Abstract
Midazolam is a benzodiazepine anticonvulsant with rapid onset and short duration of action. Midazolam is the current drug of choice for acute seizures and status epilepticus, including those caused by organophosphate nerve agents. The antiseizure activity of midazolam is thought to result from its allosteric potentiation of synaptic GABAA receptors in the brain. However, there are indications that benzodiazepines promote neurosteroid synthesis via the 18-kDa cholesterol transporter protein (TSPO). Therefore, we investigated the role of neurosteroids and their extrasynaptic GABAA receptor targets in the antiseizure activity of midazolam. Here, we used δ-subunit knockout (DKO) mice bearing a targeted deletion of the extrasynaptic receptors to investigate the contribution of the extrasynaptic receptors to the antiseizure activity of midazolam using the 6-Hz and hippocampus kindling seizure models. In both models, midazolam produced rapid and dose-dependent protection against seizures (ED50, 0.4 mg/kg). Moreover, the antiseizure potency of midazolam was undiminished in DKO mice compared with control mice. Pretreatment with PK11195 [1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide], a TSPO blocker, or finasteride, a 5α-reductase neurosteroid inhibitor, did not affect the antiseizure effect of midazolam. The antiseizure activity of midazolam was significantly reversed by pretreatment with flumazenil, a benzodiazepine antagonist. Plasma and brain levels of the neurosteroid allopregnanolone were not significantly greater in midazolam-treated animals. These studies therefore provide strong evidence that neurosteroids and extrasynaptic GABAA receptors are not involved in the antiseizure activity of midazolam, which mainly occurs through synaptic GABAA receptors via direct binding to benzodiazepine sites. This study reaffirms midazolam’s use for controlling acute seizures and status epilepticus.
Introduction
Midazolam is a benzodiazepine antiseizure agent with rapid onset and short duration of action (Mandrioli et al., 2008). It is administered intravenously or intramuscularly to control acute seizures and status epilepticus (Galvin and Jelinek, 1987; Silbergleit et al., 2011; Reddy, 2014). Midazolam is considered the drug of choice for persistent acute seizures and status epilepticus (SE), especially those caused by organophosphate nerve agents (McDonough et al., 2009). Due to superior pharmacokinetics, midazolam is being considered as a replacement for diazepam in the military antidote kit for nerve agents (Reddy and Reddy, 2015). The Rapid Anticonvulsant Medication Prior to Arrival Trial (RAMPART) has confirmed the antiseizure efficacy of midazolam in SE to be equivalent or noninferior to lorazepam in prehospital settings (Silbergleit et al., 2012). However, the molecular mechanisms underlying midazolam action are not well understood.
The antiseizure activity of midazolam is thought to result from its allosteric potentiation of synaptic GABAA receptors. GABAA receptors are ligand-gated chloride channels composed of five subunits from several classes. The major isoforms consist of 2α-, 2β-, and 1γ- or δ-subunits. The GABAA receptor mediates two types of inhibition, characterized as synaptic (phasic) and extrasynaptic (tonic) inhibition. Synaptic release of GABA results in the activation of low-affinity γ-containing synaptic receptors, whereas high-affinity δ-containing extrasynaptic receptors are persistently activated by the ambient GABA levels. Benzodiazepines bind to γ-containing synaptic receptors, leading to allosteric potentiation of GABA-gated hyperpolarization of the neuron (Rovira and Ben-Ari, 1993; Mihic et al., 1994; Kucken et al., 2003; Sarto-Jackson and Sieghart, 2008).
Additional mechanisms may also contribute to the pharmacological actions of midazolam. Benzodiazepines bind to other targets, including TSPO, a 19-kDa cholesterol transporter protein, which was formerly called a peripheral-type benzodiazepine receptor (Gavish et al., 1999) or mitochondrial diazepam-binding receptor (McCauley et al., 1995; Reddy and Kulkarni, 1996; Papadopoulos et al., 2006). Midazolam likely may interact with TSPO, a key protein in the biosynthesis of neurosteroids. Evidence suggests that midazolam can augment neurosteroidogenesis and, as a result, can increase the levels of endogenous neurosteroids (Matsumoto et al., 1994; So et al., 2010; Tokuda et al., 2010; Dhir and Rogawski, 2012). Allopregnanolone and related neurosteroids are powerful antiseizure agents (Reddy, 2011). However, it is unclear whether midazolam acts as a functional TSPO ligand to affect endogenous neurosteroid synthesis and produce pharmacological effects typical of neurosteroids. There is some evidence in support of functional TSPO ligand activity (Serra et al., 1999; So et al., 2010; Tokuda et al., 2010). Midazolam is shown to enhance steroidogenesis in Leydig cells (So et al., 2010) and in hippocampal neurons in brain slices (Tokuda et al., 2010). These studies used cultured cells, and steroidogenesis was only apparent at midazolam levels above 150 µM, which is 3-fold higher than levels associated with antiseizure activity. Therefore, the extent to which neurosteroids contribute to midazolam action remains unclear.
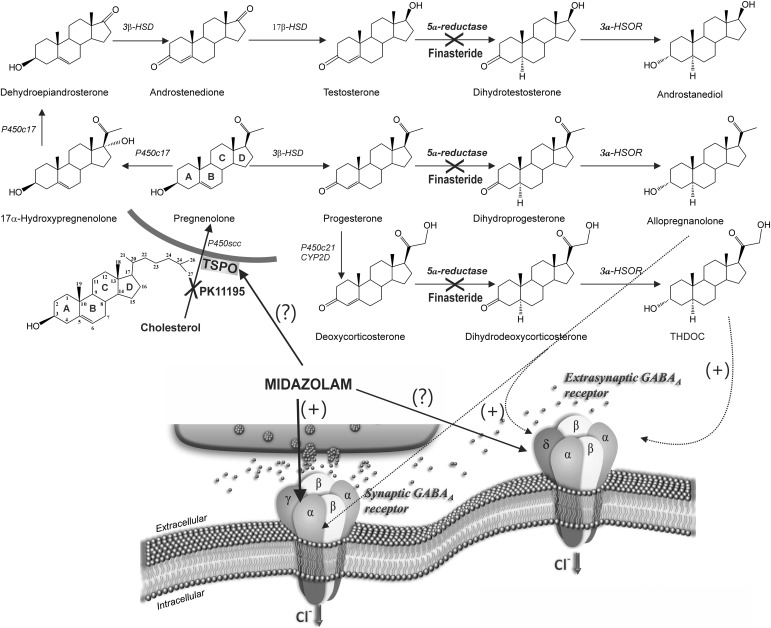
Neurosteroids are synthesized within the brain from cholesterol and steroid hormone precursors (Fig. 1). Allopregnanolone, androstanediol, and allotetrahydrodeoxycorticosterone are the prototypical endogenous neurosteroids (Carver and Reddy, 2013). The initial step in neurosteroidogenesis is the conversion of cholesterol to pregnenolone by the mitochondrial enzyme P450scc (cytochrome P450 cholesterol side-chain cleavage enzyme) (Fig. 1). Access of cholesterol to P450scc requires the steroidogenic acute regulatory protein, which functions to transfer cholesterol from the outer mitochondrial membrane to the inner membrane where P450scc is located (Stoffel-Wagner, 2003). TSPO likely functions as a complex with the steroidogenic acute regulatory protein and a voltage-dependent anion channel at the outer mitochondrial membrane (Korneyev et al., 1993; Jefcoate 2002; Papadopoulos et al., 2006; Midzak et al., 2011a,b). TSPO is highly expressed in tissue where steroidogenesis occurs, including many regions in the brain (Papadopoulos et al., 2006). Therefore, activation of TSPO by certain ligands (e.g., benzodiazepines) may facilitate increased production of neurosteroids that act on GABAA receptor targets (Kita and Furukawa, 2008; Rupprecht et al., 2009; Nothdurfter et al., 2012). Neurosteroids rapidly alter neuronal excitability through direct interaction with GABAA receptors (Hosie et al., 2007; Wu et al., 2013), thereby producing robust seizure protection (Reddy, 2011). Neurosteroids potentiate both synaptic and extrasynaptic GABAA receptors and appear to induce greater effects on extrasynaptic δ-subunit receptors that mediate tonic inhibition (Wohlfarth et al., 2002; Stell et al., 2003; Belelli et al., 2009; Brickley and Mody, 2012; Carver and Reddy, 2013; Carver et al., 2014). The net antiseizure action of midazolam could be due to a combination of its direct action on synaptic GABAA receptors along with the indirect actions mediated by neurosteroids that can activate both synaptic and extrasynaptic receptors. However, the precise role of TSPO and extrasynaptic receptors in midazolam activity remains unclear.
Fig. 1.
Neurosteroid biosynthetic pathways and potential sites of modulation by midazolam. The upper panel shows blockade sites of PK11195 and finasteride in the neurosteroid biosynthesis pathway in the brain. The prototype endogenous neurosteroids allopregnanolone (3α-hydroxy-5α-pregnane-20-one), androstanediol (5α-androstan-3α,17β-diol), and allotetrahydrodeoxy-corticosterone (THDOC) are synthesized from cholesterol and steroid hormone precursors. Activation TSPO by appropriate ligands (e.g., benzodiazepines) facilitates the intramitochondrial flux of cholesterol and thereby increases the availability of cholesterol to the cytochrome P450SCC, an enzyme located in the inner mitochondrial membrane that catalyzes cholesterol side-chain cleavage to yield pregnenolone. Pregnenolone is the first key intermediate for neurosteroid biosynthesis. PK11195 inhibits the TSPO transport protein, prohibiting cholesterol from entering the mitochondrial membrane. The steroids progesterone, deoxycorticosterone, and testosterone are further reduced into neurosteroids by the common 5α-reductase enzymatic pathway. Finasteride blocks the 5α-reductase, thereby preventing the synthesis of neurosteroids allopregnanolone, androstanediol, and THDOC. The bottom panel shows the potential GABAA receptor mechanisms for midazolam. Midazolam binds to the benzodiazepine site located on the γ-containing synaptic GABAA receptors. The extent of the drug to increase the rate of δ-extrasynaptic GABAA receptor opening remains unclear. 3α-HSOR, 3α-hydroxysteroid-oxidoreductase; HSD, hydroxysteroid dehydrogenase.
In the present study, we sought to investigate the role of endogenous neurosteroids and the extrasynaptic GABAA receptors in the antiseizure actions of midazolam using δ-subunit knockout (DKO) mice bearing a targeted null mutation of the δ-subunit gene that abrogates the expression and function of extrasynaptic GABAA receptors. Our results indicate that indirect actions mediated by neurosteroids and the extrasynaptic (δ-subunit) GABAA receptors are not involved in the antiseizure activity of midazolam. Instead, its direct activity occurs mainly at synaptic GABAA receptors.
Materials and Methods
Animals.
Adult male and female C57BL/6 mice, 25–30 g each, were used in this study. Adult GABAA receptor DKO mice were also used (Mihalek et al., 1999; Carver et al., 2014). All strains were maintained on a hybrid C57BL/6-129SV background. All mice were housed four to a cage with access to food and water ad libitum. The mice were housed in an environmentally controlled animal facility with a 12-hour light/dark cycle. The animals were cared for in strict compliance with the guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal procedures were performed in a protocol approved by the university’s Institutional Animal Care and Use Committee. Sex differences are apparent in seizure susceptibility and protective effects of many antiseizure agents (Reddy, 2014). To identify the role of neurosteroid mechanisms in the antiseizure action of midazolam, we used male mice in the 6-Hz model. We used fully kindled female mice to study the role of extrasynaptic GABAA receptors in the antiseizure action of midazolam.
6-Hz Model of Epilepsy.
To study seizure protection activity of midazolam, we used the 6-Hz electrical stimulation model, which represents a psychomotor model for intractable complex partial seizures that are highly sensitive to anticonvulsants that positively modulate GABAA receptors (Barton et al., 2001; White et al., 2002; Kaminski et al., 2004). The 6-Hz model was carried out according to a previously described protocol (Brown et al., 1953; Barton et al., 2001). Corneal stimulation (0.2-millisecond duration monopolar rectangular pulses at 6 Hz for 3 seconds) was delivered by a constant-current device (ECT Corneal Electrode system; Ugo Basile, Comerio, Italy). To find the CC99 value (stimulation current causing seizures in 99% of animals), different current intensities in the range of 5–40 mA were administered to separate groups of animals. A fixed current intensity of 32 mA (CC99 value) was used to subsequently allow direct comparison with the data obtained in the aforementioned studies. Ocular anesthetic (0.5% tetracaine) was applied to the corneas 15 minutes before stimulation, and the corneal electrodes were wetted with 0.9% saline just before stimulation. During stimulation, mice were briefly held and released into the observation cage (28 × 20 × 15 cm) immediately after current application. The following parameters were noted in each mouse: 1) brief intense motor agitation (<3 seconds), such as wild running, jumping, and cage climbing; 2) stunned posture; 3) limbic seizure activity manifested as rearing (bipedal standing), forelimb automatic movements and clonus, twitching of the vibrissae, and straub tail; and 4) unilateral or bilateral clonic seizures. The cumulative duration of the seizure activity ranged from 13 to 65 seconds in untreated animals. At the end of a seizure, animals immediately resumed their normal exploratory behavior. The occurrence of seizures and behavioral seizure severity were used as indices of seizure susceptibility. A mouse was considered to be protected if it failed to display seizures as described earlier or resume its normal exploratory behavior within 10 seconds of the start of stimulation.
Hippocampus Kindling Model of Epilepsy.
To study seizure protection activity of midazolam, we used the hippocampus kindling model, which is the best model of human temporal lobe epilepsy characterized by progressive complex partial seizures with secondary generalization (Goddard et al., 1969; Albright and Burnham, 1980). A mild focal, nonconvulsant electrical stimulus to the hippocampus on a daily basis leads to the development of a kindled state exhibiting electrographic and behavioral seizures. In mouse kindling, the focal electroencephalogram after-discharge (AD) models complex partial seizures, whereas the behavioral motor seizure stages 4/5 models generalized seizures. Electrode implantation and stimulation procedures for mouse hippocampus kindling were performed as described previously (Reddy and Mohan, 2011; Reddy et al., 2012). Mice were anesthetized by an intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). A twisted bipolar stainless steel wire electrode (model MS303/1; Plastic Products, Roanoke, VA) was stereotaxically implanted in the right hippocampus (2.9 mm posterior and 3.0 mm lateral to bregma, and 3.0 mm below the dorsal surface of the skull) using the Franklin and Paxinos atlas (Franklin and Paxinos, 1997) and anchored with dental acrylic to four jeweler’s screws placed in the skull. A period of 7–10 days was allowed for recovery. The stimulation paradigm consisted of 1-millisecond duration, bipolar, square current pulses delivered at 60 Hz for 1 second using a kindling stimulator (A-M Systems, Sequim, WA). The afterdischarge threshold was determined by stimulating at 5-minute intervals beginning with an intensity of 25 µA and increasing in steps of 25 µA until an AD of at least 5 seconds was obtained. Stimulation on subsequent days used a stimulation intensity of 125% threshold value. The AD was recorded from the hippocampus electrode with a Grass CP511 preamplifier (Astro-Med, West Warwick, RI) and stored in digital form using Axoscope 8.1 software (Axon Instruments, Foster City, CA). AD duration was the total duration of hippocampal electrographic spike activity (amplitude >2× baseline) occurring in a rhythmic pattern at a frequency of 1 Hz. The day of afterdischarge threshold determination was considered day 0 of kindling. Stimulation was continued on a 5 days/wk schedule each afternoon. Seizure activity after each stimulation was rated according to the criterion of Racine (1972) as modified for the mouse: stage 0, no response or behavior arrest; stage 1, chewing or head nodding; stage 2, chewing and head nodding; stage 3, forelimb clonus; stage 4, bilateral forelimb clonus and rearing; and stage 5, falling. Kindling stimulation was delivered daily until stage 5 seizures were elicited on 3 consecutive days. Mice were used for drug testing when they consistently exhibited stage 5 seizures after stimulation, which is considered the “fully kindled” state.
Determination of Plasma and Brain Neurosteroid Levels.
To determine plasma and brain neurosteroid levels attained at midazolam doses that protect against seizures, allopregnanolone concentrations were determined 15 minutes after administration of 2.5× ED50 doses of midazolam (1 mg/kg i.p.) in mice. Animals were anesthetized with an injection of ketamine (100 mg/kg)-xylazine (10 mg/kg) solution and ∼0.5 ml of carotid blood was collected in heparinized tubes. The plasma was separated by centrifugation at 12,000g for 10 minutes and stored at −20°C. Brains were rapidly dissected, and cortex and hippocampus samples were isolated by microdissection under a microscope. Brain tissue samples were homogenized in Triton-phosphate buffer solution. The concentration of allopregnanolone was analyzed by liquid chromatography–mass spectrometry as previously described (Reddy et al., 2004). Plasma and brain levels of allopregnanolone were estimated using 3β-methyl-allopregnanolone as internal standard. The steroid and internal standard were extracted with 0.9 ml of hexane. Each sample was analyzed using the atmospheric pressure chemical ionization technique under acidic conditions in a Triple Quad system (Applied Biosystems, Foster City, CA) under the multiple reaction monitoring detection modes.
Test Drugs and Treatment Protocols.
Midazolam (Akorn, Inc., Lake Forest, IL) was diluted in sterile saline. Finasteride (Steraloids Inc., Newport, RI) and other drugs for injection were made in 20% β-cyclodextrin in saline. Drug solutions were administered subcutaneously or intraperitoneally in a volume equaling 1% of the animal’s body weight. To examine the ability of midazolam to suppress seizure activity, the drug was administered 15 minutes prior to 6-Hz stimulation in naïve mice or kindling stimulations in fully kindled mice. Midazolam is a benzodiazepine site agonist at synaptic receptors containing the α1/2/3/5-subunits in combination with any of the β-subunits and the γ2-subunit (Whiting et al., 2000; Mohler et al., 2002; Kucken et al., 2003). Three different inhibitors, PK11195 [1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide], finasteride, and flumazenil, were selected for pharmacological intervention of midazolam response in both 6-Hz and kindling models (Fig. 1). PK11195 is a specific TSPO ligand used to verify TSPO-mediated neurosteroid production (Auta et al., 1993; Reddy and Kulkarni, 1996; Kita et al., 2004; Rupprecht et al., 2009). Finasteride is a 5α-reductase inhibitor that blocks the bioconversion of dihydroprogesterone into allopregnanolone and related precursors into neurosteroids (see Fig. 1). Finasteride blocks the synthesis of neurosteroids such as allopregnanolone and related 5α-reduced pregnane and androstane analogs that modulate GABAA receptor function (Finn et al., 2006; Mukai et al., 2008; Gangisetty and Reddy, 2010). Flumazenil is a benzodiazepine site antagonist at GABAA receptors and is used to reverse benzodiazepine overdose (Haefely, 1988; Hoffman and Warren, 1993; Khan et al., 2000).
Data Analysis.
Group data are expressed as the mean ± S.E.M. To construct dose-response curves, midazolam and test drugs were tested at several doses spanning the dose producing 50% protection (ED50). ED50 values and their corresponding 95% confidence limits (CLs) were determined by log-probit analysis using the Litchfield and Wilcoxon method (PHARM/PCS version 4.2; Micro-Computer Specialists, Philadelphia, PA). Differences in kindling seizure stages between groups were compared with the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U test. Comparison of means of the AD duration between groups was made with a one-way analysis of variance, followed by an unpaired two-tailed Student’s t test. Comparison of the mean percentage inhibition of seizure stage and AD duration in fully kindled animals was made by Wilcoxon signed rank-test and paired two-tailed Student’s t test, respectively. ED50 values of test drugs in the kindling model were determined by nonlinear curve fitting using the Levenberg-Marquardt algorithm to a logistic equation where the maximum inhibition was assumed to be 100%. In all statistical tests, the criterion for statistical significance was P < 0.05.
Results
Seizure Expression in 6-Hz Model in Mice.
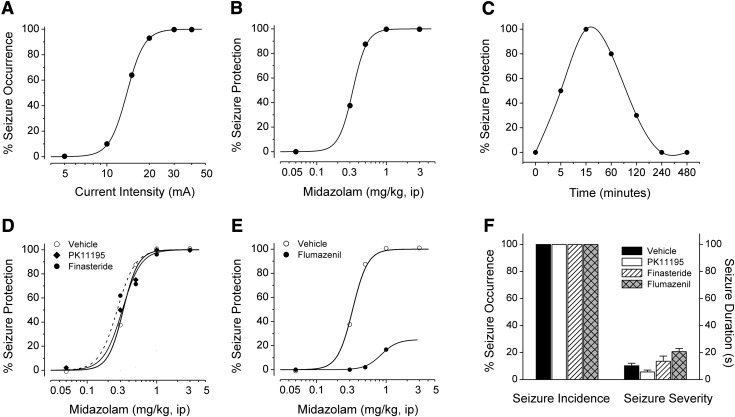
We used the 6-Hz test to assess the antiseizure effect of midazolam. In control or naïve mice, corneal stimulation with currents in the range of 5–40 mA resulted in a current-dependent increase in the fraction of mice reaching the designated seizure endpoint (Fig. 2A). The median convulsant current value (CC50), the current to produce seizure occurrence in 50% of the animals, was 10 mA (95% CL, 9–12 mA). The CC99 value is 32 mA. Thus, the pharmacological studies were carried out with 32-mA stimulation, which is 3.1 times the CC50 value and always resulted in seizure behavior.
Fig. 2.
Antiseizure activity of midazolam in the 6-Hz model in mice: influence of neurosteroidogenesis blockers PK11195 and finasteride, and the benzodiazepine antagonist flumazenil. (A) Current–seizure occurrence curve at various current intensities in 6-Hz stimulation model. The EC99 value of 32 mA was derived from this curve. (B) Dose-response relationship for protective activity of midazolam in the 6-Hz seizure test. (C) Time course for protection by midazolam in the 6-Hz seizure test. Midazolam was administered 15 minutes before electrical stimulation. Points signify percentage of animals protected from seizures within a group of six to eight at a given dose or time. (D) Effect of neurosteroidogenic inhibitors PK11195 and finasteride on the protective effect of midazolam in the 6-Hz seizure test. PK11195 (15 mg/kg) and finasteride (50 mg/kg) were administered intraperitoneally 30 and 60 minutes prior to midazolam injection, respectively. Points signify percentage of animals without seizures within groups of six to eight. ED50 values are listed in Table 1. (E) Effect of benzodiazepine antagonist flumazenil on the antiseizure effect of midazolam in the 6-Hz seizure test. Flumazenil (10 mg/kg) was given intraperitoneally 30 minutes prior to midazolam injection. Points signify percentage of animals without seizures within groups of six to eight. Curves are arbitrary fits to the data. ED50 values are listed in Table 1. (F) Effect of test inhibitors alone on seizure susceptibility in the 6-Hz test. Percent seizure occurrence considers visible symptoms of any length or magnitude as a full seizure. Seizure duration measures mean seizure length ± S.E.M. of data from six to eight animals.
Antiseizure Activity of Midazolam in the 6-Hz Seizure Model.
Pretreatment with midazolam produced dose-dependent protection in the 6-Hz test (Fig. 2B). The ED50 value derived from the experiment in Fig. 2B is presented in Table 1. The estimated ED50 value for reduction of seizures is 0.4 mg/kg. We additionally assessed the time course of action of midazolam in the 6-Hz model using a 2.5× ED50 dose. As shown in Fig. 2C, midazolam (1 mg/kg i.p.) showed a maximal protective effect within 15 minutes of injection, and significant protection up to 2 hours; minimal protection was evident at 4 hours. Midazolam at doses of 3 mg/kg and higher produced significant motor impairment. The pharmacological inhibitor studies were carried out with a dose of 1 mg/kg, which did not cause motor impairment, but always resulted in 100% seizure protection in the 6-Hz model.
TABLE 1.
Antiseizure ED50 values (milligrams per kilogram) of midazolam in the 6-Hz and kindling models of epilepsy in WT and DKO mice
Numbers in parentheses are 95% confidence intervals.
| Drug | 6-Hz Model WT Mice | Kindling Model WT Mice | Kindling Model DKO Mice |
|---|---|---|---|
| Midazolam | 0.40 (0.11–0.69) | 0.55 (0.36–0.91) | 0.6 (0.4–0.97) |
| Midazolam+PK11195 | 0.36 (0.21–0.52) | ND | ND |
| Midazolam+finasteride | 0.36 (0.17–0.55) | ND | ND |
| Midazolam+flumazenil | >2.0 (ND)* | ND | ND |
ND, not determined (blockers were tested by time-course profiles; see Fig.5).
P < 0.01 versus midazolam alone.
Antiseizure Activity of Midazolam in the 6-Hz Model Occurs via a Neurosteroid-Independent Pathway.
There are two major mechanisms by which midazolam can possibly produce seizure protection: 1) direct potentiation of synaptic GABAA receptor–mediated inhibition, and 2) indirect potentiation via interaction with TSPO cholesterol transporter and elevation of neurosteroid biosynthesis (Fig. 1). To directly test the TSPO-linked neurosteroid pathway, the antiseizure activity of midazolam was examined in the presence of PK11195, a functional TSPO inhibitor (Le Fur et al., 1983; Auta et al., 1993; Romeo et al., 1993; Bitran et al., 2000; Kita et al., 2004). Pretreatment with PK11195 (15 mg/kg i.p.) produced little or no effect on the antiseizure activity of midazolam, as reflected by the similar dose-protection curves (Fig. 2D). The ED50 values are listed in Table 1. The potency ratio of PK11195+midazolam (ED50, 0.36; CL, 0.2–0.52 mg/kg) versus midazolam alone (ED50, 0.4; CL, 0.11–0.69 mg/kg) was not significant as calculated by the Litchfield and Wilcoxon χ2 test (P > 0.05). To further test whether 5α-reduced neurosteroid synthesis is involved in the antiseizure activity of midazolam, we used the 5α-reductase inhibitor and neurosteroid synthesis inhibitor finasteride (Finn et al., 2006). Pretreatment with finasteride (50 mg/kg i.p.) did not affect the antiseizure activity of midazolam, demonstrated by dose-protection curves similar to the control (Fig. 2D). The potency ratio of finasteride+midazolam (ED50, 0.36; CL, 0.17–0.55 mg/kg) versus midazolam (ED50, 0.4; CL, 0.11–0.69 mg/kg) was also not significant as per the Litchfield and Wilcoxon χ2 analysis. By themselves, PK11195 (15 mg/kg i.p.) and finasteride (50 mg/kg i.p.) did not affect the seizure occurrence and seizure severity in the 6-Hz test (Fig. 2F). These results indicate that midazolam does not appear to protect against 6-Hz seizures through the TSPO-linked neurosteroid pathway.
Benzodiazepine Antagonist Flumazenil Inhibits the Antiseizure Activity of Midazolam in the 6-Hz Model.
To provide direct evidence for the involvement of synaptic GABAA receptors in the antiseizure activity of midazolam, the benzodiazepine site antagonist flumazenil, which competitively blocks midazolam binding to γ2-containing synaptic GABAA receptors (Haefely, 1988; Hoffman and Warren, 1993; Khan et al., 2000; Mohler et al., 2002; Kucken et al., 2003), was used as a definitive pharmacological blocker. Pretreatment with flumazenil (10 mg/kg i.p.) significantly reduced the antiseizure effect of midazolam, reflected by reduced maximal protective responses, even at higher doses of midazolam (Fig. 2E). Flumazenil caused ∼70% inhibition of maximal protection of 1 mg/kg diazepam. The potency ratio of flumazenil+midazolam (ED50, <3 mg/kg) versus midazolam (ED50, 0.4; CL, 0.11–0.69 mg/kg) differs significantly as calculated by the Litchfield and Wilcoxon χ2 test (P < 0.05). Flumazenil alone (10 mg/kg i.p.) did not significantly affect the seizure occurrence or seizure severity in the 6-Hz test (Fig. 2F). These results indicate that midazolam protects against 6-Hz seizures via synaptic GABAA receptors by binding to specific benzodiazepine sites on the GABAA receptor channel complex.
Antiseizure Activity of Midazolam in the Hippocampus Kindling Model in DKO Mice.
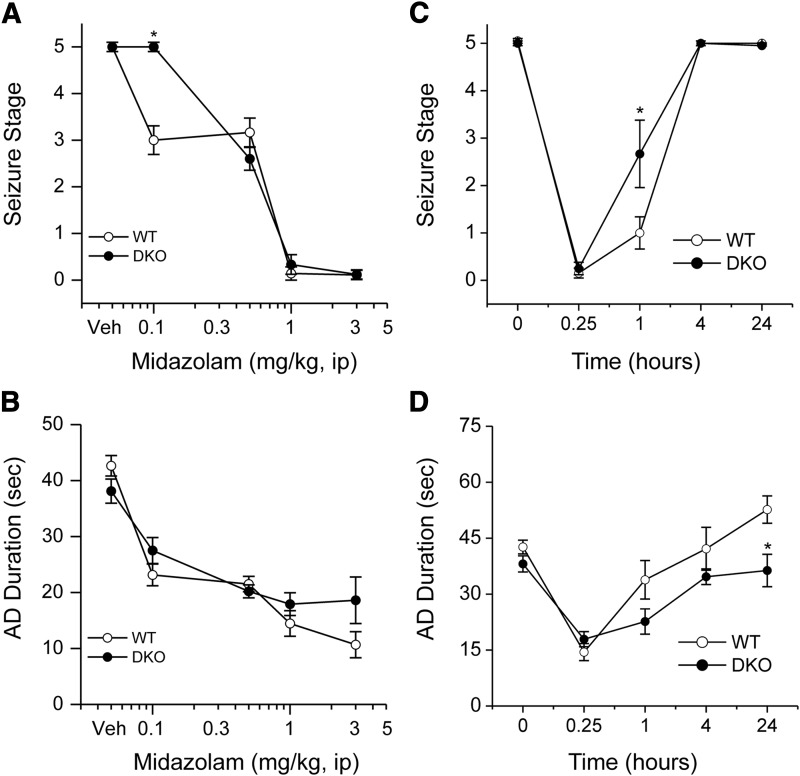
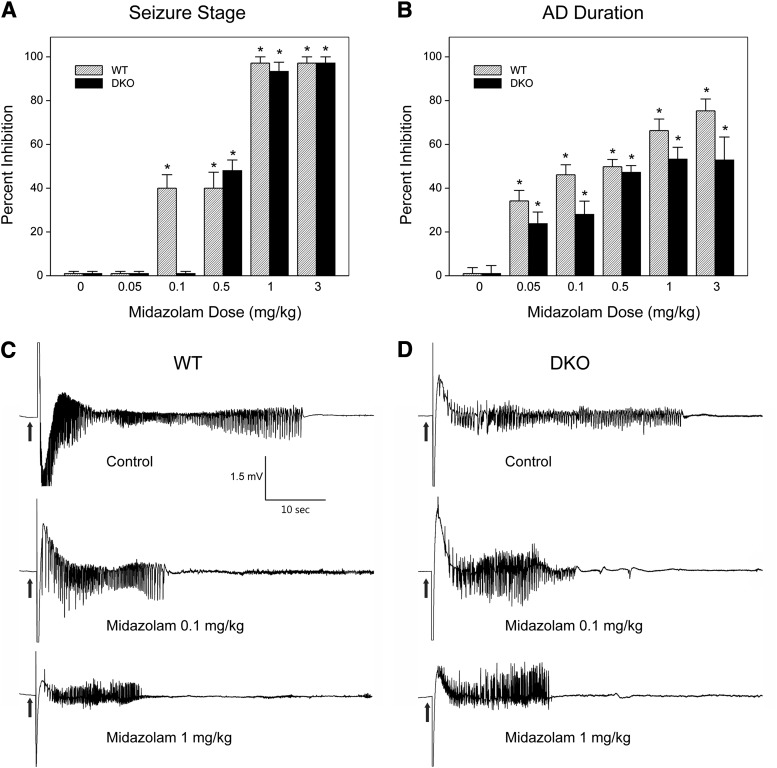
To determine the role of extrasynaptic GABAA receptors, which are also the main target of neurosteroids, in the antiseizure activity of midazolam, we used DKO mice which lack extrasynaptic receptors in the brain. Benzodiazepines with GABAA receptor modulatory activity have protective effects in the kindling model of epilepsy (Cleton et al., 1999; Reddy and Rogawski, 2010). Therefore, we selected the kindling model for characterization of the antiseizure activity of midazolam. To evaluate the systemic activity of midazolam in protecting against kindled seizures, we tested midazolam in fully kindled wild-type (WT) and DKO mice. Mice were subjected to once-daily kindling via an implanted electrode in the dentate gyrus region at 125% AD threshold until they exhibited stage 5 seizures for 3 consecutive days, which is considered the “fully kindled” state. Mice reached the fully kindled state with consistent stage 5 seizures after 12–16 stimulations. Daily kindling stimulation was associated with a steady progression of behavioral seizures and AD duration, which remained consistent once they reached the fully kindled state. The overall mean values of the initial AD threshold in WT and DKOs were 50 ± 6 μA (n = 13) and 37 ± 4 μA (n = 11), respectively. Three parameters were assessed in fully kindled mice as indices of midazolam efficacy: 1) stimulation-induced electrographic AD duration, 2) behavioral seizure intensity measured as per the Racine scale, and 3) duration of generalized seizures. As shown in Fig. 3, midazolam produced a dose-dependent suppression of behavioral seizure activity (Fig. 3A) and AD duration (Fig. 3B), with significant effects at 0.1-, 0.5-, and 1-mg/kg doses in WT mice. At the highest dose tested, behavioral seizures were nearly completely suppressed. The estimated ED50 values for suppression of seizure stage and AD duration were 0.55 and 0.76 mg/kg, respectively. The antiseizure potency of midazolam was undiminished in DKO mice compared with WT mice (Fig. 3, A and B). Although there was a modest difference in behavioral response at a lower dose (0.1 mg/kg) in DKO mice, the overall dose-response profiles of midazolam were similar between WT and DKO mice (Fig. 3, A and B). There was no significant difference in the ED50 value of midazolam in WT and DKO mice (Table 1). The percent inhibition of seizures and AD duration was also similar between the genotypes (Fig. 4, A and B), except for a modest inhibition of seizures in the WT group at the 0.1-mg/kg dose. The electrographic events are illustrated in Fig. 4. Midazolam pretreatment markedly reduced the AD duration in a dose-dependent manner in fully kindled DKO mice. There were no significant differences in the overall amplitude or extent of AD duration between midazolam-treated WT and DKO mice. Thus, these studies provide strong evidence that the extrasynaptic GABAA receptors are not required for the antiseizure effects of midazolam, which may occur through its binding to the synaptic receptors.
Fig. 3.
Inhibition of hippocampus kindled seizures by midazolam in a dose-dependent (A and B) and time-course fashion (C and D) in fully kindled WT and DKO mice. Dose-response curves for behavioral seizure stage (A) and AD duration (B) with varying doses of midazolam (0.1–3 mg/kg i.p.). Time-course curves for behavioral seizure stage (C) and AD duration (D) at various time points after midazolam (1 mg/kg i.p.). Mice that were fully kindled as described in Materials and Methods were injected intraperitoneally with vehicle or midazolam 15 minutes before stimulation. Each point represents the mean ± S.E.M. of data from six to nine animals. *P < 0.05 versus WT control. Veh, vehicle.
Fig. 4.
Antiseizure activity of midazolam in fully kindled WT and DKO mice. (A and B) Percent inhibition of fully kindled seizures and AD duration in WT (A) and DKO mice (B) by midazolam. Percent inhibition of seizure stage was calculated as 100 × (1 − S/5), where S is the seizure stage following drug treatment. Percent inhibition of AD was calculated as 100 × (1 − D/Dc), where D is the AD duration after drug treatment and Dc is the average control AD duration without any drug treatment. The overall mean control AD duration in WT and DKO was 43 ± 2.0 and 38 ± 2.1 seconds, respectively. Each bar represents the mean ± S.E.M. of values from six to eight animals. *P < 0.05 versus control. (C and D) Representative traces illustrating inhibition of AD in a fully kindled WT (C) and DKO (D) mouse by midazolam. Traces show depth of electroencephalogram recordings from a stimulating electrode in the hippocampus. Arrows indicate onset of the 1-second kindling stimulus, which is followed by the stimulus artifact. Midazolam was administered intraperitoneally 15 minutes prior to the stimulation; control trace was obtained without drug treatment.
Time Course for Seizure Protection by Midazolam in Fully Kindled DKO Mice.
The time courses for seizure protection after a 2× ED50 dose of midazolam are shown in Fig. 3, C and D. Midazolam (1 mg/kg i.p.) exhibited a rapid onset of seizure protection. In WT and DKO mice, the protection was maximal at 15 minutes and steadily diminished during the 240-minute period after the injection, as evident by its time-dependent decrease in behavioral seizures (Fig. 3C) and AD duration (Fig. 3D). On the day after midazolam treatment, all DKO mice exhibited full-blown stage 5 seizures with normal AD duration, indicating that midazolam treatment suppresses the expression of kindled seizures but does not alter the kindled state. These results are consistent with the possibility that midazolam protection is due to synaptic GABAA receptor-mediated inhibition that occurs rapidly within a few minutes.
Antiseizure Activity of Midazolam in the Hippocampus Kindling Model Occurs via a Neurosteroid-Independent Pathway in DKO Mice.
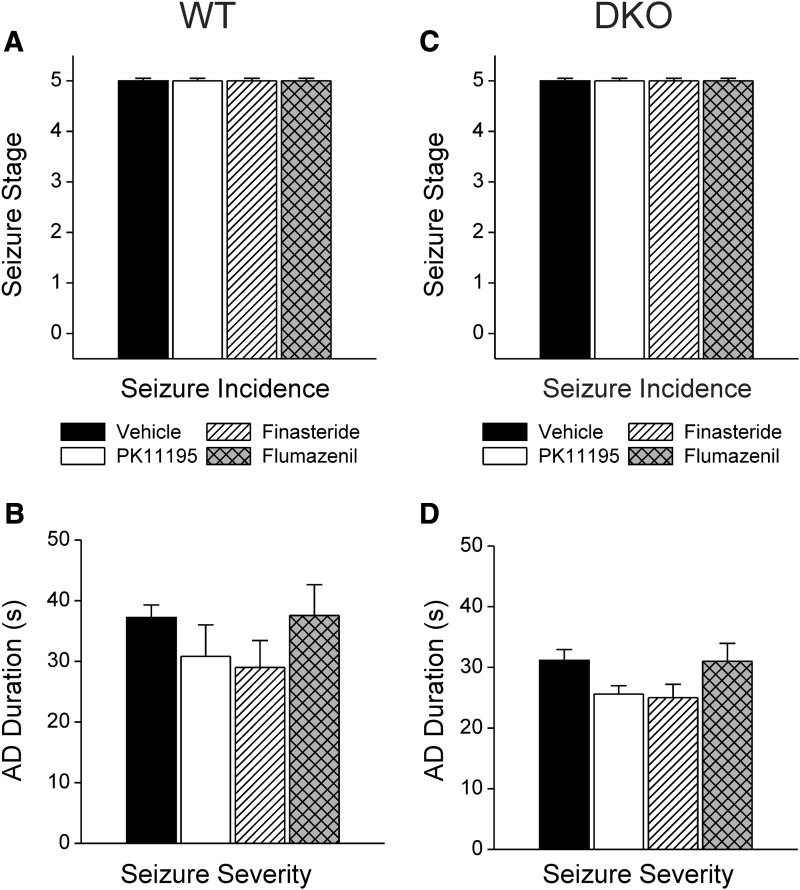
As in the 6-Hz model, we assessed the two potential mechanisms by which midazolam can suppress seizures in the kindling model in DKO mice: direct potentiation of synaptic GABAA receptors and TSPO-mediated neurosteroid biosynthesis. We examined the effects of midazolam in fully kindled WT and DKO mice with and without pretreatments of PK11195, a functional TSPO antagonist, and finasteride, a 5α-reductase inhibitor known to block the synthesis of allopregnanolone and related 5α-reduced neurosteroids. Kindled mice were treated with midazolam (1 mg/kg i.p.) with or without PK11195 pretreatment (15 mg/kg i.p.) and finasteride (50 mg/kg i.p.), and the time course for seizure protection following kindling stimulations was determined in WT (Fig. 5, A and B) and DKO (Fig. 5, C and D) mice. Pretreatment with PK11195 did not influence the protective effects of midazolam, evident in the similar time-protection curves for behavioral seizure stage (Fig. 5, A and C) and AD duration (Fig. 5, B and D) between genotypes. Pretreatment with finasteride did not diminish the antiseizure effects of midazolam in DKO mice (Fig. 5, C and D) compared with control WT mice (Fig. 5, A and B). In both genotypes, PK11195 and finasteride alone had no significant effect on kindling seizure expression (Fig. 6, A and C) or AD duration (Fig. 6, B and D). Together, these results suggest that midazolam protection against kindling seizures is not mediated through TSPO-linked production of 5α-reduced neurosteroids.
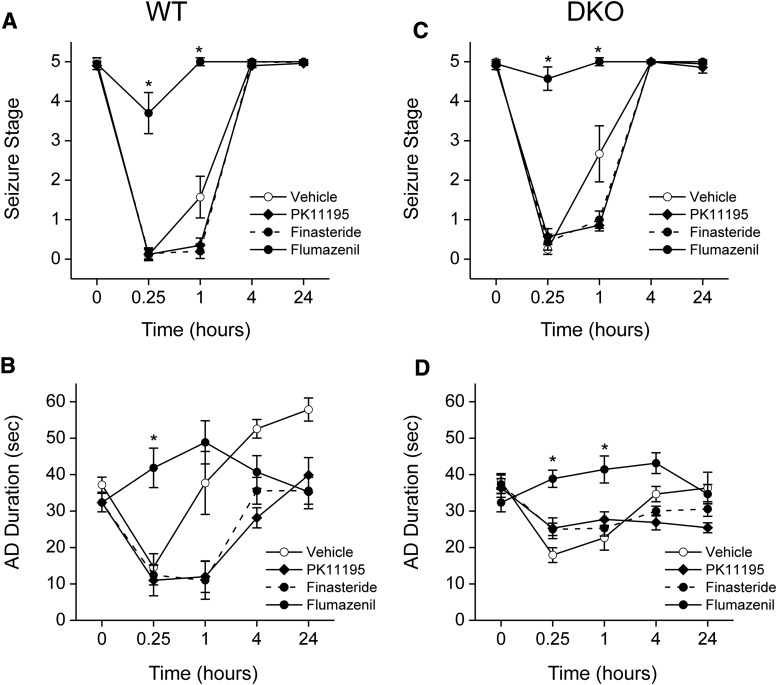
Fig. 5.
Effect of neurosteroid synthesis inhibitors and flumazenil on the antiseizure activity of midazolam in fully kindled WT and DKO mice. The extent of inhibition of midazolam-induced time-dependent protection of seizure activity by PK11195, finasteride, and flumazenil in WT (A and B) and DKO (C and D) mice. Test inhibitors PK11195 (15 mg/kg), finasteride (50 mg/kg), and flumazenil (10 mg/kg) were administered intraperitoneally 30 or 60 minutes prior to midazolam injection. The interval between midazolam injection and the kindling stimulus is plotted on the abscissa, and the seizure stage of animals is plotted on the ordinate. Each point represents the mean ± S.E.M. of data from six to nine animals. *P < 0.05 versus vehicle or midazolam control group.
Fig. 6.
Effect of pharmacological inhibitors PK11195, finasteride, and flumazenil alone on behavioral seizure stage (A and B) and electrographic AD duration (C and D) in fully kindled WT and DKO mice. Test inhibitors PK11195 (15 mg/kg), finasteride (50 mg/kg), and flumazenil (10 mg/kg) were administered intraperitoneally without midazolam 30 or 60 minutes prior to stimulation. Each point represents the mean ± S.E.M. of data from six to nine animals.
Flumazenil Inhibits the Antiseizure Activity of Midazolam in the Hippocampus Kindling Model in DKO Mice.
To confirm whether the protective effects of midazolam against kindled seizures in DKO mice occur via the drug’s ability to bind to the benzodiazepine site of synaptic GABAA receptors, pharmacological studies were conducted with the benzodiazepine site antagonist flumazenil. In kindled DKO mice, flumazenil (10 mg/kg i.p.) alone did not affect the behavioral seizures (Fig. 6C) or AD duration (Fig. 6D) compared with the control WT group (Fig. 6, A and B). Similar to WT mice, DKO animals pretreated with flumazenil displayed a significant (P < 0.01), time-dependent blockade of midazolam’s antiseizure effects and a greater AD duration, leading to drastically worsened seizure protection. Together, these results indicate that midazolam’s seizure protection results from synaptic GABAA receptor inhibition via specific, flumazenil-sensitive benzodiazepine sites. These results are consistent with earlier reports of flumazenil-sensitive antiseizure action of diazepam in the kindling model (Gangisetty and Reddy, 2010). Similar to midazolam, the protective effect of diazepam (1 mg/kg i.p.) against kindling behavioral seizure stage (0.16 ± 0.12) and electrographic AD duration (8 ± 2 seconds) was significantly reduced by pretreatment with flumazenil (seizure stage, 4.5 ± 0.34; AD duration, 43 ± 7 seconds).
Effect of Midazolam on Neurosteroid Levels in Plasma and Brain.
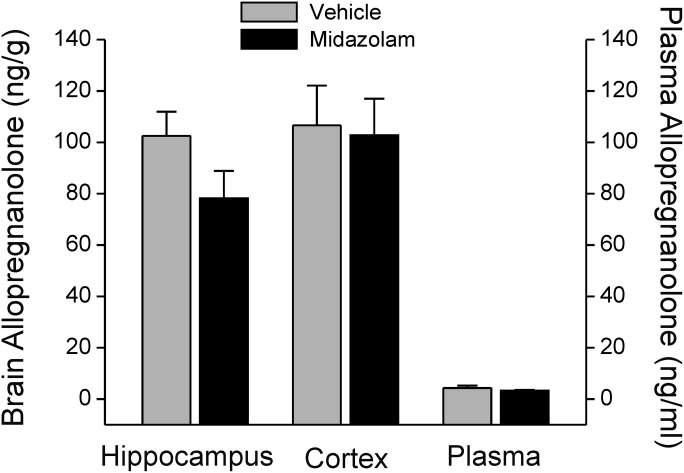
To provide direct support for the lack of involvement of neurosteroids in the antiseizure action of midazolam, plasma and brain levels of the neurosteroid allopregnanolone were determined by liquid chromatography–mass spectrometry in animals pretreated with midazolam. Allopregnanolone levels are commonly measured by mass spectrometry assay (Reddy et al., 2004; Porcu et al., 2009; Snelling et al., 2014). Allopregnanolone levels were determined 30 minutes after injection with an antiseizure dose of midazolam (1 mg/kg i.p.) in mice. As shown in Fig. 7, midazolam treatment was not associated with a significant increase in plasma allopregnanolone levels (4.3 ± 0.96 ng/ml) compared with the control (3.3 ± 0.34 ng/ml). Allopregnanolone levels in the hippocampus were not significantly higher in the midazolam-treated group (102 ± 10 ng/g) relative to the untreated control group (79 ± 11 ng/g). There were no significant differences in the cortex allopregnanolone levels achieved with an anticonvulsant dose of midazolam in mice (Fig. 7). Thus, antiseizure doses of midazolam are not associated with significant change in plasma and brain neurosteroid levels.
Fig. 7.
Allopregnanolone levels in midazolam-treated mice. Brain and plasma levels of allopregnanolone were measured using liquid chromatography–mass spectrometry assay. Plasma and brain samples were collected 30 minutes after midazolam (1 mg/kg i.p.) injection. Vehicle signifies drug-free animals. Values represent mean concentrations ± S.E.M. of data from six to eight animals.
Discussion
The principal findings of the study are that neurosteroid synthesis is not greatly affected by midazolam, and the drug’s antiseizure activity was unaffected by the neurosteroid synthesis inhibitors PK11195 and finasteride. This is shown for the first time using two distinct seizure models: the 6-Hz test triggering generalized psychomotor seizures, and hippocampus kindling specifically eliciting limbic seizures. In the latter approach, we demonstrated that the antiseizure activity of midazolam is undiminished in DKO mice, which lack extrasynaptic forms of the GABAA receptors, thus definitively proving that the δ-containing extrasynaptic GABAA receptors are not required for the antiseizure activity of midazolam. Moreover, the seizure protection of midazolam was eliminated by pretreatment of animals with the benzodiazepine site antagonist flumazenil. Taken together, these results indicate that the antiseizure action of midazolam occurs through a neurosteroid-independent, nonextrasynaptic receptor pathway, and is mediated by the drug’s direct action on the benzodiazepine site of synaptic GABAA receptors. However, there are certain aspects that must be considered regarding the interpretation of the findings. Since the relationship between animal seizure models and human epilepsy or SE is not well defined, it cannot be directly concluded that extrasynaptic receptors do not participate in midazolam’s ability to control seizures in the clinical setting. Neurosteroids are powerful endogenous antiseizure agents, most likely due to their direct ability to potentiate GABA neurotransmission in the brain via GABAA receptors. This study also cannot rule out the neurosteroid involvement in the etiology of seizures in humans as it was intended to unravel the extent of such mechanisms in the antiseizure actions of midazolam. Neurosteroids are more relevant in seizures related to hormonal aspects such as catamenial epilepsy and stress (Reddy, 2014).
In the present study, midazolam displayed dose-dependent protection against seizures in both 6-Hz and kindling models; the potency of the benzodiazepine in the 6-Hz test and kindling model is similar to, and largely corresponds with, its potencies as a GABAA receptor modulator. In the 6-Hz model, pretreatment with PK11195 or finasteride (ED50, 0.36 mg/kg each) did not elicit any significant change in seizure protection compared with midazolam alone (ED50, 0.4 mg/kg). Animals pretreated with flumazenil, however, displayed a dose-dependent blockade of midazolam’s anticonvulsant effects, leading to drastically worsened seizure protection (ED50, >3 mg/kg). When given alone, the inhibitors did not affect seizures. The data from the kindling model displayed a similar trend, showing a negligible difference in the antiseizure responses between the control midazolam group and groups pretreated with PK11195 or finasteride. However, flumazenil pretreatment significantly inhibited the antiseizure effect of midazolam. Inhibitors alone also yielded no protection in the kindling model. Additionally, plasma and brain levels of allopregnanolone were similar between vehicle and midazolam groups. These results demonstrate a lack of enhanced endogenous neurosteroid synthesis, mediated by either a TSPO or 5α-reductase pathway, following antiseizure doses of midazolam. The extrasynaptic δ-containing GABAA receptors do not contribute to the antiseizure action of midazolam, which is possibly mediated by its direct binding to benzodiazepine sites on the γ-containing postsynaptic GABAA receptors.
Our data suggest that there is little difference in the antiseizure response of midazolam between WT and DKO, and that δ-subunit extrasynaptic receptors are not major contributors to the antiseizure effects of midazolam. However, antiseizure effects of midazolam are slightly reduced in DKO mice at few low doses. This subtle difference may not account for the particularly effective antiseizure action of midazolam in DKO mice. Such minor differences could be due to compensational or other developmental limitations apparent in transgenic mice, such as the DKO mouse model that exhibits subunit plasticity due to germline deletion of the δ-subunit (Spigelman et al., 2002).
Synaptic GABAA receptors mediate the antiseizure activity of benzodiazepines. GABAA receptors are pentameric in structure, with five subunits that form a central Cl− ion channel (Fig. 1). There are 19 subunits (α1–6, β1–3, γ1–3, δ, ε, θ, ρ1–2). Each subunit has four transmembrane segments, with both the amino and carboxy terminals located extracellularly. These extracellular domains form the primary recognition sites for GABA and allosteric recognition sites for benzodiazepines and neurosteroids (Hosie et al., 2007; Carver and Reddy, 2013). Subunit composition and receptor location determine sensitivity to benzodiazepines. A typical benzodiazepine-sensitive, postsynaptic GABAA receptor consists of two α1-, 2-, 3-, or 5-subunits, two β2- or β3-subunits (or one each), and a γ2-subunit (Mihic et al., 1994; Kucken et al., 2003). Midazolam binds to these receptors and acts as a positive allosteric modulator by augmenting the inhibition in neurons. The benzodiazepine-sensitive receptors mediate phasic inhibition through the generation of fast, rapidly desensitizing inhibitory postsynaptic currents in neurons. However, benzodiazepines do not bind to extrasynaptic GABAA receptors that mediate tonic inhibition. These extrasynaptic receptors contain the δ-subunit, rather than the γ-subunit characteristic of synaptic GABAA receptors (Farrant and Nusser, 2005; Carver and Reddy, 2013). Receptors containing α4,α5, or α6 are commonly located at perisynaptic or extrasynaptic sites. Receptor isoforms with α4-, α6-, or δ-subunits are insensitive to benzodiazepines such as diazepam and midazolam, whereas those with α1-, α2-, α3-, α5-, or γ2-subunits are benzodiazepine sensitive. Due to their synaptic location, these isoforms undergo dynamic plasticity (such as internalization or subunit switching) in response to prolonged seizures or SE, resulting in benzodiazepine resistance that becomes a refractory condition as time elapses.
SE is a neurologic emergency characterized by continuous seizure activity for 5–30 minutes or more, and these seizures respond to benzodiazepines if given within 5–20 minutes. Presently, three benzodiazepines are primarily used in the management of acute seizures and SE: diazepam, lorazepam, and midazolam. Similar to diazepam and lorazepam, midazolam is an effective anticonvulsant for the treatment of SE (Alldredge et al., 2001; Shorvon and Ferlisi, 2011; Silbergleit et al., 2012). A meta-analysis has confirmed that midazolam by any route is superior to diazepam (McMullan et al., 2010). Midazolam offers several unique advantages over other benzodiazepines, including its rapid onset of action, short duration, water solubility, and extended shelf life (Mandrioli et al., 2008). Midazolam has a maximum shelf life of 2–3 years at room temperature. Midazolam is administered intravenously or intramuscularly to terminate acute seizures and SE, including those caused by nerve agents (Galvin and Jelinek, 1987; Silbergleit et al., 2011, 2012). Midazolam is likely to replace diazepam in the nerve agent treatment regimen because of its superior pharmacokinetic features (Reddy and Reddy, 2015). It is available in a water-soluble form for formulation of aqueous injectable products. At pH < 5, midazolam is deprotonated and converts to an open-ring configuration due to acid-catalyzed hydrolysis of the 4,5-double bond of the diazepine ring (Orive et al., 1989). These physicochemical features are attractive to formulate midazolam into aqueous injectable products with longer shelf lives. Midazolam also has some of the significant limitations found in other benzodiazepines. Chiefly, it must be administered within 10–20 minutes, after which there is little or no protection against seizures, and progressive neurologic damage occurs (Goodkin et al., 2009; Apland et al., 2014). This timeline is often not practical in many cases because it can take a minimum of 30–60 minutes to get medical intervention. Midazolam has an extremely short half-life (1.5–2.5 hours), and therefore needs frequent boluses or continuous infusion. Repeated high doses of midazolam may result in sedation, respiratory depression, and tolerance. Seizures lasting longer than 5–20 minutes may not respond to midazolam due to internalization of GABAA receptors (Goodkin et al., 2005; Naylor et al., 2005).
In conclusion, the results from this study indicate that the antiseizure effects of midazolam are not mediated by endogenous neurosteroids such as allopregnanolone. Our results with transgenic DKO mice provide strong evidence that the extrasynaptic (δ-subunit) GABAA receptors are not required for the antiseizure effects of midazolam. Rather, the antiseizure activity of midazolam is directly mediated by synaptic (γ2-subunit) GABAA receptors through specific benzodiazepine binding sites. These studies further reaffirm that midazolam is an effective antiseizure agent for controlling acute seizures and SE.
Acknowledgments
The authors thank the Reddy laboratory staff, especially Xin Wu, Chase Carver, and Darren Abbas for technical support.
Abbreviations
- AD
after-discharge
- CC99
stimulation current causing seizures in 99% of animals
- CL
confidence limits
- DKO
δ-subunit knockout
- P450scc
cytochrome P450 cholesterol side-chain cleavage enzyme
- PK11195
1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide
- SE
status epilepticus
- TSPO
cholesterol transporter protein
- WT
wild type
Authorship Contributions
Participated in research design: S. D. Reddy, D. S. Reddy.
Conducted experiments: S. D. Reddy, Younus, Clossen, D. S. Reddy.
Performed data analysis: S. D. Reddy, D. S. Reddy.
Wrote or contributed to the writing of the manuscript: S. D. Reddy, D. S. Reddy.
Footnotes
This work supported in part by the CounterACT Program, National Institutes of Health, Office of the Director and the National Institute of Neurologic Disorders and Stroke [Grant U01-NS083460]. The authors have no competing financial interests. The views expressed in this article are those of the authors and do not reflect the official policy of the National Institutes of Health or the U.S. Government.
References
- Albright PS, Burnham WM. (1980) Development of a new pharmacological seizure model: effects of anticonvulsants on cortical- and amygdala-kindled seizures in the rat. Epilepsia 21:681–689. [DOI] [PubMed] [Google Scholar]
- Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, Gottwald MD, O’Neil N, Neuhaus JM, Segal MR, et al. (2001) A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med 345:631–637. [DOI] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, Rossetti F, Miller SL, Braga MF. (2014) The limitations of diazepam as a treatment for nerve agent-induced seizures and neuropathology in rats: comparison with UBP302. J Pharmacol Exp Ther 351:359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auta J, Romeo E, Kozikowski A, Ma D, Costa E, Guidotti A. (1993) Participation of mitochondrial diazepam binding inhibitor receptors in the anticonflict, antineophobic and anticonvulsant action of 2-aryl-3-indoleacetamide and imidazopyridine derivatives. J Pharmacol Exp Ther 265:649–656. [PubMed] [Google Scholar]
- Barton ME, Klein BD, Wolf HH, White HS. (2001) Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res 47:217–227. [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. (2009) Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci 29:12757–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Foley M, Audette D, Leslie N, Frye CA. (2000) Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology (Berl) 151:64–71. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Mody I. (2012) Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron 73:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WC, Schiffman DO, Swinyard EA, Goodman LS. (1953) Comparative assay of an antiepileptic drugs by psychomotor seizure test and minimal electroshock threshold test. J Pharmacol Exp Ther 107:273–283. [PubMed] [Google Scholar]
- Carver CM, Reddy DS. (2013) Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology (Berl) 230:151–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Wu X, Gangisetty O, Reddy DS. (2014) Perimenstrual-like hormonal regulation of extrasynaptic δ-containing GABAA receptors mediating tonic inhibition and neurosteroid sensitivity. J Neurosci 34:14181–14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleton A, Van der Graaf PH, Ghijsen W, Voskuyl R, Danhof M. (1999) Mechanism-based modeling of adaptive changes in the pharmacodynamics of midazolam in the kindling model of epilepsy. Pharm Res 16:1702–1709. [DOI] [PubMed] [Google Scholar]
- Dhir A, Rogawski MA. (2012) Role of neurosteroids in the anticonvulsant activity of midazolam. Br J Pharmacol 165:2684–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6:215–229. [DOI] [PubMed] [Google Scholar]
- Finn DA, Beadles-Bohling AS, Beckley EH, Ford MM, Gililland KR, Gorin-Meyer RE, Wiren KM. (2006) A new look at the 5α-reductase inhibitor finasteride. CNS Drug Rev 12:53–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. (1997) The mouse brain in stereotaxic coordinates, Ed. 1st Academic Press, New York. [Google Scholar]
- Galvin GM, Jelinek GA. (1987) Midazolam: an effective intravenous agent for seizure control. Arch Emerg Med 4:169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangisetty O, Reddy DS. (2010) Neurosteroid withdrawal regulates GABA-A receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience 170:865–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavish M, Bachman I, Shoukrun R, Katz Y, Veenman L, Weisinger G, Weizman A. (1999) Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev 51:629–650. [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. (1969) A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol 25:295–330. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Kapur J. (2009) The impact of diazepam’s discovery on the treatment and understanding of status epilepticus. Epilepsia 50:2011–2018. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Yeh JL, Kapur J. (2005) Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci 25:5511–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefely W. (1988) The preclinical pharmacology of flumazenil. Eur J Anaesthesiol Suppl 2 (Suppl):25–36. [PubMed] [Google Scholar]
- Hoffman EJ, Warren EW. (1993) Flumazenil: a benzodiazepine antagonist. Clin Pharm 12:641–656, quiz 699–701. [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. (2007) Neurosteroid binding sites on GABA(A) receptors. Pharmacol Ther 116:7–19. [DOI] [PubMed] [Google Scholar]
- Jefcoate C. (2002) High-flux mitochondrial cholesterol trafficking, a specialized function of the adrenal cortex. J Clin Invest 110:881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski RM, Livingood MR, Rogawski MA. (2004) Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia 45:864–867. [DOI] [PubMed] [Google Scholar]
- Khan GM, Smolders I, Ebinger G, Michotte Y. (2000) Flumazenil prevents diazepam-elicited anticonvulsant action and concomitant attenuation of glutamate overflow. Eur J Pharmacol 407:139–144. [DOI] [PubMed] [Google Scholar]
- Kita A, Furukawa K. (2008) Involvement of neurosteroids in the anxiolytic-like effects of AC-5216 in mice. Pharmacol Biochem Behav 89:171–178. [DOI] [PubMed] [Google Scholar]
- Kita A, Kohayakawa H, Kinoshita T, Ochi Y, Nakamichi K, Kurumiya S, Furukawa K, Oka M. (2004) Antianxiety and antidepressant-like effects of AC-5216, a novel mitochondrial benzodiazepine receptor ligand. Br J Pharmacol 142:1059–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneyev A, Pan BS, Polo A, Romeo E, Guidotti A, Costa E. (1993) Stimulation of brain pregnenolone synthesis by mitochondrial diazepam binding inhibitor receptor ligands in vivo. J Neurochem 61:1515–1524. [DOI] [PubMed] [Google Scholar]
- Kucken AM, Teissére JA, Seffinga-Clark J, Wagner DA, Czajkowski C. (2003) Structural requirements for imidazobenzodiazepine binding to GABA(A) receptors. Mol Pharmacol 63:289–296. [DOI] [PubMed] [Google Scholar]
- Le Fur G, Vaucher N, Perrier ML, Flamier A, Benavides J, Renault C, Dubroeucq MC, Guérémy C, Uzan A. (1983) Differentiation between two ligands for peripheral benzodiazepine binding sites, [3H]RO5-4864 and [3H]PK 11195, by thermodynamic studies. Life Sci 33:449–457. [DOI] [PubMed] [Google Scholar]
- Mandrioli R, Mercolini L, Raggi MA. (2008) Benzodiazepine metabolism: an analytical perspective. Curr Drug Metab 9:827–844. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Ogata M, Koga K, Shigematsu A. (1994) Effect of peripheral benzodiazepine receptor ligands on lipopolysaccharide-induced tumor necrosis factor activity in thioglycolate-treated mice. Antimicrob Agents Chemother 38:812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley LD, Park CH, Lan NC, Tomich JM, Shively JE, Gee KW. (1995) Benzodiazepines and peptides stimulate pregnenolone synthesis in brain mitochondria. Eur J Pharmacol 276:145–153. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Van Shura KE, LaMont JC, McMonagle JD, Shih TM. (2009) Comparison of the intramuscular, intranasal or sublingual routes of midazolam administration for the control of soman-induced seizures. Basic Clin Pharmacol Toxicol 104:27–34. [DOI] [PubMed] [Google Scholar]
- McMullan J, Sasson C, Pancioli A, Silbergleit R. (2010) Midazolam versus diazepam for the treatment of status epilepticus in children and young adults: a meta-analysis. Acad Emerg Med 17:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzak A, Akula N, Lecanu L, Papadopoulos V. (2011a) Novel androstenetriol interacts with the mitochondrial translocator protein and controls steroidogenesis. J Biol Chem 286:9875–9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzak A, Rone M, Aghazadeh Y, Culty M, Papadopoulos V. (2011b) Mitochondrial protein import and the genesis of steroidogenic mitochondria. Mol Cell Endocrinol 336:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, et al. (1999) Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA 96:12905–12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Whiting PJ, Klein RL, Wafford KA, Harris RA. (1994) A single amino acid of the human GABAA receptor γ2 subunit determines benzodiazepine efficacy. J Biologic Chem 269:32768–32773. [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. (2002) A new benzodiazepine pharmacology. J Pharmacol Exp Ther 300:2–8. [DOI] [PubMed] [Google Scholar]
- Mukai Y, Higashi T, Nagura Y, Shimada K. (2008) Studies on neurosteroids XXV. Influence of a 5α-reductase inhibitor, finasteride, on rat brain neurosteroid levels and metabolism. Biol Pharm Bull 31:1646–1650. [DOI] [PubMed] [Google Scholar]
- Naylor DE, Liu H, Wasterlain CG. (2005) Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 25:7724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothdurfter C, Rupprecht R, Rammes G. (2012) Recent developments in potential anxiolytic agents targeting GABAA/BzR complex or the translocator protein (18kDa) (TSPO). Curr Top Med Chem 12:360–370. [DOI] [PubMed] [Google Scholar]
- Orive MM, Gallo B, Alonso RM, Vicente F, Viré JC, Patriarche GJ. (1989) Spectrophotometric study of the acid-base equilibria of an imidazobenzodiazepine, midazolam, and its determination in pharmaceutical formulations. Mikrochim Acta I:181–190. [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, et al. (2006) Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 27:402–409. [DOI] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, Morrow AL. (2009) Simultaneous quantification of GABAergic 3α,5α/3α,5β neuroactive steroids in human and rat serum. Steroids 74:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294. [DOI] [PubMed] [Google Scholar]
- Reddy DS. (2011) Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Front Endocrinol (Lausanne) 2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. (2014) Clinical pharmacology of current antiepileptic drugs. Int J Pharm Sci Nanotech 7:2305–2319. [Google Scholar]
- Reddy DS, Castaneda DC, O’Malley BW, Rogawski MA. (2004) Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther 310:230–239. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. (1996) Role of GABA-A and mitochondrial diazepam binding inhibitor receptors in the anti-stress activity of neurosteroids in mice. Psychopharmacology (Berl) 128:280–292. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Mohan A. (2011) Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. J Neurosci 31:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Gould J, Gangisetty O. (2012) A mouse kindling model of perimenstrual catamenial epilepsy. J Pharmacol Exp Ther 341:784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SD, Reddy DS. (2015) Midazolam as an anticonvulsant antidote for organophosphate intoxication–A pharmacotherapeutic appraisal. Epilepsia (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. (2010) Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res 89:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo E, Cavallaro S, Korneyev A, Kozikowski AP, Ma D, Polo A, Costa E, Guidotti A. (1993) Stimulation of brain steroidogenesis by 2-aryl-indole-3-acetamide derivatives acting at the mitochondrial diazepam-binding inhibitor receptor complex. J Pharmacol Exp Ther 267:462–471. [PubMed] [Google Scholar]
- Rovira C, Ben-Ari Y. (1993) Developmental study of benzodiazepine effects on monosynaptic GABAA-mediated IPSPs of rat hippocampal neurons. J Neurophysiol 70:1076–1085. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schüle C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F, et al. (2009) Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science 325:490–493. [DOI] [PubMed] [Google Scholar]
- Sarto-Jackson I, Sieghart W. (2008) Assembly of GABAA receptors. Mol Membr Biol 25:302–310. [DOI] [PubMed] [Google Scholar]
- Serra M, Madau P, Chessa MF, Caddeo M, Sanna E, Trapani G, Franco M, Liso G, Purdy RH, Barbaccia ML, et al. (1999) 2-Phenyl-imidazo[1,2-a]pyridine derivatives as ligands for peripheral benzodiazepine receptors: stimulation of neurosteroid synthesis and anticonflict action in rats. Br J Pharmacol 127:177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorvon S, Ferlisi M. (2011) The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain 134:2802–2818. [DOI] [PubMed] [Google Scholar]
- Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, Barsan W, NETT Investigators (2012) Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med 366:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbergleit R, Lowenstein D, Durkalski V, Conwit R, Neurological Emergency Treatment Trials (NETT) Investigators (2011) RAMPART (Rapid Anticonvulsant Medication Prior to Arrival Trial): a double-blind randomized clinical trial of the efficacy of intramuscular midazolam versus intravenous lorazepam in the prehospital treatment of status epilepticus by paramedics. Epilepsia 52 (Suppl 8):45–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelling C, Tanchuck-Nipper MA, Ford MM, Jensen JP, Cozzoli DK, Ramaker MJ, Helms M, Crabbe JC, Rossi DJ, Finn DA. (2014) Quantification of ten neuroactive steroids in plasma in Withdrawal Seizure-Prone and -Resistant mice during chronic ethanol withdrawal. Psychopharmacology (Berl) 231:3401–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So EC, Chang YT, Hsing CH, Poon PW, Leu SF, Huang BM. (2010) The effect of midazolam on mouse Leydig cell steroidogenesis and apoptosis. Toxicol Lett 192:169–178. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. (2002) Behavior and physiology of mice lacking the GABAA-receptor delta subunit. Epilepsia 43 (Suppl 5):3–8. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. (2003) Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA 100:14439–14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel-Wagner B. (2003) Neurosteroid biosynthesis in the human brain and its clinical implications. Ann N Y Acad Sci 1007:64–78. [DOI] [PubMed] [Google Scholar]
- Tokuda K, O’Dell KA, Izumi Y, Zorumski CF. (2010) Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. J Neurosci 30:16788–16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HS, Woodhead JH, Wilcox KS. (2002) General principles: discovery and preclinical development of antiepileptic drugs, in Antiepileptic drugs, Ed. 5th (Levy RH, Mattson RH, Meldrum BS. eds) pp 36–48, Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- Whiting P, Wafford KA, McKernan RM. (2000) Pharmacologic subtypes of GABAA receptors based on subunit composition, in GABA in the Nervous System: The View at Fifty Years (Martin DL, Olsen RW. eds) pp 113–126, Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. (2002) Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci 22:1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gangisetty O, Carver CM, Reddy DS. (2013) Estrous cycle regulation of extrasynaptic δ-containing GABA(A) receptor-mediated tonic inhibition and limbic epileptogenesis. J Pharmacol Exp Ther 346:146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]