Abstract
Amiloride, a diuretic used in the treatment of hypertension and congestive heart failure, and 2-guanidine-4-methylquinazoline (GMQ) are guanidine compounds that modulate acid-sensing ion channels. Both compounds have demonstrated affinity for a variety of membrane proteins, including members of the Cys-loop family of ligand-gated ion channels, such as the heteromeric GABA-A αβγ receptors. The actions of these guanidine compounds on the homomeric GABA-A ρ1 receptor remains unclear, especially in light of how many GABA-A αβγ receptor modulators have different effects in the GABA-A ρ1 receptors. We sought to characterize the influence of amiloride and GMQ on the human GABA-A ρ1 receptors using whole-cell patch-clamp electrophysiology. The diuretic amiloride potentiated the human GABA-A ρ1 GABA-mediated current, whereas GMQ antagonized the receptor. Furthermore, a GABA-A second transmembrane domain site, the intersubunit site, responsible for allosteric modulation in the heteromeric GABA-A receptors mediated amiloride’s positive allosteric actions. In contrast, the mutation did not remove GMQ antagonism but only changed the guanidine compound’s potency within the human GABA-A ρ1 receptor. Through modeling and introduction of point mutations, we propose that the GABA-A ρ1 intersubunit site plays a role in mediating the allosteric effects of amiloride and GMQ.
Introduction
GABA is the major inhibitory neurotransmitter in the vertebrate brain, and targets the ionotropic GABA-A receptors, members of the Cys-loop receptor superfamily (Olsen and Sieghart, 2008). GABA-A receptors can be found as homomeric or heteromeric pentamers, consisting of a combination of α, β, γ, δ, ε, π, ρ, and θ subunits. Of these, the GABA-A ρ1 receptor can form functional homomeric anion-selective ion channels found in the retina (Jones and Palmer, 2011; Ng et al., 2011). Furthermore, the GABA-A ρ1 receptor has a more distinct pharmacological profile than the heteromeric GABA-A receptors, which complicates studies that focus on characterizing the receptor’s role in brain function. The GABA-A ρ1 receptor is insensitive to modulators of the heteromeric GABA-A receptor such as bicuculine, benzodiazepines, and barbiturates (Korpi et al., 2002), and the selective antagonist TPMPA [(1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid] inhibits GABA-A ρ1 receptors, which has no effect on heteromeric GABA-A receptors. Picrotoxin and zinc can antagonize both the heteromeric and homomeric GABA-A receptors (Dong and Werblin, 1996). Key residues at the 6′ and 15′ positions in the second transmembrane domain of the GABA-A receptors have been shown to convey the antagonistic effect of picrotoxin (Xu et al., 1995; Dibas et al., 2002).
The observed mixed pharmacology extends to endogenous guanidine compounds, which have exhibited different modulatory effects on the heteromeric GABA-A receptors than GABA-A ρ1 receptor (Neu et al., 2002; Chebib et al., 2009). In the inherited disorder guanidinoacetate methyltransferase deficiency, there is a buildup of guanidinoacetate, the immediate precursor to creatine that can directly activate the heteromeric GABA-A receptors (Neu et al., 2002). Where guanidinoacetic acid acts as an agonist in the heteromeric GABA-A receptors, this compound antagonizes the GABA-A ρ1 receptor (Chebib et al., 2009). Furthermore, guanidine compounds that modulate the acid-sensing ion channel influence Cys-loop receptor activity. The inhibitory effect of the diuretic amiloride, an acid-sensing ion channel antagonist, on GABA-A receptors was first reported in the frog sensory neurons (Inomata et al., 1988), and subsequent studies focused on amiloride in GABA-A αβγ receptors showed that the guanidine compound competitively antagonized the receptors with a 10-fold increased potency in GABA-A α6-containing receptors (Fisher, 2002). In the inhibitory glycine receptor, amiloride exhibited competitive antagonism in receptors expressed in rat spinal neurons and inferior colliculus (Li et al., 2003a,b; Tang et al., 2006). Furthermore, a recent report outlined the antagonistic effects of the guanidine compound 2-guanidine-4-methylquinazoline (GMQ) on the heteromeric GABA-A αβγ receptors (Xiao et al., 2013). The actions of both amiloride and GMQ in the human (h) GABA-A ρ1 receptor remain unclear.
Here, we have characterized the activities of amiloride and GMQ on the human GABA-A ρ1 receptors transiently expressed in HEK293T cells using whole-cell patch-clamp electrophysiology. Amiloride potentiated GABA-mediated current in a concentration-dependent manner. Because of this potentiation and based on modeling amiloride interaction with a bacterial Cys-loop receptor, we introduced mutations within the channel’s second transmembrane (TM) domain to probe which site, the TM2 domain 6′, 15′, and 19′ residues, was responsible for amiloride’s actions. A mutation at the 15′ residue, within the receptor’s intersubunit site, suggested that amiloride and the other guanidine compound, GMQ, interact with the receptor at this site. We propose that these guanidine compounds modulate GABA-A ρ1 receptor activity through the receptor’s intersubunit site like other Cys-loop receptor allosteric modulators. This suggests that these guanidine compounds can be used as the template in the design of human GABA-A ρ1 receptor allosteric modulators.
Materials and Methods
Cell Culture and Expression of Human GABA-A ρ Receptors.
Wild-type and mutant hGABA-A ρ1 receptor cDNA was cotransfected in human embryonic kidney cells containing the SV40 T-antigen (HEK-293T; American Type Culture Collection, Manassas, VA). The HEK293T cell line was maintained in T25 flasks at 37°C in a 5% CO2 water-jacketed incubator and cultured in Dulbecco’s modified Eagle’s medium (Life Technologies, Grand Island, NY), with 10% fetal bovine serum (Phenix Research, Candler, NC) and 1% penicillin-streptomycin (Mediatech, Inc., Manassas, VA). Upon reaching 80% confluency, HEK293T cells were plated on glass coverslips 2–4 hours in preparation for transfection. Cells were cotransfected with pNEGFP-EU (2 μg) and human GABA-A ρ1 cDNA (2 μg) were mixed with 5–6 μl TransIT-293 transfection reagent (Mirus Bio, LLC, Madison, WI) according manufacturer's instructions. Whole-cell patch-clamp electrophysiology was performed 18–24 hours after transfection.
Site-Directed Mutagenesis of Human GABA-A ρ Receptor.
Human GABA-A ρ1 cDNA, subcloned in pCDNA3.1, was a generous gift from Glenn Dillon (West Virginia University). Enhanced green fluorescent protein cDNA (in the pNGFP-EU mammalian expression vector) was a kind gift from Eric Gouaux (Vollum Institute, Portland OR). QuikChange Lightning Site-Directed Mutagenesis kit (Agilent Technologies, Santa Clara, CA) was used to perform mutations at the 6′, 15′, and 19′ residues of the GABA-A ρ1 receptor. After polymerase chain reaction, mutated DNA was ligated into plasmid cDNA3.1 for expression in human embryonic kidney cells.
Electrophysiology.
Borosilicate glass capillary tubes (Sutter Instrument Company, Novato, CA) were pulled to a resistance of 8–12 MΩ using a pipette puller (P-87 pipette puller, Sutter Instrument Co.). Recording patch electrodes were filled with internal solution consisting of (in mM): CsCl (120), tetraethylammonium chloride (20), CaCl2 (1), MgCl2 (2) EGTA (11), HEPES (10), and adjusted to pH 7.2 using N-methyl-d-glucamine (Chebib et al., 2009). Cells were perfused with external solution containing (in mM): NaCl (137), KCl (5.4) CaCl2 (1.8) MgCl2 (1) HEPES (5), adjusted to pH 7.4 (Chebib et al., 2009). Amiloride hydrochloride hydrate, GMQ, picrotoxin, and GABA were obtained from Sigma-Aldrich (St. Louis, MO). Stock solutions of each compound were made and stored at −20°C. On the day of experimentation, ligand stock solutions were thawed and diluted to needed concentrations. For the high concentrations (1 mM) of GMQ, picrotoxin, and amiloride, stock solutions were made using dimethylsulfoxide. Solution exchange was achieved via an array of capillary tubes that are arranged perpendicularly on an inverted fluorescent microscope. Solution flow was controlled electronically using computer driven polytetrafluoroethylene valves using a ValveLink8.2 controller (AutoMate Scientific, Berkeley, CA). Recorded currents were obtained using an Axopatch 200B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA) and were filtered and sampled at 5 and 10 kHz, respectively. Data were collected using pClamp 10.0 (Molecular Devices, Sunnyvale, CA) software. Patch-clamped cells were held at a constant voltage of −70 mV during all experiments. Current was analyzed online using Clampfit 10.0, and the resulting data were analyzed offline using Origin 8.1 (OriginLab, Northampton, MA).
Cells were bathed continuously in external solution in the absence of ligand. Upon successful establishment of the whole-cell patch-clamp configuration, test solution was applied for 5 seconds. Between exposures, cells were washed with external solution for 1.5 minutes to allow for complete recovery from the previously test solution. In both amiloride and GMQ experiments, the GABA EC50 control was established (two consecutive exposures that differed by <10% peak current amplitude) before assessing the guanidine compound effect on the receptor. If failure to re-establish the control peak current amplitude after exposure to the nonproton ligand occurred, the recording was aborted. Both GMQ and amiloride were applied in the absence of GABA to assess direct activation of the GABA-A ρ1 receptor. A positive response a guanidine compound was assigned only if the resulting peak current amplitude was greater than a pre-established cutoff of 20 pA. In the absence of GABA, amiloride exhibited minimal direct activation activity (Supplemental Fig. 1). In coapplication studies, the amiloride potentiation was assessed using 10, 30, and 100 μM amiloride in the presence of increasing GABA concentrations and was normalized to GABA EC100 concentration on the wild-type hGABA-A ρ1 receptor. Both amiloride and GMQ were coapplied in increasing concentrations with the control EC50 GABA on all constructs. Concentration response profiles were fit to a dose-response function using OriginLab 8.1.
Amiloride Docking.
An amiloride coordinate file was generated using the online server PRODRG (Schuttelkopf and van Aalten, 2004). Modeling of amiloride docking with a Cys-loop receptor crystal structure was produced using the molecular docking algorithm based on complementarity principles in PatchDock (Schneidman-Duhovny et al., 2005). The ethanol-bound Gloeobacter violaceus pentameric ligand-gated ion channel (GLIC) protein crystal structure (PDB ID 4HFE) (Sauguet et al., 2013) was used as the receptor molecule, whereas the PRODRG generated amiloride coordinate file was used as the ligand. The clustering root mean square deviation was set to 1.5 Å, whereas the complex type was set to the protein-small ligand option, as suggested. Of the resulting models, one result showed amiloride docking with the intersubunit site and was used as a starting point for the described studies. The model was presented graphically using Pymol (Schrodinger LLC, New York, NY).
Data Analysis.
Maximum peak current amplitude in each whole cell electrophysiological experiment was obtained and normalized to the maximum peak current amplitude elicited by a GABA control. Data are presented as the mean ± S.E.M. of the indicated patch-clamped HEK293T cells. Statistical significance was determined using unpaired Student’s t test.
Results
Amiloride, the prototypical acid-sensing ion channel (ASIC) antagonist, acts as a competitive antagonist for the heteromeric GABA-A αβγ receptors (Inomata et al., 1988; Fisher, 2002; Liu et al., 2010). The activity of amiloride on the GABA-A ρ1 receptor remains unclear. Thus we sought to determine the intrinsic activity of amiloride on the homomeric human GABA-A ρ1 receptors. We transiently transfected cDNA encoding the wild-type human GABA-A ρ1 receptor in HEK293T cells. The wild-type human GABA-A ρ1 receptor had a GABA EC50 of 9.4 ± 0.6 μM with a Hill coefficient of 1.1 ± 0.2 (Table 1). The described wild-type GABA-A ρ1 receptor EC50 is within the range of reported GABA-A ρ1 receptor GABA EC50 values expressed in the HEK293T cell line (Kusama et al., 1993; Amin and Weiss, 1994; Greka et al., 2000; Harrison and Lummis, 2006; Chebib et al., 2009).
TABLE 1.
Amiloride increases the affinity of the wild-type human GABA-A ρ1 receptor for GABA
Summary of EC50 and Hill coefficient values for GABA and coapplication of increasing concentrations of GABA and amiloride (30, 100, and 300 µM) on the wild-type human GABA-A ρ1 receptor. n ≥ 4 cells.
| Compound | EC50 | nH |
|---|---|---|
| μM | ||
| GABA | 9.4 ± 0.1 | 1.1 ± 0.2 |
| + 30 μM amiloride | 7.8 ± 0.5* | 1.0 ± 0.1 |
| + 100 μM amiloride | 6.4 ± 0.6** | 1.4 ± 0.2 |
| + 300 μM amiloride | 5.9 ± 0.9** | 0.9 ± 0.1 |
| + 10 μM GMQ | 7.2 ± 0.6** | 1.9 ± 0.3 |
| + 100 μM GMQ | 4.8 ± 0.9** | 2.6 ± 0.7 |
nH, Hill coefficient.
P < 0.05; **P < 0.01.
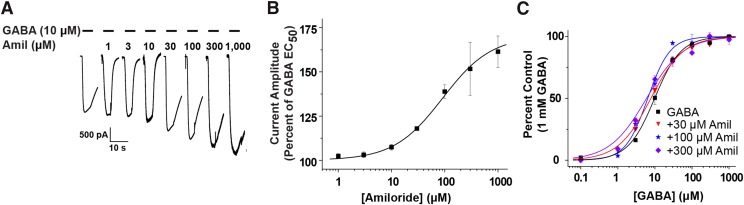
Initially, we used an amiloride concentration-response profile experiment using coapplication of 10 μM GABA as our control and increasing concentrations of amiloride (Fig. 1A). Amiloride did not exhibit an inhibitory effect on the expressed human GABA-A ρ1 receptor (Fig. 1A). When coapplied in increasing concentrations from 1 to 1000 μM amiloride, there was a 52% increase in peak current amplitude at the maximum concentration of amiloride compared with the control (Fig. 1B). We calculated that the amiloride EC50 was 77.0 ± 6.3 μM and a Hill coefficient of 1.6 ± 0.2 (n ≥ 4) (Fig. 1B; Table 2). At the lower concentrations of amiloride (1, 3, and 10 μM) tested, we observed minimal increase in GABA-mediated peak current amplitude, but there were noticeable changes in the recordings profile (Fig. 1A). Our results suggest that amiloride enhances GABA-mediated current generated by the human GABA-A ρ1 receptor, which is in contrast with heteromeric GABA-A receptor where the ASIC nonproton ligand is a competitive antagonist.
Fig. 1.
(A) Representative traces of wild-type hGABA-A ρ1 receptor currents generated by coapplication of GABA EC50 (10 μM) with increasing concentrations of amiloride (Amil). (B) Potentiating amiloride concentration-response profile is shown. Data represents the mean ± S.E.M. of 10 μM GABA and coapplication of increasing amiloride 1, 3, 10, 30, 100, 300, and 1000 μM) of n ≥ 4 individual cells. The amiloride EC50 was 77.0 ± 6.3 µM with a Hill coefficient of 1.6 ± 0.2. (C) GABA concentration-response profiles in the absence and presence of amiloride are shown. A leftward shift of the GABA EC50 is depicted from the GABA EC50 (9.4 ± 0.1 μM GABA, control), 30 μM amiloride (7.8 ± 0.5 μM GABA), 100 μM (6.4 ± 0.6 μM GABA), 300 μM (5.9 ± 0.9 μM GABA) (Table 1). Each recording was normalized to the cell’s maximal response (1 mM GABA).
TABLE 2.
GABA and allosteric modulator sensitivity in wild type and mutant GABA-A ρ1 receptors
Summary of EC50/IC50 and Hill coefficient values for GABA and coapplication amiloride, GMQ, and picrotoxin on the wild-type hGABAA-ρ1, hGABA-A ρ1 T6′F, hGABA-A ρ1 I15′N, and hGABA-A ρ1 N19′D mutant receptors. n ≥ 4 cells.
| Receptor | EC50 | nH | Amiloride EC50/IC50 | Amiloride nH | GMQ IC50 | GMQ nH | PTX IC50 | PTX nH |
|---|---|---|---|---|---|---|---|---|
| μM | μM | μM | μM | |||||
| WT ρ1 | 9.4 ± 0.1 | 1.1 ± 0.2 | (EC50) 77.0 ± 6.3 | 1.6 ± 0.2 | 13.2 ± 0.6 | 1.4 ± 0.1 | 4.8 ± 0.2 | 1.3 ± 0.1 |
| ρ1(T6′F) | 8.0 ± 1.7 | 0.6 ± 0.1 | (IC50) 277.3 ± 88.6 | 1.2 ± 0.3 | 0.4 ± 0.1*** | 0.8 ± 0.2 | N.D. | N.D. |
| ρ1(I15′N) | 2.9 ± 0.6*** | 1.0 ± 0.2 | N.D. | N.D. | 630.5 ± 58.4*** | 1.5 ± 0.2 | 63.4 ± 12.0** | 0.6 ± 0.1 |
| ρ1(N19′D) | 7.4 ± 1.5 | 1.0 ± 0.2 | (IC50) 66.4 ± 9.4 | 0.4 ± 0.02 | N.D. | N.D. | N.D | N.D |
N.D., not determined.
**P < 0.01; ***P < 0.001.
Because amiloride exhibited positive allosteric modulation, we considered that amiloride may have directly activated the wild-type GABA-A ρ1 receptor. Direct activation of the receptor was observed at 1 mM amiloride, which resulted in a modest response (Supplemental Fig. 1). In line with the activity indicative of a positive allosteric modulator, amiloride may influence the apparent GABA affinity of GABA-A ρ1 receptors. To assess this effect, we generated GABA concentration-response profiles in the presence of amiloride (30, 100, and 300 μM) (Fig. 1C). The resulting GABA concentration-response profiles were compared with control GABA concentration-response profile for the wild-type human GABA-A ρ1 receptor. Increasing amiloride concentrations revealed an overall leftward shift of the calculated GABA EC50 values, from the control value of 9.4 ± 0.1 µM with a Hill coefficient of 1.1 ± 0.2 to a GABA EC50 value of 5.9 ± 0.9 µM and Hill coefficient of 0.9 ± 0.1 in the presence of 300 µM amiloride (n ≥ 5; Fig. 1C; Table 1). These results suggest that amiloride acts as a positive allosteric modulator of the human GABA-A ρ1 receptor and could interact with another site within the receptor.
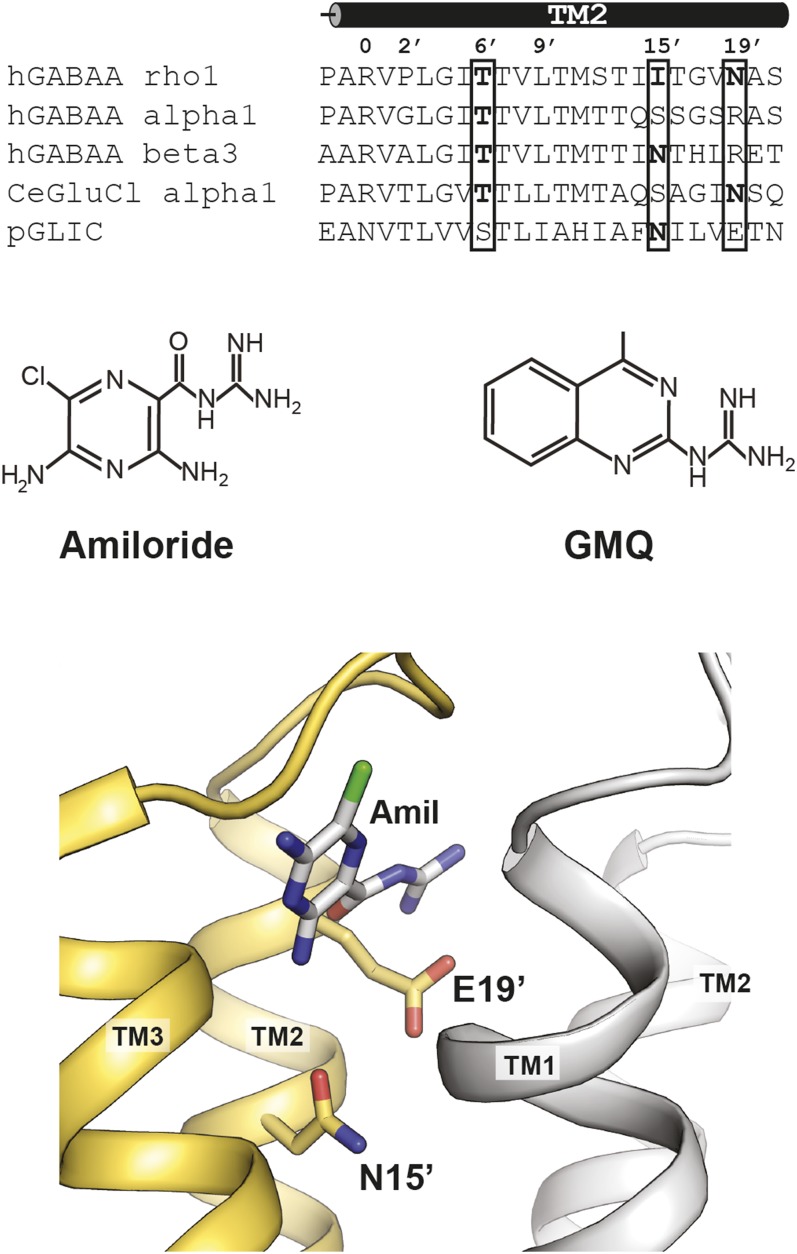
There are multiple sites within the Cys-loop receptors that could serve as the amiloride binding site. We focused our attention on the TM2 domains. A comparison of the second transmembrane domains of the hGABA-A α1, β3, ρ1, Caenorhabditis elegans glutamate chloride (GluCl) α1 subunit, and GLIC subunit revealed multiple residues that could be responsible for amiloride allosteric modulation (Fig. 2). To narrow our focus more, we generated a model of amiloride docked in a Cys-loop receptor crystal structure. We used the solved structure of the ethanol-bound GLIC protein structure as our model using the online server PatchDock (Fig. 2). One result revealed an amiloride molecule within the GLIC intersubunit cavity between the transmembrane domains of neighboring subunits. Furthermore, two residues stood out within the intersubunit site: the TM2 15′ and 19′ residues, which had amino acid side chains that protrude into the intersubunit cavity. Furthermore, we focused on sites that are responsible for allosteric modulation. Thus we included the TM2 T6′F mutation in our study, a mutation that influences channel gating and abolishes picrotoxin sensitivity. In GABA-A ρ1, the TM2 15′ position is an isoleucine. The other mammalian subunits shown have a serine (GABA-A α1) or asparagine (GABA-A β3) at this position. Based on our modeling, both 15′ and 19′ residues extend into the intersubunit site, and we hypothesized that these residues could be integral in mediating amiloride and GMQ activity (Fig. 2). We introduced a TM2 N19′D mutation in an effort to preserve the size of the residue. These allosteric modulator sites could play a role in mediating the effects of amiloride or GMQ on the human GABA-A ρ1 receptor and are suitable candidates for serving as the amiloride allosteric modulatory site.
Fig. 2.
Sequence alignment of the second transmembrane domain among select Cys-loop receptor subunits. The residues are labeled according to Miller notation (Miller, 1989). Chemical structure of guanidine compounds amiloride and GMQ. Model of amiloride interacting with the intersubunit site of ethanol sensitive GLIC (Sauguet et al., 2013). Compound docked utilizing PatchDock online server.
To assess the role of allosteric sites on guanidine compound influence, we generated the hGABA-A ρ1 T6′F, the I15′N, and N19′D mutants. The latter mutations would address if the guanidinium group of amiloride interacts within the intersubunit site where the TM2 15′ and 19′ residues reside, as suggested in the model (Fig. 1). We began our studies by establishing the GABA EC50 for each of the mutant receptors. Using whole-cell patch-clamp electrophysiology, we applied increasing concentrations of GABA (1 to 1000 μM) for 5 seconds followed by 2 minutes of an active wash (Supplemental Fig. 2). Upon electrophysiological examination of the hGABA-A ρ1 (T6′F), (I15′N) and (N19′D) mutant receptors, each mutant affected the observed GABA EC50 values. The hGABA-A ρ1 (T6′F) mutant receptors places five phenylalanine residues within the channel lining domain. In heteromeric receptors, including five phenylalanine residues at the TM2 6′ position failed to yield functional receptors (Gonzales et al., 2008). In contrast, we were able to generate GABA-mediated current and determined a GABA EC50 value for the mutant GABA-A ρ1 T6′F receptor. The hGABA-A ρ1 (T6′F) mutant receptor had a calculated GABA EC50 was 8.0 ± 1.7 μM and had a Hill coefficient of 0.6 ± 0.1, which were not significantly different from wild-type GABA-A ρ1 receptors (Table 2). The hGABA-A ρ1 I15′N mutant receptor displayed significantly increased agonist potency, with a GABA EC50 value of 2.9 ± 0.6 μM and a Hill coefficient of 1.0 ± 0.2 (Table 2). There was less of an effect on the GABA EC50 value for the hGABA-A ρ1 (N19′D) mutant receptor. The GABA EC50 shifted to 7.4 ± 1.5 μM and a Hill coefficient of 1.0 ± 0.2 (Table 2).
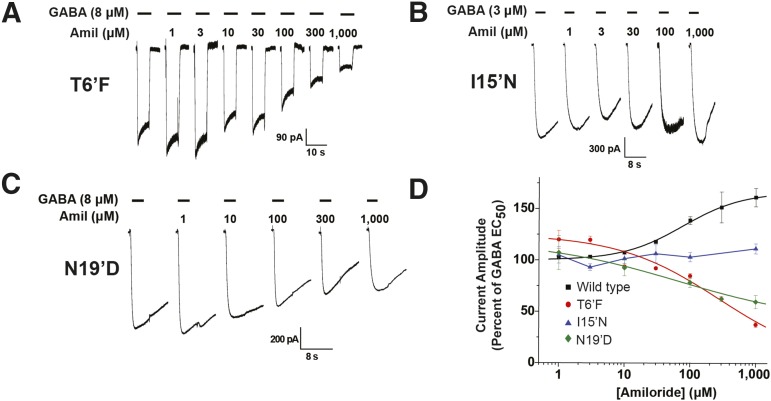
Because amiloride potentiated GABA-mediated current in the wild-type hGABA-A ρ1 receptor (Fig. 1, A and B), we examined amiloride’s activity in mutant hGABA-A ρ1 receptors. Instead of the amiloride potentiating effect observed in the wild-type hGABA-A ρ1 receptor, amiloride antagonized the hGABA-A ρ1 T6′F and N19′D mutant receptor’s GABA-mediated current, with an IC50 of 277.3 ± 88.6 µM and a Hill coefficient of 1.2 ± 0.3 and an IC50 of 66.4 ± 9.4 with a Hill coefficient of 0.4 ± 0.02, respectively (Fig. 3, A and D; Table 2). The hGABA-A ρ1 T6′F mutation resulted in a 10-fold increase in amiloride potency compared with the reported amiloride potency in the heteromeric GABA-A receptor composed of α6β3γ2, whereas the hGABA-A ρ1 N19′D mutation resulted in a 2-fold decrease in potency (Drafts and Fisher, 2004). Furthermore, amiloride failed to exhibit potentiation or antagonism in the hGABA-A ρ1 I15′N mutant receptors (Fig. 3, B and D). The loss of both potentiation and antagonism activity observed in the wild-type hGABA-A ρ1 receptor and the antagonistic intrinsic activity observed in both the hGABA-A ρ1 T6′F mutant receptor and the N19′D mutant receptor suggests that the 15′ residue might be the site of interaction for amiloride within the hGABA-A ρ1 receptor.
Fig. 3.
Human GABA-A ρ1 receptor containing the TM2 6′, 15′, and 19′ mutations display altered response profiles to amiloride. Comparison of amiloride response profile of wild-type hGABA-A ρ1 and hGABA A ρ1 T6′F, I15′N, or N19′D mutant receptors. All constructs were transiently expressed in HEK293T cells. (A) Representative traces of hGABA-A ρ1 T6′F mutant receptor currents elicited upon coapplication of 8 μM GABA (EC50) with increasing concentrations of amiloride. (B) Representative traces of hGABA-A ρ1 I15′N mutant receptor currents elicited upon coapplication of GABA EC50 (3 μM) with increasing concentrations of amiloride. All activation currents generated by applying GABA and amiloride test solutions for 5 seconds. (C) Representative traces of hGABA-A ρ1 N19′D mutant receptor currents elicited upon coapplication of GABA EC50 (8 μM) with increasing concentrations of amiloride. (D) Comparison of normalized concentration-response profiles of amiloride inhibition in wild-type hGABA-A ρ1 and hGABA-A ρ1 I15′N, hGABA-A T6′F, and hGABA-A N19′D mutant receptors compared with control the respective control concentration of GABA. The determined amiloride EC50 was 44.57 ± 14.24 μM for the wild-type hGABA-A ρ1 receptor. IC50 values of 277.3 ± 88.6 μM with a Hill coefficient of 1.2 ± 0.3 and 66.4 ± 9.4 μM with a Hill coefficient of 0.4 ± 0.02 were obtained for hGABA-A ρ1 T6′F and hGABA-A ρ1 N19′D mutant receptor, respectively. The hGABA-A ρ1 I15′N mutant receptor displayed an absence of amiloride modulation, and thus no EC50 or IC50 was obtained. Data are presented as the mean ± S.E.M., with a sample size of n ≥ 5 cells.
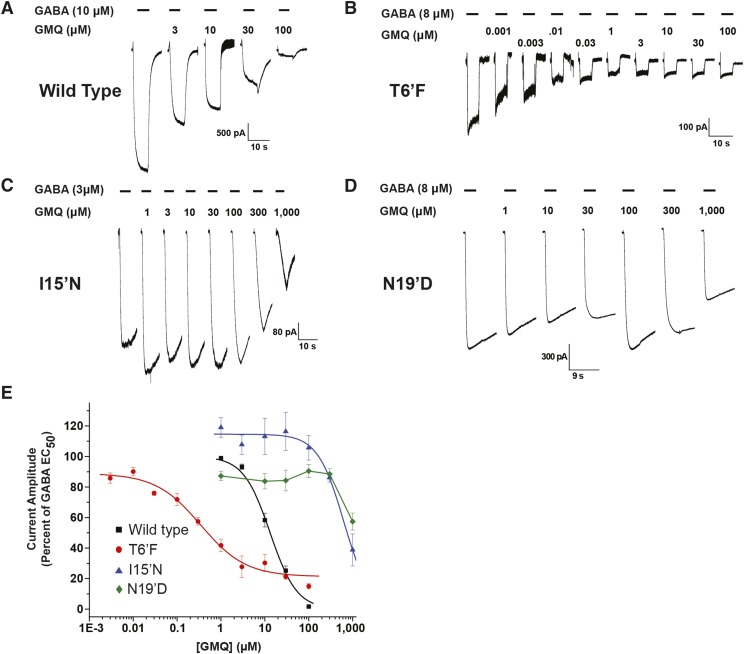
Because of amiloride’s displayed potentiation of the GABA-mediated current, we considered that the other ASIC ligand that antagonized heteromeric GABA-A receptors, GMQ, may have similar activity in the GABA-A ρ1 receptor. Like amiloride, GMQ is a ringed molecule with a guanidine group (Fig. 2). To assess GMQ activity in wild-type hGABA-A ρ1 receptors, we generated concentration-response profiles of GMQ in the presence of the approximate GABA EC50 (10 μM). The guanidine compound GMQ antagonized the GABA-mediated response in a concentration-dependent manner with a determined GMQ IC50 value of 13.2 ± 0.6 and Hill coefficient of 1.4 ± 0.1 (n ≥ 4) (Fig. 4, A and D; Table 2). Upon coapplication of 30 μM GMQ, we observed a rebound current at the end of the GABA-mediated current. This is similar to picrotoxin’s activity on the perch GABA ρ1A receptor, where the plant alkaloid exhibited a rebound current (Qian et al., 2005). Similar to our amiloride studies, we generated GABA concentration-response profiles in the presence of 10 and 100 μM GMQ to assess its influence on GABA potency (Supplemental Fig. 3). Despite the inhibition in current exhibited by GMQ, the guanidine compound decreased the GABA EC50 values (increased potency) (Table 1). In the presence of 10 and 100 μM GMQ, the GABA EC50 was reduced to 7.2 ± 0.6 μM with a Hill coefficient of 1.9 ± 0.3 and 4.8 μM ± 0.9 with a Hill coefficient of 2.6 ± 0.7, respectively (Table 1). Unlike amiloride, however, the presence of GMQ decreased the efficacy of GABA on the hGABA-A ρ1 receptor (Supplemental Fig. 3). This decrease in efficacy, along with the inhibition in GABA-induced current, suggests GMQ may act as a negative allosteric modulator.
Fig. 4.
Human GABA-A ρ1 receptor containing the 15′ and 6′ mutations display altered response profiles to GMQ. (A) Representative traces of hGABA-A ρ1 receptor currents elicited upon coapplication of GABA EC50 with increasing concentrations of GMQ. (B) Representative traces of hGABA-A ρ1 T6′F mutant receptor currents elicited upon coapplication of GABA EC50 with increasing concentrations of GMQ. (C) Representative traces hGABA-A ρ1 I15′N mutant receptor currents elicited upon coapplication of GABA EC50 with increasing concentrations of GMQ. All activation currents generated by applying GABA and GMQ test solutions for 5 seconds. (D) Representative traces of hGABA-A ρ1 N19′D mutant receptor currents elicited upon coapplication of GABA EC50 with increasing concentrations of GMQ. (E) Comparison of normalized concentration-response profiles of GMQ inhibition in wild-type hGABA-A ρ1 and hGABA-A ρ1 I15′N and hGABA-A T6′F mutant receptors compared with the respective control concentration of GABA. The determined GMQ IC50 was 13.2 ± 0.6 μM with a Hill coefficient of 1.4 ± 0.1 for the wild-type hGABA-A ρ1 receptor, 0.4 ± 0.1 μM with a Hill coefficient of 0.8 ± 0.2 for the hGABA-A ρ1 T6′F mutant receptor, and 630.5 ± 58.4 μM with a Hill coefficient of 1.5 ± 0.2 for the hGABA-A ρ1 I15′N mutant receptor. We were unable to fit the data for the N19′D mutation, and thus no IC50 was obtained. Data are presented as the mean ± S.E.M., with a sample size of n ≥ 5 cells.
We examined the effect of GMQ on the hGABA-A ρ1 (T6′F), (I15′N), and (N19′D) mutant receptors. First, we applied increasing concentrations of GMQ with the hGABA-A ρ1 (T6′F) GABA EC50 (Fig. 4, B and E). The guanidine compound GMQ had an IC50 of 0.4 ± 0.1 μM and a Hill coefficient of 0.8 ± 0.2, a 33-fold increase in potency compared with the wild-type receptor (wild-type IC50 13.2 μM) (Table 2). Furthermore, the IC50 reported here was similar to that obtained when assessing GMQ potency in the heteromeric GABA-A receptor (0.39 ± 0.05 μM) (Table 2) (Xiao et al., 2013). In contrast, the hGABA-A ρ1 I15′N receptor exhibited a significant decrease in the GMQ IC50 value (630.5 ± 58.4 μM) with a Hill coefficient of 1.5 ± 0.2 (n ≥ 5) (Fig. 4, C and E; Table 2). The hGABA-A ρ1 (I15′N) GMQ IC50 value was an approximate 48-fold reduction in potency (Fig. 4E). The hGABA-A ρ1 N19′D mutant receptor also displayed a marked decrease in the potency of GMQ, with an estimated IC50 value greater than 1 mM (Fig. 4, D and E).
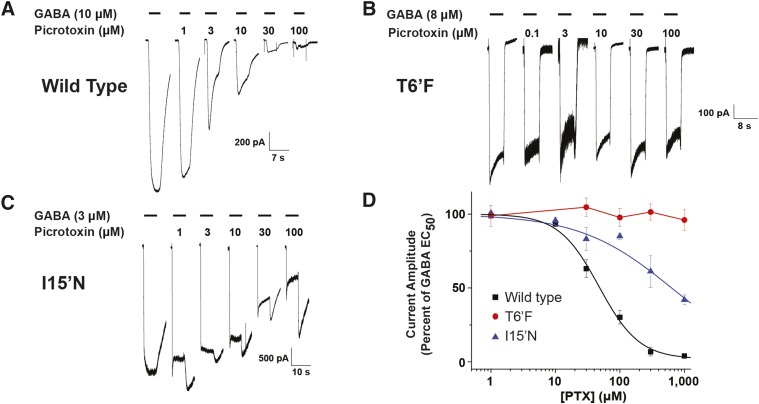
Both amiloride and GMQ activity were influenced by the TM2 mutations at the 6′, 15′, and 19′ positions. Because the GABA-A ρ1 T6′F mutation resulted in changes in amiloride and GMQ potency, we sought to characterize picrotoxin’s actions on the hGABA-A ρ1 receptor mutations as a comparison for these guanidine allosteric modulators. Exposure of the expressed wild-type hGABA-A ρ1 receptors to a GABA EC50 concentration and increasing picrotoxin concentrations exhibited similar responses to those previously reported (Alakuijala et al., 2005) (Fig. 5A). Picrotoxin had an IC50 concentration of 4.8 ± 0.2 μM and a Hill coefficient of 1.3 ± 0.1 for the wild-type hGABA-A ρ1 (Table 2). The described IC50 value obtained here is similar to values reported in the literature (Abbott et al., 2012). Whole-cell patch-clamp assessment of the picrotoxin antagonism in the hGABA-A ρ1 T6′F mutant confirmed that the mutant receptor was insensitive to picrotoxin, because increasing the concentration of picrotoxin was unable to antagonize the GABA evoked current (Fig. 5, B and D). In the hGABA-A ρ1 (I15′N) mutant receptor, there was a significant decrease in the picrotoxin potency with an IC50 of 63.4 ± 12.0 and a Hill coefficient of 0.6 ± 0.1 (Fig. 5, C and D; Table 2).
Fig. 5.
Comparison of picrotoxin inhibitory response profiles of wild-type hGABA-A ρ1, hGABA-A ρ1 I15′N mutant receptor, and hGABA-A ρ1 T6′F mutant receptor. All constructs were transiently expressed in HEK293T cells. (A) Representative traces of wild-type hGABA-A ρ1 receptor. (B) Representative traces of picrotoxin inhibition of the hGABA-A ρ1 T6′F mutant receptor. (C) Representative traces of picrotoxin inhibition of the hGABA-A ρ1 I15′N mutant receptor. All activation currents generated by 5-second exposures to increasing concentrations of picrotoxin coapplied with the receptor’s respective GABA EC50 concentrations. (D) Comparison of normalized concentration-response profiles of picrotoxin inhibition in wild-type hGABA-A ρ1 and hGABA-A ρ1 I15′N and hGABA-A ρ1 T6′F mutant receptors. Because of the difference in EC50 among each construct, each response was compared with the respective control concentration of GABA. The determined picrotoxin IC50 was 4.8 ± 0.2 μM with a Hill coefficient of 1.3 ± 0.1 for wild-type hGABA-A ρ1 receptor and 63.4 ± 12.0 with a Hill coefficient of 0.6 ± 0.1 for the hGABA-A ρ1 I15′N mutant receptor. The hGABA-A ρ1 T6′F mutant receptor displayed insensitivity toward picrotoxin, and thus no IC50 was obtained. Data are presented as the mean ± S.E.M., with a sample size of n ≥ 5 cells.
Discussion
Here we have demonstrated that mutations within the second transmembrane domain of the human GABA-A ρ1 receptor alter the allosteric modulatory properties of amiloride and GMQ. Additionally, a single residue in the GABA-A ρ1 receptor intersubunit site, the I15′N mutation, abolished positive allosteric modulation produced by amiloride. The compounds amiloride and GMQ have both been found to inhibit the heteromeric GABA-A receptors (Fisher, 2002; Xiao et al., 2013). Mutagenesis experiments conducted on the heteromeric GABA-A receptor affected the competitive inhibition of GMQ, but had no effect on the inhibition exhibited by amiloride, suggesting that these inhibitory actions are mediated through distinct sites (Xiao et al., 2013).
The observed amiloride potentiation in GABA-A ρ1 receptor was concentration-dependent with the apparent enhancement occurring at concentrations greater than 10 μM. At the modest amiloride concentrations, we observed a change in the GABA-mediated current profiles, similar to heteromeric GABA-A receptors (Fig. 1). At higher amiloride concentrations (>30 µM), the typical wild-type recording profile returned. Amiloride’s actions on the hGABA-A ρ1 receptor were similar to actions of allosteric modulators on heteromeric GABA-A receptors (Rho et al., 1996). The change in receptor desensitization kinetics is similar to the plant alkaloid picrotoxin effects on deactivation kinetics of the GABA-A ρ receptor (Goutman and Calvo, 2004). Both of these allosteric modulators act at the GABA-A TM2 15′ position. Furthermore, if amiloride’s activity on the human GABA-A ρ1 receptor was similar to pentobarbital, the diuretic should be able to directly gate the channel (Rho et al., 1996). Here, amiloride activated the channel modestly in the wild-type hGABA-A ρ1 receptor (Supplemental Fig. 1). However, barbiturates do not influence wild-type GABA-A ρ receptors, requiring a single point mutation to confer the sensitivity (Amin, 1999). These ligands require a substitution of the GABA-A ρ1 TM2 15′ position to an asparagine, the same amino acid at the GABA-A β2- and β3-subunit 15′ sites.
Based on our modeling and electrophysiological data, we considered that an allosteric modulating amiloride site of interaction may be located away from the GABA agonist site. One such site was the intersubunit site, located between neighboring subunits’ transmembrane domains where ethanol binds (Sauguet et al., 2013) and where propofol inhibits GLIC-mediated current (Ghosh et al., 2013). The side chains of the 15′ and 19′ residues protrude into the intersubunit cavity. The resulting model containing a single amiloride molecule docked near this intersubunit site in the GLIC crystal structure provided further support for our focus on this site. However convincing the molecular docking model and electrophysiology are, there remains a possibility that mutations within the intersubunit site may be involved in amiloride or GMQ allosterism and not direct binding. Additional work is necessary to determine if the intersubunit site is the site of action for amiloride and GMQ in the GABA-A ρ1 receptor.
When the 15′ residue was mutated from an isoleucine to a cysteine in the prokaryotic pLGIC homolog, propofol modulation switched from inhibition to potentiation (Ghosh et al., 2013). Charge and/or size of the functional group at the TM2 domain 15′ residue influenced propofol modification of the GLIC channel (Ghosh et al., 2013). In another example, the TM2 domain bends when glutamate chloride channels bound by ivermectin and stabilization of the second and third transmembrane domains occurs (Althoff et al., 2014). Thus to elucidate the site of interaction of amiloride, we mutated two residues that protrude into the intersubunit site (TM2 15′ and 19′ residues). Previous studies analyzed the influence of alcohol on the GABA-A ρ1 receptor and that the TM2 15′ (I15′) was integral for inhibition (Mihic et al., 1997). Furthermore, the TM2 15′ position has been implicated in the action of etomidate through the GABA-A β-subunit (Belelli et al., 1997) and anesthetics through the GABA-A α receptor subunit (Mascia et al., 2000). Consistent with these changes in amiloride allosteric modulation after the I15′N mutation, we propose that the intersubunit site is critical for amiloride potentiation in the human GABA-A ρ1 receptor.
The hGABA-A ρ1 TM2 6′ position was implicated in picrotoxin inhibition of several Cys-loop receptors, including the GABA-A ρ1 receptor (Pribilla et al., 1992; Gurley et al., 1995; Zhang et al., 1995). This residue has also been implicated in neurosteroid inhibition of the GABA-A ρ1 receptor, but with the appearance of a tail current similar to picrotoxin inhibition and channel blocking (Li et al., 2007). In the hGABA-A ρ1 T6′F and N19′D mutant receptors, amiloride antagonized the GABA-mediated current similar to what was observed in heteromeric GABA-A receptors (Fisher, 2002). The threonine at the TM2 6′ position is conserved among the inhibitory Cys-loop receptors (Fig. 2). Because we observed amiloride inhibition in these mutant receptors, perhaps the TM2 6′ position is not involved with direct amiloride binding within hGABA-A ρ1 receptor. Instead, the hGABA-A ρ1 T6′F mutation alters gating of the channel and converts the amiloride potentiation to inhibition, similar to the N19′D mutation. The hGABA-A ρ1 I15′N mutant receptor ameliorated amiloride potentiation without amiloride antagonism (Fig. 3). This change in amiloride activity in response to a mutation at the hGABA-A ρ1 TM2 domain 15′ position was similar to changes in both barbiturate and neurosteroid activity after mutagenesis of the channel’s TM2 15′ residue. When mutated to a serine, this residue introduces barbiturate sensitivity to the otherwise barbiturate-insensitive hGABA-A ρ1 receptor (Belelli et al., 1999).
The allosteric intersubunit can accommodate a large range of compounds. Resolved Cys-loop receptor structures have molecules bound within the intersubunit site that range in size from ethanol (84 Da) (Sauguet et al., 2013) to ivermectin (875 Da) (Hibbs and Gouaux, 2011). Amiloride is approximately 266 Da in size could fit into this intersubunit cavity. Furthermore, a simple reduction in 15′ site side chain volume (I to N, from 101 to 63.7 Å3) may not account for the loss of allosterism. However, this mutation increased amino acid side chain polarity. The asparagine side chain may mimic the presence of the amiloride or GMQ guanidinium group, resulting in loss of activity (amiloride) or decreased potency (GMQ) due to steric hindrance between protein and ligand. Furthermore, this is reflected in the increase in GABA potency in the presence of amiloride (Fig. 2; Table 1). The GABA-A ρ1 I15′N mutant receptor GABA EC50 approaches the same value as the wild-type receptor GABA EC50 in the presence of 300 μM amiloride (Fig. 3B; Table 2). One possible explanation for this activity is that the I15′N mutation mimics, when activated, the receptor in the presence of amiloride (Supplemental Fig. 2), thus preventing amiloride potentiation. This would suggest that the 15′ residue of the hGABA-A ρ1 receptor is integral in the potentiation activity of amiloride on this receptor. The isoleucine (whose amino acid R-group faces into the intersubunit site) creates an environment conducive for amiloride interaction. This same residue fails to accommodate a barbiturate or anesthetic, such as etomidate, to interact with this GABA-A ρ1 intersubunit site (Stewart et al., 2014).
Furthermore, GABA-A αβγ receptors are antagonized by GMQ in a competitive manner (Xiao et al., 2013). Here, GMQ inhibited the hGABA-A ρ1 receptor (Fig. 4) and the intersubunit mutations reduced GMQ potency (a 47-fold reduction) (Fig. 4E). The I15′N mutation reduced picrotoxin potency but to a lesser degree (13-fold reduction). Removing this hydrophobic side chain and replacing it with the polar side chain of asparagine reduces GMQ potency. We considered that GMQ may inhibit via the picrotoxin site found on the cytoplasmic side of the GABA-A ρ1 channel pore based upon the rebound current observed during GMQ antagonism. The observed GMQ effects in our study, such as the slight rebound current at the end of GABA and GMQ coapplication, are similar to the picrotoxin recordings of the GABA perch ρ1A receptor (Qian et al., 2005). The GABA-A ρ1 T6′F mutation resulted in a significant decrease in the IC50 and was similar to that observed in the GABA-A αβγ receptors (Fig. 4) (Xiao et al., 2013). The GABA-A ρ1 T6′F mutation eliminated picrotoxin activity but enhanced GMQ potency (a 33-fold increase). Based on this, the phenylalanine at the TM2 6′ position may have improved GMQ interaction at this site. However, additional studies are necessary to confirm that this TM2 6′ position is a GMQ binding site.
Our results describe the allosteric modulation of the human GABA-A ρ1 receptor by the guanidine compounds amiloride and GMQ. A single point mutation, the hGABA-A ρ1 I15′N, eliminated amiloride-positive allosteric modulation and provides compelling evidence to suggest that the TM2 15′ position (within the intersubunit site) contributes to the amiloride allosteric modulation site or to a guanidine compound binding site. Furthermore, the GABA-A ρ1 I15′N reduced GMQ potency. Both amiloride and GMQ increased GABA potency in the wild-type hGABA-A ρ1 receptor, which suggests that these guanidine compounds are allosteric modulators. These results provide support for the continued exploration of the molecular determinants of amiloride and GMQ allosterism in the GABA-A ρ1 receptor.
Supplementary Material
Acknowledgments
The authors thank Dr. Glenn Dillon for the generous gift of the human GABA-A ρ1 cDNA and Eric Gouaux for the kind gift of the enhanced green fluorescent protein cDNA. The authors also thank Rachel N. Smith and Amruta S. Agharkar for helpful discussions during the preparation of the manuscript.
Abbreviations
- ASIC
acid-sensing ion channel
- GLIC
Gloeobacter violaceus pentameric ligand-gated ion channel
- GMQ
2-guanidine-4-methylquinazoline
- hGABA
human GABA
- TM
transmembrane
- TPMPA
(1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid
Authorship Contributions
Participated in research design: Snell, Gonzales.
Conducted experiments: Snell.
Performed data analysis: Snell, Gonzales.
Wrote or contributed to the writing of the manuscript: Snell, Gonzales.
Footnotes
H.D.S. is supported by a training grant from the National Institutes of Health National Institute on Aging [Grant T32-AG020494]. This study was supported by the Welch Foundation [Grant BK-1736]; and the American Heart Association [Beginning Grant-In-Aid Grant 12BGIA8820001].
This work was presented at the following workshops: Snell HD and Gonzales EB (2014) Amiloride and GMQ allosteric modulation of the GABA-A ρ1 receptor: influences of the intersubunit site. University of North Texas Health Science Center 21st annual Research Appreciation Day; 2014 Apr 12; Fort Worth, TX; Snell HD and Gonzales EB (2014) Amiloride and GMQ allosteric modulation of the GABA-A ρ1 receptor: influences of the intersubunit site. Experimental Biology (EB) National Conference; 2014 Apr 26–30; San Diego, CA; Snell HD and Gonzales EB (2014) Amiloride and GMQ allosteric modulation of the GABA-A ρ1 receptor: influences of the intersubunit site. Society for the Advancement of Chicanos and Native Americans in Science (SACNAS) National Conference; 2014 Oct 16–18; Los Angeles, CA; and Snell HD and Gonzales EB (2014) Amiloride and GMQ allosteric modulation of the GABA-A ρ1 receptor: influences of the intersubunit site. Society for Neuroscience (SFN) National Conference; 2014 Nov 15–19; Washington, DC.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Abbott CJ, Percival KA, Martin PR, Grünert U. (2012) Amacrine and bipolar inputs to midget and parasol ganglion cells in marmoset retina. Vis Neurosci 29:157–168. [DOI] [PubMed] [Google Scholar]
- Alakuijala A, Palgi M, Wegelius K, Schmidt M, Enz R, Paulin L, Saarma M, Pasternack M. (2005) GABA receptor rho subunit expression in the developing rat brain. Brain Res Dev Brain Res 154:15–23. [DOI] [PubMed] [Google Scholar]
- Althoff T, Hibbs RE, Banerjee S, Gouaux E. (2014) X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors. Nature 512:333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin J. (1999) A single hydrophobic residue confers barbiturate sensitivity to gamma-aminobutyric acid type C receptor. Mol Pharmacol 55:411–423. [PubMed] [Google Scholar]
- Amin J, Weiss DS. (1994) Homomeric rho 1 GABA channels: activation properties and domains. Receptors Channels 2:227–236. [PubMed] [Google Scholar]
- Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. (1997) The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci USA 94:11031–11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Pau D, Cabras G, Peters JA, Lambert JJ. (1999) A single amino acid confers barbiturate sensitivity upon the GABA rho 1 receptor. Br J Pharmacol 127:601–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebib M, Gavande N, Wong KY, Park A, Premoli I, Mewett KN, Allan RD, Duke RK, Johnston GA, Hanrahan JR. (2009) Guanidino acids act as rho1 GABA(C) receptor antagonists. Neurochem Res 34:1704–1711. [DOI] [PubMed] [Google Scholar]
- Dibas MI, Gonzales EB, Das P, Bell-Horner CL, Dillon GH. (2002) Identification of a novel residue within the second transmembrane domain that confers use-facilitated block by picrotoxin in glycine alpha 1 receptors. J Biol Chem 277:9112–9117. [DOI] [PubMed] [Google Scholar]
- Dong CJ, Werblin FS. (1996) Use-dependent and use-independent blocking actions of picrotoxin and zinc at the GABAC receptor in retinal horizontal cells. Vision Res 36:3997–4005. [DOI] [PubMed] [Google Scholar]
- Drafts BC, Fisher JL. (2004) Structural determinants of the pharmacological properties of the GABAA receptor alpha6 subunit. J Pharmacol Exp Ther 309:1108–1115. [DOI] [PubMed] [Google Scholar]
- Fisher JL. (2002) Amiloride inhibition of gamma-aminobutyric acid(A) receptors depends upon the alpha subunit subtype. Mol Pharmacol 61:1322–1328. [DOI] [PubMed] [Google Scholar]
- Ghosh B, Satyshur KA, Czajkowski C. (2013) Propofol binding to the resting state of the gloeobacter violaceus ligand-gated ion channel (GLIC) induces structural changes in the inter- and intrasubunit transmembrane domain (TMD) cavities. J Biol Chem 288:17420–17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales EB, Bell-Horner CL, Dibas MI, Huang RQ, Dillon GH. (2008) Stoichiometric analysis of the TM2 6′ phenylalanine mutation on desensitization in alpha1beta2 and alpha1beta2gamma2 GABA A receptors. Neurosci Lett 431:184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutman JD, Calvo DJ. (2004) Studies on the mechanisms of action of picrotoxin, quercetin and pregnanolone at the GABA rho 1 receptor. Br J Pharmacol 141:717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greka A, Lipton SA, Zhang D. (2000) Expression of GABA(C) receptor rho1 and rho2 subunits during development of the mouse retina. Eur J Neurosci 12:3575–3582. [DOI] [PubMed] [Google Scholar]
- Gurley D, Amin J, Ross PC, Weiss DS, White G. (1995) Point mutations in the M2 region of the alpha, beta, or gamma subunit of the GABAA channel that abolish block by picrotoxin. Receptors Channels 3:13–20. [PubMed] [Google Scholar]
- Harrison NJ, Lummis SC. (2006) Locating the carboxylate group of GABA in the homomeric rho GABA(A) receptor ligand-binding pocket. J Biol Chem 281:24455–24461. [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E. (2011) Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata N, Ishihara T, Akaike N. (1988) Effects of diuretics on GABA-gated chloride current in frog isolated sensory neurones. Br J Pharmacol 93:679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Palmer MJ. (2011) Pharmacological analysis of the activation and receptor properties of the tonic GABA(C)R current in retinal bipolar cell terminals. PLoS ONE 6:e24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Gründer G, Lüddens H. (2002) Drug interactions at GABA(A) receptors. Prog Neurobiol 67:113–159. [DOI] [PubMed] [Google Scholar]
- Kusama T, Spivak CE, Whiting P, Dawson VL, Schaeffer JC, Uhl GR. (1993) Pharmacology of GABA rho 1 and GABA alpha/beta receptors expressed in Xenopus oocytes and COS cells. Br J Pharmacol 109:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Jin X, Covey DF, Steinbach JH. (2007) Neuroactive steroids and human recombinant rho1 GABAC receptors. J Pharmacol Exp Ther 323:236–247. [DOI] [PubMed] [Google Scholar]
- Li Y, Li YF, Xu TL. (2003a) Amiloride inhibition of glycinergic miniature IPSCs in mechanically dissociated rat spinal neurons. Neurosci Lett 349:17–20. [DOI] [PubMed] [Google Scholar]
- Li YF, Li Y, Xu TL. (2003b) Inhibition of glycine response by amiloride in rat spinal neurons. Neurosci Lett 345:173–176. [DOI] [PubMed] [Google Scholar]
- Liu F, Zhang M, Tang ZQ, Lu YG, Chen L. (2010) Inhibitory effects of amiloride on the current mediated by native GABA(A) receptors in cultured neurons of rat inferior colliculus. Clin Exp Pharmacol Physiol 37:435–440. [DOI] [PubMed] [Google Scholar]
- Mascia MP, Trudell JR, Harris RA. (2000) Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci USA 97:9305–9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP. (1997) Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389:385–389. [DOI] [PubMed] [Google Scholar]
- Miller C. (1989) Genetic manipulation of ion channels: a new approach to structure and mechanism. Neuron 2:1195–1205. [DOI] [PubMed] [Google Scholar]
- Neu A, Neuhoff H, Trube G, Fehr S, Ullrich K, Roeper J, Isbrandt D. (2002) Activation of GABA(A) receptors by guanidinoacetate: a novel pathophysiological mechanism. Neurobiol Dis 11:298–307. [DOI] [PubMed] [Google Scholar]
- Ng CK, Kim HL, Gavande N, Yamamoto I, Kumar RJ, Mewett KN, Johnston GA, Hanrahan JR, Chebib M. (2011) Medicinal chemistry of ρ GABAC receptors. Future Med Chem 3:197–209. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. (2008) International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev 60:243–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribilla I, Takagi T, Langosch D, Bormann J, Betz H. (1992) The atypical M2 segment of the beta subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J 11:4305–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Pan Y, Zhu Y, Khalili P. (2005) Picrotoxin accelerates relaxation of GABAC receptors. Mol Pharmacol 67:470–479. [DOI] [PubMed] [Google Scholar]
- Rho JM, Donevan SD, Rogawski MA. (1996) Direct activation of GABAA receptors by barbiturates in cultured rat hippocampal neurons. J Physiol 497:509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauguet L, Howard RJ, Malherbe L, Lee US, Corringer PJ, Harris RA, Delarue M. (2013) Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat Commun 4:1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ. (2005) PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res 33:W363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüttelkopf AW, van Aalten DM. (2004) PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D Biol Crystallogr 60:1355–1363. [DOI] [PubMed] [Google Scholar]
- Stewart DS, Pierce DW, Hotta M, Stern AT, Forman SA. (2014) Mutations at beta N265 in γ-aminobutyric acid type A receptors alter both binding affinity and efficacy of potent anesthetics. PLoS ONE 9:e111470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ZQ, Lu YG, Zhou KQ, Xu TL, Chen L. (2006) Amiloride attenuates glycine-induced currents in cultured neurons of rat inferior colliculus. Biochem Biophys Res Commun 350:900–904. [DOI] [PubMed] [Google Scholar]
- Xiao X, Zhu MX, Xu TL. (2013) 2-Guanidine-4-methylquinazoline acts as a novel competitive antagonist of A type γ-aminobutyric acid receptors. Neuropharmacology 75:126–137. [DOI] [PubMed] [Google Scholar]
- Xu M, Covey DF, Akabas MH. (1995) Interaction of picrotoxin with GABAA receptor channel-lining residues probed in cysteine mutants. Biophys J 69:1858–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Pan ZH, Zhang X, Brideau AD, Lipton SA. (1995) Cloning of a gamma-aminobutyric acid type C receptor subunit in rat retina with a methionine residue critical for picrotoxinin channel block. Proc Natl Acad Sci USA 92:11756–11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.