Abstract
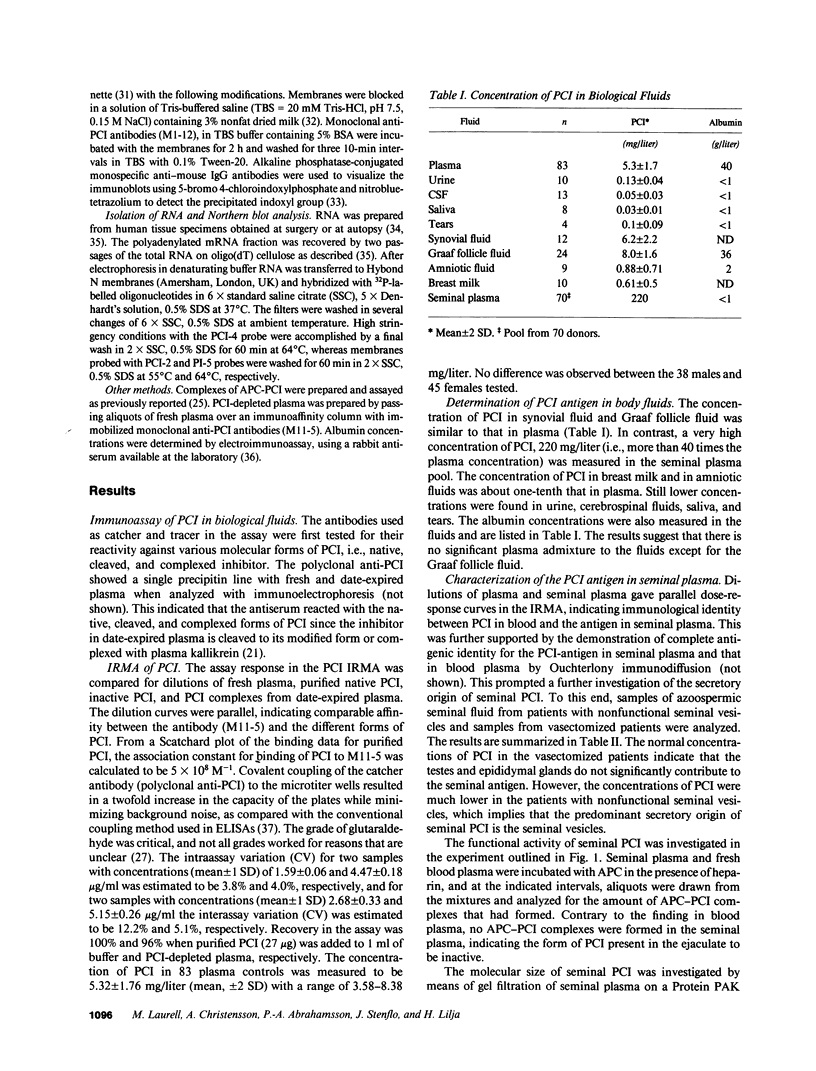
An assay was developed for the measurement of human protein C inhibitor antigen (PCI) in blood plasma and other biological fluids. Both native PCI, modified inhibitor, and complexes of inhibitor with activated protein C or plasma kallikrein could be measured with the assay. Inhibitor antigen concentrations were found to be very high in seminal plasma (greater than 200 mg/liter), more than 40 times the concentration of PCI found in blood plasma. The inhibitor in seminal plasma was unable to form complexes with activated protein C. Gel filtration and immunoblotting findings indicated that the inhibitor in seminal plasma is present in a high molecular mass complex or cleaved to its modified form. As PCI antigen was absent from seminal plasma of patients with dysfunctional seminal vesicles, the seminal vesicle glands would appear to be the major source of seminal plasma PCI, a conclusion supported by immunohistochemical demonstration of the presence of PCI epitopes in the secretory epithelium of the seminal vesicles. Specific PCI immunoreactivity was also shown to be present in the testes, the epididymis glands, and the prostate, suggesting the inhibitor to have a complex or multiple function in the male reproductive system. Conclusive evidence of a local synthesis of PCI in the four male sex glands was provided by Northern blot analysis of RNA from these organs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angles-Cano E., Sultan Y. A solid-phase fibrin immunoassay for the specific detection of monoclonal antibodies against different epitopic determinants of tissue-plasminogen activators. J Immunol Methods. 1984 Apr 13;69(1):115–127. doi: 10.1016/0022-1759(84)90283-7. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chapdelaine P., Paradis G., Tremblay R. R., Dubé J. Y. High level of expression in the prostate of a human glandular kallikrein mRNA related to prostate-specific antigen. FEBS Lett. 1988 Aug 15;236(1):205–208. doi: 10.1016/0014-5793(88)80315-6. [DOI] [PubMed] [Google Scholar]
- Chmielewska J., Rånby M., Wiman B. Kinetics of the inhibition of plasminogen activators by the plasminogen-activator inhibitor. Evidence for 'second-site' interactions. Biochem J. 1988 Apr 15;251(2):327–332. doi: 10.1042/bj2510327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Comp P. C., Jacocks R. M., Ferrell G. L., Esmon C. T. Activation of protein C in vivo. J Clin Invest. 1982 Jul;70(1):127–134. doi: 10.1172/JCI110584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T. The regulation of natural anticoagulant pathways. Science. 1987 Mar 13;235(4794):1348–1352. doi: 10.1126/science.3029867. [DOI] [PubMed] [Google Scholar]
- Esmon C. T. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J Biol Chem. 1989 Mar 25;264(9):4743–4746. [PubMed] [Google Scholar]
- España F., Berrettini M., Griffin J. H. Purification and characterization of plasma protein C inhibitor. Thromb Res. 1989 Aug 1;55(3):369–384. doi: 10.1016/0049-3848(89)90069-8. [DOI] [PubMed] [Google Scholar]
- Gültekin H., Heermann K. H. The use of polyvinylidenedifluoride membranes as a general blotting matrix. Anal Biochem. 1988 Aug 1;172(2):320–329. doi: 10.1016/0003-2697(88)90451-4. [DOI] [PubMed] [Google Scholar]
- Heeb M. J., España F., Geiger M., Collen D., Stump D. C., Griffin J. H. Immunological identity of heparin-dependent plasma and urinary protein C inhibitor and plasminogen activator inhibitor-3. J Biol Chem. 1987 Nov 25;262(33):15813–15816. [PubMed] [Google Scholar]
- Heeb M. J., Griffin J. H. Physiologic inhibition of human activated protein C by alpha 1-antitrypsin. J Biol Chem. 1988 Aug 25;263(24):11613–11616. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hunt L. T., Dayhoff M. O. A surprising new protein superfamily containing ovalbumin, antithrombin-III, and alpha 1-proteinase inhibitor. Biochem Biophys Res Commun. 1980 Jul 31;95(2):864–871. doi: 10.1016/0006-291x(80)90867-0. [DOI] [PubMed] [Google Scholar]
- Kazama Y., Niwa M., Yamagishi R., Takahashi K., Sakuragawa N., Koide T. Specificity of sulfated polysaccharides to accelerate the inhibition of activated protein C by protein C inhibitor. Thromb Res. 1987 Oct 15;48(2):179–185. doi: 10.1016/0049-3848(87)90414-2. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Laurell M., Carlson T. H., Stenflo J. Monoclonal antibodies against the heparin-dependent protein C inhibitor suitable for inhibitor purification and assay of inhibitor complexes. Thromb Haemost. 1988 Oct 31;60(2):334–339. [PubMed] [Google Scholar]
- Laurell M., Stenflo J. Protein C inhibitor from human plasma: characterization of native and cleaved inhibitor and demonstration of inhibitor complexes with plasma kallikrein. Thromb Haemost. 1989 Nov 24;62(3):885–891. [PubMed] [Google Scholar]
- Lilja H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J Clin Invest. 1985 Nov;76(5):1899–1903. doi: 10.1172/JCI112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja H., Abrahamsson P. A., Lundwall A. Semenogelin, the predominant protein in human semen. Primary structure and identification of closely related proteins in the male accessory sex glands and on the spermatozoa. J Biol Chem. 1989 Jan 25;264(3):1894–1900. [PubMed] [Google Scholar]
- Lilja H., Abrahamsson P. A. Three predominant proteins secreted by the human prostate gland. Prostate. 1988;12(1):29–38. doi: 10.1002/pros.2990120105. [DOI] [PubMed] [Google Scholar]
- Lilja H., Oldbring J., Rannevik G., Laurell C. B. Seminal vesicle-secreted proteins and their reactions during gelation and liquefaction of human semen. J Clin Invest. 1987 Aug;80(2):281–285. doi: 10.1172/JCI113070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlar R. A., Griffin J. H. Deficiency of protein C inhibitor in combined factor V/VIII deficiency disease. J Clin Invest. 1980 Nov;66(5):1186–1189. doi: 10.1172/JCI109952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijers J. C., Chung D. W. Evidence for a glycine residue at position 316 in human protein C inhibitor. Thromb Res. 1990 Jul 15;59(2):389–393. doi: 10.1016/0049-3848(90)90142-y. [DOI] [PubMed] [Google Scholar]
- Meijers J. C., Kanters D. H., Vlooswijk R. A., van Erp H. E., Hessing M., Bouma B. N. Inactivation of human plasma kallikrein and factor XIa by protein C inhibitor. Biochemistry. 1988 Jun 14;27(12):4231–4237. doi: 10.1021/bi00412a005. [DOI] [PubMed] [Google Scholar]
- Polakoski K. L., McRorie R. A. Boar acrosin. II. Classification, inhibition, and specificity studies of a proteinase from sperm acrosomes. J Biol Chem. 1973 Dec 10;248(23):8183–8188. [PubMed] [Google Scholar]
- Polakoski K. L., McRorie R. A., Williams W. L. Boar acrosin. I. Purification and preliminary characterization of a proteinase from boar sperm acrosomes. J Biol Chem. 1973 Dec 10;248(23):8178–8182. [PubMed] [Google Scholar]
- Pratt C. W., Macik B. G., Church F. C. Protein C inhibitor: purification and proteinase reactivity. Thromb Res. 1989 Mar 15;53(6):595–602. doi: 10.1016/0049-3848(89)90149-7. [DOI] [PubMed] [Google Scholar]
- Radtke K. P., Stief T. W., Heimburger N. A new and simple isolation procedure for human protein C inhibitor. Evidence for a second inhibitor for activated protein C present in human plasma. Biol Chem Hoppe Seyler. 1988 Sep;369(9):965–974. doi: 10.1515/bchm3.1988.369.2.965. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Schleuning W. D., Fritz H. Some characteristics of highly purified boar sperm acrosin. Hoppe Seylers Z Physiol Chem. 1974 Feb;355(2):125–130. doi: 10.1515/bchm2.1974.355.1.125. [DOI] [PubMed] [Google Scholar]
- Stief T. W., Radtke K. P., Heimburger N. Inhibition of urokinase by protein C-inhibitor (PCI). Evidence for identity of PCI and plasminogen activator inhibitor 3. Biol Chem Hoppe Seyler. 1987 Oct;368(10):1427–1433. doi: 10.1515/bchm3.1987.368.2.1427. [DOI] [PubMed] [Google Scholar]
- Stump D. C., Thienpont M., Collen D. Purification and characterization of a novel inhibitor of urokinase from human urine. Quantitation and preliminary characterization in plasma. J Biol Chem. 1986 Sep 25;261(27):12759–12766. [PubMed] [Google Scholar]
- Stump D. C., Thienpont M., Collen D. Urokinase-related proteins in human urine. Isolation and characterization of single-chain urokinase (pro-urokinase) and urokinase-inhibitor complex. J Biol Chem. 1986 Jan 25;261(3):1267–1273. [PubMed] [Google Scholar]
- Suzuki K., Deyashiki Y., Nishioka J., Kurachi K., Akira M., Yamamoto S., Hashimoto S. Characterization of a cDNA for human protein C inhibitor. A new member of the plasma serine protease inhibitor superfamily. J Biol Chem. 1987 Jan 15;262(2):611–616. [PubMed] [Google Scholar]
- Suzuki K., Nishioka J., Hashimoto S. Protein C inhibitor. Purification from human plasma and characterization. J Biol Chem. 1983 Jan 10;258(1):163–168. [PubMed] [Google Scholar]
- Suzuki K., Nishioka J., Kusumoto H., Hashimoto S. Mechanism of inhibition of activated protein C by protein C inhibitor. J Biochem. 1984 Jan;95(1):187–195. doi: 10.1093/oxfordjournals.jbchem.a134583. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]









