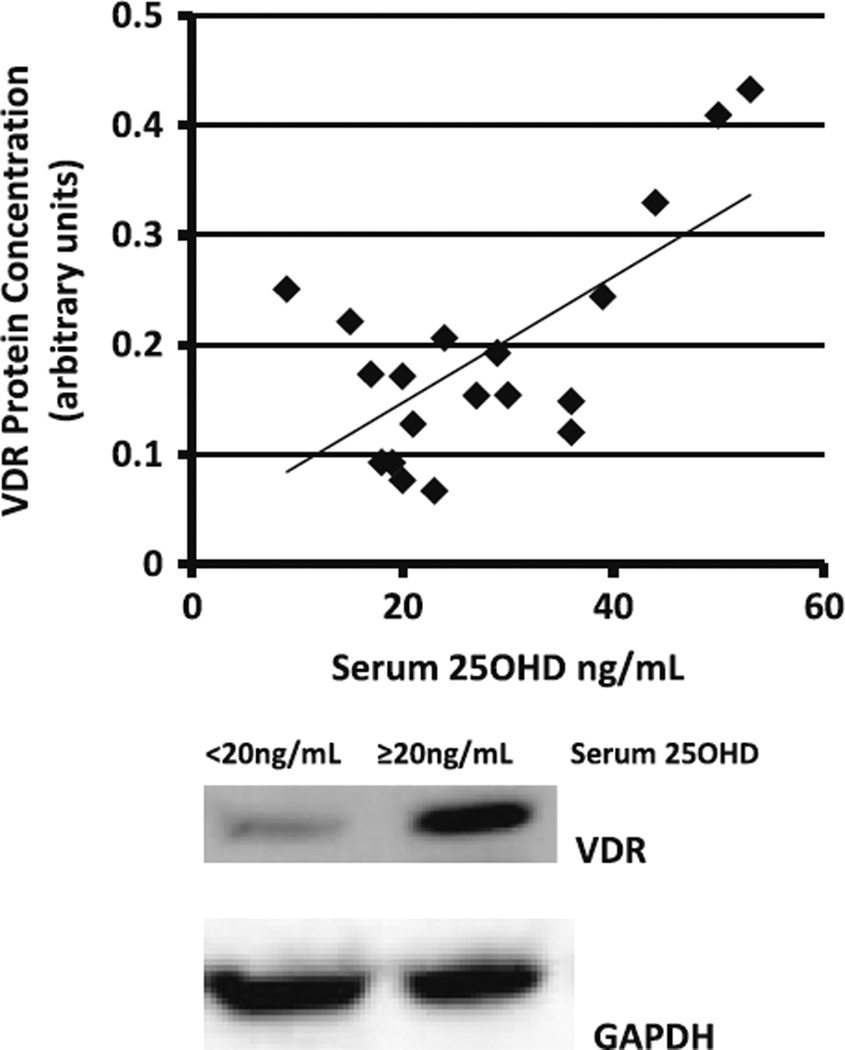
There was no significant correlation between baseline serum 25OHD concentration and VDR mRNA expression in 20 older mobility-limited men and women (male = 8, female = 12) at baseline (P = 0.47). However, serum 25OHD concentrations were positively associated with VDR protein concentration by immunoblot (R = 0.67; P = 0.0028; Fig. 6a). A similar relationship was noted when subjects were divided based on vitamin D status with vitamin D sufficient subjects (n = 12) having greater VDR concentrations versus those who were vitamin D insufficient/ deficient (n = 8, P = 0.02; Fig. 6b).
Fig. 6.
Association between serum vitamin D and VDR. a Serum 25OHD (ng/mL) strongly associated with VDR protein concentration (R = 0.67; P = 0.0028) in older mobility-limited adults (n = 20) as demonstrated by Pearson correlational analysis. b Muscle cell VDR protein concentrations were significantly higher in vitamin D sufficient (n = 12) versus vitamin D insufficient/deficient older mobility-limited adults (n = 8, P = 0.02) as determined by Student’s t test. Statistical significance between groups, *P ≤ 0.05
Discussion
Much of the work to date examining the interaction between vitamin D and skeletal muscle has been conducted in non-human muscle cells, whereas data in human skeletal muscle are scarce. Our study found that human primary myoblasts treated with 1,25OH2D3 for 18 h significantly increased expression of VDR and its target CYP24A1 gene in a dose-dependent manner and that vitamin D3 for 16 weeks resulted in a persistent increase in VDR gene expression in older adult muscle tissue. Together, these findings not only support data in non-human muscle cells suggesting a direct effect of 1,25OH2D3 by way of the VDR, but also demonstrate sustained increases in VDR gene expression in human muscle tissue with longer term vitamin D3 supplementation.
There have been several recent studies in murine C2C12 muscle cells, murine primary myotubes, and adult mouse muscle tissue demonstrating a stimulatory effect of 25OHD and 1,25OH2D3 treatment over periods lasting 2–4 days on the expression of VDR and/or its target CYP24A1, a gene which is translated to a protein responsible for local 1,25OH2D3 degradation [9, 10, 13]. Girgis et al. [10, 13] demonstrated that murine C2C12 cells express VDR, CYP27B1 (1-α-hydroxylase), CYP24A1, and vitamin D-binding protein at the transcript level. These investigations demonstrated increased VDR mRNA following 2 days (48 h) of treatment with 1,25OH2D3 with concomitant increases in CYP24A1. Notably, our study using human primary myoblasts exposed to a shorter 18-h period of 1,25OH2D3 treatment at a lower maximal dose of 1 nmol 1,25OH2D3, showed similar findings to the previous non-human studies [10, 13]. Our data reveals a dose-dependent gene expression of VDR and CYP24A1 following treatment with 1,25OH2D3 which corroborates and adds to earlier findings of the presence of VDR protein and 24-hydroxylase enzyme in cloned human muscle cells [6].
In addition, our vitamin D3 intervention study showed a 20 % increase in human skeletal muscle VDR gene expression in older, mobility-limited, vitamin D-insufficient women supplemented with vitamin D3 versus placebo for 16 weeks. In a previous report in the same intervention study, our group demonstrated a relatively similar rise (approximately 30 %) in intramyonuclear VDR protein concentration by immunohistochemistry in the muscle samples after 16 weeks [15]. Further analysis of our VDR immunohistochemical staining of muscle cross-sections in the present study found no difference in non-nuclear VDR protein signal in myofibers after 16 weeks of vitamin D3 supplementation versus placebo. This null finding in nonnuclear VDR protein concentration may indicate a predominantly nuclear effect. Another potential explanation is that a non-nuclear effect may occur in the short term. Binding of 1,25OH2D3 to a putative non-nuclear VDR has been proposed to occur within seconds to minutes and induce fast, non-transcriptional responses [18, 19]. Further, short treatment with 1,25OH2D3 has induced reverse translocation of the VDR from the nucleus to the plasma membrane in C2C12 and chick skeletal muscle within 24 h [9, 20, 21]. Therefore, the possibility that there may be a more immediate non-nuclear VDR effect or acute translocation of the VDR requires further investigation in a shorter term human clinical intervention study.
Finally, our present study confirmed a strong positive association between intramuscular VDR protein concentration by immunoblotting and serum 25OHD concentration, consistent with a previous study using different methodology to evaluate VDR protein content [15]. In our sample of older women and men, an association between circulating 25OHD concentration and intramuscular VDR gene expression was not significant. Based on our in vitro myoblast study and our 4-month intervention study, VDR mRNA concentrations increased with supplemental vitamin D. As we saw no association between circulating 25OHD concentration and intramuscular VDR gene expression in the cross-sectional analysis, it may be that VDR mRNA level responds to the availability of vitamin D. Another possibility is that this analysis was conducted in a group of older subjects at the start of a study; thus, results were limited by its cross-sectional design and the null finding may be due to lack of statistical power to see an association in this small sample. A larger study is needed to clarify this finding.
The strengths of our study include providing important additional information on the effects of different formulations of vitamin D on VDR and CYP24A1 gene expression in human primary myoblasts and on VDR gene expression over a 16-week period in adult human muscle tissue. Further studies are needed to explain the sustained effects on VDR gene expression. Limitations of the study include the small sample size and the fact that these studies were conducted as secondary analyses. Nevertheless, our data are fairly consistent with data in non-human cells lines and animal models. A larger confirmatory human study would be important to verify these findings.
In summary, our study noted increases in VDR and CYP24A1 gene expression in human myoblasts following short-term treatment with 1,25OH2D3 as shown in nonhuman cells lines. Our data also demonstrated a persistent increase in VDR gene expression after 16 weeks of vitamin D3 supplementation in older female muscle tissue biopsies. Together, these findings suggest that treatment with vitamin D compounds results in sustained increases VDR gene expression in human skeletal muscle. Further translational studies are needed to examine both the acute and long-term effects of vitamin D3 supplementation on key skeletal muscle biological pathways and how these effects influence muscle function.
Acknowledgments
USDA Agricultural Research Service, under Agreement Nos. 58-1950-7-707 (to BDH and LC) and 58-1950-0-014 (to RAF); The Dairy Research Institute (R.A.F.), Boston Claude D. Pepper Older Americans Independence Center (1P30AG031679 to R.A.F.), the Boston Nutrition/Obesity Research Center (DK046200 to R.A.F.), and an NHLBI pre-doctoral training grant (T32HL69772 to RMP). Any opinions, findings, conclusion, or recommendation expressed in this publication are those of the author(s) and do not necessarily reflect the views of the U.S. Department of Agriculture.
Footnotes
Conflict of Interest Rachele Pojednic, Lisa Ceglia, Karl Olsson, Thomas Gustafsson, Alice Lichtenstein, Bess Dawson-Hughes, and Roger Fielding declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5).
Contributor Information
Rachele M. Pojednic, Email: rachele.pojednic@joslin.harvard.edu, Nutrition, Exercise Physiology and Sarcopenia Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, 711 Washington St., Boston, MA 02111, USA.
Lisa Ceglia, Division of Endocrinology, Diabetes and Metabolism, Tufts Medical Center, Boston, MA, USA; Bone Metabolism Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
Karl Olsson, Division of Clinical Physiology, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden.
Thomas Gustafsson, Division of Clinical Physiology, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden.
Alice H. Lichtenstein, Cardiovascular Nutrition Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA
Bess Dawson-Hughes, Bone Metabolism Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
Roger A. Fielding, Nutrition, Exercise Physiology and Sarcopenia Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, 711 Washington St., Boston, MA 02111, USA
References
- 1.Annweiler C, Schott AM, Montero-Odasso M, Berrut G, Fantino B, Herrmann FR, Beauchet O. Cross-sectional association between serum vitamin D concentration and walking speed measured at usual and fast pace among older women: the EPIDOS study. J Bone Miner Res. 2010;25:1858–1866. doi: 10.1002/jbmr.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, Dawson-Hughes B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or = 60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 3.Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, Knol DL, Lips P. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 4.Schubert L, DeLuca HF. Hypophosphatemia is responsible for skeletal muscle weakness of vitamin D deficiency. Arch Biochem Biophys. 2010;500:157–161. doi: 10.1016/j.abb.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem. 1985;260:8882–8891. [PubMed] [Google Scholar]
- 6.Costa EM, Blau HM, Feldman D. 1,25-Dihydroxyvitamin D3 receptors and hormonal responses in cloned human skeletal muscle cells. Endocrinology. 1986;119:2214–2220. doi: 10.1210/endo-119-5-2214. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stahelin HB, Dick W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33:19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- 8.Ceglia L, da Silva Morais M, Park LK, Morris E, Harris SS, Bischoff-Ferrari HA, Fielding RA, Dawson-Hughes B. Multi-step immunofluorescent analysis of vitamin D receptor loci and myosin heavy chain isoforms in human skeletal muscle. J Mol Histol. 2010;41:137–142. doi: 10.1007/s10735-010-9270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srikuea R, Zhang X, Park-Sarge OK, Esser KA. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: potential role in suppression of myoblast proliferation. Am J Physiol Cell Physiol. 2012;303:C396–C405. doi: 10.1152/ajpcell.00014.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girgis CM, Mokbel N, Cha KM, Houweling PJ, Abboud M, Fraser DR, Mason RS, Clifton-Bligh RJ, Gunton JE. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology. 2014;155:3227–3237. doi: 10.1210/en.2014-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, DeLuca HF. Is the vitamin d receptor found in muscle? Endocrinology. 2011;152:354–363. doi: 10.1210/en.2010-1109. [DOI] [PubMed] [Google Scholar]
- 12.Bischoff-Ferrari HA, Borchers M, Gudat F, Durmuller U, Stahelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19:265–269. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 13.Girgis CM, Clifton-Bligh RJ, Mokbel N, Cheng K, Gunton JE. Vitamin D Signaling regulates proliferation, differentiation and myotube size in C2C12 skeletal muscle cells. Endocrinology. 2013;155:347–357. doi: 10.1210/en.2013-1205. [DOI] [PubMed] [Google Scholar]
- 14.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 15.Ceglia L, Niramitmahapanya S, Morais MD, Rivas DA, Harris SS, Bischoff-Ferrari H, Fielding RA, Dawson-Hughes B. A randomized study on the effect of vitamin D3 supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab. 2013;98:E1927–E1935. doi: 10.1210/jc.2013-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chale A, Cloutier GJ, Hau C, Phillips EM, Dallal GE, Fielding RA. Efficacy of whey protein supplementation on resistance exercise-induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults. The journals of gerontology. Biol Sci Med Sci A. 2013;68:682–690. doi: 10.1093/gerona/gls221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceglia L, Rivas DA, Pojednic RM, Price LL, Harris SS, Smith D, Fielding RA, Dawson-Hughes B. Effects of alkali supplementation and vitamin D insufficiency on rat skeletal muscle. Endocrine. 2013;44:454–464. doi: 10.1007/s12020-013-9976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buitrago C, Pardo VG, Boland R. Role of VDR in 1alpha,25-dihydroxyvitamin D-dependent non-genomic activation of MAPKs, Src and Akt in skeletal muscle cells. J Steroid Biochem Mol Biol. 2013;136:125–130. doi: 10.1016/j.jsbmb.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Boland RL. VDR activation of intracellular signaling pathways in skeletal muscle. Mol Cell Endocrinol. 2011;347:11–16. doi: 10.1016/j.mce.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Buitrago C, Boland R. Caveolae and caveolin-1 are implicated in 1alpha,25(OH)2-vitamin D3-dependent modulation of Src, MAPK cascades and VDR localization in skeletal muscle cells. J Steroid Biochem Mol Biol. 2010;121:169–175. doi: 10.1016/j.jsbmb.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Capiati D, Benassati S, Boland RL. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J Cell Biochem. 2002;86:128–135. doi: 10.1002/jcb.10191. [DOI] [PubMed] [Google Scholar]