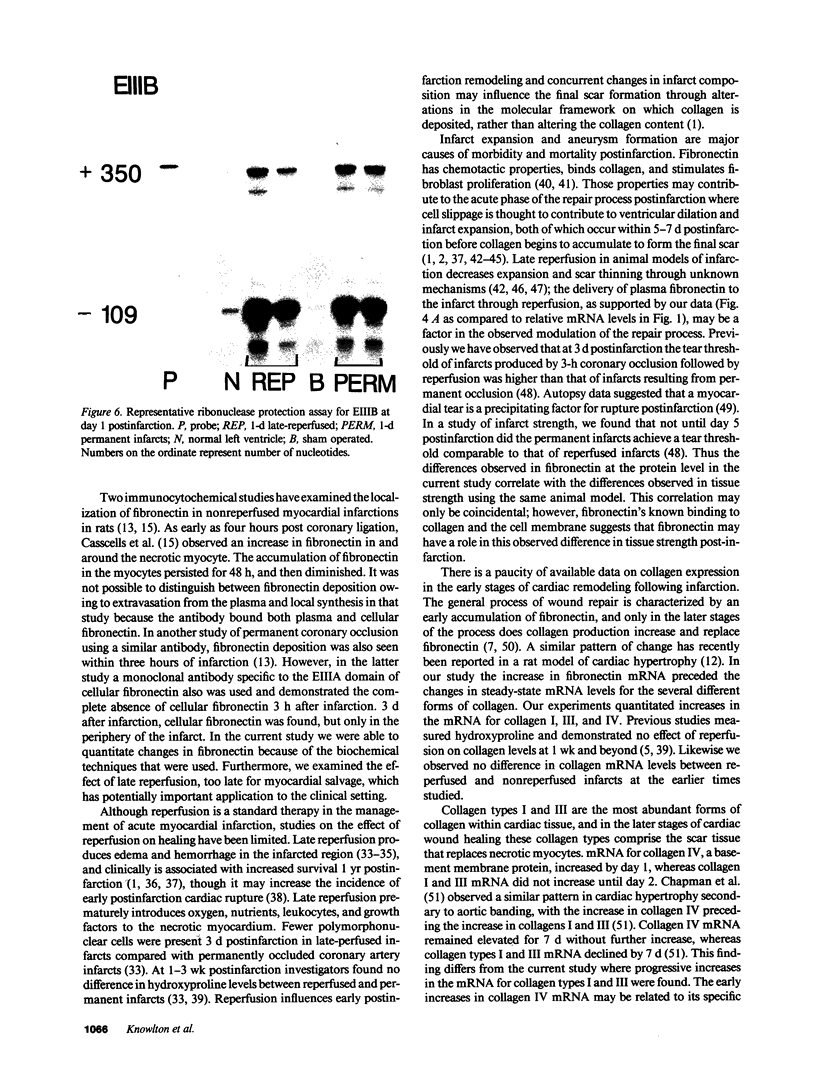
Abstract
The expression of fibronectin in the repair process after myocardial infarction was studied using two protocols of coronary occlusion in the rabbit: a permanent occlusion or 3 h of occlusion followed by reperfusion (too late for salvage). We found a rapid and progressive increase in cardiac fibronectin expression in the infarcted region of the ventricle. Steady-state mRNA levels for fibronectin increased 13- and 16-fold, respectively, in the permanent and reperfused infarcts 1 d postinfarction. Immunological detection of the protein with a polyclonal antibody against plasma fibronectin showed significant increases of the protein fibronectin in the infarcted myocardium by day 3 in the reperfused group and by day 5 in the permanent coronary occlusion group. Ribonuclease protection assays established the induction of EIIIB containing fibronectin mRNA in both models by day 1 and use of a monoclonal antibody showed an increase in the EIIIA isoform 2 d postinfarction. Increases in steady-state mRNA levels for several collagen types were found in both groups, but these changes occurred after those noted for fibronectin. Thus fibronectin mRNA and protein expression increased rapidly postinfarction suggesting a functional role in the repair process.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahumada G. G., Rennard S. I., Figueroa A. A., Silver M. H. Cardiac fibronectin: developmental distribution and quantitative comparison of possible sites of synthesis. J Mol Cell Cardiol. 1981 Jul;13(7):667–678. doi: 10.1016/0022-2828(81)90274-1. [DOI] [PubMed] [Google Scholar]
- Bonaduce D., Petretta M., Villari B., Breglio R., Conforti G., Montemurro M. V., Lanzillo T., Morgano G. Effects of late administration of tissue-type plasminogen activator on left ventricular remodeling and function after myocardial infarction. J Am Coll Cardiol. 1990 Dec;16(7):1561–1568. doi: 10.1016/0735-1097(90)90301-5. [DOI] [PubMed] [Google Scholar]
- Borsi L., Carnemolla B., Castellani P., Rosellini C., Vecchio D., Allemanni G., Chang S. E., Taylor-Papadimitriou J., Pande H., Zardi L. Monoclonal antibodies in the analysis of fibronectin isoforms generated by alternative splicing of mRNA precursors in normal and transformed human cells. J Cell Biol. 1987 Mar;104(3):595–600. doi: 10.1083/jcb.104.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunwald E. Myocardial reperfusion, limitation of infarct size, reduction of left ventricular dysfunction, and improved survival. Should the paradigm be expanded? Circulation. 1989 Feb;79(2):441–444. doi: 10.1161/01.cir.79.2.441. [DOI] [PubMed] [Google Scholar]
- Carnemolla B., Borsi L., Zardi L., Owens R. J., Baralle F. E. Localization of the cellular-fibronectin-specific epitope recognized by the monoclonal antibody IST-9 using fusion proteins expressed in E. coli. FEBS Lett. 1987 May 11;215(2):269–273. doi: 10.1016/0014-5793(87)80160-6. [DOI] [PubMed] [Google Scholar]
- Casscells W., Kimura H., Sanchez J. A., Yu Z. X., Ferrans V. J. Immunohistochemical study of fibronectin in experimental myocardial infarction. Am J Pathol. 1990 Oct;137(4):801–810. [PMC free article] [PubMed] [Google Scholar]
- Chapman D., Weber K. T., Eghbali M. Regulation of fibrillar collagen types I and III and basement membrane type IV collagen gene expression in pressure overloaded rat myocardium. Circ Res. 1990 Oct;67(4):787–794. doi: 10.1161/01.res.67.4.787. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Connelly C. M., Leppo J. A., Weitzman P. W., Vogel W. M., Apstein C. S. Effect of coronary occlusion and reperfusion on myocardial blood flow during infarct healing. Am J Physiol. 1989 Aug;257(2 Pt 2):H365–H374. doi: 10.1152/ajpheart.1989.257.2.H365. [DOI] [PubMed] [Google Scholar]
- Connelly C. M., McLaughlin R. J., Vogel W. M., Apstein C. S. Reversible and irreversible elongation of ischemic, infarcted, and healed myocardium in response to increases in preload and afterload. Circulation. 1991 Jul;84(1):387–399. doi: 10.1161/01.cir.84.1.387. [DOI] [PubMed] [Google Scholar]
- Connelly C. M., Vogel W. M., Wiegner A. W., Osmers E. L., Bing O. H., Kloner R. A., Dunn-Lanchantin D. M., Franzblau C., Apstein C. S. Effects of reperfusion after coronary artery occlusion on post-infarction scar tissue. Circ Res. 1985 Oct;57(4):562–577. doi: 10.1161/01.res.57.4.562. [DOI] [PubMed] [Google Scholar]
- Connelly C., Vogel W. M., Hernandez Y. M., Apstein C. S. Movement of necrotic wavefront after coronary artery occlusion in rabbit. Am J Physiol. 1982 Nov;243(5):H682–H690. doi: 10.1152/ajpheart.1982.243.5.H682. [DOI] [PubMed] [Google Scholar]
- Contard F., Koteliansky V., Marotte F., Dubus I., Rappaport L., Samuel J. L. Specific alterations in the distribution of extracellular matrix components within rat myocardium during the development of pressure overload. Lab Invest. 1991 Jan;64(1):65–75. [PubMed] [Google Scholar]
- Eghbali M., Blumenfeld O. O., Seifter S., Buttrick P. M., Leinwand L. A., Robinson T. F., Zern M. A., Giambrone M. A. Localization of types I, III and IV collagen mRNAs in rat heart cells by in situ hybridization. J Mol Cell Cardiol. 1989 Jan;21(1):103–113. doi: 10.1016/0022-2828(89)91498-3. [DOI] [PubMed] [Google Scholar]
- Eghbali M., Czaja M. J., Zeydel M., Weiner F. R., Zern M. A., Seifter S., Blumenfeld O. O. Collagen chain mRNAs in isolated heart cells from young and adult rats. J Mol Cell Cardiol. 1988 Mar;20(3):267–276. doi: 10.1016/s0022-2828(88)80059-2. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Van de Water L., Dvorak H. F., Hynes R. O. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989 Aug;109(2):903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force T., Kemper A., Leavitt M., Parisi A. F. Acute reduction in functional infarct expansion with late coronary reperfusion: assessment with quantitative two-dimensional echocardiography. J Am Coll Cardiol. 1988 Jan;11(1):192–200. doi: 10.1016/0735-1097(88)90189-1. [DOI] [PubMed] [Google Scholar]
- Fujiwara H., Onodera T., Tanaka M., Fujiwara T., Wu D. J., Kawai C., Hamashima Y. A clinicopathologic study of patients with hemorrhagic myocardial infarction treated with selective coronary thrombolysis with urokinase. Circulation. 1986 Apr;73(4):749–757. doi: 10.1161/01.cir.73.4.749. [DOI] [PubMed] [Google Scholar]
- Geft I. L., Fishbein M. C., Hashida J., Ninomiya K., Nishizawa S., Haendchen R., Venkatesh N., Y-Rit J., Yano J., Ganz W. Effects of late coronary artery reperfusion after myocardial necrosis is complete. Am Heart J. 1984 Apr;107(4):623–629. doi: 10.1016/0002-8703(84)90306-5. [DOI] [PubMed] [Google Scholar]
- Genovese C., Rowe D., Kream B. Construction of DNA sequences complementary to rat alpha 1 and alpha 2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry. 1984 Dec 4;23(25):6210–6216. doi: 10.1021/bi00320a049. [DOI] [PubMed] [Google Scholar]
- Hale S. L., Kloner R. A. Left ventricular topographic alterations in the completely healed rat infarct caused by early and late coronary artery reperfusion. Am Heart J. 1988 Dec;116(6 Pt 1):1508–1513. doi: 10.1016/0002-8703(88)90736-3. [DOI] [PubMed] [Google Scholar]
- Higginson L. A., Beanlands D. S., Nair R. C., Temple V., Sheldrick K. The time course and characterization of myocardial hemorrhage after coronary reperfusion in the anesthetized dog. Circulation. 1983 May;67(5):1024–1031. doi: 10.1161/01.cir.67.5.1024. [DOI] [PubMed] [Google Scholar]
- Hochman J. S., Bulkley B. H. Pathogenesis of left ventricular aneurysms: an experimental study in the rat model. Am J Cardiol. 1982 Jul;50(1):83–88. doi: 10.1016/0002-9149(82)90012-1. [DOI] [PubMed] [Google Scholar]
- Hochman J. S., Choo H. Limitation of myocardial infarct expansion by reperfusion independent of myocardial salvage. Circulation. 1987 Jan;75(1):299–306. doi: 10.1161/01.cir.75.1.299. [DOI] [PubMed] [Google Scholar]
- Honan M. B., Harrell F. E., Jr, Reimer K. A., Califf R. M., Mark D. B., Pryor D. B., Hlatky M. A. Cardiac rupture, mortality and the timing of thrombolytic therapy: a meta-analysis. J Am Coll Cardiol. 1990 Aug;16(2):359–367. doi: 10.1016/0735-1097(90)90586-e. [DOI] [PubMed] [Google Scholar]
- Jugdutt B. I., Amy R. W. Healing after myocardial infarction in the dog: changes in infarct hydroxyproline and topography. J Am Coll Cardiol. 1986 Jan;7(1):91–102. doi: 10.1016/s0735-1097(86)80265-0. [DOI] [PubMed] [Google Scholar]
- Kawahara E., Mukai A., Oda Y., Nakanishi I., Iwa T. Left ventriculotomy of the heart: tissue repair and localization of collagen types I, II, III, IV, V, VI and fibronectin. Virchows Arch A Pathol Anat Histopathol. 1990;417(3):229–236. doi: 10.1007/BF01600138. [DOI] [PubMed] [Google Scholar]
- Knowlton A. A., Brecher P., Apstein C. S. Rapid expression of heat shock protein in the rabbit after brief cardiac ischemia. J Clin Invest. 1991 Jan;87(1):139–147. doi: 10.1172/JCI114963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton A. A., Burrier R. E., Brecher P. Rabbit heart fatty acid-binding protein. Isolation, characterization, and application of a monoclonal antibody. Circ Res. 1989 Oct;65(4):981–988. doi: 10.1161/01.res.65.4.981. [DOI] [PubMed] [Google Scholar]
- Kurkinen M., Condon M. R., Blumberg B., Barlow D. P., Quinones S., Saus J., Pihlajaniemi T. Extensive homology between the carboxyl-terminal peptides of mouse alpha 1(IV) and alpha 2(IV) collagen. J Biol Chem. 1987 Jun 25;262(18):8496–8499. [PubMed] [Google Scholar]
- Lerman R. H., Apstein C. S., Kagan H. M., Osmers E. L., Chichester C. O., Vogel W. M., Connelly C. M., Steffee W. P. Myocardial healing and repair after experimental infarction in the rabbit. Circ Res. 1983 Sep;53(3):378–388. doi: 10.1161/01.res.53.3.378. [DOI] [PubMed] [Google Scholar]
- Liau G., Yamada Y., de Crombrugghe B. Coordinate regulation of the levels of type III and type I collagen mRNA in most but not all mouse fibroblasts. J Biol Chem. 1985 Jan 10;260(1):531–536. [PubMed] [Google Scholar]
- Mann J. M., Roberts W. C. Rupture of the left ventricular free wall during acute myocardial infarction: analysis of 138 necropsy patients and comparison with 50 necropsy patients with acute myocardial infarction without rupture. Am J Cardiol. 1988 Nov 1;62(13):847–859. doi: 10.1016/0002-9149(88)90881-8. [DOI] [PubMed] [Google Scholar]
- Olivetti G., Capasso J. M., Meggs L. G., Sonnenblick E. H., Anversa P. Cellular basis of chronic ventricular remodeling after myocardial infarction in rats. Circ Res. 1991 Mar;68(3):856–869. doi: 10.1161/01.res.68.3.856. [DOI] [PubMed] [Google Scholar]
- Pfeffer M. A., Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990 Apr;81(4):1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- Przyklenk K., Connelly C. M., McLaughlin R. J., Kloner R. A., Apstein C. S. Effect of myocyte necrosis on strength, strain, and stiffness of isolated myocardial strips. Am Heart J. 1987 Dec;114(6):1349–1359. doi: 10.1016/0002-8703(87)90536-9. [DOI] [PubMed] [Google Scholar]
- Roberts C. S., Schoen F. J., Kloner R. A. Effect of coronary reperfusion on myocardial hemorrhage and infarct healing. Am J Cardiol. 1983 Sep 1;52(5):610–614. doi: 10.1016/0002-9149(83)90036-x. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Patel R. S., Fonda D., Hynes R. O. Multiple sites of alternative splicing of the rat fibronectin gene transcript. EMBO J. 1987 Sep;6(9):2573–2580. doi: 10.1002/j.1460-2075.1987.tb02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhonin B. V., Domogatsky S. P., Idelson G. L., Koteliansky V. E. Participance of fibronectin and various collagen types in the formation of fibrous extracellular matrix in cardiosclerosis. J Mol Cell Cardiol. 1988 Jun;20(6):501–508. doi: 10.1016/s0022-2828(88)80077-4. [DOI] [PubMed] [Google Scholar]
- Shekhonin B. V., Guriev S. B., Irgashev S. B., Koteliansky V. E. Immunofluorescent identification of fibronectin and fibrinogen/fibrin in experimental myocardial infarction. J Mol Cell Cardiol. 1990 May;22(5):533–541. doi: 10.1016/0022-2828(90)90955-2. [DOI] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki I., Chobanian A. V., Brecher P. Biosynthesis of fibronectin by rabbit aorta. J Biol Chem. 1991 Sep 15;266(26):17686–17694. [PubMed] [Google Scholar]
- Thompson N. L., Bazoberry F., Speir E. H., Casscells W., Ferrans V. J., Flanders K. C., Kondaiah P., Geiser A. G., Sporn M. B. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988;1(1):91–99. doi: 10.3109/08977198809000251. [DOI] [PubMed] [Google Scholar]
- Trotter K. M., Beezhold D. H., Lause D. B. Modulation of macrophage fibronectin secretion by LPS. J Leukoc Biol. 1989 Jun;45(6):515–522. doi: 10.1002/jlb.45.6.515. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivaldi M. T., Eyre D. R., Kloner R. A., Schoen F. J. Effects of methylprednisolone on collagen biosynthesis in healing acute myocardial infarction. Am J Cardiol. 1987 Aug 1;60(4):424–425. doi: 10.1016/0002-9149(87)90277-3. [DOI] [PubMed] [Google Scholar]
- Weisman H. F., Bush D. E., Mannisi J. A., Weisfeldt M. L., Healy B. Cellular mechanisms of myocardial infarct expansion. Circulation. 1988 Jul;78(1):186–201. doi: 10.1161/01.cir.78.1.186. [DOI] [PubMed] [Google Scholar]
- Welch M. P., Odland G. F., Clark R. A. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol. 1990 Jan;110(1):133–145. doi: 10.1083/jcb.110.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]