Abstract
Cardiac gene therapy has emerged as a promising option to treat advanced heart failure (HF). Advances in molecular biology and gene targeting approaches are offering further novel options for genetic manipulation of the cardiovascular system. The aim of this study was to improve cardiac function in chronic HF by overexpressing constitutively active inhibitor-1 (I-1c) using a novel cardiotropic vector generated by capsid reengineering of adeno-associated virus (BNP116). One month after a large anterior myocardial infarction, 20 Yorkshire pigs randomly received intracoronary injection of either high-dose BNP116.I-1c (1.0 × 1013 vector genomes (vg), n = 7), low-dose BNP116.I-1c (3.0 × 1012 vg, n = 7), or saline (n = 6). Compared to baseline, mean left ventricular ejection fraction increased by 5.7% in the high-dose group, and by 5.2% in the low-dose group, whereas it decreased by 7% in the saline group. Additionally, preload-recruitable stroke work obtained from pressure–volume analysis demonstrated significantly higher cardiac performance in the high-dose group. Likewise, other hemodynamic parameters, including stroke volume and contractility index indicated improved cardiac function after the I-1c gene transfer. Furthermore, BNP116 showed a favorable gene expression pattern for targeting the heart. In summary, I-1c overexpression using BNP116 improves cardiac function in a clinically relevant model of ischemic HF.
Introduction
Significant progresses in treatment of acute cardiac diseases, including acute myocardial infarction (MI) and decompensated heart failure (HF), have increased survival rates dramatically. However, patients who survive the acute phase suffer from chronic HF, and population studies show growing numbers of this demographic.1 Despite the improved care for these patients, mortality of chronic HF is high and remains to be the main cause of death in the developed world.1 The primary course of rescue for these patients continues to be cardiac transplantation. However, this choice suffers from a lack of sufficient organ supply and is highly invasive. Together with an aging population, application of cardiac transplant will be highly limited, and alternative treatments with less invasive and widely applicable means are needed.
Gene therapy is emerging as a promising therapeutic approach for treating chronic HF, supported by a growing number of positive preclinical studies2 and a recent successful result in a phase 2 study (CUPID trial) targeting the cardiac sarcoplasmic/endoplasmic reticulum Ca2+ ATPase pump (SERCA2a).3 The appropriate combination of vector and gene are key for successful gene therapy, and advances in understanding the molecular mechanisms offer several therapeutic options.4 Adeno-associated virus (AAV) is the vector selected for the CUPID trial and has the advantage of being nonpathogenic, having a long expression profile compared to other viral vectors such as adenovirus.5 However, a reduced efficacy in patients with high neutralizing antibody (NAb) titers to AAV has been demonstrated, limiting the broad application of this approach for all the patients.6 Recently, “biological nanoparticles” designed to mimic key physicochemical properties of virion shells with cardiotropism were developed using capsid reengineering techniques.7 Of particular interest, a chimeric vector of AAV-2 and 8 (BNP116) displayed predominant muscle tropism together with an altered antigenic profile8 and thus holds significant promise for cardiac targeting while altering the antigenicity. Although a mouse study demonstrated high transduction in the heart and markedly reduced off-target expression in the major organs,8 the efficacy in more developed animals remains to be elucidated.
HF may result from multiple causes, but defective cardiac Ca2+ homeostasis is an important final common pathway.9,10 We have recently reported that AAV-9 mediated overexpression of constitutively active inhibitor-1 (I-1c), a potential target for cardiac gene transfer, can preserve cardiac function in a swine model of ischemic HF.11 Our goals in this study were (i) to establish the utility of a novel cardiotropic vector, BNP116, for cardiac gene transfer in a clinically relevant animal model, and (ii) to demonstrate further the efficacy of I-1c in a large animal model of ischemic HF. BNP116 was developed as a chimera of AAV-2/AAV-8, which readily traverses the blood vasculature and selectively transduces cardiac and whole-body skeletal muscle tissues with high efficiency while detargeting the liver and the lungs.8
Results
One month after MI, pigs developed chronic HF as evidenced by impaired cardiac function with left ventricular (LV) dilation (end diastolic volume: 40.8 ± 5.7 ml versus 81.7 ± 17.3 ml, P < 0.001, end systolic volume: 12.1 ± 2.8 ml versus 49.9 ± 15.0 ml, P < 0.001, LV ejection fraction (EF): 70.5 ± 3.6% versus 39.9 ± 6.8%, P < 0.001, before MI versus 1 month post-MI, respectively). A total of 20 pigs were randomized to receive high-dose BNP116.I-1c (high-dose group; 1.0 × 1013 vector genomes (vg), n = 7), low-dose BNP116.I-1c (low-dose group; 3.0 × 1012 vg, n = 7), or saline (control group, n = 6). Due to the relatively high prevalence of NAb to BNP116 in pigs, all animals with NAb titers <1:8 were included in either the high-dose or low-dose groups and not in the control group. Randomization was performed to match baseline characteristics between the groups with priority between the high-dose group and the control group. One pig each in the high-dose and the low-dose group died within 24 hours after the injection. The cause of deaths was uncertain, but no signs of recent infarction were found in postmortem examination. One pig in the control group was excluded due to the severe cardiac depression at 3 months due to vascular puncture induced intrathoracic bleeding.
Cardiac function and volumes
Cardiac function and volumes were evaluated with a high-fidelity conductance catheter, Swan-Ganz catheter, and blinded three dimensional echocardiography (3DE) analyses (Figure 1). Excellent 3DE reproducibility allowed us to evaluate accurately the post-MI LV dilatation. Intra- and interobserver intraclass correlation for LV volumes were 0.99 (P < 0.001) and 0.93 (P < 0.001), respectively. Before gene transfer, functional and volumetric parameters were similar between the high-dose and the control groups. However, the low-dose group tended to have a better function and smaller volumes with statistically different stroke volumes from the control group (Table 1). In the control group, average of relative changes to the baseline in mean LVEF was –7% while both end diastolic volume and end systolic volume increased about 40% during the 2 months between gene delivery and follow-up. In contrast, gene transfer of I-1c improved LVEF by an average of 5.7% in the high-dose group and by 5.2% in the low-dose group without statistical significance (P = 0.23 and P = 0.27, respectively) and suppressed LV dilatation (Figure 2). LV end diastolic volume and LV end systolic volume changes were significantly lower in the low-dose group compared to the control group. Other hemodynamic parameters consistently indicated improved cardiac function after the gene transfer (Figure 2). It is notable that the high-dose group had significantly increased stroke volume while preload-recruitable stroke work was also increased, indicating that it was not due to the alteration in afterload, but to the improved cardiac performance. Consistent with the LV volumes, heart weights and scar sizes were smaller in the groups that received gene therapy (Figure 3).
Figure 1.
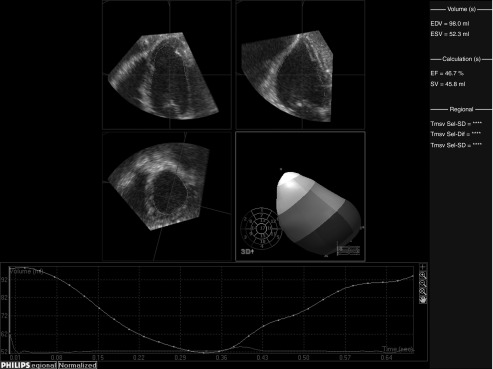
Representative three dimensional echocardiography (3DE) analysis of left ventricular volumes and ejection fraction. Representative color 3DE movies from each group are provided in Supplementary Material (Supplementary Movies S1–S3). EDV, end diastolic volume; EF, ejection fraction; ESV, end systolic volume; SV, stroke volume.
Table 1. Left ventricular characteristics before and after gene transfer.
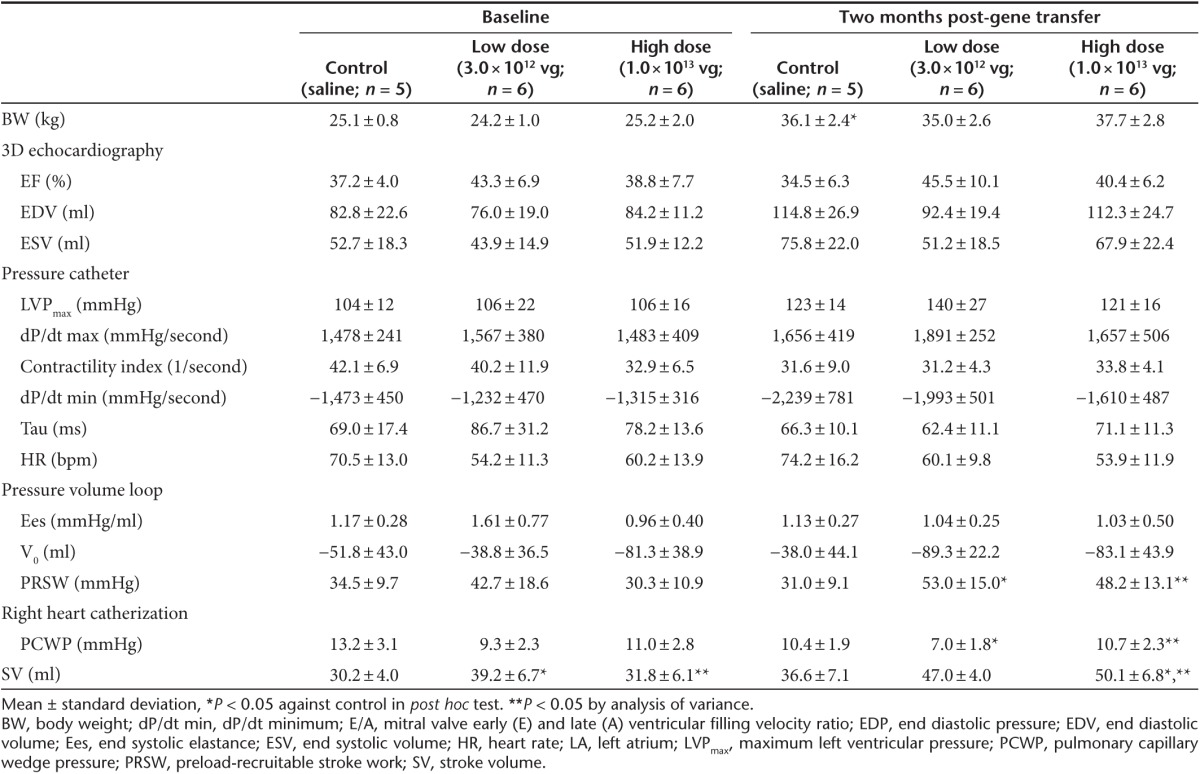
Figure 2.
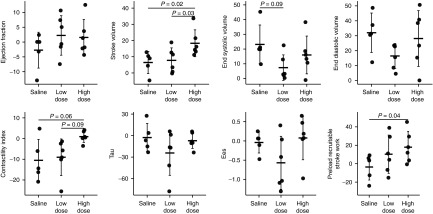
Absolute changes of structural and functional parameters before and 2 months after gene transfer. Absolute changes of each animal are shown in dots. The bars represent mean ± SD. I-1c-treated pigs showed improved cardiac function after gene transfer with attenuated ventricular dilatation compared with saline treated pigs. Contractility index = (dP/dt maximum)/(P at dP/dt maximum−left ventricular end diastolic pressure); Ees, end systolic elastance.
Figure 3.
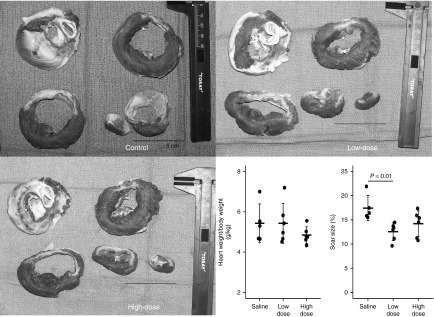
Heart weight and scar size 2 months after gene transfer. Scar size of the low-dose group was significantly smaller than saline-treated pigs. The bars represent mean ± SD. Representative pictures of cross-sectioned heart stained with triphenyl tetrazolium chloride are shown for different groups. Color images are available in Supplementary Figure S2.
I-1c distribution
mRNA levels of I-1c in the heart remote to the scar was low and required relatively high numbers of cycles to be detected by real-time quantitative polymerase chain reaction. I-1c mRNA levels in the brain, liver, lung, and kidney were compared to the pigs who received AAV-9.I-1c from the previously published study11 by relative quantitation to the heart in individual animals. Lung was the major organ with extra cardiac gene expression followed by the liver (Figure 4). Only one pig each in the AAV-9 and BNP116 high-dose groups had detectable copies of I-1c mRNA in the brain, and no pigs had detectable levels of I-1c genome in the kidney. Accurate quantitation of I-1c mRNA levels may not be possible due to the low mRNA level, however, the BNP116 group seemed to have similar to lower levels of off-target gene expression relative to the AAV-9 group. Histological analyses of major organs revealed no significant changes due to BNP116.I1c injection (Supplementary Figure S1). Cardiac transduction efficiency was also evaluated by injection of BNP116.green fluorescent protein (GFP) in four pigs and it was similar to AAV-9.GFP (n = 3) (% green fluorescent myocytes at the anterior wall: 64.2 ± 9.3% versus 63.0 ± 9.5%, P = 0.86).
Figure 4.
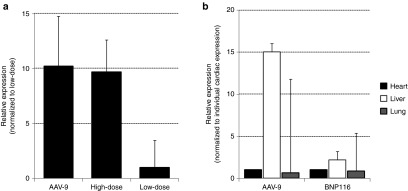
I-1c mRNA expression in the heart, lung, and liver. Real-time quantitative polymerase chain reaction was performed on animals in the present study and AAV-9.I-1c-treated animals from ref. 11 (n = 6). (a) Relative I-1c mRNA levels in the heart (remote to the scar) from different groups. (b) Relative I-1c mRNA expression in heart, lung, and liver. Expression was normalized to myocardium remote to the scar. The data are mean ± SD. AAV, adeno-associated virus.
Prevalence of NAb in pig and human sera
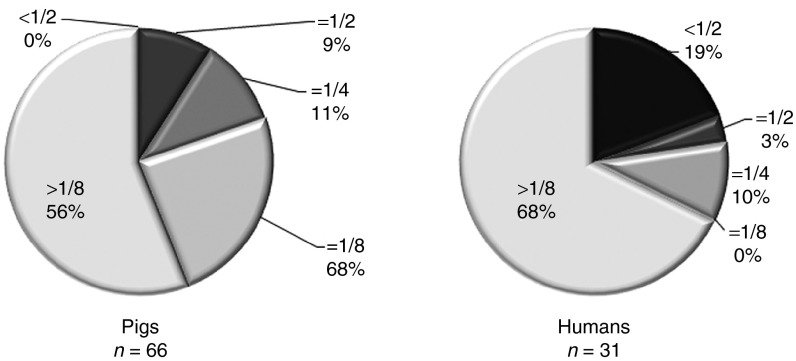
Of the 66 pigs analyzed for NAb against BNP116 in this study, only 9% had inhibitory titers of less than 1:2. Due to the relatively high prevalence of NAbs against BNP116, we included pigs with NAb titers up to less than 1:8. To evaluate the clinical utility of this vector, the prevalence of NAb to BNP116 in the human population was investigated in sera from 31 different individuals. Although the seroprevalence of more than 1:8 was higher in humans, those with NAb titers of less than 1:2 were also more prevalent in human compared to the pigs (Figure 5).
Figure 5.
Neutralizing antibody titers against BNP116 in pigs and humans. Percentage of patients and pigs with the indicated NAb titers, defined as the highest serum dilution resulting ≥50% inhibition of transduction when compared to no serum controls.
Discussion
In this study, we demonstrated that BNP116.I-1c gene transfer improves cardiac function and possibly prevents post-MI LV dilatation in a well-established model of chronic ischemic cardiomyopathy. The beneficial impact of I-1c gene transfer on cardiac function was revalidated in the most clinically relevant model together with a promising capability of a novel vector for clinical application. Furthermore, the intracoronary delivery route used in this study is readily available in the clinical practice, and the less invasive nature offers potential benefit over other delivery methods for patients with severe HF.
I-1c as a therapeutic tool
Aberrant calcium handling is a consistent finding in failing hearts in human and experimental studies.9,10 Several key molecules have been shown to be responsible for the abnormal calcium cycling.4 Among them, the SERCA2a/phospholamban complex plays a pivotal role in mediating intracellular calcium homeostasis, and decreased SERCA2a activity is strongly associated with HF regardless of etiology.4 Inhibitor-1 (I-1) is one of the SERCA2a/phospholamban-complex upstream regulators, which is also closely linked to the β-adrenoceptor system.12 Upon phosphorylation of the Thr35 site by PKA, I-1 suppresses protein phosphatase-1 (PP-1) activity, thereby maintaining higher phospholamban phosphorylation and leads to increased SERCA2a activity.12,13 I-1 expression as well as phosphorylation of I-1 Thr35 are shown to be decreased in HF together with increased PP-1 activity.14,15,16 Heart-specific overexpression of I-1 showed detrimental effects on cardiac function in mice, and it was accompanied by increased PP-1 levels.17 This may be due to the overall effect of phosphorylation at other sites such as I-1 Ser6718 and Thr7519 which are shown to result in increased PP-1 activity. The rational for overexpressing I-1c is that it is truncated to 65 amino acids from 171 and lacks other phosphorylation sites that negatively impact cardiac function. In addition, Thr 35 is replaced by phosphomimetic asparatic acid (T35D) and takes constitutively active form, thus leading to decreased PP-1 activity. In fact, several studies showed potential therapeutic benefit of I-1c on HF in experimental animal studies.11,20,21,22 Our study is consistent with these results and further confirms the efficacy of I-1c overexpression. As was the case in the AAV-9.I-1c gene transfer,11 the level of I-1c detected in the heart was relatively low. However, the level of I-1 protein in the heart is originally low,13 and we have previously shown that a similar level of I-1c expression is sufficient to positively influence cardiac function.11 Due to the higher variability than we expected, we were not able to show statistically significant improvement in LVEF after gene transfer. While our study may have been underpowered to show improvement in LVEF as an endpoint, we have demonstrated improvements in multiple functional parameters suggesting a favorable effect of I-1c gene transfer on post-MI heart. Although dP/dt maximum showed similar changes between the high-dose and control groups, the load dependence of this parameter has been well recognized23 and, when normalized with isovolumic developed pressure, the improvement in contractitliy index was significant in the high-dose group. Moreover, preload-recruitable stroke work is the established parameter of load independent measures of LV contractility,23,24 and improvement in this parameter was found after high-dose BNP116.I-1c gene transfer. We saw a significantly lower LV volume increase in only the low-dose group. In addition, scar size at 3 months was significantly smaller in the low-dose group compared to control. Although there may be an inhibitory effect of scar expansion by I-1c gene transfer, it is likely that the baseline scar size was already smaller in the low-dose group considering the baseline differences in various parameters. In contrast, baseline characteristics of the high-dose and control groups were similar, and thus, the changes in the parameters would mainly derive from the difference in the treatment.
A novel vector for cardiac gene delivery
Recently, AAV vectors have emerged as a useful tool for therapeutic gene transfer.5 Lack of pathogenicity and long-term expression are attractive features of AAV over other viral vectors for treating HF. Several AAV serotypes have been found, and advances in understanding of AAV biology unraveled that the diversity in capsid surface topology is responsible for different tissue tropism, antigenicity, and receptor affinity.7 Accordingly, attempts to produce novel vectors with clinically useful profiles were made by reengineering the capsid surface of AAV.8,25 Among the large numbers of chimeric AAV libraries generated by different laboratories, some show significant promise in specific therapeutic purpose. For instance, muscular gene therapy using a chimeric AAV has already entered the clinical trial.26 In the present study, we chose a chimeric vector generated from AAV-2 and AAV-8, and demonstrated the promising efficacy of this novel vector for cardiac gene transfer. To characterize the gene distribution profile of BNP116 in the swine we compared the I-1c mRNA levels in multiple organs with pigs that have received AAV-9.I-1c. AAV-9 has a natural cardiac tropism with lower affinity to transduce liver than other serotypes27 and provides efficient cardiac gene transfer.28 In our study, BNP116 showed a similar or better expression profile compared to AAV-9 for detargeting major organs. Our distribution data in pigs are consistent with the results of Asokan et al.8 in mice who showed that cardiac and diaphragm muscles were transduced with high efficiency whereas low levels of vector genome copies were recovered from other major organs, such as brain, lung, and spleen. Importantly, cardiac tropism is a great advantage for clinical application, since it could minimize the extracardiac expression, which is especially important for genes that may affect major organ functions. Additionally, the total viral load to achieve sufficient gene expression may be reduced.
Another important advantage of capsid reengineering is an altered antigenic profile. The presence of NAbs against AAV as a result of previous exposure can significantly limit effective gene transfer.6 Because the high titers of NAb against AAV-1 was the major reason for study exclusion in CUPID trial,3 a different antigenic profile is a very attractive feature that can expand the candidates for clinical gene transfer. By reengineering the receptor footprint on the AAV capsid surface, BNP116 obtained a unique antigenic profile compared to the parental viruses.8 It has also been shown that BNP116 was only modestly neutralized after the exposure to anti-AAV-2 serum, suggesting different antigenicity to the natural AAV serotypes. However, in contrast to our expectations, pigs had relatively high titers of NAb for BNP116 probably from infection with a virus with a similar capsid while they were raised together. Due to the high prevalence of NAb against BNP116 in pigs, we chose NAb titers < 1:8 as an inclusion criteria for the treatment group. While it needs further investigation whether this titer of NAb may alter the efficacy of cardiac gene transduction, lower efficacy of AAV-1.SERCA2a gene transfer in patients with only NAb = 1:229 suggests that the inhibition of expression by NAb even at the low levels. Interestingly, despite the high NAb prevalence for BNP116 in pigs, the human sero prevalence was lower. Up to 20% of humans with NAb ≤ 1:2 is a promising advantage for future clinical application. A possible option would be to screen the patients prior to gene therapy for NAb against multiple AAV serotypes including BNP116 and choose the best vector considering the NAb titer and tropism, etc. While this vector may not be applicable to many of the patients, it can definitely broaden the candidates pool for AAV-mediated gene therapy.
In summary, I-1c overexpression using a novel chimeric AAV vector, BNP116, improved cardiac function in a swine model of ischemic HF. The optimal dose for BNP116.I-1c remains to be determined due to the difference in baseline characteristics in the low-dose group and relatively high NAb titers for BNP116 in the pig population. However, the positive results achieved with a simple delivery method in a clinically relevant model offers significant promise for clinical translation.
Materials and Methods
BNP116.I-1c construction. BNP116 vector packaging the cytomegalovirus-driven sc-CMV.I-1c transgene cassette was produced and purified using previously published procedures.8,30 sc-CMV.I-1c is a self-complementary vector construct encoding a constitutively active and truncated form of I-1 (first 65 amino acids), which is generated by using a 5′ primer homologous to the I-1 coding sequence with a flanking SalI site before the initiation start site (CGCCGCTGGTCGACCTGACCGGGAGCCATGGAG) and a 3′ primer (GTGGAGACATGCGGCCGCTCATGACAAGGTGGA) containing a Not-I site and translational stop site following the codon for amino acid 65. This was subcloned (SalI-NotI) into pBluescript SK II and amplified. The construct was then used to convert threonine-35 to aspartic acid-35 with T7, T3, and a mutant primer engineered with appropriate nucleotide changes GGCAGGG(T3G)(C3T)GGGGCGGC. I-1c sequence analysis indicates that there are no alternative reading frames.
Animal protocols. The experimental protocols complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and standards of United States regulatory agencies. They were approved by the Institutional Animal Care and Use Committee of the Icahn School of Medicine at Mount Sinai. Yorkshire pigs (18–22 kg) were premedicated using intramuscular Telazol (8.0 mg/kg, Fort Dodge, IA). After the placement of an intravenous line, animals were intubated and ventilated with 100% oxygen. General anesthesia was maintained with intravenous propofol (8–10 mg/kg/hour) throughout the procedure. Electrocardiograms and pulse oximeter measurements were recorded at 5-minute intervals. Continuous monitoring with an intravenous saline infusion was maintained for a period of 30 minutes to stabilize the hemodynamic status. Then all animals underwent echocardiographic assessment, followed by hemodynamic measurement. All pigs received proximal left anterior descending coronary artery (LAD) balloon occlusion for 2 hours followed by reperfusion. Cardiac performance was evaluated at 1 month after MI and pigs were randomized to receive either high-dose BNP116.I-1c (3 × 1012 vg), low-dose BNP116.I-1c (1 × 1013 vg), or saline injection. Two months after the injection (3 months), cardiac performance was re-evaluated to determine the efficacy of gene transfer. Then the pigs were euthanized and tissue samples were collected for histology and biodistribution analysis. Seven pigs received reporter gene injection to assess the cardiac transduction efficiency using BNP116.GFP (n = 4) and AAV-9.GFP (n = 3). These pigs were euthanized 1 month after the gene delivery and % green fluorescent cardiomyocytes were counted.
Cardiac performance assessment. A Philips iE-33 ultrasound system (Philips Medical Systems, Andover, MA) was used to acquire echocardiographic data with a multifrequency imaging transducer. Complete Doppler transthoracic echocardiographic studies were performed. Images were recorded during end-expiratory breath-hold in the standard LV apical view. 3DE datasets were acquired from four to seven consecutive cardiac cycles using an R-wave-triggered mode. An average of three data sets were acquired per animal to ensure optimal data sets. Postacquisition image analyses were performed offline using a custom software package (Q-lab; Phillips Medical Systems) by two blinded investigators independently. LV volumes were calculated using 3D full-volume algorithms including a semiautomated border detection system. The endocardial detection accuracy was checked and optimized manually (Figure 1). Analysis was performed in two different sequences and an average of two measurements were used for final data analyses. The methods for pressure measurement were previously described in detail.31 Briefly, percutaneous punctures were performed to establish the vascular access to the femoral artery and femoral vein. Then, heparin (100 IU/kg intravenously) was administered to maintain an activated coagulation time of 200–300 seconds. A Swan-Ganz catheter (Edwards Lifesciences LLC, Irvine, CA) was advanced to the main pulmonary artery and pressure measurements were taken. Approximately 0.25 × body weight (kg) ml of cold saline were injected into the inferior vena cava to obtain cardiac output by the thermodilution method. Subsequently, an 11-Fr balloon catheter was advanced to the inferior vena cava for preload alterations. A 7-Fr, 12-electrode, dual-field conductance catheter (Millar Instruments, Houston, TX) was advanced into the LV for assessment of LV pressure–volume relationships. MPVS Ultra (Millar Instruments) was used to acquire analog data and convert it to digital data. Data analysis was performed using iox2 (Emka Technologies, Falls Church, VA). The conductance catheter gain factor α was calculated as the ratio of conductance-derived cardiac output to that measured by thermodilution. Parallel conductance was adjusted using the end diastolic volume obtained from 3DE. Contractility index was defined by the software as (dP/dt max)/(pressure at dP/dt max−LV end diastolic pressure).23
MI creation. After cardiac performance evaluation was completed, a bolus of atropine (0.05 mg/kg) and amiodarone (1–3 mg/kg) were given intravenously or intramuscularly. A 1,000-ml saline bag mixed with atropine (0.1 mg/kg), amiodarone (3 mg/kg), and potassium acetate (20 mEq) was continuously infused at the rate of 300 ml/hour for the duration of the procedure. A 7-Fr hockey-stick catheter (Cordis, Miami, FL) was advanced to the left coronary artery. After the coronary angiogram, a 0.014-inch guide wire (Abbott, Park, IL) was advanced into the LAD and 8-mm-long, 4.0-mm VOYAGER over-the-wire balloon (Abbott) was advanced to the proximal part of the coronary artery. The balloon was then inflated to 3–4 atm for 120 minutes followed by reperfusion. In case of malignant arrhythmia, direct current shock was applied immediately with continuous chest compression. After confirmation of hemodynamic stability, animals were allowed to recover. Intramuscular injections of nitroglycerine and furosemide were administered. Intravenous saline with amiodarone, atropine, and potassium acetate infusion was decreased to 50 ml/hour and was given overnight. The animals were housed in their cages, and examined daily for any signs of pain or distress.
Intracoronary gene delivery. Following the cardiac performance measurements, 1 µg/kg/minute of nitroglycerin injection was started through an intravenous route until the end of the injection procedure to enhance the efficacy of gene transfer.32 The left coronary artery was cannulated with a 5Fr hockey stick catheter (CordisVista Brite Tip; Miami, FL) and two 0.014-inch coronary guide wires (Abbott HI-TORQUE ADVANCE, Santa Clara, CA) were introduced in LAD and left circumflex coronary artery to fix the position of the catheter at the left main trunk. Fifteen milliliters of the virus or saline solution diluted 1:1 in blood were infused into the proximal part of the left main coronary artery at 1 ml/minute followed immediately by a 5 ml flush of saline/blood solution at the same flow rate. Then the left coronary artery catheter is removed and the catheter was placed in the right coronary artery. Similarly, 5 ml of the diluted BNP116 or saline solution in blood 1:1 was infused into the right coronary artery at 1 ml/minute followed immediately by a 5 ml flush. The visual guidance of the procedures are available in Ishikawa et al.33
In vitro neutralization assay for BNP116. 293T cells were plated on the day of the assay in Dulbecco's modified Eagle's medium with 1% penicillin/streptomycin. The assay was performed 4–5 hours after plating. In a separate 96-well plate, stepwise twofold dilutions of pig sera were loaded with Dulbecco's modified Eagle's medium for pig samples and in Immunoglobulin Depleted Serum for human samples resulting in a total volume of 40 µl. Following the serum dilution, 40 µl of Dulbecco's modified Eagle's medium containing 8 × 108 vg of BNP116-CBALuc were added to each well. The mixture was incubated at 37 °C for 30 minutes and then 20 µl of the mixture was added per well in triplicate. Twenty-four hours later, the transduction efficiency was assessed by analyzing luciferase activity. The percentage of inhibition was calculated relative to no serum control. The luciferase assay was performed as previously described.34 Briefly, the cells were lysed in the wells by adding equal volume of 2× lysis buffer (10 mmol/l Tris–hydrochloride pH 8.0, 150 mmol/l NaCl, 1% (wt/vol) NP40, 10 mmol/l dithiothreitol). Twenty microliters of the cell lysate were then mixed with 100 µl of luciferase assay buffer (25 mmol/l Tricine–hydrochloride pH 7.8, 5 mmol/l magnesium sulfate, 0.5 mmol/l ethylenediaminetetraacetic acid (pH 8.0), 3.3 mmol/l dithiothreitol, 0.5 mmol/l ATP pH ~7–8 (A26209; Sigma-Aldrich, St Louis, MO), 1 mg/ml bovine serum albumin, 0.05 mg/ml D-luciferin (cat. no.: LUCK-100; Gold Biotechnology, St Louis, MO), 0.05 mmol/l CoA (cat. no.: 13787, United States Biological, Swampscott, MA) and the luminescence was read in a luminescence counter (Microbeta Trilux 1450 LSC and Luminescence Counter; Perkin Elmer, Carlsbad, CA). The titer of neutralizing antibody was categorized into <1:2, =1:2, =1:4, = 1:8, and >1:8, reflecting the highest dilution factor to exhibit ≥50% inhibition of transduction compared to no serum controls. These dilution factors are shown in a circular graph representing the percentage of investigated population. The protocol for testing NAb prevalence in human serum samples was reviewed and approved by the local Institutional Review Board.
Postmortem histology. Hearts were explanted, weighed, and sectioned into six slices. One layer from the papillary muscle level was stored for biodistribution studies. Additionally, liver, lung, brain, and kidney were collected to assess off-target effect. The remainder of the heart was stained with triphenyl-tetrazolium chloride to delineate the scar area from viable tissue and the size of the infarction was assessed by digital planimetry.
Real-time quantitative polymerase chain reaction (qPCR) detection of I-1C mRNA. Total RNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA) followed by DNase I treatment (Qiagen, Valencia, CA). Total RNA was reverse transcribed using the high-capacity cDNA Reverse Transcription kit (Applied Biosystems, ABI) according to the manufacturer's protocol. Quantitative PCR reactions were performed with Power SYBR Green Master Mix using an ABI Prism 7500 Real Time PCR System. The real-time qPCR assay detects a 125 bp sequence unique to I-1c and the number of copies of I-1c was quantified using serial dilutions of a plasmid containing the target sequences as standards. Primers for the I-1c were designed with the Primer Express software based on the human protein sequence (NP 006732.3; forward: CGTGCCCCTGCTGGAA; reverse: TCCGGTCCTCGTCGATCTC). Because relatively high numbers of cycles were required for detection, accurate quantification of the vector genomes was challenging. Quantitation of the I-1c mRNA detected in the lung and liver, relative to the heart sample remote from the scar region, was calculated in the same animal to evaluate the off-target transductions. Samples of AAV-9.I-1c gene transferred animals11 were used for the comparison to BNP116.I-1c.
Statistical analysis. Data are expressed as means ± standard deviations. Study sample size was determined by using an α value of 0.05, a power of 80% to detect a 6% absolute increase of LVEF in the assumption of 3.5% standard deviation with 1 control per treatment animal. Comparison between before MI and 1 month after MI data was performed by paired t-tests. Comparisons between the three groups were performed by analysis of variance at baseline and for the absolute changes overtime, followed by Tukey post hoc test. In the presence of statistically significant differences in the analysis of variance test of baseline comparisons, the change scores between the time points were adjusted by the baseline values. Intra- and interclass correlation coefficients were analyzed to assess the agreement of two repeated measurements for 3DE volume analysis. A P value of <0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Representative movies of 3DE analyses from each group are provided in mov. files. Figure S1. Hematoxylin & eosin staining of organs. Figure S2. Color images of heart cross sections (Figure 3). Movie S1. Control. Movie S2. High-dose BNP116.I-1c. Movie S3. Low-dose BNP116.I-1c.
Acknowledgments
The authors thank Lauren Leonardson (Icahn School of Medicine at Mount Sinai) for providing excellent technical assistance and expertise. This work is supported by Leducq Foundation (R.J.H.), by NIH R01 HL117505, HL 119046 and P50 HL112324 (to R.J.H.), P01 HL112761, R01 AI072176, and R01 AR064369 (to R.J.S.), and HL26057 and HL64018 (E.G.K.). J.A. was supported by Fundacion Alfonso Martin-Escudero. E.G.K., R.J.S., and R.J.H. are scientific cofounders of NanoCor Therapeutics. NanoCor is planning to commercialize I-1c for future therapeutic use. R.J.H., R.J.S., and E.G.K. are scientific cofounders of Nanocor Co., which is developing AAV-9.I-1c and BNP116.I-1c for the treatment of heart failure.
Supplementary Material
Hematoxylin & eosin staining of organs.
Color images of heart cross sections (Figure 3).
Control.
High-dose BNP116.I-1c.
Low-dose BNP116.I-1c.
References
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Tilemann L, Ladage D, Aguero J, Leonardson L, Fish K, et al. Cardiac gene therapy in large animals: bridge from bench to bedside. Gene Ther. 2012;19:670–677. doi: 10.1038/gt.2012.3. [DOI] [PubMed] [Google Scholar]
- Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) Investigators Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar RJ. Potential of gene therapy as a treatment for heart failure. J Clin Invest. 2013;123:53–61. doi: 10.1172/JCI62837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak CA, Byrne BJ. AAV vectors for cardiac gene transfer: experimental tools and clinical opportunities. Mol Ther. 2011;19:1582–1590. doi: 10.1038/mt.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokan A. Reengineered AAV vectors: old dog, new tricks. Discov Med. 2010;9:399–403. [PMC free article] [PubMed] [Google Scholar]
- Asokan A, Conway JC, Phillips JL, Li C, Hegge J, Sinnott R, et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat Biotechnol. 2010;28:79–82. doi: 10.1038/nbt.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ Res. 2012;110:1646–1660. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase Y, Hajjar RJ. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases. Nat Clin Pract Cardiovasc Med. 2008;5:554–565. doi: 10.1038/ncpcardio1301. [DOI] [PubMed] [Google Scholar]
- Fish KM, Ladage D, Kawase Y, Karakikes I, Jeong D, Ly H, et al. AAV9.I-1c delivered via direct coronary infusion in a porcine model of heart failure improves contractility and mitigates adverse remodeling. Circ Heart Fail. 2013;6:310–317. doi: 10.1161/CIRCHEARTFAILURE.112.971325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou P, Hajjar RJ, Kranias EG. Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J Mol Cell Cardiol. 2009;47:365–371. doi: 10.1016/j.yjmcc.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittköpper K, Dobrev D, Eschenhagen T, El-Armouche A. Phosphatase-1 inhibitor-1 in physiological and pathological β-adrenoceptor signalling. Cardiovasc Res. 2011;91:392–401. doi: 10.1093/cvr/cvr058. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Mishra S, Rastogi S, Imai M, Habib O, Sabbah HN. Cardiac SR-coupled PP1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am J Physiol Heart Circ Physiol. 2003;285:H2373–H2381. doi: 10.1152/ajpheart.00442.2003. [DOI] [PubMed] [Google Scholar]
- Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, et al. Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol. 2002;22:4124–4135. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Armouche A, Pamminger T, Ditz D, Zolk O, Eschenhagen T. Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc Res. 2004;61:87–93. doi: 10.1016/j.cardiores.2003.11.005. [DOI] [PubMed] [Google Scholar]
- El-Armouche A, Wittköpper K, Degenhardt F, Weinberger F, Didié M, Melnychenko I, et al. Phosphatase inhibitor-1-deficient mice are protected from catecholamine-induced arrhythmias and myocardial hypertrophy. Cardiovasc Res. 2008;80:396–406. doi: 10.1093/cvr/cvn208. [DOI] [PubMed] [Google Scholar]
- Sahin B, Shu H, Fernandez J, El-Armouche A, Molkentin JD, Nairn AC, et al. Phosphorylation of protein phosphatase inhibitor-1 by protein kinase C. J Biol Chem. 2006;281:24322–24335. doi: 10.1074/jbc.M603282200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez P, Mitton B, Waggoner JR, Kranias EG. Identification of a novel phosphorylation site in protein phosphatase inhibitor-1 as a negative regulator of cardiac function. J Biol Chem. 2006;281:38599–38608. doi: 10.1074/jbc.M604139200. [DOI] [PubMed] [Google Scholar]
- Pathak A, del Monte F, Zhao W, Schultz JE, Lorenz JN, Bodi I, et al. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res. 2005;96:756–766. doi: 10.1161/01.RES.0000161256.85833.fa. [DOI] [PubMed] [Google Scholar]
- Chen G, Zhou X, Florea S, Qian J, Cai W, Zhang Z, et al. Expression of active protein phosphatase 1 inhibitor-1 attenuates chronic beta-agonist-induced cardiac apoptosis. Basic Res Cardiol. 2010;105:573–581. doi: 10.1007/s00395-010-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou P, Rodriguez P, Ren X, Zhou X, Qian J, Sadayappan S, et al. Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ Res. 2009;104:1012–1020. doi: 10.1161/CIRCRESAHA.108.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass DA, Maughan WL, Guo ZM, Kono A, Sunagawa K, Sagawa K. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation. 1987;76:1422–1436. doi: 10.1161/01.cir.76.6.1422. [DOI] [PubMed] [Google Scholar]
- Little WC, Cheng CP, Mumma M, Igarashi Y, Vinten-Johansen J, Johnston WE. Comparison of measures of left ventricular contractile performance derived from pressure-volume loops in conscious dogs. Circulation. 1989;80:1378–1387. doi: 10.1161/01.cir.80.5.1378. [DOI] [PubMed] [Google Scholar]
- Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, Camp AS, et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther. 2012;20:443–455. doi: 10.1038/mt.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bish LT, Morine K, Sleeper MM, Sanmiguel J, Wu D, Gao G, et al. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum Gene Ther. 2008;19:1359–1368. doi: 10.1089/hum.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Fuess S, Storm TA, Gibson GA, Mctiernan CF, Kay MA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, et al. Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease (CUPID) Trial Investigators Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase ½ clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Chemaly ER, Tilemann L, Fish K, Ladage D, Aguero J, et al. Assessing left ventricular systolic dysfunction after myocardial infarction: are ejection fraction and dP/dt(max) complementary or redundant. Am J Physiol Heart Circ Physiol. 2012;302:H1423–H1428. doi: 10.1152/ajpheart.01211.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakikes I, Hadri L, Rapti K, Ladage D, Ishikawa K, Tilemann L, et al. Concomitant intravenous nitroglycerin with intracoronary delivery of AAV1.SERCA2a enhances gene transfer in porcine hearts. Mol Ther. 2012;20:565–571. doi: 10.1038/mt.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Ladage D, Tilemann L, Fish K, Kawase Y, Hajjar RJ. Gene transfer for ischemic heart failure in a preclinical model. J Vis Exp. 2011. [DOI] [PMC free article] [PubMed]
- Rapti K, Louis-Jeune V, Kohlbrenner E, Ishikawa K, Ladage D, Zolotukhin S, et al. Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol Ther. 2012;20:73–83. doi: 10.1038/mt.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hematoxylin & eosin staining of organs.
Color images of heart cross sections (Figure 3).
Control.
High-dose BNP116.I-1c.
Low-dose BNP116.I-1c.