Abstract
Background
In the Netherlands about 18,000 procedures with implant removal are performed annually following open or closed reduction and fixation of fractures, of which 30-80% concern the foot, ankle and lower leg region. For clean surgical procedures, the rate of postoperative wound infections (POWI) should be less than ~2%. However, rates of 10-12% following implant removal have been reported, specifically after foot, ankle and lower leg fractures. Currently, surgeons individually decide if antibiotics prophylaxis is given, since no guideline exists. This leads to undesirable practice variation. The aim of the study is to assess the (cost-)effectiveness of a single intravenous gift of Cefazolin prior to implant removal following surgical fixation of foot, ankle and/or lower leg fractures.
Methods
This is a double-blind randomized controlled trial in patients scheduled for implant removal following a foot, ankle or lower leg fracture. Primary outcome is a POWI within 30 days after implant removal. Secondary outcomes are quality of life, functional outcome and costs at 30 days and 6 months after implant removal. With 2 x 250 patients a decrease in POWI rate from 10% to 3.3% (expected rate in clean-contaminated elective orthopaedic trauma procedures) can be detected (Power = 80%, 2-sided alpha = 5%, including 15% lost to follow up).
Discussion
If administration of prophylactic antibiotics prior to implant removal reduces the infectious complication rate, this will offer a strong argument to adopt this as standard practice of care. This will consequently lead to less physical and social disabilities and health care use. A preliminary, conservative estimation suggests yearly cost savings in the Netherlands of € 3.5 million per year.
Trial registration
This study is registered at Clinicaltrials.gov (NCT02225821) and the Netherlands Trial Register (NTR4393) and was granted permission by the Medical Ethical Review Committee of the Academic Medical Centre on October 7 2014.
Keywords: Antibiotic prophylaxis, Postoperative wound infection, Implant removal, Fracture surgery, Functional outcome
Background
Open or closed reduction followed by internal fixation is a frequently performed operation for lower extremity fractures. Indications for implant removal in adult patients include symptomatic hardware (i.e. pain, thin overlying skin and restricted motion), implant failure (breakage, loosening), or a persistent infectious complication of the index procedure (infection or fistula). Following successful surgical procedures for extremity fractures, implant removal is not a routinely indicated procedure. However, removal of implants causing symptoms can result in pain relief and a high rate of patient satisfaction [1, 2].
In the Netherlands about 18,000 implant removals are performed annually, of which 30-80% in the foot, ankle and lower leg region [3]. Literature on implant removal is sparse, but studies show most of the implants removed are following lower extremity injuries, especially below the knee (Table 1).
Table 1.
Studies on implant removal and the portion of implant removal from the foot-ankle and lower leg region
| Study (year) | N of cases | N of IR FAL (%) |
|---|---|---|
| Raahave (1967) [4] | 269 | 109 (41) |
| Richards (1992) [5] | 88 | 25 (28) |
| Sanderson (1992) [6] | 188 | 92 (49) |
| Minkowitz (2007) [7] | 60 | 42 (70) |
| Vos (2012) [2] | 284 | 89 (31) |
| Backes (2015) [8] | 512 | 404 (79) |
N; Number, IR; implant removal, FAL; foot- ankle or lower leg.
In addition, there is only a small amount of literature available on the risk of postoperative wound infection (POWI) following implant removal (Table 2). For ‘clean’ procedures the rate of POWI should be less than ~2% [11]. However, POWI rates of about 10-12%, specifically after foot, ankle and/or lower leg fractures, have been observed both by us and others in studies in which patients with implant removal due to an active wound infection were excluded [2, 8]. In syndesmotic screw removal 9.2% of POWI were observed and in calcaneal implant removal following fracture surgery without postoperative complications in dislocated closed calcaneal fractures 19% of POWI were observed [9, 10]. Preoperative prophylactic antibiotics might be beneficial to reduce the incidence of infectious complications following implant removal.
Table 2.
Implant removal and incidence postoperative wound infections
| Study (year) | N of cases | N of IR in FAL | N of POWI in FAL (%) |
|---|---|---|---|
| Raahave (1967) [4] | 269 | 109 | 4 (3.7) |
| Richards (1992) [5] | 88 | 25 | 0 (0) |
| Sanderson (1992) [6] | 188 | 92 | 12 (13) |
| Minkowitz (2007) [7] | 60 | 42 | 0 (0) |
| Schepers (2011) [9] | 76 | 76 | 7 (9.2) |
| Backes (2013) [10] | 228 | 69 | 6 (9) |
| Vos (2012) [2] | 284 | 89 | 9 (11) |
| Backes (2015) [ [8] |
512 | 403 | 49 (12.2) |
N; Number, IR; implant removal NA; not available, POWI; postoperative wound infection, FAL; foot- ankle and lower leg.
To date, only evidence exists on the effectiveness of prophylactic antibiotics in internal fixation with implants, but not in implant removal to prevent POWI [12]. In the Netherlands antibiotic prophylaxis is not routinely administered prior to implant removal as it is considered a clean procedure. Surgeons decide upon themselves if antibiotics are administered prior to implant removal, which is based on expert opinion as no evidence based guideline exists. This results in a undesirable practice variation.
Our aim is to study the (cost-)effectiveness of a single intravenous gift of Cefazolin prior to implant removal following surgical fixation of foot, ankle and/or lower leg fractures. The primary outcome is the incidence of POWI and secondary outcomes are health-related quality of life, functional outcome, health care utilization including transmural care, and costs from a health care and societal perspective.
Methods
This double blind randomised controlled trial will randomise between pre-operative administration of a single gift of Cefazolin or sodium chloride 0.9% in patients scheduled for elective implant removal below the knee. Twenty one centers will participate, including two Level 1 trauma centers.
Participants
The eligible study population will consist of all consecutive adult patients who are planned for elective implant removal following fracture treatment of the foot, ankle and/or lower leg.
Inclusion criteria
Patients ≥18 years and ≤75 years of all ethnic backgrounds
Scheduled implant removal following foot, ankle and/or lower leg surgery
Exclusion criteria
Removal and adding osteosynthesis material during the same procedure
Active wound infection or (plate) fistula
Antibiotic treatment at the time of implant removal for a concomitant disease or infection
A medical history of an allergic reaction to a cephalosporin, penicillin, or any other β-lactam antibiotic
Known kidney disease (or known eGFR <60 ml/min/1.73 m2)
Pregnancy and lactation
Immunosuppressant use in organ transplantation or rheumatoid joint disease
Interventions
After obtaining informed consent in the outpatient clinic, patients are contacted for a pre-operative assessment of functional status and health-related quality of life by way of self-administered questionnaires before surgery.
At the day of surgery, patients will be randomly assigned web-based in a 1:1 allocation ratio to one of the following study arms:
antibiotic prophylaxis: a single intravenous (iv) gift of 1000 mg Cefazolin in 10 cc of NaCl 0.9% (intervention group) or
no antibiotic prophylaxis: a single iv gift of 10 cc NaCl 0.9%.After implant removal, patients are routinely assessed within four weeks postoperatively at the outpatient clinic (Figure 1). They are instructed to visit the outpatient clinic sooner in case of any signs of POWI, including warmth, redness, pain, swelling, drainage or a fever above 38.5 degrees Celsius. In case of a POWI, appropriate treatment is started according to protocol. In addition to the one time visit, the patient is asked to return a surgical wound healing post-discharge questionnaire by mail filled in at thirty days postoperatively. At six months after implant removal, patients are contacted by telephone or mail to fill out web-based questionnaires to assess functional outcome, QOL measurement, patient satisfaction, health care resources utilization, costs evaluation and questions on late infections (Figure 1).
Figure 1.
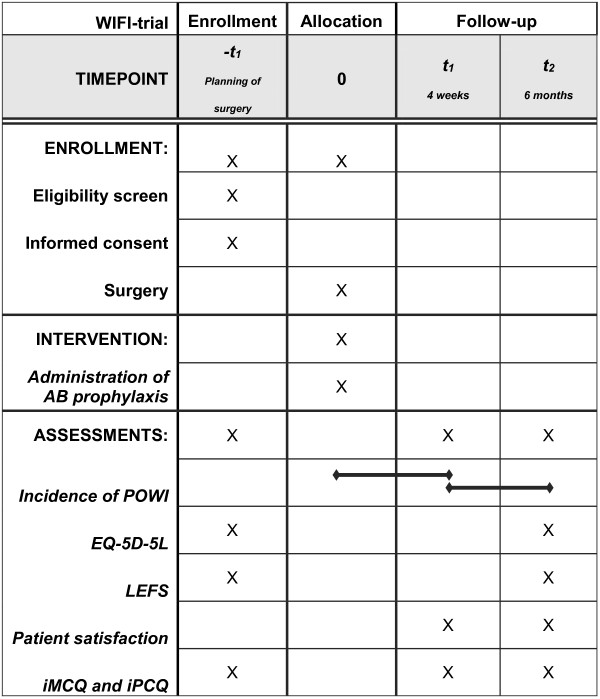
Schedule of the study procedures. AB; antibiotic, POWI; postoperative wound infection, EQ-5D; EuroQuality of Life-5D, LEFS; Lower extremity functional Scale, iMCQ; iMTA Medical Consumption Questionnaire, iPCQ; MTA Productivity Cost Questionnaire.
Randomization
Randomization will be stratified per center and will be blocked within strata. Randomization sequence is generated by a dedicated computer randomization software program and will be performed preoperatively by a theatre assistant and/or the anaesthesiologist using a dedicated, password protected, SSL–encrypted website, ensuring allocation concealment during the Time Out Procedure. Given the randomization result, the anaesthesiologist will prepare either a syringe with 1000 mg Cefazolin or with NaCl 0.9% in the operating theatre or pre-operative holding area, which is administered thirty minutes prior to surgery through a peripheral iv catheter. The iv-catheter is used routinely for either sedatives, muscle relaxants and/or pain medication.
Blinding
Importantly, the anaesthesiologist prepares the study medication in the absence of the surgeon and administers the study medication or NaCl 0.9%. Neither the patient nor the surgeon will know if the patient receives prophylactic antibiotics. During the visit to the outpatient clinic the patient is seen by a physician other than the surgeon who performed the surgery. The attending physician will document signs of POWI and will determine its presence or any special findings on physical examination. In addition, a photograph of the wound(s) will be taken by the attending physician and kept in the medical charts. This will enable an independent outcome assessment committee to judge the clinical aspect of the surgical wound, blinded for the study intervention. If the local investigator or attending physician decides unblinding is essential, (s)he will make every effort to contact the coordinating investigator before unblinding to discuss options. Otherwise, the randomization code will be unblinded after analysis of the study results.
Primary Outcome
The primary outcome variable is a POWI within 30 days after implant removal as defined by the criteria applied by the CDC [11].
Secondary Outcomes
The study will focus on the following secondary outcomes (Figure 1):
Health-related quality of life as measured by the EQ-5D questionnaire. The EQ-5D-5 L is a descriptive system of health-related quality of life states consisting of five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression [13].
Functional outcome as assessed with the Lower Extremity Functional Scale (LEFS). The LEFS is a questionnaire containing 20 questions about a person’s ability to perform everyday tasks and can be used to monitor the patient over time and to evaluate the effectiveness of an intervention [14, 15].
Patient satisfaction as measured by a ten-point Visual Analog Scale.
Health care resources utilization (including amongst others, number of visits to the general practitioner and use of home care organizations) as measured by way of a combination of the Dutch iMTA Medical Consumption Questionnaire (iMCQ) and iMTA Productivity Cost Questionnaire (iPCQ).
Costs (economic evaluation including budget impact analysis): the economic evaluation of antibiotic prophylaxis in patients scheduled for implant removal following a foot, ankle or lower leg fracture against no prophylaxis as its best alternative will be performed as a cost-effectiveness (CEA) as well as a cost-utility (CUA) analysis. The primary economic outcome in the CEA will be the costs per patient without a POWI, which closely relates to the clinical outcome measure. The CUA outcome is the costs per quality adjusted life year (QALY), which is a suitable outcome measure for priority setting during health care policy making across interventions, patient populations, and health care settings.
Sample size
Since information from prospective studies is limited, there is uncertainty about the POWI rate in current medical practice. In recent Dutch prospective studies the incidence of POWI below the knee is 11%, 12.2%, 9.2% and 19% [2, 8–10]. To be on the safe side, a POWI rate of 10% is assumed for the control group. According to the expected rate in clean-contaminated elective orthopedic procedures, a POWI rate of 3.3% for the antibiotic prophylaxis group is assumed [11]. At least 216 patients per study arm are necessary to detect this difference with a power of 80% and a two-sided alpha of 5%. An estimation of the POWI rate in the control group is planned midway, when 216 patients have been included and reached the primary outcome at 30 days post-surgery. Since only an estimation of the POWI rate of the control group is performed and no treatment effect is tested, the overall Type I error rate is maintained. This estimation will be performed by an independent statistician. To allow for an anticipated drop out of 10-15%, we will include a total of 250 patients per arm.
Based on our recent retrospective cohort studies in both an academic and non-academic hospital an annual number of 33–66 patients are expected to be included in our study for implant removal following lower leg injuries for each participating clinic [8]. With a number of 21 participating centers and an inclusion period of 1.5 years the number of study participants needed, is therefore highly feasible.
Statistical analysis
All analyses will be performed according to the intention-to-treat principle. In addition, protocol analyses will be done to check for robustness of results. A two-sided P-value < 0.05 will be considered statistically significant. In all analyses statistical uncertainties will be quantified using corresponding 95% two-sided confidence intervals. Descriptive analysis will be performed to compare baseline characteristics between patients with and without an infection. Univariate analysis will be performed for primary and secondary outcomes, followed by a multivariate logistic regression analysis to eliminate confounders. All analyses will be done using the Statistical Package for the Social Sciences (SPSS) version 19.0. (SPSS, Chicago, Illinois, USA).
Regulation statement
The study will be conducted according to the principles of the Declaration of Helsinki (version 10, 64th WMA General Assembly, Forteleza, Brazil, October 2013) and in accordance with the Medical Research Involving Human Subjects Act (WMO) and the Good Clinical Practice Guidelines (ICH-GCP).
Recruitment and consent
The patient will be informed about the WIFI-trial when he or she visits the outpatient clinic and implant removal is discussed. Documents are handed to the patient and the patient is asked to read the patient information letter. In order to be able to prepare for the elective (day care) surgery the patient is asked to participate in the trial during this visit to the outpatient clinic and will be asked to sign the informed consent form. Surgeons are asked by the coordinating investigator to check whether patients are included in the pre-operative assessment a day prior to surgery.
Benefits and risks assessment, group relatedness
Patient risks in this study are minimal and acceptable, as Cefazolin is currently used as prophylaxis in open reduction and internal fixation of fractures. Patients in both study groups will not be exposed to risks other than in current practice, since there is practice variation in the use of prophylactic antibiotics. As mentioned, currently surgeons decide upon themselves if antibiotics are administered preoperatively. We assume that the routine use of prophylactic antibiotics prior to implant removal following surgical fixation of foot, ankle and/or lower leg fractures will reduce the rate of POWI significantly (by two-thirds, from 10% to 3.3%). If our hypothesis is supported by the results of the proposed RCT, this will offer a strong argument to incorporate prophylactic use of a Cefazolin as strategy of choice in (inter)national guidelines for implant removal following fixation of ankle, foot and lower leg fractures. This could lead to less morbidity and social adverse effects in patients like pain, physical discomfort, multiple outpatient clinic visits/less healthcare consumption, work absenteeism and decreased self-confidence.
Indemnities
The institutional review board at the AMC has waived liability insurance, because no additional risk can be attributed to participation in this study.
Publication plan
The principal investigator, the study designer and the study coordinator will be named author. There will be a limit of ten authors. All others will obtain group authorship in the study group. All authors including group members are allowed to present the results.
Discussion
This RCT on wound infections following implant removal is performed in twenty-one different hospitals by a larger number of surgeons, which causes heterogeneity in patients and surgeons. However, we believe this also reflects normal practise in which antibiotic profylaxis could be beneficial. If our assumption that prophylactic antibiotics prior to implant removal reduces the infectious complication rate is confirmed by this RCT, this will offer a strong argument to adopt a single gift of antibiotic prophylaxis as standard practice of care. This will reduce the incidence of POWI and consequently will lead to less physical and social disabilities and health care use. In addition, it will decrease the rate of use of empiric broad-spectrum antibiotics (and antibiotic resistance) prescribed upon suspicion or diagnosis of a POWI. A preliminary, conservative estimation suggests yearly cost savings in the Netherlands of € 3.5 million per year.
Acknowledgements
We would like to thank M.G.W. Dijkgraaf and M.P. Merkus for their contributions to the design of the study and help in writing the study protocol.
Abbreviations
- AB
Antibiotic
- CEA
Cost-effectiveness analysis
- CUA
Cost-utility analysis
- EQ-5D
EuroQuality of Life-5D
- FAL
Foot- ankle and lower leg
- iMCQ
iMTA Medical Consumption Questionnaire
- iPCQ
MTA Productivity Cost Questionnaire
- IR
Implant removal
- Iv
Intravenous
- LEFS
Lower extremity functional Scale
- N
Number
- NA
Not available
- POWI
Postoperative wound infection
- RCT
Randomized controlled trial
- SPSS
Statistical Package for the Social Sciences
- QALY
Quality adjusted life year
- WIFI
Wound infections following implant removal
Footnotes
Competing interests
The authors declare that they have no competing interests. Funding from ZonMw (Goed Gebruik Geneesmiddelen) was received for this study. ZonMw funds health research and stimulates use of the knowledge developed to help improve health and healthcare in the Netherlands.
Authors’ contributions
All authors participated in the design of the study and the drafting of the manuscript. MB designed the study and drafted the manuscript, NWLS participated in the design of the study, helped the draft and critically revised the manuscript, JCG participated in the design of the study and critically revised the manuscript and TS designed the study, helped the draft and critically revised the manuscript. All other authors have critically revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Contributor Information
Manouk Backes, Email: m.backes@amc.nl.
Siem A Dingemans, Email: s.a.dingemans@amc.nl.
Niels WL Schep, Email: n.w.schep@amc.nl.
Frank W Bloemers, Email: fw.bloemers@vumc.nl.
Bart Van Dijkman, Email: bvdijkman@flevoziekenhuis.nl.
Frank P Garssen, Email: frgar@zha.nl.
Robert Haverlag, Email: r.haverlag@olvg.nl.
Jochem M Hoogendoorn, Email: j.hoogendoorn@mchaaglanden.nl.
Pieter Joosse, Email: p.joosse@mca.nl.
Boj Mirck, Email: bmirck@rkz.nl.
Victor Postma, Email: v.postma@mcgroep.com.
Ewan Ritchie, Email: e.ritchie@rijnland.nl.
W Herbert Roerdink, Email: roerdink@dz.nl.
Jan Bernard Sintenie, Email: sintenie@hotmail.com.
Nicolaj MR Soesman, Email: nsoesman@vlietlandziekenhuis.nl.
Nico L Sosef, Email: nsosef@spaarneziekenhuis.nl.
Bas A Twigt, Email: b.twigt@bovenij.nl.
Ruben N Van Veen, Email: r.vanveen@slaz.nl.
Alexander H Van der Veen, Email: alexander.vd.veen@catharinaziekenhuis.nl.
Romuald Van Velde, Email: rvanvelde@tergooi.nl.
Dagmar I Vos, Email: DVos@amphia.nl.
Mark R De Vries, Email: M.deVries@rdgg.nl.
Jasper Winkelhagen, Email: j.winkelhagen@westfriesgasthuis.nl.
J Carel Goslings, Email: j.c.goslings@amc.nl.
Tim Schepers, Email: t.schepers@amc.nl.
References
- 1.Williams AA, Witten DM, Duester R, Chou LB. The benefits of implant removal from the foot and ankle. J Bone Joint Surg Am. 2012;94(14):1316–20. doi: 10.2106/JBJS.J.01756. [DOI] [PubMed] [Google Scholar]
- 2.Vos DI. Implant removal after fracture healing. Facts and fiction. PhD thesis. Enschede: University Utrecht, Department of Surgery; 2013. [Google Scholar]
- 3.Vos D, Hanson B, Verhofstad M. Implant removal of osteosynthesis: The dutch practice. Results of a survey. J Trauma Manag Outcomes. 2012;6(1):6,2897. doi: 10.1186/1752-2897-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raahave D. Postoperative wound infection after implant and removal of osteosynthetic material. Acta Orthop Scand. 1976;47(1):28–35. doi: 10.3109/17453677608998968. [DOI] [PubMed] [Google Scholar]
- 5.Richards RH, Palmer JD, Clarke NM. Observations on removal of metal implants. Injury. 1992;23(1):25–8. doi: 10.1016/0020-1383(92)90120-H. [DOI] [PubMed] [Google Scholar]
- 6.Sanderson PL, Ryan W, Turner PG. Complications of metalwork removal. Injury. 1992;23(1):29–30. doi: 10.1016/0020-1383(92)90121-8. [DOI] [PubMed] [Google Scholar]
- 7.Minkowitz RB, Bhadsavle S, Walsh M, Egol KA. Removal of painful orthopaedic implants after fracture union. J Bone Joint Surg Am. 2007;89(9):1906–12. doi: 10.2106/JBJS.F.01536. [DOI] [PubMed] [Google Scholar]
- 8.Backes M, Schep NW, Luitse JS, Goslings JC, Schepers T: High rates of postoperative wound infection following elective implant removal. Under review [DOI] [PMC free article] [PubMed]
- 9.Schepers T, Van Lieshout EM, de Vries MR, Van der Elst M. Complications of syndesmotic screw removal. Foot Ankle Int. 2011;32(11):1040–4. doi: 10.3113/FAI.2011.1040. [DOI] [PubMed] [Google Scholar]
- 10.Backes M, Schep NW, Luitse JS, Goslings JC, Schepers T. Indications for implant removal following intra-articular calcaneal fractures and subsequent complications. Foot Foot Ankle Int. 2013;34(11):1521–5. doi: 10.1177/1071100713502466. [DOI] [PubMed] [Google Scholar]
- 11.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital infection control practices advisory committee. Infect Control Hosp Epidemiol. 1999;20(4):250,78. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 12.Boxma H, Broekhuizen T, Patka P, Oosting H. Randomised controlled trial of single-dose antibiotic prophylaxis in surgical treatment of closed fractures: The Dutch trauma trial. Lancet. 1996;347(9009):1133–7. doi: 10.1016/S0140-6736(96)90606-6. [DOI] [PubMed] [Google Scholar]
- 13.Rabin R, de Charro F. EQ-5D: A measure of health status from the EuroQol group. Ann Med. 2001;33(5):337–43. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 14.Binkley JM, Stratford PW, Lott SA, Riddle DL. The lower extremity functional scale (LEFS): Scale development, measurement properties, and clinical application. North american orthopaedic rehabilitation research network. Phys Ther. 1999;79(4):371–83. [PubMed] [Google Scholar]
- 15.Hoogeboom TJ, de Bie RA, den Broeder AA, van den Ende CH. The Dutch Lower Extremity Functional Scale was highly reliable, valid and responsive in individuals with hip/knee osteoarthritis: a validation study. BMC Musculoskelet Disord. 2012;13:117. doi: 10.1186/1471-2474-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pre-publication history
- The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2482/15/12/prepub


