Abstract
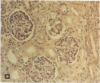
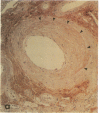
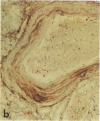
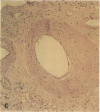
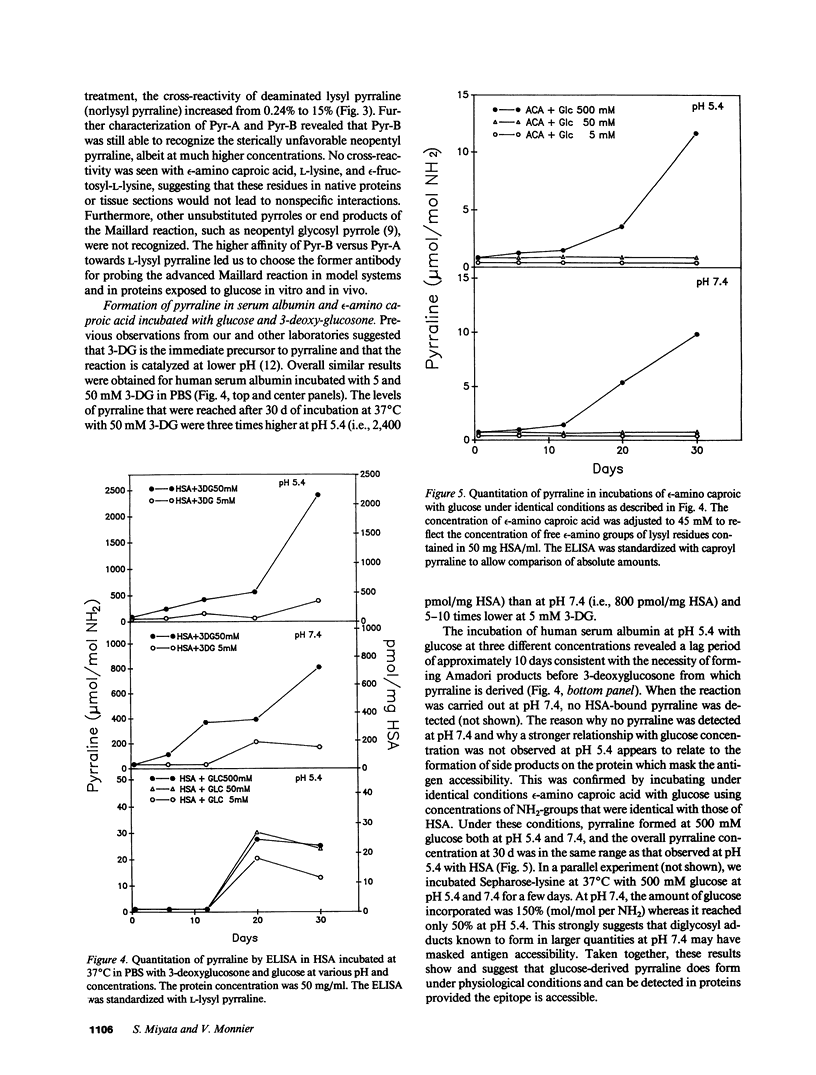
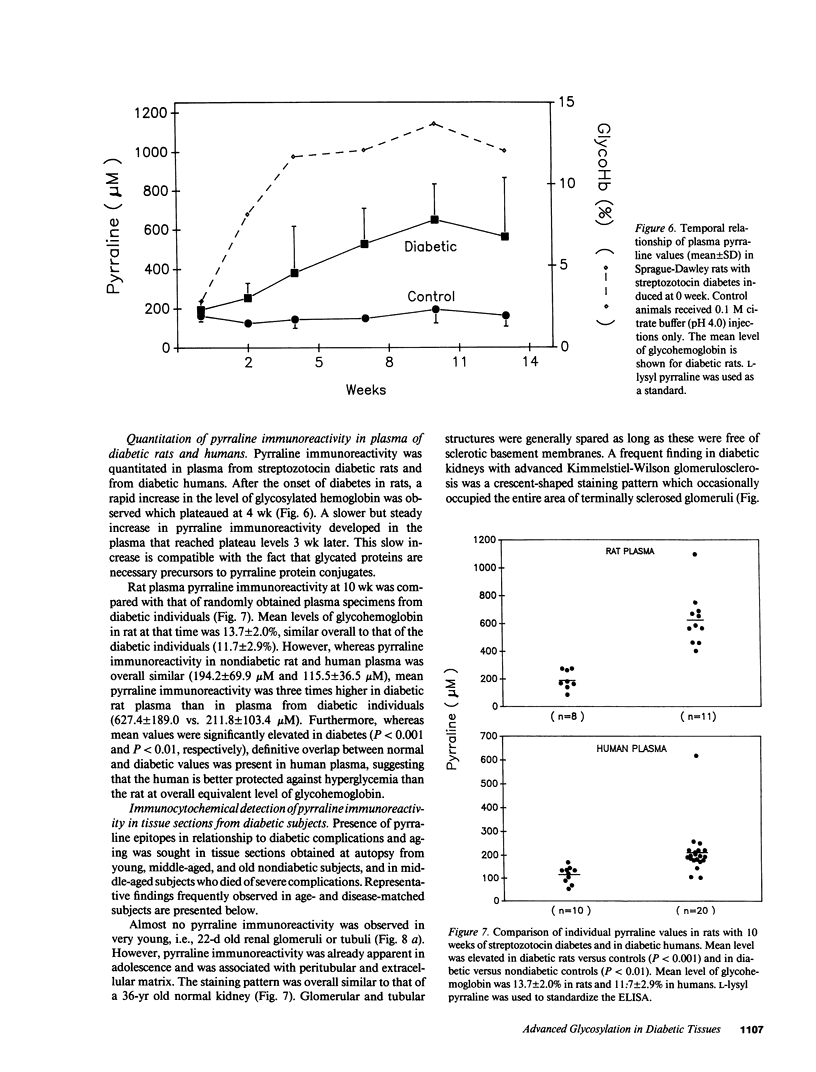
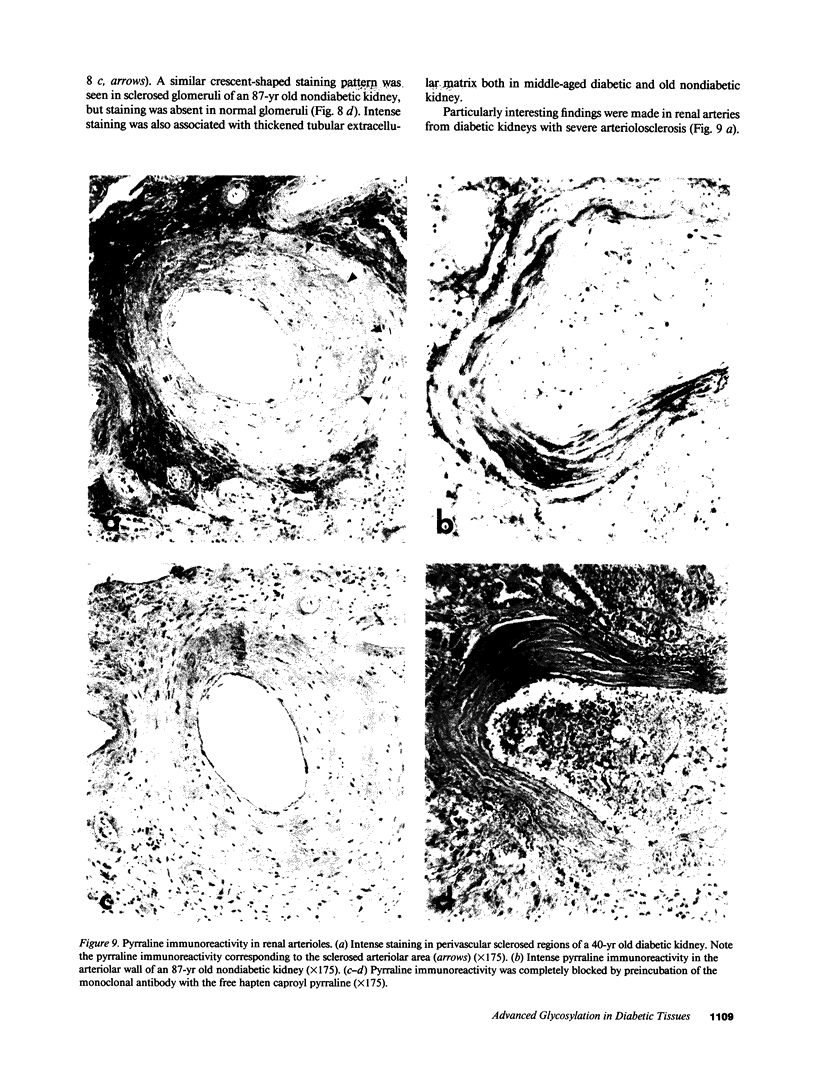
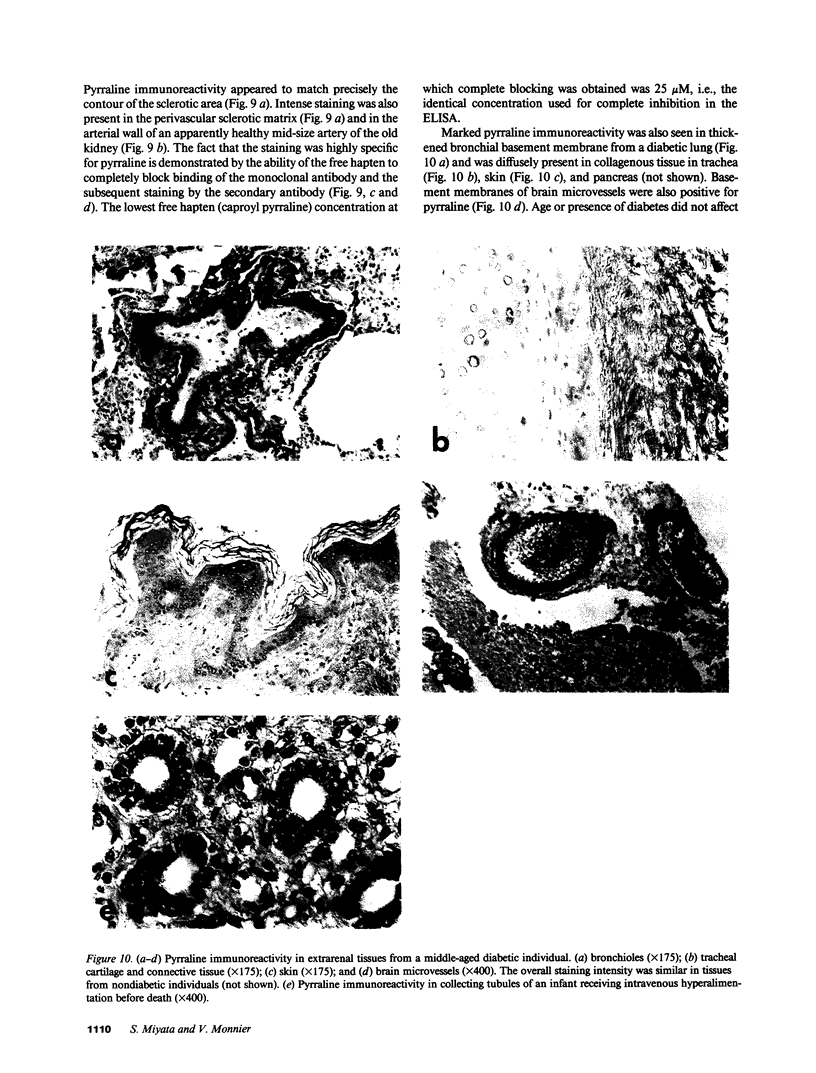
Pyrraline is one of the major Maillard compounds resulting from the reaction of glucose with amino compounds at slightly acidic pH. For in vivo studies, monoclonal pyrraline antibodies were raised after immunization of Balb/c mice with keyhole limpet hemocyamin-caproyl pyrraline conjugate. Of 660 hybridoma clones from one donor, 260 produced an antibody to the free hapten, two of which named Pyr-A and Pyr-B also cross-reacted with L-lysyl pyrraline. Using Pyr-B antibody and an ELISA, a gradual increase in pyrraline immunoreactivity was observed in serum albumin incubated with glucose or 3-deoxyglucosone. Plasma pyrraline levels increased fourfold (P less than 0.001) in Sprague-Dawley rats upon induction of diabetes with streptozotocin and were twofold increased in randomly selected plasmas from diabetic humans. Highly specific pyrraline immunoreactivity was detected in sclerosed glomeruli from diabetic and old normal kidneys as well as in renal arteries with arteriolosclerosis and in perivascular and peritubular sclerosed extracellular matrix and basement membranes. The preferential localization of pyrraline immunoreactivity in the extracellular matrix strengthens the notion that the advanced glycosylation reaction may contribute to decreased turnover and thickening of the extracellular matrix in diabetes and aging.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cagliero E., Maiello M., Boeri D., Roy S., Lorenzi M. Increased expression of basement membrane components in human endothelial cells cultured in high glucose. J Clin Invest. 1988 Aug;82(2):735–738. doi: 10.1172/JCI113655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. A., McCance D. R., Thorpe S. R., Lyons T. J., Baynes J. W. Age-dependent accumulation of N epsilon-(carboxymethyl)lysine and N epsilon-(carboxymethyl)hydroxylysine in human skin collagen. Biochemistry. 1991 Feb 5;30(5):1205–1210. doi: 10.1021/bi00219a007. [DOI] [PubMed] [Google Scholar]
- Dunn J. A., Patrick J. S., Thorpe S. R., Baynes J. W. Oxidation of glycated proteins: age-dependent accumulation of N epsilon-(carboxymethyl)lysine in lens proteins. Biochemistry. 1989 Nov 28;28(24):9464–9468. doi: 10.1021/bi00450a033. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Scheinman J. I., Mauer S. M., Michael A. F. Polyantigenic expansion of basement membrane constituents in diabetic nephropathy. Diabetes. 1983 May;32 (Suppl 2):34–39. doi: 10.2337/diab.32.2.s34. [DOI] [PubMed] [Google Scholar]
- Hayase F., Nagaraj R. H., Miyata S., Njoroge F. G., Monnier V. M. Aging of proteins: immunological detection of a glucose-derived pyrrole formed during maillard reaction in vivo. J Biol Chem. 1989 Mar 5;264(7):3758–3764. [PubMed] [Google Scholar]
- Kaetzel C. S., Mather I. H., Bruder G., Madara P. J. Characterization of a monoclonal antibody to bovine xanthine oxidase. Biochem J. 1984 May 1;219(3):917–925. doi: 10.1042/bj2190917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njoroge F. G., Sayre L. M., Monnier V. M. Detection of D-glucose-derived pyrrole compounds during Maillard reaction under physiological conditions. Carbohydr Res. 1987 Sep 15;167:211–220. doi: 10.1016/0008-6215(87)80280-x. [DOI] [PubMed] [Google Scholar]
- Oimomi M., Kitamura Y., Nishimoto S., Matsumoto S., Hatanaka H., Baba S. Age-related acceleration of glycation of tissue proteins in rats. J Gerontol. 1986 Nov;41(6):695–698. doi: 10.1093/geronj/41.6.695. [DOI] [PubMed] [Google Scholar]
- Oimomi M., Maeda Y., Hata F., Kitamura Y., Matsumoto S., Baba S., Iga T., Yamamoto M. Glycation of cataractous lens in non-diabetic senile subjects and in diabetic patients. Exp Eye Res. 1988 Mar;46(3):415–420. doi: 10.1016/s0014-4835(88)80029-0. [DOI] [PubMed] [Google Scholar]
- Roy S., Sala R., Cagliero E., Lorenzi M. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci U S A. 1990 Jan;87(1):404–408. doi: 10.1073/pnas.87.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. End-stage renal disease and diabetes catalyze the formation of a pentose-derived crosslink from aging human collagen. J Clin Invest. 1990 Feb;85(2):380–384. doi: 10.1172/JCI114449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989 Dec 25;264(36):21597–21602. [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Staros J. V., Wright R. W., Swingle D. M. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986 Jul;156(1):220–222. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]