Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, representing a spectrum of liver pathologies that include simple hepatic steatosis and the more advanced nonalcoholic steatohepatitis (NASH). The current study was conducted to determine whether pediatric NASH also results in altered disposition of acetaminophen (APAP) and its two primary metabolites, APAP-sulfate and APAP-glucuronide. Pediatric patients with hepatic steatosis (n = 9) or NASH (n = 3) and healthy patients (n = 12) were recruited in a small pilot study design. All patients received a single 1000-mg dose of APAP. Blood and urine samples were collected at 1, 2, and 4 hours postdose, and APAP and APAP metabolites were determined by high-performance liquid chromatography. Moreover, human liver tissues from patients diagnosed with various stages of NAFLD were acquired from the Liver Tissue Cell Distribution System to investigate the regulation of the membrane transporters, multidrug resistance–associated protein 2 and 3 (MRP2 and MRP3, respectively). Patients with the more severe disease (i.e., NASH) had increased serum and urinary levels of APAP-glucuronide along with decreased serum levels of APAP-sulfate. Moreover, an induction of hepatic MRP3 and altered canalicular localization of the biliary efflux transporter, MRP2, describes the likely mechanism for the observed increase in plasma retention of APAP-glucuronide, whereas altered regulation of sulfur activation genes may explain decreased sulfonation activity in NASH. APAP-glucuronide and APAP-sulfate disposition is altered in NASH and is likely due to hepatic membrane transporter dysregulation as well as altered intracellular sulfur activation.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease in the United States and many other industrialized nations (Wieckowska and Feldstein, 2008; Lomonaco et al., 2013). Although NAFLD was initially described almost 30 years ago, the true scope of the disease has only recently been understood. NAFLD encompasses a number of progressive disease stages, linked by the presence of hepatocellular lipid accumulation. The full spectrum of the disorder ranges from simple steatosis to nonalcoholic steatohepatitis (NASH), which may progress to end-stage liver diseases, such as cirrhosis and hepatocellular carcinoma (Starley et al., 2010; Lomonaco et al., 2013).
Given the disease’s close association with metabolic morbidities, such as obesity and insulin resistance, NAFLD is often regarded as the hepatic manifestation of the metabolic syndrome. The number of Americans with these three conditions (NAFLD, insulin resistance, and obesity) has increased dramatically in the last few decades and is still on the rise. In 2008, the prevalence of obese adults (body mass index >30 kg/m2) in the United States was approximately 32% and is projected to reach more than 50% by 2030 (Wang et al., 2008; Flegal et al., 2010). Similarly, the prevalence of diabetes in the United States is estimated to increase from 14% in 2007 up to 33% by 2050, therefore concomitantly increasing the incidence of metabolic comorbidities such as NAFLD (Boyle et al., 2010).
Although NAFLD cases are largely benign and manifest as simple steatosis, progression to NASH significantly increases the risk for hepatic morbidity and mortality. NASH is the most common cause of cryptogenic cirrhosis and was reported to be the most likely risk factor for developing hepatocellular carcinoma (Preiss and Sattar, 2008; Rahimi and Landaverde, 2013). The prevalence of NAFLD is estimated to be 6%–30% worldwide, whereas NASH is reported to affect up to 12.2% of the general population (Lomonaco et al., 2013; Rahimi and Landaverde, 2013). Moreover, once thought of as primarily an adult disease, NAFLD is now known to afflict children as well.
It is now recognized that NAFLD is an important pediatric liver disorder (Patton et al., 2006; Lavine et al., 2010). Like in adults, as the prevalence rates for obesity rise among pediatric patients, so does the prevalence of NAFLD. It is estimated that 17% of children in Western society are overweight; among them, up to 80% may also have NAFLD (Giorgio et al., 2013). In pediatric patients, the prevalence of NAFLD is estimated to be 9.6%, and the rate is higher among adolescents (17.3%) than in infants (0.7%) (Patton et al., 2006; Schwimmer et al., 2006; Bozic et al., 2013).
It has been well documented that NAFLD, and especially NASH, alters the expression of proteins involved in drug metabolism and disposition, particularly membrane transporters and biotransformation enzymes (Fisher et al., 2009; Gomez-Lechon et al., 2009; Hardwick et al., 2011; Lake et al., 2011). We have previously reported that experimental NASH in a rat model alters drug transporter expression, resulting in a significant shift in the disposition of the acetaminophen (APAP) metabolite acetaminophen-glucuronide (APAP-gluc), from bile to blood and urine (Lickteig et al., 2007a). Moreover, a recent study has reported altered APAP-gluc disposition in children; however, a mechanism for these observations was left to speculation (Barshop et al., 2011).
The purpose of the current study was to determine the effects of pediatric NASH on the disposition of APAP and its primary metabolites, APAP-sulfate (APAP-sulf) and APAP-gluc. In addition, the regulation of two hepatic membrane transporters, MRP2 and MRP3, and intracellular sulfur activation were investigated in NASH as potential mechanisms for altered APAP disposition. Using a small pilot study design, this investigation contributes to the current knowledge of NASH in affecting drug disposition and offers an incentive for exploring these findings in a larger, randomized-controlled setting.
Materials and Methods
Tris-HCL, EDTA, NaCl, glycerol, and NP-40 were obtained from Sigma-Aldrich (St. Louis, MO).
Clinical Subjects
Pediatric subjects (n = 12) between the ages of 12 and 18 years were recruited from a pool of NAFLD patients. All patients (non-NAFLD exempt) had undergone a prior liver biopsy as part of routine patient care. These biopsies were used to establish two distinct groups of patients (simple steatosis and NASH) based on the severity of three characteristics of NAFLD: steatosis, fibrosis, and inflammation. In addition, pediatric subjects (n = 12) between the ages of 12 and 18 years were recruited from a panel of non-NAFLD patients. Many of these subjects were patients with constipation or abdominal pain without NAFLD. All patients and their legal guardians were approached during regular office visits, and informed consent was received. To participate in the current study, each subject passed a screening evaluation based on medical history and physical examination. Subjects also had to meet basic inclusion and exclusion criteria:
Inclusion Criteria.
Liver biopsy indicating either simple steatosis or NASH
Age 12–18 years
Informed consent and assent
Exclusion Criteria.
History of significant alcohol consumption (>20 g/day)
Clinical or histologic evidence of cirrhosis
Evidence of other chronic liver disease (e.g., Dubin-Johnson syndrome)
Presence of the hepatitis B virus surface antigen or hepatitis C virus antibodies
Use of drugs historically associated with NAFLD
Use of anti-NAFLD drugs in the 3 months preceding enrollment in this study
Pregnancy or breastfeeding
History of renal dysfunction
Other disease or conditions considered by the physician to be significant (e.g., cardiovascular disease)
Inclusion and exclusion criteria were applied to non-NAFLD participants as well, with the obvious exception of NAFLD diagnosis. Non-NAFLD patients were lean and had no significant medical history. The study and all study procedures were approved by the University of Arizona Institutional Review Board (no. 00004218) before study commencement.
Visit Procedures
Subjects arrived at the Clinical and Translational Science Center at the University of Arizona Medical Center on the morning of the study after an overnight fast. Subjects were advised to avoid any product containing APAP for at least 3 days before study participation. Approximately 7 ml of blood was collected in two separate tubes, one of which was immediately sent for serum biochemistry analysis. The second blood sample and an initial urine sample were used as blank samples in the quantification of serum APAP levels by HPLC. After these baseline collections of blood and urine, subjects were given one oral dose of 1000 mg of APAP (McNeil Consumer Healthcare, Fort Washington, PA). Subjects were allowed access to food and water during the 4-hour study. Blood and urine were collected at 1, 2, and 4 hours by the attending nursing staff. Approximately 7 ml of blood was collected at each time point into a serum-separator BD Vacutainer tube (Becton Dickinson, Franklin Lakes, NJ). Samples were allowed to clot and centrifuged to obtain the serum fraction. Each sample was subdivided into multiple aliquots to avoid excessive freeze/thaw cycles. The serum and urine samples for HPLC analysis were stored at –80°C for future analyses.
Human Liver Samples
Frozen and formalin-fixed, paraffin-embedded adult human liver tissue was obtained from the Liver Tissue Cell Distribution System, coordinated through the University of Minnesota, Virginia Commonwealth University, and the University of Pittsburgh, as described previously (Hardwick et al., 2011). Briefly, all samples were scored and categorized by a medical pathologist within the Liver Tissue Cell Distribution System according to a previously validated scoring rubric developed by (Kleiner et al., 2005), and pathology was then confirmed at the University of Arizona. Donor information, including the age and gender of the donors, has been published previously (Fisher et al., 2009). The samples were diagnosed as either normal (n = 20), steatotic (n = 12), NASH with fatty liver (NASH fatty, n = 11), or NASH without fatty liver (NASH not fatty/cirrhosis, n = 11). For the purposes of this study, NASH fatty and NASH without fat were combined as one experimental group diagnosed as NASH. Those samples exhibiting >10% fatty infiltration of hepatocytes were staged as steatotic. Samples were diagnosed as NASH when >5% fatty infiltration of hepatocytes occurred with significant inflammation and fibrosis. These liver specimens were used for protein, mRNA, and immunohistochemical analyses described as follows.
Tissue Preparations
Whole-cell lysate preparations of human liver tissue were prepared from tissue homogenized in NP-40 buffer [20 mM Tris HCl, 137 mM NaCl, 10% glycerol, 1% NP-40, and 2 mM EDTA with one Protease Inhibitor Cocktail tablet (Roche, Indianapolis, IN) per 25ml) at 4°C. Homogenized tissue was then agitated at 4°C for 2 hours, centrifuged at 10,000g for 30 minutes, and the supernatant transferred to a clean collection tube. Protein concentrations were determined using the Pierce BCA Protein Quantitation Assay (Thermo Scientific, Rockford, IL) per the manufacturer’s recommendations.
MRP3 Immunoblot Analysis
Whole-cell lysate proteins (50 µg/well) were prepared in Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA) in nonreducing conditions (without β-mercaptoethanol or boiling) and separated by SDS-PAGE using 7.5% Tris-Glycine gels followed by transfer to polyvinylidine fluoride membranes. Membranes were blocked for 1 hour at room temperature using a 5% nonfat dry milk solution dissolved in phosphate buffered saline-Tween. After the membrane block, the membrane was incubated overnight in a primary antibody incubation (in 5% milk solution) using the following mouse monoclonal antibody raised against MRP3 protein: M3II-9 (Abcam, Inc., Cambridge, MA); an anti-mouse HRP-conjugated secondary (sc-2005; Santa Cruz Biotechnology, Santa Cruz, CA.) was used for detection and incubated for 1 hour at room temperature. Quantification of relative protein expression was determined using image processing and analysis with ImageJ Software (National Institutes of Health, Bethesda, MD) and normalized to total pan-Cadherin (1:7000, Abcam, Inc.).
MRP2 Liver Immunohistochemistry
Immunohistochemical staining for all proteins was performed on formalin-fixed, paraffin-embedded human liver samples as described previously (Hardwick et al., 2011). Briefly, tissue sections were deparaffinized in xylene and rehydrated in ethanol, followed by antigen retrieval in citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked with 0.3% (v/v) H2O2 in methanol for 20 minutes. Immunohistochemical staining for MRP2 was performed with the MACH3 staining kit (Biocare Medical, Concord, CA) per the manufacturer’s protocol. Samples were incubated in an MRP2 primary antibody (M2III-5; Kamiya Biomedical Company, Seattle, WA) solution overnight at 4°C. All slides were counterstained with hematoxylin (Sigma Aldrich) after color development with Betazoid DAB (Biocare Medical). All slides were imaged with a Nikon Eclipse E4000 microscope (Nikon Corporation, Tokyo, Japan) and a Sony Exwave DXC-390 camera (Sony Corporation, Tokyo, Japan).
APAP and APAP Metabolite Quantification
APAP and its primary metabolites (APAP-gluc and APAP-sulf) were analyzed in serum and urine under high-performance liquid chromatography (HPLC) conditions followed by UV detection as previously described (Lickteig et al., 2007a).
Microarray Expression Analysis of Sulfur Activation Genes
Individual Affymetrix GeneChip Human 1.0 ST Arrays (Affymetrix, La Jolla, CA) were generated from purified mRNA for each liver sample as previously described (Lake et al., 2011). The expression of 33,252 annotated and unannotated genes among three diagnosis groups (normal, steatosis, and NASH) is available in the array data set, which is accessible at the ArrayExpress public repository for microarray data (accession no. E-MEXP-3291) (http://www.webcitation.org/5zyojNu7T).
Statistical Analysis
Statistical differences between patient groups at each time point were determined using a one-way analysis of variance followed by a Bonferroni posthoc test. Immunoblot data were analyzed by a nonparametric trend analysis and described as box and whisker plots. The level of significance was set at P ≤ 0.05 for all analyses using Stata9 statistical software (Stata, College Station, TX).
Results
Patient Demographics and Serum Chemistry.
Table 1 displays study participant demographics and the results of serum biochemistry tests taken at the time of the study. Male patients were more predominant than female patients, particularly within the simple steatosis and NASH patient groups. Median ages were similar across groups. Analysis of hepatic function was measured, including total serum protein, serum albumin, conjugated (direct) and total bilirubin, alkaline phosphatase, aspartate aminotransaminase, and alanine transaminase. No statistical difference was observed in all analytes measured except aspartate aminotransaminase and alanine transaminase, which were significantly elevated in patients with NASH (Table 1). Moreover, most of the NAFLD patients recruited for the study (steatosis and NASH) were male and of a Hispanic ethnic background, indicative of the local population (data not shown).
TABLE 1.
Study participant demographics and blood chemistry
| Healthy (n = 12) | Simple Steatosis (n = 9) | NASH (n = 3) | P value | |
|---|---|---|---|---|
| Age [median (range)], yr | 15 (13–18) | 13 (12–16) | 13 (10–14) | — |
| Sex (M/F) | 5/7 | 8/1 | 3/0 | — |
| Protein, total (g/dl) | 7.1 ± 0.1 | 7.3 ± 0.1 | 7.4 ± 0.3 | 0.54 |
| Albumin (g/dl) | 4.4 ± 0.1 | 4.6 ± 0.1 | 4.6 ± 0.1 | 0.24 |
| Bilirubin, total (mg/dl) | 0.4 ± 0 | 0.5 ± 0.2 | 0.4 ± 0 | 0.72 |
| Bilirubin, direct (mg/dl) | 0.1 ± 0 | 0.1 ± 0 | nm | na |
| Alkaline phosphatase (IU/l) | 139.4 ± 27.2 | 204.1 ± 30.6 | 260 ± 13.2 | 0.08 |
| Aspartate aminotransferase (IU/l) | 21.1 ± 0.7 | 27.4 ± 3.8 | 85 ± 31.2 | <0.0001* |
| Alanine aminotrasferase (IU/l) | 16.3 ± 1.5 | 31.7 ± 6.0 | 134 ± 88.2 | 0.004* |
na, not applicable; nm, not measured.
P < 0.05 from NASH compared with healthy and simple steatosis.
Serum and Urine APAP and APAP Metabolites in Pediatric NAFLD Patients.
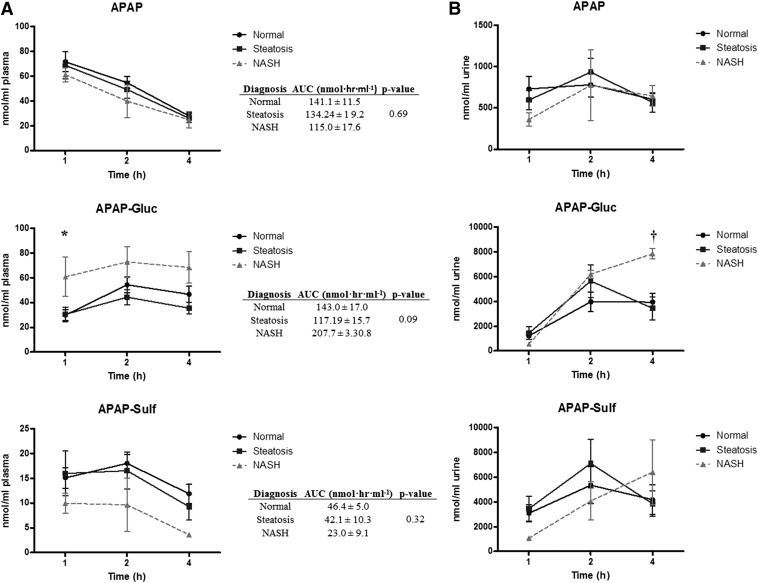
To determine the effects of NAFLD on APAP disposition, all study participants were given a single oral dose of APAP (1000 mg), followed by measurement of parent and APAP-gluc and APAP-sulf in serum and urine at 1, 2, and 4 hours postdose. The results of the analysis are described in Fig. 1. No difference was observed in APAP serum or urine levels in the three groups at any time point. The levels of APAP-sulf in serum tended to decrease at all time points measured in patients with NASH, but these data did not reach statistical significance (Fig. 1A). In contrast, serum APAP-gluc levels in children with NASH were significantly increased above normal patients at 1 hour after administration, and remained elevated compared with patients in both the normal and steatosis groups over all time points measured; however, plasma AUC values did not differ significantly across all three groups (Fig. 1A). The increase in serum APAP-gluc in NASH subjects was accompanied by a significant increase in urine excretion of APAP-gluc at 4 hours compared with patients with simple steatosis (Fig. 1B). No change in APAP-sulf concentration was detected in the urine across all subjects.
Fig. 1.
Plasma and urine APAP, APAP-gluc, and APAP-sulf in pediatric NAFLD. Plasma (A) and urine (B) concentrations of APAP, APAP-gluc, and APAP-sulf after a single oral administration of 1000 APAP. Samples were collected at 1, 2, and 4 hours postdosing and measured by HPLC-UV. Area under the plasma versus time curve (AUC) for APAP, APAP-gluc, and APAP-sulf were calculated for all three diagnosis stages and are provided as an insert to (A). P values provided for the AUC data represent the calculated ANOVA P value across all three diagnosis stages. *P < 0.05 healthy patients compared with NASH patients; †P < 0.05 patients with simple steatosis compared with NASH patients.
Hepatic MRP3 and MRP2 Regulation in Human NASH Patients.
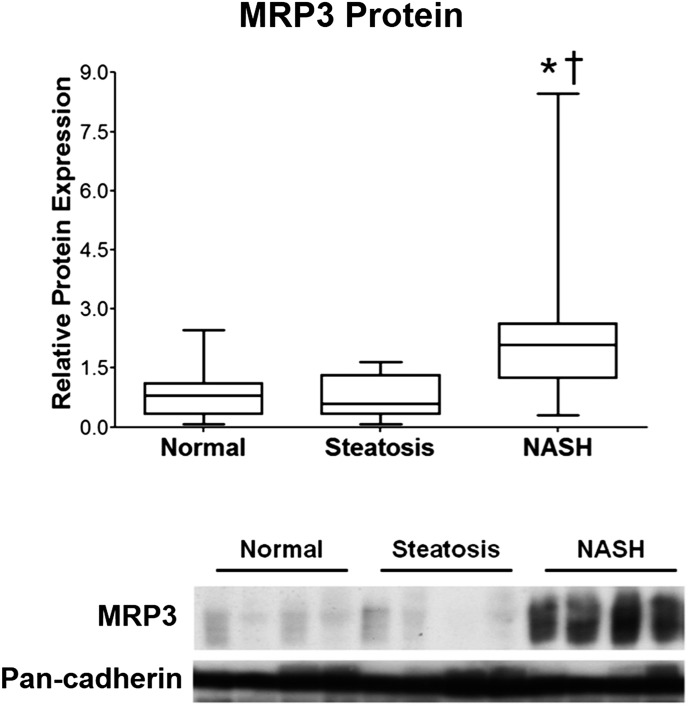
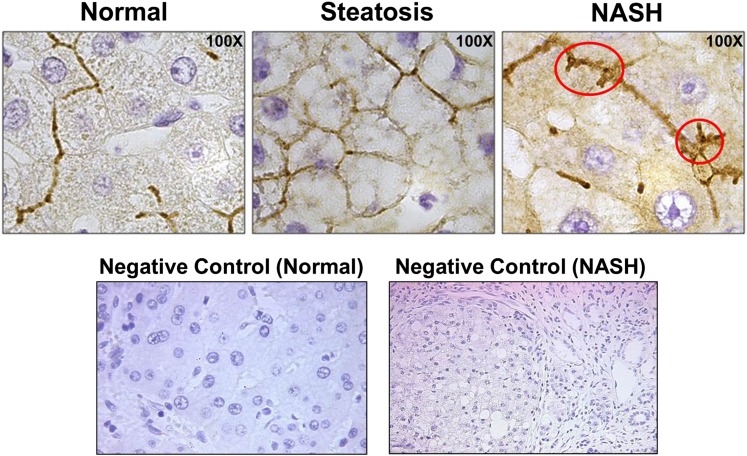
To investigate a potential molecular mechanism responsible for the observed effects of NASH on APAP-gluc disposition, the regulation of hepatic membrane transporters, MRP3 and MRP2, was determined via Western blot analysis and immunohistochemistry, respectively. The expression of the basolateral efflux transporter, MRP3, is significantly elevated in the livers of patients with NASH (Fig. 2). In contrast, the apical efflux transporter responsible for biliary efflux of APAP-gluc, MRP2, appears to be improperly localized away from the canalicular membrane of hepatocytes in NASH patients (Fig. 3) as evidenced by the lack of sharp, localized staining on the apical membrane (Fig. 3, circled), which is in contrast to what is observed in healthy and steatosis subjects.
Fig. 2.
Hepatic MRP3 protein induction in NASH. MRP3 protein was measured via immunoblot analysis from human liver samples obtained from human patients with ages ranging from 16–70 that were diagnosed as being normal, steatotic (NAFLD), or having NASH as described in Materials and Methods. * P < 0.05 healthy patients compared with NASH patients; † P < 0.05 patients with simple steatosis compared with NASH.
Fig. 3.
Hepatic MRP2 localization in patients with NASH. Immunohistochemistry was used to detect and visualize MRP2 protein localization within normal, steatosis, and NASH liver. The red circles indicate regions of perturbed localization of MRP2 on the canalicular membrane. Negative controls (performed without primary antibody) are included to demonstrate positive membrane staining. All images were taken at 100× magnification and are representative images of multiple immunohistochemical sample analyses.
Sulfur Activation and Utilization Gene Expression.
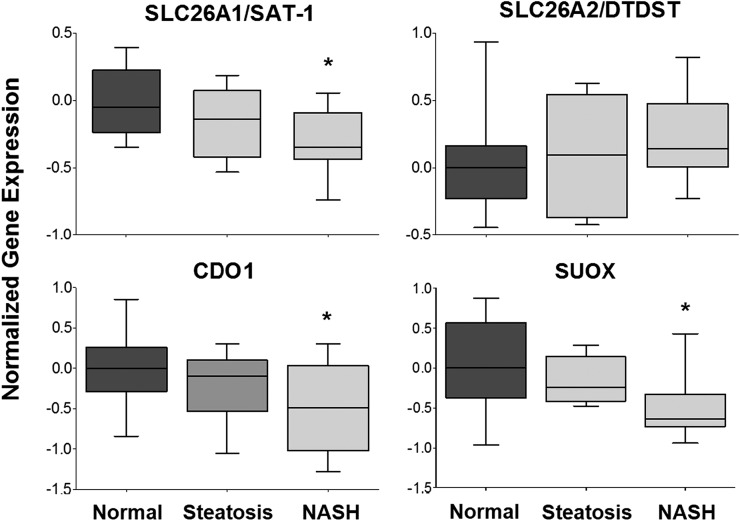
To further determine the potential for altered SULT activity in vivo, we used results from a previously validated and published microarray experiment in these same human samples to determine the expression of key players in the sulfur activation and utilization pathway (Lake et al., 2011). Results of the gene expression analysis are shown in Fig. 4. Expression of the sulfur uptake transporter, SLC26A1, is significantly reduced in NASH livers compared with normal livers; however, expression of SLC26A2 was unchanged. In addition to extracellular uptake, sulfur may also be liberated from cysteine pools in the cell through the action of cysteine dioxygenase type 1 (CDO1) resulting in sulfite which is then converted to sulfate by sulfite oxidase (SUOX) (Markovich, 2001; Wilkinson and Waring, 2002; Feng et al., 2007). CDO1 and SUOX were both significantly downregulated in NASH samples compared with normal levels, suggesting that there is a decreased potential for sulfate activation from intracellular sources.
Fig. 4.
Normalized gene expression of sulfur activation and utilization pathways. Hepatic gene expression of enzymes and transporters involved in the activation and utilization of sulfur are shown in human liver samples diagnosed as normal, steatotic, and NASH. Gene expression data were mined from a previously validated and published microarray experiment performed in a subset of aforementioned human liver samples (Kleiner et al., 2005). Data were normalized to the median of the normal diagnostic category and presented as normalized gene expression. *P < 0.05 healthy patients compared with NASH patients.
Discussion
The purpose of the current study was to investigate the effects of NASH on the disposition of APAP and its two primary metabolites, APAP-gluc and APAP-sulf, in a cohort of pediatric patients. Our results indicate that children with NASH tend to have increased retention of the metabolite APAP-gluc in systemic circulation, along with increased excretion into the urine. Moreover, we identified the dysregulation of the hepatic membrane transporters MRP2 and MRP3 as a potential mechanism for these observations. However, a significant limitation of this study is the low number of NASH patients that were recruited in the study, as well as the lack of ethnic, racial, and sexual diversity within our patient pool, which was primarily male and of Hispanic background. Therefore, the validation and significance of these findings as a potential diagnostic to distinguish patients with NASH from those with milder disease will benefit from the inclusion of a larger randomized trial in the future. Moreover, the analyses of MRP2 and MRP3 expression were performed in liver samples obtained from primarily an adult population, whereas the age range of the clinical subjects tested was 12–18 years. Developmental patterns in transporter expression are known to exist; however, recent evidence demonstrates that, at least for MRP2 and MDR1, expression appears to stabilize before puberty (Prasad et al., 2013; Mooij et al., 2014), suggesting that the expression data presented in this article may be an accurate representation of the expression profiles of the adolescents on study. Despite these limitations, the results gathered from this study warrant further consideration when administering pharmaceuticals to children with NAFLD, as they may be at higher risk for developing adverse drug reactions as a result of aberrant drug disposition.
The metabolism and disposition of APAP have been heavily investigated and well characterized. Glucuronidation and sulfonation of APAP in the liver are the predominant metabolic pathways and account for 50%–70% and 25%–35% of acetaminophen, respectively (McGill and Jaeschke, 2013). Unlike APAP parent, APAP-gluc and APAP-sulf are considerably more polar and require the aid of membrane transporters for proper efflux and excretion from the body. In healthy livers, biliary excretion of the sulfate and glucuronide conjugates of APAP is predominantly mediated by MRP2, which is localized to the apical, or canalicular membrane of hepatocytes (Xiong et al., 2000). Sinusoidal excretion of the APAP-gluc metabolite from hepatocytes is predominantly mediated by MRP3 (Manautou et al., 2005), whereas MRP4, which is also expressed on the sinusoidal membrane, appears to mediate excretion of APAP-sulf metabolites (Zamek-Gliszczynski et al., 2006).
Despite the limited number of NASH patients, our findings are consistent with previous investigations. Specifically, we have previously reported the effects of experimental NASH on the disposition of APAP-gluc (Lickteig et al., 2007a). Similar to our observations in pediatric patients, NASH rodents demonstrated an increase in plasma and urine levels of APAP-gluc (Lickteig et al., 2007a). Moreover, these findings were coupled to decreased biliary excretion of APAP-gluc. This shift from bile to plasma disposition was presumably due to an induction of the hepatic sinusoidal membrane transporter, MRP3, which we also found to be induced in human NASH as described herein. It is interesting to note, however, that although biliary excretion of APAP-gluc was decreased, MRP2 protein was induced in experimental NASH (Lickteig et al., 2007a). This anomaly was later confirmed using a rat model of NASH, in which it was noted that Mrp2 localization was altered, which subsequently led to altered ezetimibe disposition (Hardwick et al., 2012). These effects were suggested to be due to MRP2 being internalized from the canalicular membrane, which would consequently diminish MRP2-mediated excretion into the bile. Indeed, previous investigations have demonstrated that Mrp2 is reversibly internalized into intracellular compartments and mediated by the redox-sensitive balance of protein kinase C activation in experimental cholestasis (Mottino et al., 2002; Sekine et al., 2008, 2011). Oxidative stress is a central feature of NASH pathogenesis, and we have previously identified the activation of key redox-sensitive genes in both humans and rodents with NASH (Lickteig et al., 2007b; Hardwick et al., 2010). Together, our data confirm the original findings in the rodent model and suggest that MRP2 localization is altered in human NASH.
Barshop et al. (2011) previously reported that acetaminophen disposition is altered in pediatric patients with NAFLD. In this study, APAP-gluc was slightly increased in both the plasma and urine of pediatric patients with NAFLD. Our results are in agreement with these previous findings and demonstrate that the mechanistic features of transport function in pediatric NASH causes a functional disruption in APAP-gluc disposition. In contrast to our conclusions, however, the previous investigators allude to altered glucuronidation, presumably via disruption of enzymatic activity, as a mechanism for the observed increase in systemic APAP-gluc levels (Barshop et al., 2011). Although we did not investigate glucuronidation capacity in these patients, more recent findings have demonstrated that APAP glucuronidation is not altered in NASH subjects, suggesting that the observed effects of NASH on APAP-gluc disposition are less likely mediated by altered glucuronidation (Hardwick et al., 2013). In contrast, we argue that decreased MRP2 function (resulting from altered membrane localization) coupled to induction of MRP3 is the primary mechanism resulting in increased systemic exposure to APAP-gluc in these patients. However, glucuronidation capacity is known to be variable among children; therefore, further characterization of APAP glucuronidation in children with NASH is needed for a more comprehensive conclusion.
In spite of the induction in MRP3 protein expression, we did not observe a concomitant increase in serum APAP-sulf levels after APAP administration in these NASH subjects. In contrast, we report a decreasing trend in serum APAP-sulf in NASH, which is consistent with previous findings in a rodent model dosed with APAP (Lickteig et al., 2007a). These results are also in parallel with previous data demonstrating decreased pan-sulfotransferase activity in NASH despite the protein induction of several sulfotransferase isoforms in the disease (Hardwick et al., 2013). Moreover, decreased total sulfotransferase activity in humans with alcoholic and nonalcoholic liver disease was identified in an independent study (Yalcin et al., 2013). Together, these results are suggestive of disrupted cellular sulfur activation and utilization in NASH, which would ultimately limit the intracellular concentrations of the sulfonation cofactor, 3′-phosphoadenosine-5∋-phosphosulfate (PAPS). Indeed, we demonstrate altered expression of genes critically involved in sulfur utilization in this study. Specifically, we identify decreased expression of the sulfur uptake transporter, SLC26A1, as well as deceased expression of CDO1 and SUOX, which are important in liberating intracellular sulfur pools from the amino acid cysteine (Wilkinson and Waring, 2002; Feng et al., 2007). Together, these results demonstrate decreased hepatic capacity to synthesize PAPS in NASH, which may partially explain the slight decrease in serum APAP-sulf observed.
It is worth noting that, in addition to APAP-gluc being elevated compared with normal levels in healthy clinical subjects, it is also elevated in NASH patients compared with patients with simple steatosis attributable to the mechanistic features of altered transporter function only present at the later stage of the disease. This finding highlights the possible utility of using serum APAP-gluc levels as a potential biomarker to be used as a noninvasive tool for distinguishing NASH from “not NASH.” Histologic analysis of a liver biopsy remains the gold standard in diagnosing NAFLD, as it is able to assess steatosis, fibrosis, and inflammation, as well as changes in overall liver architecture (Wieckowska and Feldstein, 2008). This procedure is invasive, however, and impractical to use per standard of care. Moreover, current methods of distinguishing patients with steatosis from those with NASH lack the specificity and sensitivity to replace liver biopsy. Most NAFLD diagnoses are currently made on the basis of elevated aminotransferase levels, but normal serum aminotransferase tests may be seen in patients with both steatosis and NASH (Ipekci et al., 2003; Mofrad et al., 2003), which is consistent with our observations (Table 1). Furthermore, several investigators have reported that two-thirds of NASH patients may have normal aminotransferase levels at any given time (Delgado, 2008; Oh et al., 2008; Wieckowska and Feldstein, 2008), highlighting the need for a more effective, noninvasive means of diagnosing NASH. The clinical development of an APAP-gluc disposition test for NASH may help indicate at-risk patients for diagnostic liver biopsies or serve as a noninvasive means of tracking progression and treatment of the disease.
In conclusion, the results obtained from this preliminary pilot study demonstrate the potential for NAFLD to disrupt drug pharmacokinetics in children. Specifically, altered MRP2 localization and MRP3 induction appear to represent a primary mechanism for the increase in APAP-gluc in pediatric NAFLD patients. Although APAP-gluc is pharmacologically and/or toxicologically inactive, serious health risks may be imposed in the event of increased systemic exposure of a highly active metabolite in patients with NAFLD. Further understanding how NASH affects renal clearance mechanisms would also be highly beneficial in understanding how the disease affects all excretory routes and, of course, a larger sample size in a randomized controlled setting is warranted for further verification of these findings and their clinical implications.
Abbreviations
- APAP
N-acetyl-para-aminophenol
- APAP-gluc
N-acetyl-para-aminophenol glucuronide
- APAP-sulf
N-acetyl-para-aminophenol
- CDO1
cysteine dioxygenase type 1
- HPLC
high-performance liquid chromatography
- MRP2
multidrug resistance–associated protein 2
- MRP3
multidrug resistance–associated protein 3
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- SUOX
sulfite oxidase
Authorship Contributions
Participated in research design: Canet, Merrell, Erickson, Cherrington.
Conducted experiments: Canet, Merrell, Hardwick, Bataille, Campion, Ferreira.
Contributed new reagents: Manautou, A-Kader, Erickson, Xanthakos.
Performed data analysis: Canet, Merrell, Cherrington.
Wrote or contributed to the writing of the manuscript: Canet, Merrell, Cherrington.
Footnotes
This work was supported by the National Institutes of Health [Grants DK068039, HD062489, ES006694], the National Institutes of Health National Institute of Environmental Health Science Toxicology Training Grant [Grant ES007091], and The National Science Foundation of Arizona.
References
- Barshop NJ, Capparelli EV, Sirlin CB, Schwimmer JB, Lavine JE. (2011) Acetaminophen pharmacokinetics in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr 52:198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. (2010) Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence (Abstract). Popul Health Metr 8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozic MA, Subbarao G, Molleston JP. (2013) Pediatric nonalcoholic fatty liver disease. Nutr Clin Pract 28:448–458. [DOI] [PubMed] [Google Scholar]
- Delgado JS. (2008) Evolving trends in nonalcoholic fatty liver disease. Eur J Intern Med 19:75–82. [DOI] [PubMed] [Google Scholar]
- Feng C, Tollin G, Enemark JH. (2007) Sulfite oxidizing enzymes. Biochim Biophys Acta 1774:527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CD, Lickteig AJ, Augustine LM, Ranger-Moore J, Jackson JP, Ferguson SS, Cherrington NJ. (2009) Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos 37:2087–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303:235–241. [DOI] [PubMed] [Google Scholar]
- Giorgio V, Prono F, Graziano F, Nobili V. (2013) Pediatric non alcoholic fatty liver disease: old and new concepts on development, progression, metabolic insight and potential treatment targets (Abstract). BMC Pediatr 13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Lechón MJ, Jover R, Donato MT. (2009) Cytochrome p450 and steatosis. Curr Drug Metab 10:692–699. [DOI] [PubMed] [Google Scholar]
- Hardwick RN, Ferreira DW, More VR, Lake AD, Lu Z, Manautou JE, Slitt AL, Cherrington NJ. (2013) Altered UDP-glucuronosyltransferase and sulfotransferase expression and function during progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 41:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Canet MJ, Lake AD, Cherrington NJ. (2010) Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 38:2293–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Canet MJ, Scheffer GL, Cherrington NJ. (2011) Variations in ATP-binding cassette transporter regulation during the progression of human nonalcoholic fatty liver disease. Drug Metab Dispos 39:2395–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Street SM, Canet MJ, Cherrington NJ. (2012) Molecular mechanism of altered ezetimibe disposition in nonalcoholic steatohepatitis. Drug Metab Dispos 40:450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipekci SH, Basaranoglu M, Sonsuz A. (2003) The fluctuation of serum levels of aminotransferase in patients with nonalcoholic steatohepatitis (Abstract). J Clin Gastroenterol 36:371. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Nonalcoholic Steatohepatitis Clinical Research Network (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321. [DOI] [PubMed] [Google Scholar]
- Lake AD, Novak P, Fisher CD, Jackson JP, Hardwick RN, Billheimer DD, Klimecki WT, Cherrington NJ. (2011) Analysis of global and absorption, distribution, metabolism, and elimination gene expression in the progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 39:1954–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine JE, Schwimmer JB, Molleston JP, Scheimann AO, Murray KF, Abrams SH, Rosenthal P, Sanyal AJ, Robuck PR, Brunt EM, et al. Nonalcoholic Steatohepatitis Clinical Research Network Research Group (2010) Treatment of nonalcoholic fatty liver disease in children: TONIC trial design. Contemp Clin Trials 31:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickteig AJ, Fisher CD, Augustine LM, Aleksunes LM, Besselsen DG, Slitt AL, Manautou JE, Cherrington NJ. (2007a) Efflux transporter expression and acetaminophen metabolite excretion are altered in rodent models of nonalcoholic fatty liver disease. Drug Metab Dispos 35:1970–1978. [DOI] [PubMed] [Google Scholar]
- Lickteig AJ, Fisher CD, Augustine LM, Cherrington NJ. (2007b) Genes of the antioxidant response undergo upregulation in a rodent model of nonalcoholic steatohepatitis. J Biochem Mol Toxicol 21:216–220. [DOI] [PubMed] [Google Scholar]
- Lomonaco R, Sunny NE, Bril F, Cusi K. (2013) Nonalcoholic fatty liver disease: current issues and novel treatment approaches. Drugs 73:1–14. [DOI] [PubMed] [Google Scholar]
- Manautou JE, de Waart DR, Kunne C, Zelcer N, Goedken M, Borst P, Elferink RO. (2005) Altered disposition of acetaminophen in mice with a disruption of the Mrp3 gene. Hepatology 42:1091–1098. [DOI] [PubMed] [Google Scholar]
- Markovich D. (2001) Physiological roles and regulation of mammalian sulfate transporters. Physiol Rev 81:1499–1533. [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. (2013) Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res 30:2174–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, Shiffman ML, Stravitz RT, Sanyal AJ. (2003) Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 37:1286–1292. [DOI] [PubMed] [Google Scholar]
- Mooij MG, Schwarz UI, de Koning BAE, Leeder JS, Gaedigk R, Samsom JN, Spaans E, van Goudoever JB, Tibboel D, Kim RB, et al. (2014) Ontogeny of human hepatic and intestinal transporter gene expression during childhood: age matters. Drug Metab Dispos 42:1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottino AD, Cao J, Veggi LM, Crocenzi F, Roma MG, Vore M. (2002) Altered localization and activity of canalicular Mrp2 in estradiol-17beta-d-glucuronide-induced cholestasis. Hepatology 35:1409–1419. [DOI] [PubMed] [Google Scholar]
- Oh MK, Winn J, Poordad F. (2008) Review article: diagnosis and treatment of non-alcoholic fatty liver disease. Aliment Pharmacol Ther 28:503–522. [DOI] [PubMed] [Google Scholar]
- Patton HM, Sirlin C, Behling C, Middleton M, Schwimmer JB, Lavine JE. (2006) Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. J Pediatr Gastroenterol Nutr 43:413–427. [DOI] [PubMed] [Google Scholar]
- Prasad B, Lai Y, Lin Y, Unadkat JD. (2013) Interindividual variability in the hepatic expression of the human breast cancer resistance protein (BCRP/ABCG2): effect of age, sex, and genotype. J Pharm Sci 102:787–793. [DOI] [PubMed] [Google Scholar]
- Preiss D, Sattar N. (2008) Non-alcoholic fatty liver disease: an overview of prevalence, diagnosis, pathogenesis and treatment considerations. Clin Sci (Lond) 115:141–150. [DOI] [PubMed] [Google Scholar]
- Rahimi RS, Landaverde C. (2013) Nonalcoholic fatty liver disease and the metabolic syndrome: clinical implications and treatment. Nutr Clin Pract 28:40–51. [DOI] [PubMed] [Google Scholar]
- Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. (2006) Prevalence of fatty liver in children and adolescents. Pediatrics 118:1388–1393. [DOI] [PubMed] [Google Scholar]
- Sekine S, Ito K, Horie T. (2008) Canalicular Mrp2 localization is reversibly regulated by the intracellular redox status. Am J Physiol Gastrointest Liver Physiol 295:G1035–G1041. [DOI] [PubMed] [Google Scholar]
- Sekine S, Ito K, Saeki J, Horie T. (2011) Interaction of Mrp2 with radixin causes reversible canalicular Mrp2 localization induced by intracellular redox status. Biochim Biophys Acta 1812:1427–1434. [DOI] [PubMed] [Google Scholar]
- Starley BQ, Calcagno CJ, Harrison SA. (2010) Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 51:1820–1832. [DOI] [PubMed] [Google Scholar]
- Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. (2008) Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 16:2323–2330. [DOI] [PubMed] [Google Scholar]
- Wieckowska A, Feldstein AE. (2008) Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis 28:386–395. [DOI] [PubMed] [Google Scholar]
- Wilkinson LJ, Waring RH. (2002) Cysteine dioxygenase: modulation of expression in human cell lines by cytokines and control of sulphate production. Toxicol In Vitro 16:481–483. [DOI] [PubMed] [Google Scholar]
- Xiong H, Turner KC, Ward ES, Jansen PL, Brouwer KL. (2000) Altered hepatobiliary disposition of acetaminophen glucuronide in isolated perfused livers from multidrug resistance-associated protein 2-deficient TR(-) rats. J Pharmacol Exp Ther 295:512–518. [PubMed] [Google Scholar]
- Yalcin EB, More V, Neira KL, Lu ZJ, Cherrington NJ, Slitt AL, King RS. (2013) Downregulation of sulfotransferase expression and activity in diseased human livers. Drug Metab Dispos 41:1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Nezasa K, Tian X, Bridges AS, Lee K, Belinsky MG, Kruh GD, Brouwer KL. (2006) Evaluation of the role of multidrug resistance-associated protein (Mrp) 3 and Mrp4 in hepatic basolateral excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in Abcc3-/- and Abcc4-/- mice. J Pharmacol Exp Ther 319:1485–1491. [DOI] [PubMed] [Google Scholar]