Abstract
To test the hypothesis that direct contact between sympathetic neurons and myocytes regulates expression and function of cardiac Ca channels, we prepared cultures of neonatal rat ventricular myocytes with and without sympathetic ganglia. Contractile properties of myocytes were assessed by an optical-video system. Contractility-pCa curves showed a 60% greater increase in contractility for innervated myocytes compared with control cells at 6.3 mM [Ca]0 (n = 8, P less than 0.05). Cells grown in medium conditioned by growth of ganglia and myocytes were indistinguishable physiologically from control cells. [Bay K 8644]-contractility curves revealed a 60 +/- 10% enhancement of the contractility response at 10(-6) M for innervated cells compared with control cells. The increased response to Bay K 8644 was not blocked by alpha- or beta-adrenergic antagonists. Moreover, increased efficacy of Bay K 8644 was maintained for at least 24 h after denervation produced by removal of ganglia from the culture. Dihydropyridine binding sites were assessed with the L channel-specific radioligand 3[H]PN200-110. PN200-110 binding sites were increased by innervation (51 +/- 5 to 108 +/- 20 fmol/mg protein, P less than 0.01), with no change in KD. Peak current-voltage curves were determined by whole-cell voltage clamp techniques for myocytes contacted by a neuron, control myocytes, and myocytes grown in conditioned medium. Current density of L-type Ca channels was significantly higher in innervated myocytes (10.5 +/- 0.4 pA/pF, n = 5) than in control myocytes (5.9 +/- 0.3 pA/pF, n = 8, P less than 0.01) or myocytes grown in conditioned medium (6.2 +/- 0.2 pA/pF, n = 10, P less than 0.01). Thus, physical contact between a sympathetic neuron and previously uninnervated neonatal rat ventricular myocytes increases expression of functional L-type calcium channels as judged by contractile responses to Ca0 and Bay K 8644, as well as by electrophysiological and radioligand binding properties.
Full text
PDF

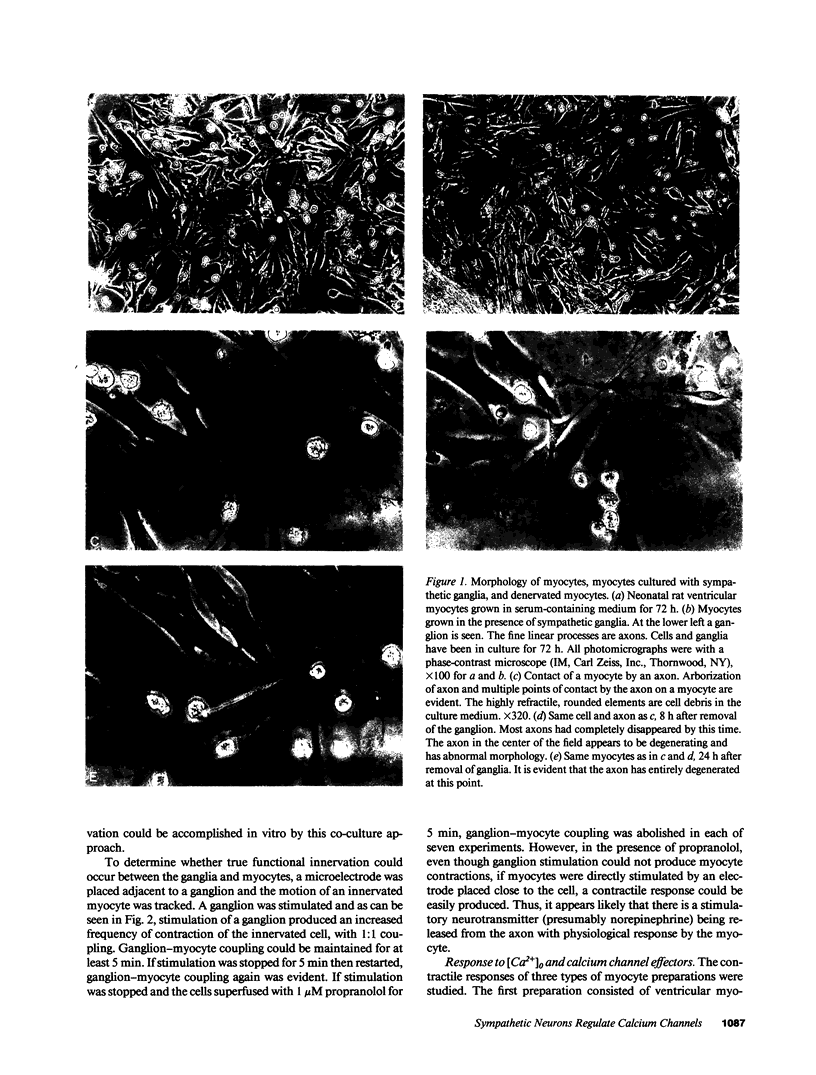
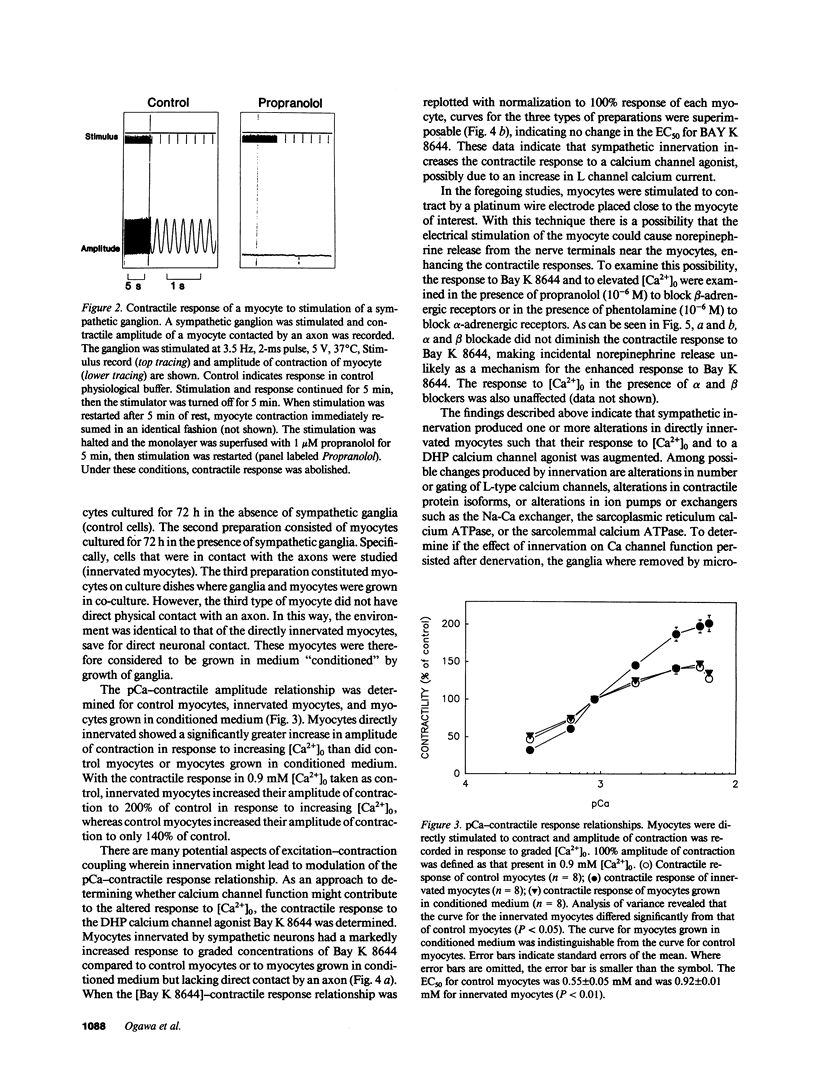
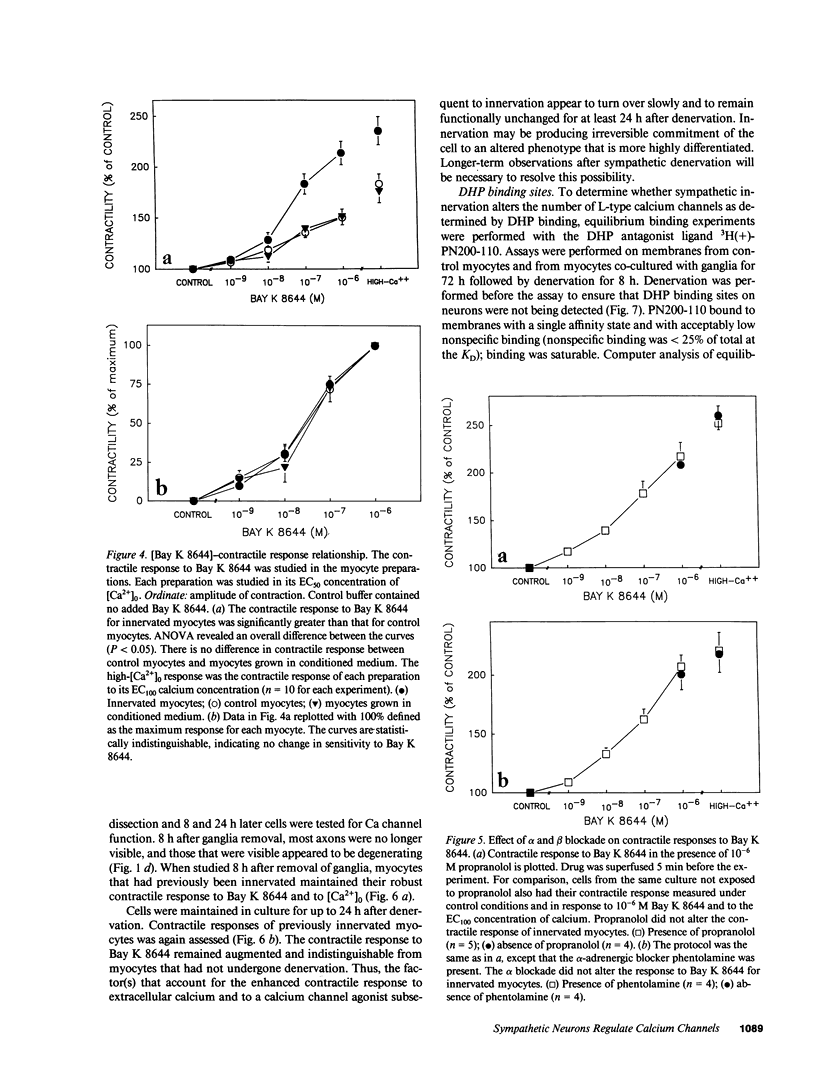
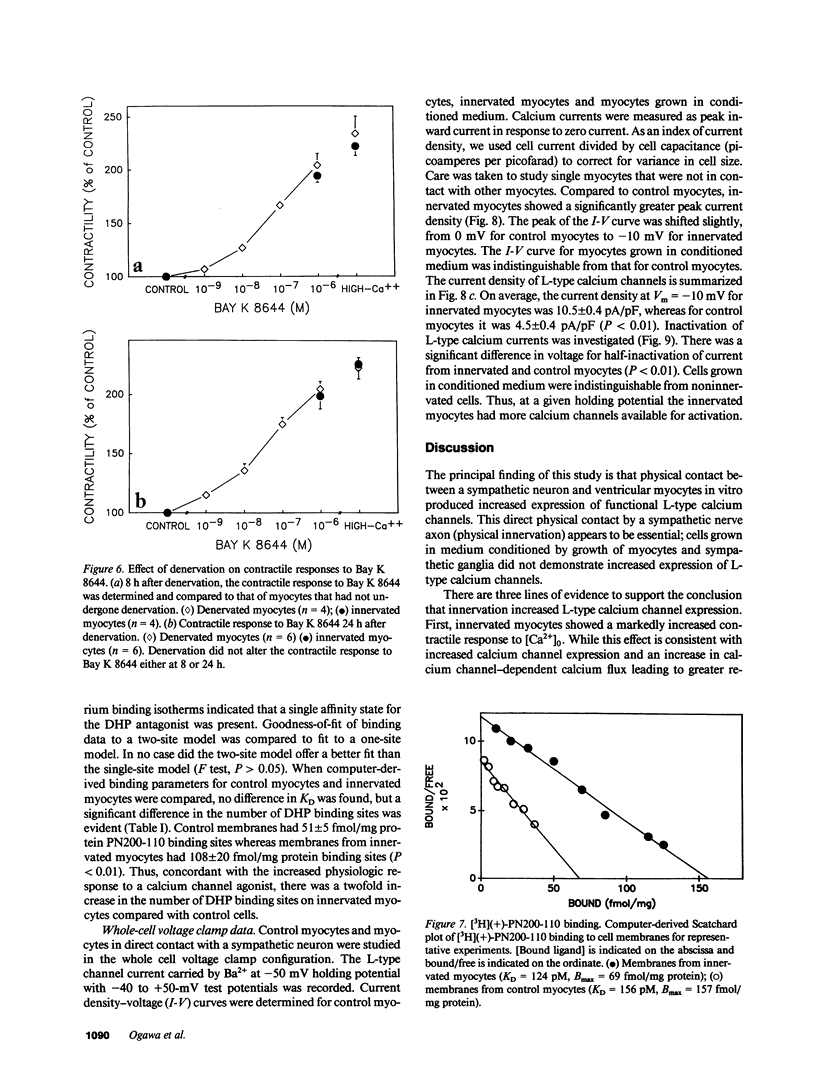
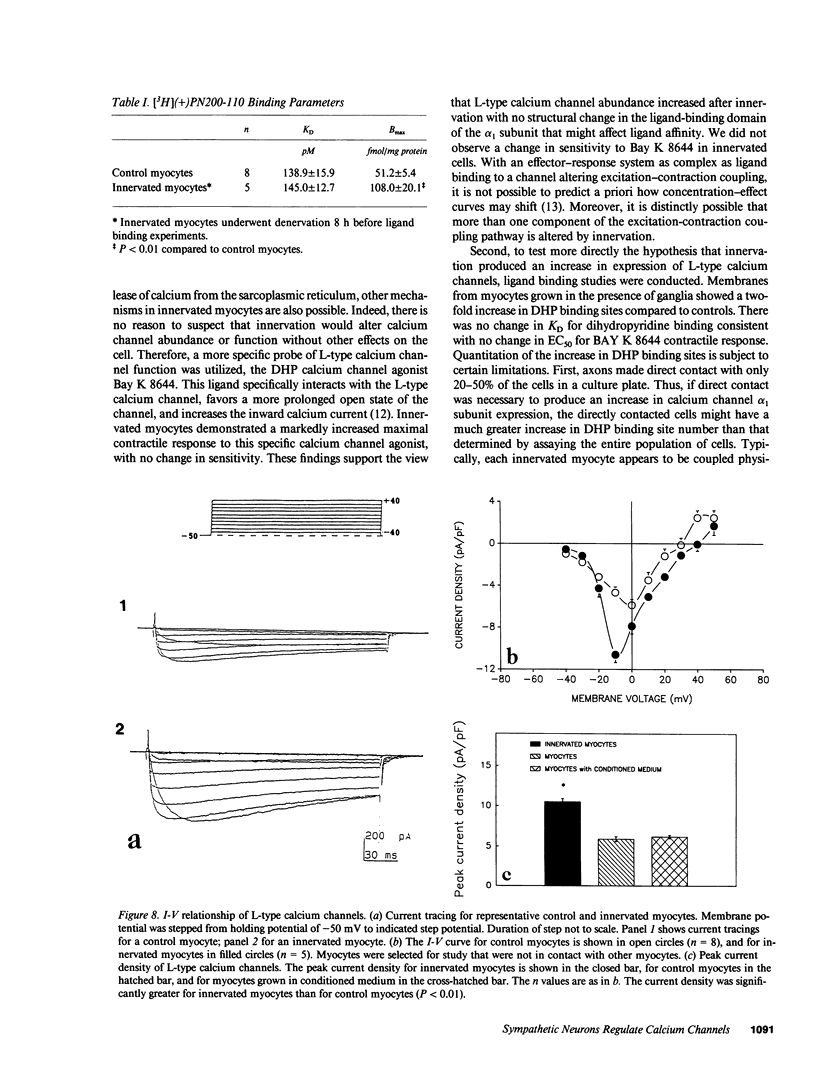
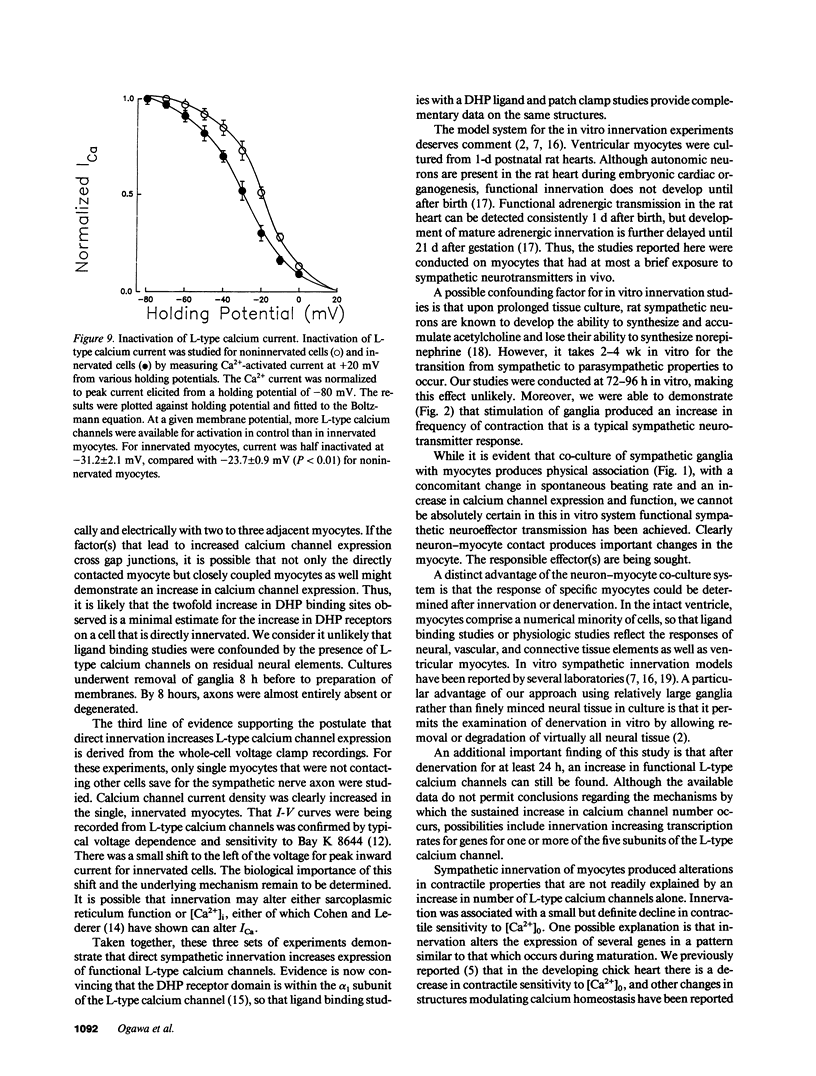

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins D. L., Marvin W. J., Jr Chronotropic responsiveness of developing sinoatrial and ventricular rat myocytes to autonomic agonists following adrenergic and cholinergic innervation in vitro. Circ Res. 1989 Jun;64(6):1051–1062. doi: 10.1161/01.res.64.6.1051. [DOI] [PubMed] [Google Scholar]
- Borzak S., Murphy S., Marsh J. D. Mechanisms of rate staircase in rat ventricular cells. Am J Physiol. 1991 Mar;260(3 Pt 2):H884–H892. doi: 10.1152/ajpheart.1991.260.3.H884. [DOI] [PubMed] [Google Scholar]
- Caffrey J. M., Brown A. M., Schneider M. D. Mitogens and oncogenes can block the induction of specific voltage-gated ion channels. Science. 1987 May 1;236(4801):570–573. doi: 10.1126/science.2437651. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Hanbauer I., Morad M. Modulation of calcium channels in cardiac and neuronal cells by an endogenous peptide. Science. 1989 Feb 3;243(4891):663–666. doi: 10.1126/science.2536955. [DOI] [PubMed] [Google Scholar]
- Chick J. D., Ritson E. B. Alcoholism: advice versus extended treatment. Br J Addict. 1989 Jul;84(7):817–818. doi: 10.1111/j.1360-0443.1989.tb03062.x. [DOI] [PubMed] [Google Scholar]
- Cohen N. M., Lederer W. J. Changes in the calcium current of rat heart ventricular myocytes during development. J Physiol. 1988 Dec;406:115–146. doi: 10.1113/jphysiol.1988.sp017372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Phosphorylation of the calcium antagonist receptor of the voltage-sensitive calcium channel by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2528–2532. doi: 10.1073/pnas.82.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorme E. M., Rabe C. S., McGee R., Jr Regulation of the number of functional voltage-sensitive Ca++ channels on PC12 cells by chronic changes in membrane potential. J Pharmacol Exp Ther. 1988 Mar;244(3):838–843. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cells from adult human, dog, cat, rabbit, rat, and frog hearts and from fetal and new-born rat ventricles. Ann N Y Acad Sci. 1978 Apr 28;307:491–522. doi: 10.1111/j.1749-6632.1978.tb41979.x. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Allen D. G. Comparison of the effects of inotropic interventions on isometric tension and shortening in isolated ferret ventricular muscle. Cardiovasc Res. 1989 Sep;23(9):748–755. doi: 10.1093/cvr/23.9.748. [DOI] [PubMed] [Google Scholar]
- Marsh J. D., Allen P. D. Developmental regulation of cardiac calcium channels and contractile sensitivity to [Ca]o. Am J Physiol. 1989 Jan;256(1 Pt 2):H179–H185. doi: 10.1152/ajpheart.1989.256.1.H179. [DOI] [PubMed] [Google Scholar]
- Marsh J. D., Barry W. H., Smith T. W. Desensitization to the inotropic effect of isoproterenol in cultured ventricular cells. J Pharmacol Exp Ther. 1982 Oct;223(1):60–67. [PubMed] [Google Scholar]
- Marsh J. D. Coregulation of calcium channels and beta-adrenergic receptors in cultured chick embryo ventricular cells. J Clin Invest. 1989 Sep;84(3):817–823. doi: 10.1172/JCI114241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin W. J., Jr, Atkins D. L., Chittick V. L., Lund D. D., Hermsmeyer K. In vitro adrenergic and cholinergic innervation of the developing rat myocyte. Circ Res. 1984 Jul;55(1):49–58. doi: 10.1161/01.res.55.1.49. [DOI] [PubMed] [Google Scholar]
- Massagué J. Transforming growth factor-alpha. A model for membrane-anchored growth factors. J Biol Chem. 1990 Dec 15;265(35):21393–21396. [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Offord J., Catterall W. A. Electrical activity, cAMP, and cytosolic calcium regulate mRNA encoding sodium channel alpha subunits in rat muscle cells. Neuron. 1989 May;2(5):1447–1452. doi: 10.1016/0896-6273(89)90190-6. [DOI] [PubMed] [Google Scholar]
- Pappano A. J. Ontogenetic development of autonomic neuroeffector transmission and transmitter reactivity in embryonic and fetal hearts. Pharmacol Rev. 1977 Mar;29(1):3–33. [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The induction of acetylcholine synthesis in primary cultures of dissociated rat sympathetic neurons. II. Developmental aspects. Dev Biol. 1977 Oct 15;60(2):473–481. doi: 10.1016/0012-1606(77)90144-0. [DOI] [PubMed] [Google Scholar]
- Rampe D., Caffrey J. M., Schneider M. D., Brown A. M. Control of expression of the 1,4-dihydropyridine receptor in BC3H1 cells. Biochem Biophys Res Commun. 1988 Apr 29;152(2):769–775. doi: 10.1016/s0006-291x(88)80104-9. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Steinberg S. F., Drugge E. D., Bilezikian J. P., Robinson R. B. Acquisition by innervated cardiac myocytes of a pertussis toxin-specific regulatory protein linked to the alpha 1-receptor. Science. 1985 Oct 11;230(4722):186–188. doi: 10.1126/science.2994230. [DOI] [PubMed] [Google Scholar]
- Strickland S., Loeb J. N. Obligatory separation of hormone binding and biological response curves in systems dependent upon secondary mediators of hormone action. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1366–1370. doi: 10.1073/pnas.78.3.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Seagar M. J., Jones J. F., Reber B. F., Catterall W. A. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyota N., Shimada Y. Isoform variants of troponin in skeletal and cardiac muscle cells cultured with and without nerves. Cell. 1983 May;33(1):297–304. doi: 10.1016/0092-8674(83)90358-6. [DOI] [PubMed] [Google Scholar]
- Varadi G., Orlowski J., Schwartz A. Developmental regulation of expression of the alpha 1 and alpha 2 subunits mRNAs of the voltage-dependent calcium channel in a differentiating myogenic cell line. FEBS Lett. 1989 Jul 3;250(2):515–518. doi: 10.1016/0014-5793(89)80787-2. [DOI] [PubMed] [Google Scholar]
- Varadi G., Orlowski J., Schwartz A. Developmental regulation of expression of the alpha 1 and alpha 2 subunits mRNAs of the voltage-dependent calcium channel in a differentiating myogenic cell line. FEBS Lett. 1989 Jul 3;250(2):515–518. doi: 10.1016/0014-5793(89)80787-2. [DOI] [PubMed] [Google Scholar]
- Vetter R., Will H., Küttner I., Kemsies C., Will-Shahab L. Developmental changes of Ca++ transport systems in chick heart. Biomed Biochim Acta. 1986;45(1-2):S219–S222. [PubMed] [Google Scholar]
- Wieczorek D. F. Regulation of alternatively spliced alpha-tropomyosin gene expression by nerve extract. J Biol Chem. 1988 Jul 25;263(21):10456–10463. [PubMed] [Google Scholar]
- Yatani A., Brown A. M. Rapid beta-adrenergic modulation of cardiac calcium channel currents by a fast G protein pathway. Science. 1989 Jul 7;245(4913):71–74. doi: 10.1126/science.2544999. [DOI] [PubMed] [Google Scholar]








