Abstract
Enzyme replacement therapy has revolutionized the treatment of the somatic manifestations of lysosomal storage diseases (LSD), although it has been ineffective in treating central nervous system (CNS) manifestations of these disorders. The development of neurotrophic vectors based on novel serotypes of adeno-associated viruses (AAV) such as AAV9 provides a potential platform for stable and efficient delivery of enzymes to the CNS. We evaluated the safety and efficacy of intrathecal delivery of AAV9 expressing α-l-iduronidase (IDUA) in a previously described feline model of mucopolysaccharidosis I (MPS I). A neurological phenotype has not been defined in these animals, so our analysis focused on the biochemical and histological CNS abnormalities characteristic of MPS I. Five MPS I cats were dosed with AAV9 vector at 4–7 months of age and followed for 6 months. Treated animals demonstrated virtually complete correction of biochemical and histological manifestations of the disease throughout the CNS. There was a range of antibody responses against IDUA in this cohort which reduced detectable enzyme without substantially reducing efficacy; there was no evidence of toxicity. This first demonstration of the efficacy of intrathecal gene therapy in a large animal model of a LSD should pave the way for translation into the clinic.
Introduction
Mucopolysaccharidosis type I (MPS I, Hurler, Scheie, Hurler-Scheie syndromes) is a recessively inherited disease caused by deficiency of a ubiquitous lysosomal enzyme, α-l-iduronidase (IDUA), which is required for the degradation of the glycosaminoglycans (GAGs) heparan sulfate and dermatan sulfate. Accumulation of these undegraded lysosomal substrates results in widespread tissue pathology, often characterized by skeletal deformities, cardiac and pulmonary disease, upper airway obstruction, and in some cases, progressive neurological disease.1 The central nervous system manifestations of MPS I vary, with profound developmental decline occurring in early childhood in severely affected patients, while those with a more mild phenotype often maintain normal intelligence.2,3,4,5,6,7 However, even the patients with attenuated disease sometimes face serious neurological complications such as communicating hydrocephalus, as well as spinal cord compression secondary to GAG storage in the meninges.
The currently available treatments for MPS I include bone marrow transplantation (BMT) and intravenous enzyme replacement therapy (ERT). Both modalities exploit the observation that the mannose-6-phosphate receptor, which is responsible for sorting lysosomal proteins from the trans-Golgi, is also expressed at the cell surface, allowing for receptor-mediated uptake and lysosomal trafficking of IDUA infused intravenously or secreted from donor-derived leukocytes following BMT.8,9 While both ERT and BMT have demonstrated efficacy against many of the somatic features of MPS I, only BMT is believed to alter the course of CNS disease, presumably through IDUA secretion by donor derived cells that migrate into the CNS.10 Despite the promise of this approach, BMT has been associated with engraftment rates as low as 50% and mortality rates in excess of 10% in MPS I patients.10 Studies in the canine model of MPS I and small clinical trials have indicated that it may be possible to instead achieve IDUA delivery throughout the CNS using intrathecal injection of the enzyme, which would allow for distribution via the cerebrospinal fluid.11,12,13,14,15,16,17,18 While intrathecal ERT provides important proof of principle for achieving enzyme distribution in CNS via the cerebrospinal fluid (CSF), it does not represent a practical treatment approach due to the need to repeatedly access the CSF for enzyme delivery for the lifetime of the patient. This leaves a significant unmet need for a safe and effective long-term treatment for the CNS manifestations of MPS I.
Gene therapy offers an attractive alternative to BMT and intrathecal ERT for targeting the CNS in MPS I. Gene transfer to the brain using adeno-associated viral (AAV) vectors has been found safe in multiple human trials, and delivery of the therapeutic transgene to quiescent cells within the CNS could provide a permanent source of secreted enzyme, obviating the need to repeatedly access the CSF.19,20,21,22 In murine MPS models, CNS-directed AAV gene transfer has demonstrated complete correction of tissue lesions and improvements in disease-specific behavioral phenotypes.23,24 The primary obstacle to this approach has been translation to large animal models. Direct intraparenchymal brain injection of AAV vectors results in transgene expression constrained to the area surrounding the injection site, with histological correction limited to the adjacent tissue.25 Although studies in both canine and feline models of lysosomal enzyme deficiencies have shown that this limitation can be overcome using multiple vector injections, this strategy is not readily scalable to the human brain.25,26 Further, intraparenchymal injections have been shown to induce a local inflammatory response that can be accompanied by elimination of transduced cells.26 A potential alternative delivery approach has been highlighted by recent studies of intrathecal AAV delivery, which show that in dogs, cats, and nonhuman primates, an AAV9 vector delivered into the CSF transduces glial and neuronal cells throughout the brain and spinal cord.24,27,28 This potential to transduce cells across the neuraxis in a large animal via a single minimally invasive injection could radically transform the therapeutic potential of AAV-mediated gene therapy for CNS disease. We hypothesized that this capacity of intrathecal AAV9 to effect such widespread CNS transduction, coupled with the ability of genetically corrected cells to secrete enzyme to cross-correct untransduced cells, would make this a highly effective approach for the long-term treatment of CNS manifestations of MPS I.
In the present study, we tested the capacity of intrathecal delivery of an AAV9 vector to correct storage lesions throughout the CNS in a large animal model of MPS I. We selected the feline MPS I model for these experiments because the CNS lesions in these animals closely resemble those of MPS I patients, with accumulation of gangliosides in neurons and GAGs in the meninges and around cerebral blood vessels.29 We found that intrathecal delivery of a vector bearing the normal feline IDUA sequence resulted in global CNS correction of the histological and biochemical features of MPS I. IDUA-specific antibody responses were elicited in the CNS of most treated animals, but were not associated with adverse clinical sequelae or loss of efficacy. Together these results strongly support the development of intrathecal AAV9-mediated gene delivery as a therapeutic approach for MPS I.
Results
Intrathecal AAV9 delivery induced robust IDUA expression in CSF and serum
Eight MPS I cats between 4 and 7 months of age were included in this study (Table 1). These animals carried a three base pair deletion in the IDUA gene, resulting in omission of a single aspartate residue.30 Three cats heterozygous for the IDUA mutation and two wild-type animals from the same colony served as unaffected controls. Five of the affected animals at ages 4–7 months were treated with a single intrathecal injection via the cisterna magna of 1012 GC/kg of an AAV9 vector bearing a codon-optimized normal feline IDUA sequence. The vector administered to two of the cats carried a chicken beta actin (CB) promoter; the other three treated animals received a vector carrying a cytomegalovirus (CMV) promoter. One additional animal assigned to receive the CB vector died under anesthesia during the pretreatment CSF collection. There were no other adverse events throughout the study period.
Table 1. Summary of study subjects.

Serum and CSF were serially collected from the treated and naive animals and assayed for IDUA enzyme activity (Figure 1). IDUA activity was not detected in samples from untreated MPS I cats. Treated animals exhibited a rapid elevation in both CSF and serum IDUA activity following vector injection, with peak activity exceeding that measured in normal cats. The CB promoter appeared to be more active, inducing higher enzyme levels in both CSF and serum. Following a peak at 21 days postinjection, CSF enzyme levels rapidly declined to near baseline in two animals, although activity remained detectable at most time points. CSF IDUA activity stabilized at approximately normal levels in the other three cats. Serum activity varied between the normal range and baseline values, although high background in the serum assay precluded accurate assessment of low levels of circulating enzyme.
Figure 1.
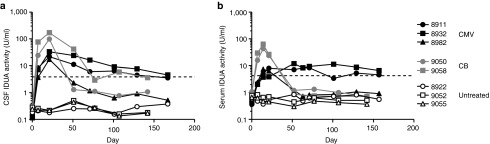
IDUA expression in CSF and serum following IT AAV9 delivery. Five MPS I cats were treated with an intracisternal injection of an AAV9 vector (1012 GC/kg) expressing feline IDUA from a CB (gray symbols) or CMV (black symbols) promoter. CSF and serum were serially collected from the treated animals as well as three untreated MPS I cats. IDUA activity was measured using the fluorogenic substrate 4MU-iduronide in CSF (a) and serum (b). All values are the mean of duplicate assays. Normal serum and CSF activity (dashed line) are the mean values from two wild-type animals.
Heterogeneous antibody responses were elicited against the therapeutic enzyme
The sharp decline in IDUA activity in some of the treated animals did not appear to be consistent with a cellular immune response against the transduced cells, as residual expression was detectable and there were no clinical signs of meningitis or encephalitis. CSF analysis revealed normal nucleated cell counts and only mildly elevated protein, which was also observed in untreated MPS I cats (Supplmentary Table S1). We suspected that this decline in CSF enzyme could be due to the induction of antibodies against IDUA. Indirect ELISA using purified feline IDUA as a target for capture of antibodies showed clear antibody responses in the CSF of some of the treated animals (Figure 2a). Within the CMV vector-treated group, CSF IDUA activity decreased in proportion to antibody titer, with animal 8982 having the highest titer corresponding to nearly undetectable CSF enzyme levels. The same was true for the animals treated with the CB vector; animal 9050 exhibited elevated antibody titers and very low CSF IDUA activity whereas 9058 maintained normal CSF IDUA and did not have a detectable antibody response. The correlation was not as clear between the groups treated with the different vectors, as the CB vector-treated animals appeared to have lower steady state CSF antibody titers overall. Considering that these animals also had higher initial enzyme expression, it is likely that the additional antigen either sequestered antibody or interfered with ELISA-based detection, resulting in lower overall titer measurements in this group. In summary there was a correlation between CSF IDUA antibody and steady state level of CSF IDUA for all animals except 8911, which developed moderate levels of CSF antibodies but retained high levels of steady state CSF enzyme.
Figure 2.

Normalization of CSF hexosaminidase activity despite a transgene-specific antibody response. CSF samples collected before vector administration and at the end of the study were tested for the presence of antibodies against IDUA by indirect ELISA (a). Titers are based on a standard curve of a serially diluted positive sample, which was arbitrarily assigned a titer of 1:1,000. (b) Total Hex activity was measured in CSF using the fluorogenic substrate MUG. Normal activity (dashed line) is the mean of two heterozygous control samples. The untreated MPS I level (dotted line) is the mean of untreated controls.
Normalization of CSF hexosaminidase (Hex) activity despite antibody induction to the therapeutic enzyme
IDUA deficiency has been shown to induce secondary elevation of activity of other lysosomal glycohydrolases. We found that elevated activity of one such enzyme, beta hexosamindase, was detectable in the CSF of MPS I cats (Figure 2b). This elevated Hex activity in CSF provided a potential noninvasive biomarker to detect aberrant cellular regulation in the CNS of MPS I cats. Following vector delivery, we observed an approximately twofold decrease in CSF Hex activity in all treated animals. Animals receiving the CB vector, which induced higher initial expression, exhibited the most rapid normalization of CSF Hex. Only one animal (8982), which had high anti-IDUA titers and very low CSF IDUA activity, exhibited incomplete normalization of CSF Hex. This demonstrated that all treated animals, even those that lost nearly all CSF enzyme activity, exhibited a persistent biochemical response to gene transfer.
Intrathecal AAV9 delivery resulted in global CNS transduction and normalization of tissue Hex activity
All treated animals were euthanized between 160 and 170 days following vector delivery. We performed quantitative PCR analysis on DNA from tissue samples, which revealed substantial vector deposition throughout the brain and spinal cord (Figure 3a). There were no differences in copy number in samples from animals with very low CSF IDUA levels, further confirming that elimination of transduced cells played no role in the decrease in circulating activity. Vector biodistribution to peripheral organs was limited, except to the liver, which contained very high vector copy numbers. All subsequent analyses of transduction activity and biochemical and pathological correction are presented in subgroups of cats with high (8911, 8932 and 9058) or low (8982 and 9050) CSF IDUA.
Figure 3.
Global CNS transduction and biochemical correction following IT gene transfer. Vector genomes were quantified in DNA extracted from CNS and peripheral tissues of treated cats by TaqMan PCR (a). Tissue samples from MPS I cats and heterozygous controls were collected from sites throughout the CNS for measurement of IDUA activity (b) and Hex activity (c). Treated animals are stratified into those with normal CSF IDUA activity (8911, 8932, 9058) and those with low CSF activity (8982, 9050). All enzyme activities are normalized to protein concentration.
IDUA activity was measured in tissue samples collected from various regions of the brain and spinal cord (Figure 3b). Activity was undetectable in untreated MPS I cats. All treated animals exhibited elevated tissue IDUA activity, exceeding that of heterozygous control animals in several brain regions. Tissue IDUA was lower in many regions of the CNS in the animals with the lowest CSF enzyme activity, although in all cases it was greater than that found in untreated MPS I cats. To evaluate tissue correction of the MPS I phenotype in the CNS, Hex activity was measured in CNS tissue lysates (Figure 3c). Similar to observations in the CSF, untreated animals had significantly elevated tissue Hex activity, which was normalized in all brain regions of treated animals, including sites with very low IDUA activity. This indicates an extremely low threshold for the expressed enzyme to effect changes in the abnormal cellular processes associated with MPS I in the CNS. We also attempted to quantify total GAG concentrations in the brain, but we found that the dimethylmethylene blue assay used for these measurements exhibited high background in brain tissue and thus could not reliably distinguish between normal and MPS I cats.
IDUA reconstitution reverses lysosomal storage lesions throughout the CNS
The CNS manifestations of MPS I are characterized histologically by intracellular accumulation of cholesterol and the gangliosides GM2 and GM3 in the brain parenchyma, with storage of GAGs prominent in the meninges and surrounding the cerebral vasculature.29,31 Untreated MPS I cats exhibited marked GM3 staining throughout multiple brain sections, which was absent in normal controls (Figure 4; Supplementary Table S2). In treated animals, GM3 storage was cleared throughout all brain regions analyzed, although scattered cells showing residual GM3 staining were visible in the animals with the lowest CSF enzyme activity. The reduction in GM3 staining correlated with a reduced frequency of abnormal neurons exhibiting distended cytoplasm visible on histopathology; while these were frequently observed in the cortex of untreated cats, they were absent in treated animals with high CSF IDUA activity, and rare in treated animals with lower CSF enzyme (Figure 5; Supplementary Table S2). Filipin staining for cholesterol revealed a similar pattern of correction, with an average of 239 ± 60 positive cells per field in untreated animals, which was reduced to an average of 2 positive cells per field in animals with high CSF IDUA activity and 38 and 52 cells per field in treated animals with low CSF IDUA activity (Figure 5; Supplementary Table S2). We also stained for LIMP2, a lysosomal integral membrane protein, which has been shown to accumulate in neurons in murine models of MPS.24 This marker also showed considerable lysosomal storage in the brains of MPS I cats that was almost completely normalized following intrathecal vector administration in all treated animals (Figure 5; Supplementary Table S2).
Figure 4.

Reversal of brain ganglioside storage. Immunostaining for the ganglioside GM3 was performed on tissue sections from four brain regions in untreated MPS I cats (a–d), treated cats with high CSF IDUA activity (e–h) and low CSF activity (i–l), as well as normal controls (m–p). Scale bar = 500 µm.
Figure 5.

Correction of neuronal storage lesions. Sections from the cerebral cortex were stained with H&E (a,f,k,p). Arrows indicate the distended neuron cell bodies typical of MPS I. The treated cats were stratified into those with low CSF IDUA activity and those with high activity. Cholesterol accumulation was evaluated in matched brain sections by filipin staining in untreated (b,c), treated (g,h,l,m) and normal control (q,r) cats. Immunostaining for the lysosomal integral membrane protein LIMP2 was performed on corresponding sections from each animal (d,e,i,j,n,o,s,t). Scale bar = 500 µm (4×), 200 µm (10×), or 100 µm (H&E).
To evaluate correction of storage pathology in the meninges and perivascular spaces, Alcian blue staining for GAGs was performed on cortical tissue sections (Figure 6). The untreated animals exhibited significant GAG accumulation in the thickened meninges, as well as in perivascular cells. This GAG storage was almost completely reversed in all treated animals.
Figure 6.
Reduced GAG storage in the cerebral vasculature and meninges. Cortical brain sections were stained for GAGs using Alcian blue. Low magnification images (a,d,g,j) show the cerebral cortex with the associated meninges. High magnification images show representative cortical blood vessels (b,e,h,k) and segments of meninges (c,f,i,l). Scale bars = 500 µm (left column) and 100 µm (middle and right columns).
Intrathecal AAV9 delivery corrects somatic lesions
It has previously been reported that AAV9 delivery into the CSF in a murine MPS model results in both CNS and peripheral organ transduction, with corresponding correction of peripheral pathology.24,27 It has also been demonstrated that this distribution of vector to the periphery occurred after intrathecal administration in primates and dogs, an observation confirmed by our PCR analysis in the present study.27,29 Given the distribution of vector to the periphery and significant serum IDUA activity, we evaluated somatic tissues for evidence of disease correction. Quantitative analysis of GAGs in liver and spleen showed normalization in the liver of all treated animals, whereas splenic GAG storage was corrected in animals which exhibited the lowest antibody titers in their treatment groups (8911, 8932, 9058) but not in those that had the highest antibody titers within their treatment group and had reduced CSF enzyme activity (Supplementary Figure S1). This is consistent with IDUA being expressed primarily from the heavily transduced liver, with cross correction of distant organs such as the spleen occurring only in the absence of an interfering antibody response. Histological evaluation of GAG storage by Alcian blue stain confirmed correction in the liver and spleen, as well as improvement in the heart and lungs, which was more pronounced in the animals with lower antibody titers (Figure 7). The kidney appeared to have significant GAG storage that did not respond as readily to treatment.
Figure 7.

Correction of somatic lesions. Sections from heart, lung, liver, spleen, and kidney were stained with Alcian blue. Animals are stratified according to antibody response and circulating enzyme activity as in Figure 6. Scale bar = 100 µm.
Serum levels of the covalent complex formed by thrombin and the protease inhibitor heparin cofactor II (HCII) have been proposed as a biomarker in MPS I, as formation of this complex is catalyzed by GAGs such as dermatan sulfate.32,33 We saw reductions in serum HCII-thrombin in all treated animals, consistent with reduction in peripheral GAGs (Supplementary Figure S2). The response appeared less robust in the two animals with high residual spleen GAGs.
Intrathecal AAV9 does not induce an inflammatory response in the CNS
One report has suggested that intrathecal AAV9 delivery can result in a robust inflammatory response and cellular infiltration.34 We evaluated hematoxylin and eosin stained tissue sections from cerebrum and cerebellum for evidence of cellular infiltrates (Supplementary Figure S3). We also immunostained tissue sections for GFAP, an astrocyte intermediate filament protein that is highly upregulated in the context of inflammation (Supplementary Figure S4; Supplementary Table S2). We observed no evidence of cellular infiltration or astrocyte activation in any of the treated animals based on standard histopathology or GFAP staining.
Discussion
The goal of this study was to evaluate the feasibility of intrathecal gene therapy for treating CNS manifestations of MPS I in an authentic large animal model as a necessary step toward human trials. A previously described feline model of MPS I was further characterized for biochemical and histological abnormalities as a prelude to an evaluation of gene transfer. Our study demonstrated diffuse CNS pathology in this model, including neuronal storage lesions staining strongly for gangliosides and cholesterol as well as pronounced meningeal and perivascular GAG storage, consistent with previous reports.29 We also found accumulation of the lysomomal membrane protein LIMP2 and elevation of hexosaminidase activity in the brains of MPS I cats, as has been observed in other species.24 We found that a single intrathecal administration of AAV9 expressing normal feline IDUA resulted in virtually complete correction of the histological and biochemical features of MPS I in the CNS. We could not directly assess the clinical consequences of treatment because MPS I cats do not have a well-defined neurological phenotype, although mouse studies suggest that correction of CNS storage pathology correlates with normalization of cognitive deficits.23
Intrathecal AAV9 delivery could potentially be applied to many lysosomal storage diseases affecting the CNS. However, MPS I may represent a particularly tractable target due to the extremely low levels of enzyme required to correct lysosomal storage. In vitro studies have shown that IDUA concentrations below 1 pM can reduce GAG accumulation in MPS I patient fibroblasts, which would predict extremely efficient cross correction of untransduced cells by enzyme secreted from even a small number of genetically modified cells.9 MPS I is also an important target due to the substantial CNS morbidity in this disease and the limited efficacy and availability of treatment options. BMT is currently the only therapeutic option with the potential to modify the course of cognitive decline in patients with a severe phenotype, but this comes with high morbidity and mortality. Other CNS manifestations such as communicating hydrocephalus and spinal cord compression also cause debilitating symptoms and necessitate surgical interventions such as ventriculoperitoneal shunting or spinal laminectomy.2,3,4,5,6,7 Our results demonstrate that intrathecal AAV9 administration can reverse storage lesions in both the brain parenchyma and the surrounding meninges, indicating the potential to benefit patients with a variety of CNS sequelae of MPS I. In addition to the unmet clinical need in MPS I and strong preclinical data, this disease presents some practical benefits as a target for human trials. The ability to measure enzyme activity in the CSF would allow for direct confirmation of transgene expression, and a variety of potential biomarkers could be useful for assessing biological activity. In this study, we found that CSF hexosaminidase activity was elevated in MPS I animals and was normalized in proportion to histological CNS correction, making this a strong candidate for a biomarker in human trials. Longitudinal evaluation of the MRI findings typical of MPS I—enhanced white matter signal intensity, enlarged perivascular spaces and meningeal thickening—could also provide useful correlates of therapeutic activity.5
We found that a variable antibody response against normal feline IDUA was elicited by intrathecal AAV9-mediated expression. This might be expected, given that nearly all MPS I patients develop antibodies against the enzyme during the course of ERT. The heterogeneity of clinical responses and high prevalence of antibodies make it difficult to discern a clear effect of antibodies on therapeutic outcomes in humans, but studies in MPS I dogs indicate that tissue correction from systemic ERT is significantly reduced by high antibody titers.35 A study examining the effect of antibody titer on the efficacy of intrathecal ERT in canine MPS I likewise showed that antibodies reduce treatment efficacy in the CNS.36 Our results suggest that while anti-IDUA antibodies impact the efficiency of tissue correction, cross correction of untransduced cells remains reasonably effective in the setting of an antibody response. Some degree of residual efficacy after antibody induction against IDUA could also be explained by intracellular enzyme production alone; however, a previous study demonstrated that intracisternal AAV9 delivery at the dose tested transduces only a small fraction of cells in the feline brain, suggesting that widespread reversal of storage lesions is due to cross-correction. Most critically, development of an antibody response against IDUA in the CSF was not associated with adverse clinical events or histological evidence of inflammation. Together these data strongly support the safety and efficacy of this approach, even with the risk of developing antibodies against the therapeutic enzyme. Given the correlation we observed between antibody titer and histological evidence of efficacy, measuring CSF antibody titers may be informative for predicting clinical outcomes in human trials. Long-term follow up of patients treated with systemic ERT has shown that antibody titers fall to baseline levels in most patients within two years of treatment initiation, suggesting that a similar decline in titer may be seen in the context of gene therapy, which would further support the potential for robust long-term clinical benefit.37 Long-term evaluation of AAV9 intrathecal gene therapy in feline MPS I is currently underway.
Here, we have demonstrated proof-of-principle that intrathecal AAV9 delivery can globally correct CNS manifestations of MPS I in a high-fidelity large animal model, even when antibodies are elicited against the therapeutic enzyme. The excellent tolerability and efficacy of this approach support progression into human trials for MPS I. These promising results also suggest that this approach may serve as a broad platform for the treatment of other lysosomal storage diseases affecting the CNS.
Materials and Methods
Vector production. The feline IDUA sequence was isolated as described (Wang et al., manuscript in preparation). A codon-optimized version of the cDNA was cloned into expression constructs flanked by AAV2 terminal repeats that contained the CMV or CB promoter, a chimeric intron, and an SV40 or rabbit globin polyadenylation sequence. The constructs were packaged in an AAV9 capsid by triple transfection of 293 cells and purified as previously described.38
Animal procedures. The MPS I cat colony was maintained at the University of Pennsylvania School of Veterinary Medicine under NIH and USDA guidelines for the care and use of animals in research. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Approximately 3 ml of blood was collected from the jugular vein for serum analysis. For intrathecal injections, propofol-anesthetized animals were intubated, and the suboccipital region was clipped of hair and scrubbed. Using sterile technique, a suboccipital puncture was performed with a 22 gauge spinal needle. Placement was confirmed by CSF return. After collecting 1–2 ml of CSF, vector diluted in sterile phosphate-buffered saline (PBS) (1–2 ml) was slowly injected by hand. The animals were monitored to confirm complete recovery after the procedure. Euthanasia was by an intravenous overdose of sodium barbiturate, 80 mg/kg.
Enzyme assays. Tissue, serum, and CSF samples were immediately frozen on dry ice and stored at −80 degrees until analysis. Serum and CSF were used directly in IDUA and Hexosaminidase (Hex) assays. Tissue samples were homogenized in lysis buffer (0.2 % Triton-X100, 0.9% NaCl, pH 4.0), and briefly sonicated. Samples were then freeze-thawed and clarified by centrifugation. Protein concentrations were determined by Bradford assay. IDUA activity was measured by incubating sample diluted in 0.1 ml water with 0.1 ml of 100 mmol/l 4MU-iduronide (Toronto Research Chemicals, Toronto, Canada; Glycosynth, Warrington, England) in IDUA buffer (0.15 mol/l NaCl, 0.05% Triton-X100, 0.1 mol/l sodium acetate, pH 3.58) at 37 degrees for 1–3 hours. The reaction was stopped by addition of 2 ml 290 mmol/l glycine, 180 mmol/l sodium citrate, pH 10.9. The liberated 4MU was quantified by comparing fluorescence to standard dilutions of 4MU. Units are given as nmol 4MU liberated per hour per mg of protein (tissues) or per ml of serum or CSF. Hex assays were performed as described.39
HCII-thrombin western blot. 0.5 µl of serum in 1× LDS buffer was separated on a 4–12% Bis-Tris polyacrylamide gel (Novex) in MOPS buffer at 120 V for 2 hours. Protein was transferred to a PVDF membrane at 30 V for 1.5 hours. The membrane was blocked for 1 hour in 5% NFDM, and then incubated overnight at 4 degrees in 5% NFDM containing a 1:25,000 dilution of HRP-conjugated goat anti-HCII antibody (Enzyme Research, South Bend, IN). The blot was washed and developed with a chemiluminescent substrate (Thermo Scientific, Waltham, MA).
GAG assay. Tissue samples were processed as for enzyme assays. Tissue GAGs were quantified using the Blyscan assay (Biocolor, Carrickfergus, UK) according to the manufacturer's instructions.
ELISA for detection of antibodies to feline IDUA. A C-terminal his-tag was added to the feline IDUA cDNA by PCR. The tagged cDNA was cloned into an expression cassette driven by a CB promoter. This plasmid was transfected into six 90% confluent 10 cm plates of HEK 293 cells using lipofectamine 2000 (Invitrogen, Carlsbad, CA). Supernatant was collected twice at 24-hour intervals; each time the supernatant pH was immediately titrated to pH 5.8, which vastly increased enzyme stability, and stored at 4 degrees. The enzyme was purified on a 1 ml HisTrap FF column (GE). The eluted fractions were immediately adjusted to pH 5.8. The fractions containing purified feline IDUA were identified by enzyme assay and SDS–PAGE. The purified protein was incubated at 3 µg/ml in PBS, pH 5.8 on polystyrene ELISA plates overnight at 4 degrees. The plates were washed twice in PBS, pH 5.8, blocked in 3% BSA, pH 5.8, and then incubated with diluted samples for one hour at room temperature. The plates were washed five times, incubated 1 hour with a 1:10,000 dilution of HRP-conjugated goat anti-feline IgG (Peirce, Rockford, IL) in blocking solution, washed five times, and developed using TMB substrate.
Quantitative PCR. Quantification of vector genomes in tissue was performed as previously described.38
Histology. Brains were divided into left and right hemisphere. The right half was sliced and, except for the distal occipital part, fixed in paraformaldehyde to be further processed for GM3 and cholesterol detection. Slices from the left half were used for all other stains as well as for PCR analysis and enzyme assays. For PCR and enzyme analysis, samples were collected from the frontal, temporal and occipital cortices as well as medulla, hippocampus, and cerebellum. Hippocampus samples included the entire dissected hippocampus, spanning from CA1 to the DG.
Hematoxylin and eosin staining (H&E). H&E staining was performed on 6 µm sections from formalin-fixed paraffin-embedded tissues according to standard protocols.
GM3 immunohistochemistry. Brain slices were fixed overnight in 4% paraformaldehyde/PBS, equilibrated sequentially in 15% and 30% sucrose, and frozen in OCT embedding medium.
Immunostaining was performed on 30 µm thick floating cryosections as described31 using monoclonal antibody DH2 (Glycotech, Gaithersburg, MD) as primary antibody followed by a biotinylated secondary anti-mouse antibody (Jackson Immunoresearch, West Grove, PA) and detection with a Vectastain Elite ABC kit (Vector Labs, Burlingame, CA). Stained sections were transferred onto glass slides and mounted with Fluoromount G (Electron Microscopy Sciences, Hatfield, PA).
GAG histochemistry. Brain samples were fixed overnight in methacarn (60% methanol, 30% chloroform, 10% glacial acetic acid) at 4 °C, dehydrated through an ethanol series and xylene, and paraffin embedded. Deparaffinized 6 µm sections were stained in 1% Alcian Blue (Sigma, St Louis, MO)/0.1 N HCl (pH1.0) for 15 minutes, rinsed in water for 2–3 minutes, and counterstained with Nuclear Fast Red (Vector Labs).
Filipin stain. Cholesterol was detected on 30 µm thick cryosections prepared as described for GM3 immunohistochemistry. Floating sections were stained with filipin (Sigma, 10 µg/ml prepared from 3 mg/ml stock solution) for 1.5 hours. After washing in PBS (2 × 5 minutes), sections were mounted with Fluoromount G.
Immunofluorescence. Immunostaining was performed on 6 µm sections from formalin-fixed paraffin-embedded tissue samples. Sections were deparaffinized through an ethanol and xylene series, boiled in a microwave for 6 minutes in 10 mmol/l citrate buffer (pH 6.0) for antigen retrieval, and blocked with 1% donkey serum in PBS + 0.2% Triton for 15 minutes followed by sequential incubation with primary (1 hour) and labeled secondary (45 minutes) antibodies diluted in blocking buffer. Primary antibodies used were rabbit antibodies against GFAP (Abcam, Cambridge, UK; 1:1,000) and LIMP2 (Novus Biologicals, Littleton, CO; 1:200), and FITC- or TRITC-labeled donkey anti-rabbit (Jackson Immunoresearch) served as secondary antibody.
Morphometric analyses. Images taken for quantification purposes were acquired using either a 4× (GM3), 10× (filipin), or 20× objective (GFAP, LIMP2, H&E) depending on the lowest magnification that still allowed accurate visualization of neurons or astrocytes. Images for GFAP and LIMP2 were taken from the area directly below the cerebral cortex surface and include the cerebral molecular layer; all other images were centered on the middle to upper neuron layers of the cortex. The images shown for LIMP2, filipin, and H&E stained sections correspond to the region depicted in the second column of Figure 4. Five images from each brain were taken for analyzing storage in H&E sections as well as GM3 and filipin staining which showed low variation between different brain parts, while a total of 20 images (10 each from two different sections) were acquired for GFAP and LIMP2 analyses. For quantification of GM3 and filipin positive neurons, ImageJ software (Rasband W.S., National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/) was used to first threshold images, i.e., to mark positively stained cells, and then to count the number of these cells with the “Analyze Particles” tool of ImageJ. GFAP- and LIMP2-positive cells as well as storage-positive H&E-stained neurons were counted manually.
SUPPLEMENTARY MATERIAL Figure S1. Peripheral GAG clearance following intrathecal AAV9 delivery. Figure S2. Reduced serum heparin cofactor II-thrombin complex concentration following intrathecal gene therapy. Figure S3. Absence of inflammation or cellular infiltration in treated animals. Figure S4. Absence of astrocyte activation in treated animals. Table S1. CSF analysis in treated and control MPS I cats. Table S2. Quantification of histopathology and GM3, LIMP2, filipin and GFAP positive cells per field in matched brain sections.
Acknowledgments
We thank Hongwei Yu (University of Pennsylvania, Gene Therapy Program) for expert histology services and Jenny Greig (University of Pennsylvania, Gene Therapy Program) for assistance with figure preparation. We also acknowledge the support of the Vector, Immunology, and Animal Models Cores of the Gene Therapy Program. This work was funded by NIH grants P40-OD010939 and DK25759 (M.E.H.) and ReGenX (J.M.W.). J.M.W. is an advisor to ReGenX Biosciences and Dimension Therapeutics, and is a founder of, holds equity in, and receives grants from ReGenX Biosciences and Dimension Therapeutics; in addition, he is a consultant to several biopharmaceutical companies and is an inventor on patents licensed to various biopharmaceutical companies. M.E.H. is a stockholder of BioMarin Pharmaceuticals.
Supplementary Material
Peripheral GAG clearance following intrathecal AAV9 delivery.
Reduced serum heparin cofactor II-thrombin complex concentration following intrathecal gene therapy.
Absence of inflammation or cellular infiltration in treated animals.
Absence of astrocyte activation in treated animals.
CSF analysis in treated and control MPS I cats.
Quantification of histopathology and GM3, LIMP2, filipin and GFAP positive cells per field in matched brain sections.
References
- Xing M, Parker EI, Moreno-De-Luca A, Harmouche E, Terk MR. Radiological and clinical characterization of the lysosomal storage disorders: non-lipid disorders. Br J Radiol. 2014;87:14. doi: 10.1259/bjr.20130467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachur E, Del Maestro R. Mucopolysaccharidoses and spinal cord compression: case report and review of the literature with implications of bone marrow transplantation. Neurosurgery. 2000;47:223–8; discussion 228. doi: 10.1097/00006123-200007000-00046. [DOI] [PubMed] [Google Scholar]
- Taccone A, Tortori Donati P, Marzoli A, Dell'Acqua A, Gatti R, Leone D. Mucopolysaccharidosis: thickening of dura mater at the craniocervical junction and other CT/MRI findings. Pediatr Radiol. 1993;23:349–352. doi: 10.1007/BF02011954. [DOI] [PubMed] [Google Scholar]
- Vijay S, Wraith JE. Clinical presentation and follow-up of patients with the attenuated phenotype of mucopolysaccharidosis type I. Acta Paediatr. 2005;94:872–877. doi: 10.1111/j.1651-2227.2005.tb02004.x. [DOI] [PubMed] [Google Scholar]
- Zafeiriou DI, Batzios SP. Brain and spinal MR imaging findings in mucopolysaccharidoses: a review. AJNR Am J Neuroradiol. 2013;34:5–13. doi: 10.3174/ajnr.A2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y, Hamaguchi A, Nozaki I, Iizuka T, Sasagawa T, Shima Y, et al. Cervical pachymeningeal hypertrophy as the initial and cardinal manifestation of mucopolysaccharidosis type I in monozygotic twins with a novel mutation in the alpha-L-iduronidase gene. J Neurol Sci. 2011;302:121–125. doi: 10.1016/j.jns.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Ahmed A, Whitley CB, Cooksley R, Rudser K, Cagle S, Ali N, et al. Neurocognitive and neuropsychiatric phenotypes associated with the mutation L238Q of the α-L-iduronidase gene in Hurler-Scheie syndrome. Mol Genet Metab. 2014;111:123–127. doi: 10.1016/j.ymgme.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahms NM, Lobel P, Kornfeld S. Mannose 6-phosphate receptors and lysosomal enzyme targeting. J Biol Chem. 1989;264:12115–12118. [PubMed] [Google Scholar]
- Kakkis ED, Matynia A, Jonas AJ, Neufeld EF. Overexpression of the human lysosomal enzyme alpha-L-iduronidase in Chinese hamster ovary cells. Protein Expr Purif. 1994;5:225–232. doi: 10.1006/prep.1994.1035. [DOI] [PubMed] [Google Scholar]
- Aldenboven M, Boelens F, de Koning TF. The clinical outcome of Hurler syndrome after stem cell transplantation. Biology of Blood and Marrow Transplantation. 2008;14:485–498. doi: 10.1016/j.bbmt.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Chen A, Vogler C, McEntee M, Hanson S, Ellinwood NM, Jens J, et al. Glycosaminoglycan storage in neuroanatomical regions of mucopolysaccharidosis I dogs following intrathecal recombinant human iduronidase. APMIS. 2011;119:513–521. doi: 10.1111/j.1600-0463.2011.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson P, Ellinwood NM, Dierenfeld A, Kline K, Parkes J, Hanson S, et al. Intrathecal enzyme replacement therapy treats meningeal storage and spinal cord compression in MPS I dogs. Mol Genet Metab. 2010;99:S15–S15. [Google Scholar]
- Dickson P, Naylor D, Mlikotic A, Victoroff A, Chen A, Passage M, et al. Initial experience with intrathecal recombinant human alpha-L-iduronidase for spinal cord compression in two mucopolysaccharidosis I patients. Mol Genet Metab. 2008;93:S19–S19. [Google Scholar]
- Dickson PI, Ellinwood NM, Hanson S, Vite C, Passage M, Le S, et al. Intrathecal enzyme replacement therapy may stabilize or reverse signs of spinal cord compression in MPS I dogs. Mol Genet Metab. 2009;98:70–70. [Google Scholar]
- Dickson PI, Hanson S, McEntee MF, Vite CH, Vogler CA, Mlikotic A, et al. Early versus late treatment of spinal cord compression with long-term intrathecal enzyme replacement therapy in canine mucopolysaccharidosis type I. Mol Genet Metab. 2010;101:115–122. doi: 10.1016/j.ymgme.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson PI, Naylor D, Mlikotic A, Victoroff A, Chen A, Passage M, et al. Intrathecal recombinant human a-L-iduronidase alleviates spinal cord compression symptoms and is well-tolerated in attenuated MPS I patients. Mol Genet Metab. 2008;93:247–247. [Google Scholar]
- Kakkis E, McEntee M, Vogler C, Le S, Levy B, Belichenko P, et al. Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I. Mol Genet Metab. 2004;83:163–174. doi: 10.1016/j.ymgme.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Munoz-Rojas MV, Vieira T, Costa R, Fagondes S, John A, Jardim LB, et al. Intrathecal enzyme replacement therapy in a patient with mucopolysaccharidosis type I and symptomatic spinal cord compression. Am J Med Genet A. 2008;146A:2538–2544. doi: 10.1002/ajmg.a.32294. [DOI] [PubMed] [Google Scholar]
- Mandel RJ, Burger C. Clinical trials in neurological disorders using AAV vectors: promises and challenges. Curr Opin Mol Ther. 2004;6:482–490. [PubMed] [Google Scholar]
- McPhee SW, Janson CG, Li C, Samulski RJ, Camp AS, Francis J, et al. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med. 2006;8:577–588. doi: 10.1002/jgm.885. [DOI] [PubMed] [Google Scholar]
- Mittermeyer G, Christine CW, Rosenbluth KH, Baker SL, Starr P, Larson P, et al. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson's disease. Hum Gene Ther. 2012;23:377–381. doi: 10.1089/hum.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Wolf DA, Lenander AW, Nan Z, Belur LR, Whitley CB, Gupta P, et al. Direct gene transfer to the CNS prevents emergence of neurologic disease in a murine model of mucopolysaccharidosis type I. Neurobiol Dis. 2011;43:123–133. doi: 10.1016/j.nbd.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurigot V, Marco S, Ribera A, Garcia M, Ruzo A, Villacampa P, et al. Whole body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy. J Clin Invest. 2013;123:3254–3271. doi: 10.1172/JCI66778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vite CH, McGowan JC, Niogi SN, Passini MA, Drobatz KJ, Haskins ME, et al. Effective gene therapy for an inherited CNS disease in a large animal model. Ann Neurol. 2005;57:355–364. doi: 10.1002/ana.20392. [DOI] [PubMed] [Google Scholar]
- Ciron C, Desmaris N, Colle MA, Raoul S, Joussemet B, Vérot L, et al. Gene therapy of the brain in the dog model of Hurler's syndrome. Ann Neurol. 2006;60:204–213. doi: 10.1002/ana.20870. [DOI] [PubMed] [Google Scholar]
- Gray SJ, Nagabhushan Kalburgi S, McCown TJ, Jude Samulski R. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 2013;20:450–459. doi: 10.1038/gt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher T, Dubreil L, Colle MA, Maquigneau M, Deniaud J, Ledevin M, et al. Intracisternal delivery of AAV9 results in oligodendrocyte and motor neuron transduction in the whole central nervous system of cats. Gene Ther. 2014;21:522–528. doi: 10.1038/gt.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vite CH, Wang P, Patel RT, Walton RM, Walkley SU, Sellers RS, et al. Biodistribution and pharmacodynamics of recombinant human alpha-L-iduronidase (rhIDU) in mucopolysaccharidosis type I-affected cats following multiple intrathecal administrations. Mol Genet Metab. 2011;103:268–274. doi: 10.1016/j.ymgme.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Li CM, Simonaro CM, Wan Q, Haskins ME, Desnick RJ, et al. Identification and characterization of the molecular lesion causing mucopolysaccharidosis type I in cats. Mol Genet Metab. 1999;67:106–112. doi: 10.1006/mgme.1999.2860. [DOI] [PubMed] [Google Scholar]
- McGlynn R, Dobrenis K, Walkley SU. Differential subcellular localization of cholesterol, gangliosides, and glycosaminoglycans in murine models of mucopolysaccharide storage disorders. J Comp Neurol. 2004;480:415–426. doi: 10.1002/cne.20355. [DOI] [PubMed] [Google Scholar]
- Langford-Smith KJ, Mercer J, Petty J, Tylee K, Church H, Roberts J, et al. Heparin cofactor II-thrombin complex and dermatan sulphate:chondroitin sulphate ratio are biomarkers of short- and long-term treatment effects in mucopolysaccharide diseases. J Inherit Metab Dis. 2011;34:499–508. doi: 10.1007/s10545-010-9254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall DR, Colobong KE, Hemmelgarn H, Sinclair GB, Hetty E, Thomas A, et al. Heparin cofactor II-thrombin complex: a biomarker of MPS disease. Mol Genet Metab. 2008;94:456–461. doi: 10.1016/j.ymgme.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Samaranch L, San Sebastian W, Kells AP, Bringas J, Pivirotto P, Forsayeth J, et al. Immune response activation and neurotoxicity after intrathecal infusion of AAV9 vector encoding a non-self reporter protein in the non-human primate. Mol Ther. 2013;21:S227–S227. [Google Scholar]
- Dickson P, Peinovich M, McEntee M, Lester T, Le S, Krieger A, et al. Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J Clin Invest. 2008;118:2868–2876. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson PI, Ellinwood NM, Brown JR, Witt RG, Le SQ, Passage MB, et al. Specific antibody titer alters the effectiveness of intrathecal enzyme replacement therapy in canine mucopolysaccharidosis I. Mol Genet Metab. 2012;106:68–72. doi: 10.1016/j.ymgme.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakavanos R, Turner CT, Hopwood JJ, Kakkis ED, Brooks DA. Immune tolerance after long-term enzyme-replacement therapy among patients who have mucopolysaccharidosis I. Lancet. 2003;361:1608–1613. doi: 10.1016/S0140-6736(03)13311-9. [DOI] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Bell P, Lin J, Grant RL, Siegel DL, et al. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther. 2011;22:1389–1401. doi: 10.1089/hum.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendeler M, Sandhoff K. Hexosaminidase assays. Glycoconj J. 2009;26:945–952. doi: 10.1007/s10719-008-9137-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peripheral GAG clearance following intrathecal AAV9 delivery.
Reduced serum heparin cofactor II-thrombin complex concentration following intrathecal gene therapy.
Absence of inflammation or cellular infiltration in treated animals.
Absence of astrocyte activation in treated animals.
CSF analysis in treated and control MPS I cats.
Quantification of histopathology and GM3, LIMP2, filipin and GFAP positive cells per field in matched brain sections.




