Abstract
Mucopolysaccharidosis type I (MPS I) is a progressive lysosomal storage disorder with systemic and central nervous system (CNS) involvement due to deficiency of α-l-iduronidase (IDUA). We previously identified a receptor-binding peptide from apolipoprotein E (e) that facilitated a widespread delivery of IDUAe fusion protein into CNS. In this study, we evaluated the long-term CNS biodistribution, dose-correlation, and therapeutic benefits of IDUAe after systemic, sustained delivery via hematopoietic stem cell (HSC)-mediated gene therapy with expression restricted to erythroid/megakaryocyte lineages. Compared to the highest dosage group treated by nontargeted control IDUAc (165 U/ml), physiological levels of IDUAe in the circulation (12 U/ml) led to better CNS benefits in MPS I mice as demonstrated in glycosaminoglycan accumulation, histopathology analysis, and neurological behavior. Long-term brain metabolic correction and normalization of exploratory behavior deficits in MPS I mice were observed by peripheral enzyme therapy with physiological levels of IDUAe derived from clinically attainable levels of HSC transduction efficiency (0.1). Importantly, these levels of IDUAe proved to be more beneficial on correction of cerebrum pathology and behavioral deficits in MPS I mice than wild-type HSCs fully engrafted in MPS I chimeras. These results provide compelling evidence for CNS efficacy of IDUAe and its prospective translation to clinical application.
Introduction
Lysosomal storage disorders are a group of inherited metabolic conditions with defects in lysosomal functions and more than 65% affecting the central nervous system (CNS).1 Mucopolysaccharidosis type I (MPS I), one of the most common lysosomal storage disorders, is caused by mutations in the gene encoding a lysosomal enzyme, α-l-iduronidase (IDUA), and subsequent accumulation of dermatan sulfate and heparan sulfate.2 The abnormal accumulation of these complex sugars known as glycosaminoglycans (GAG) in the lysosomes leads to progressive multiorgan damage. Patients with severe form of MPS I have an early-onset, rapidly progressive disease that is accompanied by CNS complications and premature death if untreated.3 Currently, there is no successful therapy to restore CNS abnormalities, although allogeneic hematopoietic stem cell (HSC) transplantation performed before 2 years of age has shown amelioration of CNS manifestations in some MPS I patients presumably due to donor-derived brain macrophages originated from donor HSC-derived monocytes.4,5,6 Enzyme therapy has been restricted to patients without neurological manifestations due to the inability of lysosomal enzymes to penetrate the CNS.6,7
Clinical trials with HSC-mediated gene therapy via autologous transplantation have demonstrated potential life-long therapeutic benefits without the morbidity and mortality of allogeneic transplantation.8 It has been reported recently that lentiviral (LV) mediated HSC gene therapy completely corrected CNS disease manifestations in MPS I mice with gene transfer efficiency of ~15 vector copies per HSC,9 suggesting that enzyme dose is critical to CNS efficacy. The frequencies of successfully engrafted and transduced HSC in gene therapy clinical trials have increased from the original value of ≤0.1 copy/genome to ~4 copy/genome recently.10,11 High transgene dosage would be necessary to achieve clinical efficacy; but it will also increase the risk for proviral integration-associated mutagenesis, which has become a major limitation in gene therapy.12,13,14 We have shown that supraphysiological levels of IDUA in the circulation can be achieved with moderate gene dosage (0.3 copy per HSC) when utilizing maturing red blood cells and/or megakaryocytic lineage for IDUA expression, leading to complete correction in all organs tested except the brain due to the apparent inability of IDUA to penetrate the blood–brain barrier (BBB) in therapeutically significant amounts.15,16
The BBB, mainly formed by brain capillary endothelial cells, restricts the delivery of most macromolecules from the blood into the brain except for those that are endorsed by one of the receptor-mediated transcytosis systems on the BBB.17 LDL receptor-related protein 1 (LRP-1) and very low-density lipoprotein receptor are abundantly expressed on the BBB18,19 and bind apolipoproteins (apo) to facilitate their transcytosis. Our recent studies have identified a receptor-binding peptide from apoE that facilitated a widespread CNS delivery of IDUA-e1 fusion protein (IDUAe) via the LRP-1-mediated transcytosis, while retaining appropriate lysosomal enzyme trafficking and catalytic function.20 The therapeutic potential was indicated by normalization of brain β-hexosaminidase levels and GAG accumulation 5 months after continuous systemic exposure to IDUAe at high levels in circulation. However, the ultimate benefits on neurological function and CNS pathology of MPS I are still to be determined, and the minimum dose of IDUAe that would enable the enzyme to cross the BBB and to treat the neurological deficits is still unknown.
Regardless of variations in therapeutic strategies for CNS treatment, two main questions remain. First, if and how therapeutic enzyme can reach the brain parenchyma, for which we have found a promising solution in previous report by using a receptor-binding peptide(s) derived from apoE.20 In this study, we aim to investigate the second question, i.e., what quantity and distribution within brain parenchyma are sufficient to make a significant impact on the functional progression of the CNS disease. Both LRP-1 and very low-density lipoprotein receptor are also abundantly expressed on neurons, astrocytes, and pericytes,21,22,23 thereby likely providing for broad distribution of IDUAe into the brain and significantly influence its therapeutic efficacy. Here, we explored the threshold levels of plasma IDUAe required for long-term CNS benefits in a MPS I mouse model, in comparison to a nontargeting control enzyme (IDUA fused with a myc-tag; IDUAc). Five months after systemic delivery of IDUAe produced from erythroid/megakaryocytic cells derived from LV-mediated HSC gene transfer, metabolic correction and normalization of behavioral deficits were accomplished in MPS I mice with physiological levels of IDUAe and relatively low copy number (0.1 copy/genome). The results from this preclinical study provide compiling evidence for CNS efficacy of IDUAe protein and its further translation to clinical application.
Results
Long-term systemic delivery of IDUAe increases IDUA activities in the brain of MPS I mice with correlation to plasma enzyme dosages
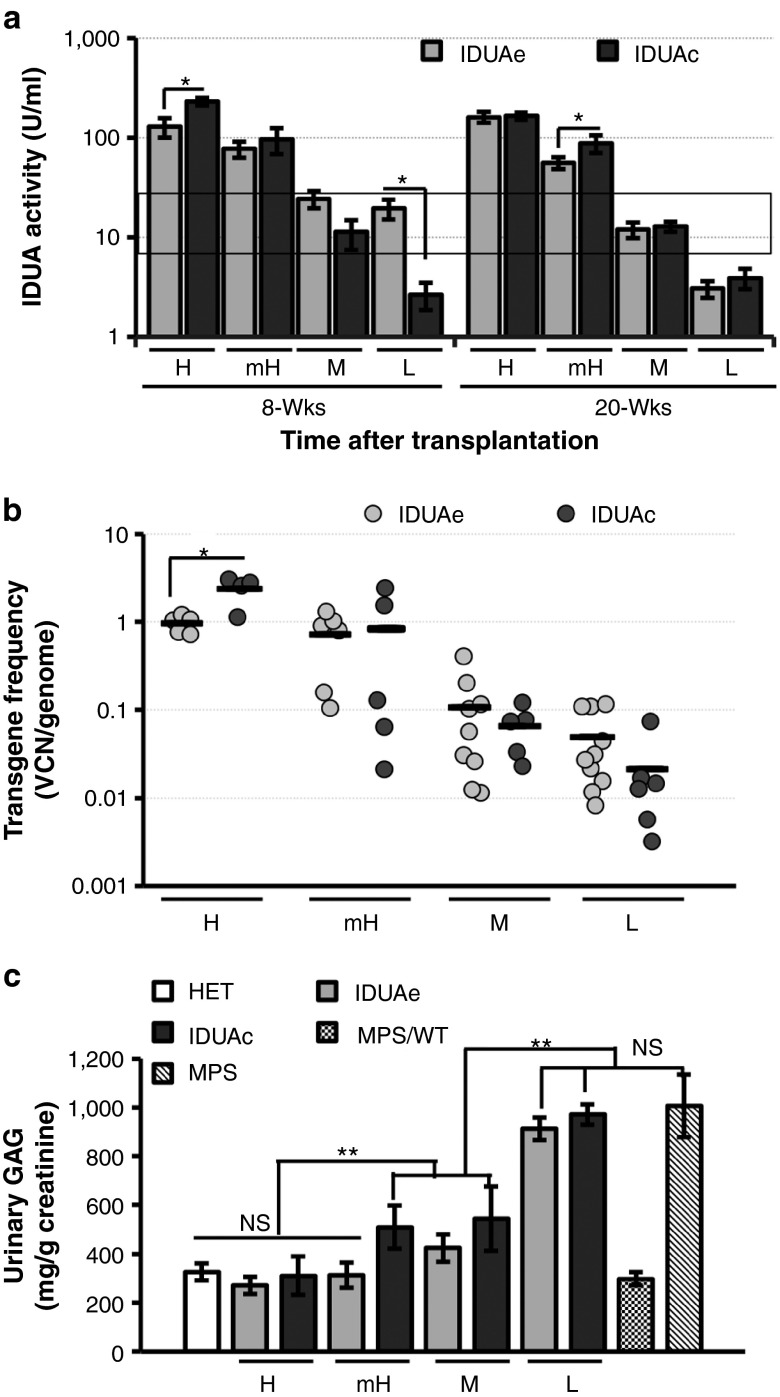
To assess long-term brain delivery of peripheral IDUA-myc-e1 (i.e., IDUAe) at different dosages, we conducted LV-mediated HSC gene transfer in MPS I mice with varying multiplicities of infection (2–50) using a hybrid promoter to restrict the expression of IDUAe or IDUAc in erythroid and megakaryocytic lineages.15,16 Sustained and varying amounts of plasma IDUA levels were detected in all IDUAe- and IDUAc-treated MPS I animals (Supplementary Figure S1) with normal total blood counts (Supplementary Table S1). Based on peripheral IDUA activities at 5 months posttransplantation, both IDUAe- and IDUAc-treated mice were divided into four plasma enzyme-dosage groups, including high (H; mean of 160 U/ml for IDUAe or 165 U/ml for IDUAc with all 20-week plasma activities >100 U/ml), moderate-to-high (mH; mean of 55 or 80 ranging from 31 to 95), moderate (M; mean of 12 or 13 ranging from 6 to 24), and low subgroups (L; mean of 3 or 4 ranging from 0.5 to 5) (Figure 1a and Supplementary Table S2). The plasma IDUA activities in the moderate groups (IDUAe-M or IDUAc-M groups) are comparable to those observed in normal carrier and wild-type animals. Vector copy numbers (VCN) in bone marrow were determined by quantitative PCR (qPCR), resulting in the mean of 0.96 copy/genome for IDUAe-H or 2.4 for IDUAc-H, 0.7 or 0.8 for mH groups, 0.1 or 0.07 for M groups, and 0.05 or 0.02 for L-dosage groups, respectively (Figure 1b). Urinary GAG levels approached or were completely normalized to normal heterozygous levels in MPS I mice treated with moderate or higher dosages of IDUAe and those with only the highest dosage of IDUAc, suggesting that a better systemic metabolic correction could be obtained by IDUAe than IDUAc treatment (Figure 1c). Normal GAG levels were observed in urine of MPS I animals transplanted with wild-type bone marrow cells (MPS/WT) that serve as treatment controls resembling patients with allogeneic bone marrow transplantation treatment. The data also demonstrate a loose correlation of gene dose with peripheral IDUA enzyme activity in the circulation.
Figure 1.
Long-term expression of fusion IDUA in various MPS I chimera groups after HSC-mediated gene transfer using lineage-specific LV. (a) Plasma IDUA levels at 8 and 20 weeks (wks) after transplantation. MPS I mice (8–10 weeks old) were transplanted with MPS I Lin− cells that were transduced with LV-KIe1-iG (IDUAe) or LV-KImyc-iG (IDUAc) at different MOI. Based on their plasma IDUA levels at 20 wks, mice were divided into four enzyme dosage groups including high (H; >100 U/ml), medium/high (mH; 25–100 U/ml), medium (M; 6–24 U/ml), and low (L; <5 U/ml) groups. Data were derived from 5–10 mice per group. Black rectangle indicates plasma IDUA levels in heterozygote (HET) and wild-type mice. (b) IDUA transgene frequencies (VCN/genome) determined by real-time quantitative PCR using bone marrow 6–7 months after transplantation. Black lines represent mean frequencies for each subgroup. (c) Urinary GAG levels quantified by a binding assay with 1,9-dimethylmethylene blue chloride dye 5 months after transplantation (n = 5–8 per group). HSC, hematopoietic stem cell; IDUA, α-l-iduronidase; LV, lentiviral; MPS/WT, MPS I animals transplanted with wild-type bone marrow cells; MOI, multiplicities of infection; VCN, vector copy number. *P ≤ 0.05; **P < 0.01; NS, no significance.
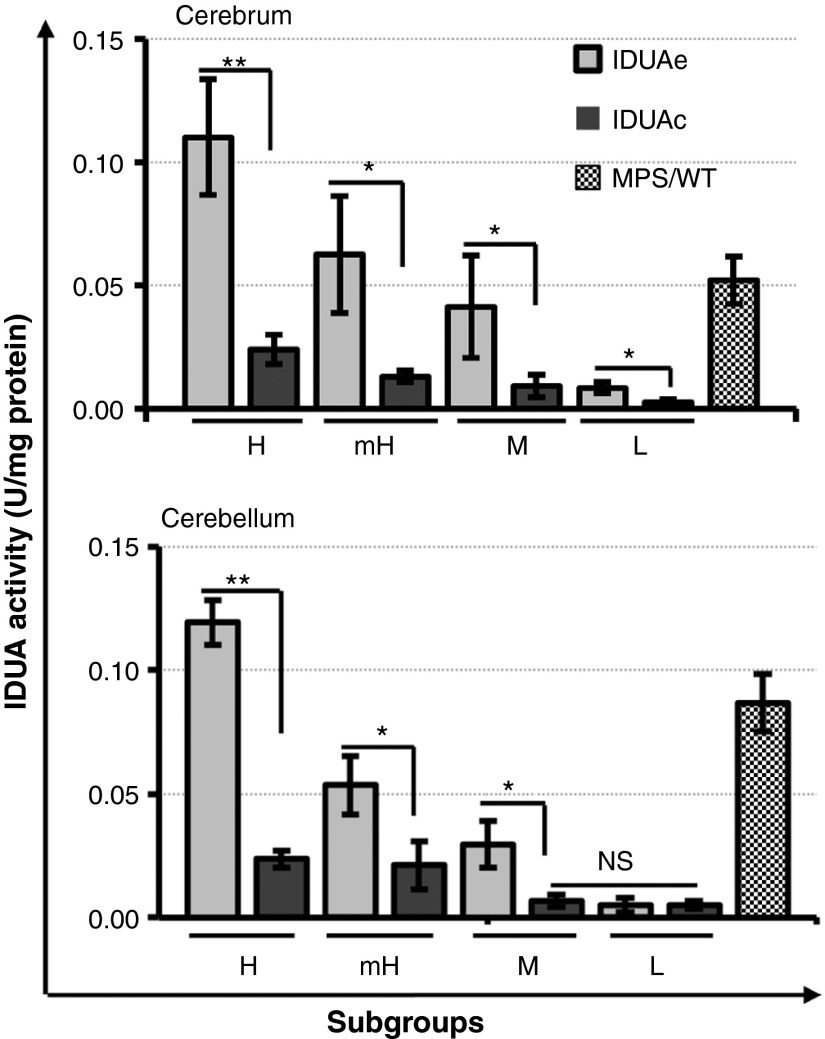
To examine the efficiency of brain delivery of BBB-targeted IDUAe, we evaluated IDUA enzyme activity in brain tissues obtained from well-perfused animals treated with either IDUAe or IDUAc controls (Figure 2). In addition to strong correlations with plasma enzyme dosages, IDUA activities in IDUAe-treated MPS I mice were significantly higher than those found in forebrain tissues of IDUAc controls at all dosage groups (3.5–5-fold) and in cerebellum (2.5–6-fold) at dosages higher than moderate. Barely detectable levels of IDUA were found in forebrain of IDUAc-L group and in cerebellum of IDUAe-L, IDUAc-M, and IDUAc-L groups. The MPS/WT group exhibited IDUA levels between IDUAe-M and IDUAe-mH groups in forebrain tissues and between mH and H groups in cerebellum tissues. These results demonstrate that long-term delivery of IDUAe could elevate brain functional IDUA levels in MPS I animals at high (~4% of normal), moderate-to-high (2%), and moderate (1%) enzyme-dosage groups, despite constant consumption or inactivation of the enzyme along the delivery path.
Figure 2.
Enzyme activities in brain cerebrum and cerebellum. Two pieces of the brain cortex and one piece from the left cerebellum dissected from perfused animals were tested for IDUA activity in triplicate. Data are generated from 5–12 animals in each group. Undetectable level of IDUA activity (<0.003 U/mg) was found in brain and cerebellum of untreated MPS I mice. IDUA activities in normal heterozygous mice were 4.3 ± 0.5 U/mg in cerebrum and 3.3 ± 0.4 U/mg in cerebellum. *P ≤0.05; **P < 0.001; NS, no significance. IDUA, α-l-iduronidase; MPS I, mucopolysaccharidosis type I.
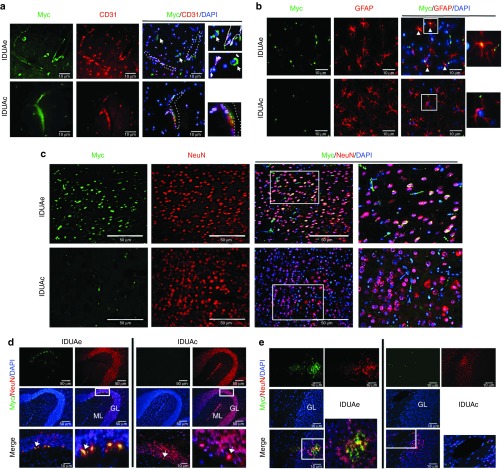
Peripheral delivery of IDUAe attains widespread protein distribution in brain of treated MPS I animals 6 months after gene therapy
To assess the brain distribution of full-length IDUAe protein, we performed immunofluorescent microscopy analysis with anti-myc antibody for the myc-tag fused at the C-terminus of IDUAe or the control IDUAc using animals from high enzyme-dosage groups (Figure 3a–c). In control IDUAc-treated brain, myc-positive signals were observed sporadically on capillary-like structures (Figure 3a). However, in IDUAe-treated mice, strong myc staining was detected not only along the lining of blood vessels and at the abluminal side of the BBB-forming capillary endothelial cells labeled by CD31 marker but also in nearby neuron-like cells. These observations indicate a continuous passage of IDUAe, but not IDUAc, from the blood stream through the BBB into the brain parenchyma. Colocalization of myc+ signals with NeuN+ (a marker for neurons) or GFAP+ (a marker for astrocytes) staining was observed throughout cerebral cortex of IDUAe-treated mice but not IDUAc control mice that expressed even higher enzyme levels in circulation (Figure 3b,c). These results document CNS delivery of full-length IDUAe from the peripheral circulation into neurons and astrocytes.
Figure 3.
Widespread distribution of IDUAe, not IDUAc, was detected in brain cerebrum and cerebellum of treated MPS I animals. Seven months after HSC-mediated gene therapy with erythroid/megakaryocyte specific LV, frozen sections from forebrain or cerebellum were obtained from perfused animals of high dosage groups. (a–c) Representative images of cerebral cortex from IDUAe-treated (IDUAe) or IDUAc-treated (IDUAc) MPS I animals showing immunofluorescence staining with antibodies against myc-tag (green), CD31 (red) or NeuN (red), or GFAP (red). Sections were counterstained with DAPI for nuclei staining (blue). White dashed lines indicate the location of blood vessels, and white arrows show myc+ neuron-like cells (in a) or Myc+/GFAP+ astrocytes (in b). Areas within white boxes are amplified and showed on the right. (d–e) Representative images of cerebellar lobes are shown after coimmunofluorescence staining with anti-myc tag (green) for full-length modified protein (IDUAc or IDUAe), anti-NeuN (red) antibodies for neuronal cells, and DAPI (blue) for nuclei. Areas within white boxes are amplified and showed on the bottom (in d) or the right (in e) with white arrows indicating Purkinje cells. Scale bar = 50 µm in a–d or 10 µm in e. DAPI, 4, 6-diamidino-2-phenylindole; GL, granular layer; HSC, hematopoietic stem cell; IDUA, α-l-iduronidase; LV, lentiviral; ML, molecular layer; MPS I, mucopolysaccharidosis type I.
Purkinje neurons in the cerebellum are one of the major storage sites for GAG in untreated MPS I animals and are also abundant with LRP-1 receptor. Unlike in IDUAc-treated mice, enzyme-containing Purkinje cells were detected only in IDUAe-treated MPS I mice as large, myc+/NeuN+ cells along the Purkinje layer located between molecular and granular layers (Figure 3d). Myc+ signals were also observed to a less degree in non-Purkinje cells (in the molecular and granular layers) (Figure 3e). These results indicate a widespread, Purkinje cell-prone distribution of IDUAe protein in the cerebellum of IDUAe-H animals.
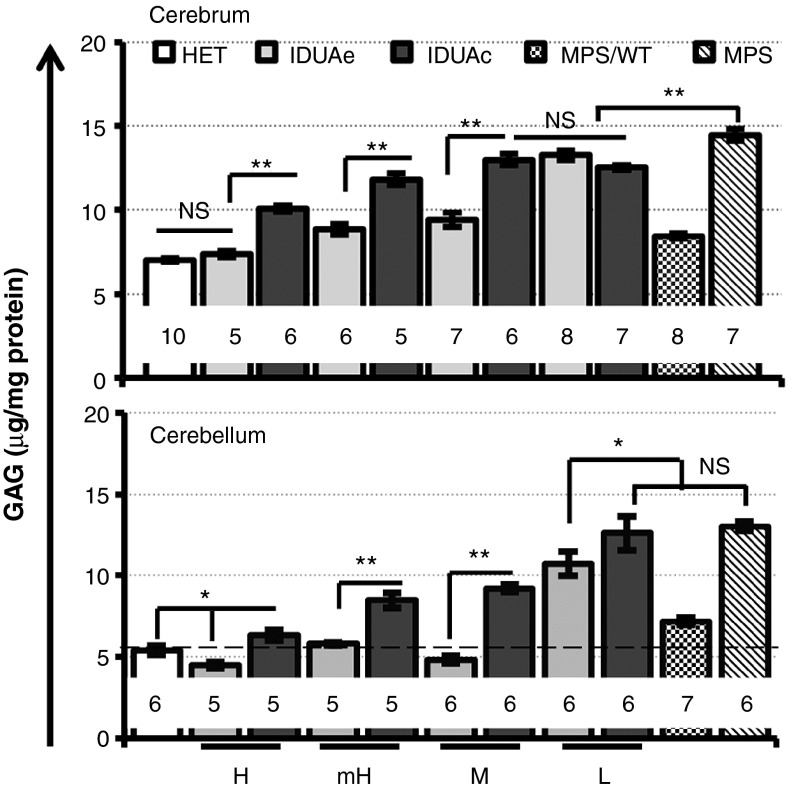
CNS delivery of peripheral IDUAe leads to metabolic correction in cerebrum and cerebellum of MPS I animals with different threshold dosages
To assess therapeutic efficacy of IDUAe on the diseased brain after long-term peripheral delivery at different enzyme dosages, we measured GAG levels in cerebrum and cerebellum of all dosage groups treated with IDUAe or IDUAc 6–7 months after transplantation (Figure 4a). In the cerebrum, elevated GAG levels in MPS I mice (2.1-fold higher than nondisease heterozygous levels) were completely normalized by IDUAe treatment in the MPS/IDUAe-H group. Significantly more GAG reduction was observed in IDUAe-treated mice than that in IDUAc-treated mice within all but L-dosage groups. The GAG levels in MPS/WT treatment group (8.4 ± 0.12 µg/mg) were similar to those detected in IDUAe-mH groups (8.8 ± 0.33). Moreover, the abnormal GAG levels in cerebellum of MPS I mice (2.4-fold of normal levels) was fully corrected by long-term IDUAe treatment in all but L-dosage groups (i.e., ≥ 6 U/ml plasma enzyme activities). MPS I mice treated with only moderate levels of IDUAe exhibited better GAG reduction than those treated by IDUAc with the highest dosage in both cerebrum and cerebellum. In addition, GAG accumulation was only partially resolved in the highest dosage group treated by IDUAc (i.e., mean of 165 U/ml) or allogeneic transplantation controls (MPS/WT). These results demonstrate that complete metabolic correction could be achieved in cerebrum of MPS I mice by peripheral delivered IDUAe at high dosages (plasma IDUA > 100 U/ml) and in cerebellum at moderate dosages (plasma IDUA ≥ 6 U/ml).
Figure 4.
Metabolic correction in MPS I cerebrum and cerebellum of IDUAe-treated animals. Two pieces of forebrain cortex or one piece from the left cerebellum dissected from perfused animals were tested for GAG levels. N for each group is indicated under each bar; dotted line represents mean of GAG levels in normal HET animals. Error bars represent SEM. *P < 0.05; **P < 0.001; NS, no significance. HET, heterozygote; IDUA, α-l-iduronidase; MPS I, mucopolysaccharidosis type I.
Significant improvement in brain pathology of IDUAe-treated animals
To evaluate the brain pathology in response to systemic IDUAe treatment, we scored the percentage of brain vasculatures associated with perivascular cells that were distended and contained massive vacuoles (Vac+ vessels) in cerebral cortex of IDUAe- or IDUAc-treated animals (Figure 5a,b). The percentage of Vac+ vessels was significantly reduced from 43% in untreated MPS I to ~30% in IDUAe-L and most IDUAc-treated groups, then further reduced to a plateau of ≤19% in IDUAe-treated groups with moderate or higher dosages. The levels of storage pathology in IDUAe-M, which were not completely corrected to normal levels (6%), were significantly better (P < 0.01) than those in IDUAc-H animals (27%) as well as those in MPS/WT controls (27%). Interestingly, brain pathology in IDUAe-L group was comparable to those observed in IDUAc-treated animals at enzyme dosages of moderate and higher levels.
Figure 5.
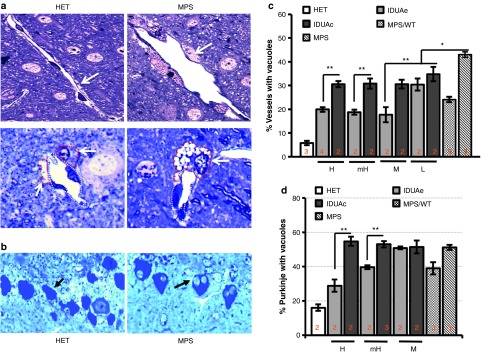
Improvement in pathology of cerebrum and cerebellum among different dosage groups of MPS I animals treated with IDUAe or IDUAc. Histopathology analysis of (a,b) forebrain cortex and (c,d) cerebellum was performed in Epon-embeded tissue sections (0.5 µm) with toluidine blue staining. (a) Representative images showing brain large vasculatures (top) or microvasculatures (bottom). Dotted lines outline either brain vascular endothelia cells (white) or perivascular cells (red) (arrows). (b) Percentages of blood vessels that are associated with vacuolated perivascular cells in forebrain. (c) Representative images of cerebellum showing Purkinje cells (arrow) without or with one or more large vacuoles. (d) Percentages of vacuolated Purkinje cells. The scoring data are shown as mean ± SD from >6 sections of 2–4 slides of 2–4 mice per group, and the percentages of all sections contributed to statistical analysis. *P < 0.05; **P < 0.001; NS, no significance. IDUA, α-l-iduronidase; MPS I, mucopolysaccharidosis type I.
We also conducted histological examination of Purkinje layers in cerebellar tissues by scoring more than 400 Purkinje cells for those with large vacuoles derived from abnormal lysosomal storage (Figure 5c,d). The percentages of vacuolated Purkinje cells were 3.2-fold elevated from 16% in normal controls to 51% in untreated MPS I mice, with no significant reduction found in any IDUAc-treated mice. The IDUAe-treated mice exhibited a clear dose-correlated reduction with a threshold of significance observed in mH groups (40%), which was significantly reduced further (P < 0.001) in IDUAe-H animals (29%). Allogeneic transplantation treatment controls (i.e., MPS/WT) showed similar levels of storage in Purkinje cells (39%) to those found in IDUAe-mH groups (P = 0.66). These results demonstrate that pathological improvement can be obtained in cerebral cortex by physiological levels of plasma IDUAe (≥6 U/ml) and in cerebellum by supraphysiological levels of IDUAe (mean of 55 U/ml; i.e., two-fold of WT) in the circulation.
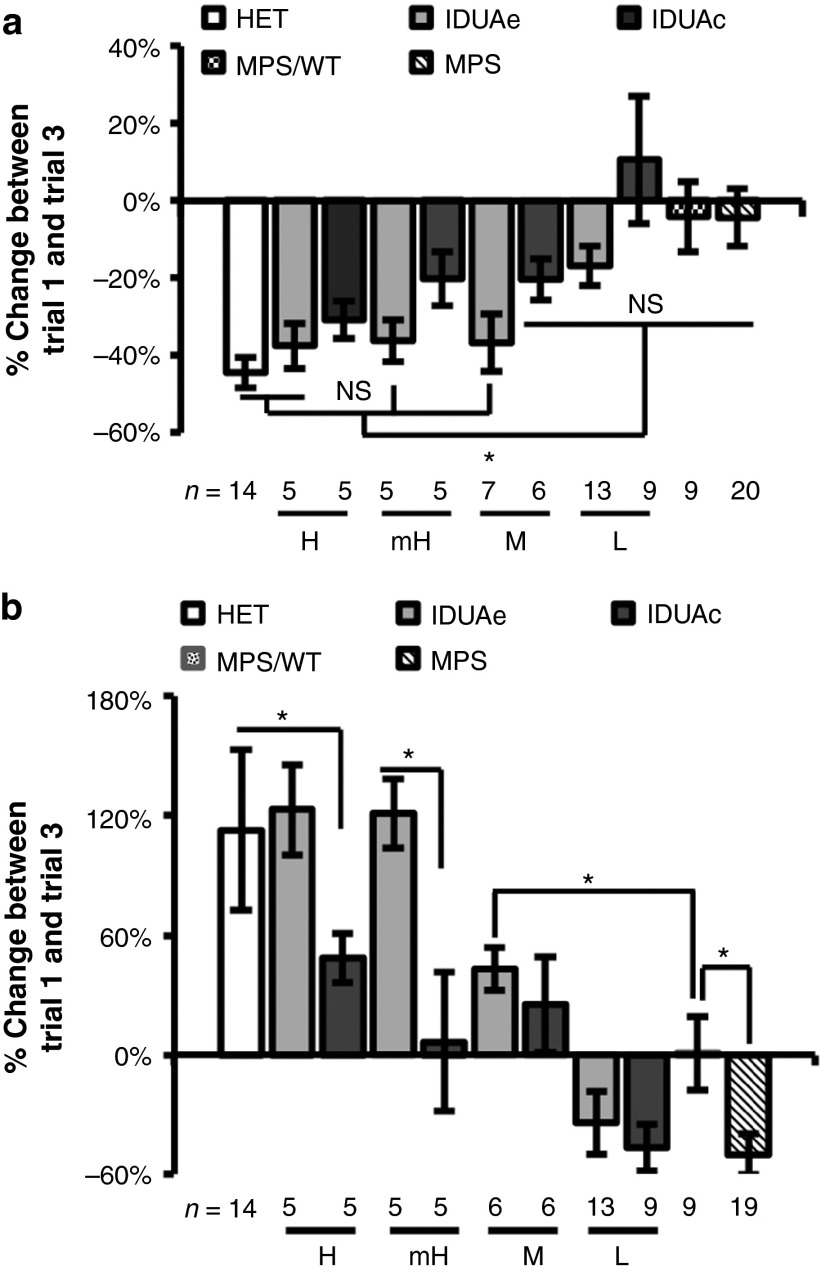
Normalization of behavioral deficits was achieved in IDUAe-treated MPS I animals during repeated open-field test
To determine the effect of IDUAe on neurological functions in MPS I mice, we conducted a repeated open-field test which has been previously utilized to evaluate murine exploratory behavior and nonaversive/nonassociative memory in an unknown environment.24 Mice were exposed to the same open-field for three repeated trials with a 30-minute intertrial interval (Figure 6). The difference between the first and third trials was determined for frequency of horizontal grid crossing and time spent on grooming. The normal (heterozygous) mice showed a 44% reduction in horizontal activity and 113% increase in grooming activity. However, the MPS I mice showed minimum reduction (4%) in horizontal activity and a 50% reduction in grooming activity. For total horizontal activity, no significant difference from normal behavior was observed in IDUAe-H (P = 0.52), -mH (P = 0.40), -M (P = 0.48) groups as well as IDUAc-H (P = 0.29) groups (Figure 6a). Partial restoration of this exploratory activity was detected in IDUAe-L and IDUAc-mH and IDUAc-M groups. No improvement was shown in IDUAc-L and MPS/WT (P = 0.99) treatment controls, in comparison to untreated MPS I mice. For grooming activity, a complete correction to normal behavior was observed in the IDUAe-H (123%; P = 0.80) and -mH (121%; P = 0.78) dosage groups with significant improvement and strong dosage correlation found in other IDUAe-treated groups (Figure 6b). Improvement in grooming behavior was also detected in IDUAc-treated mice at dosages higher than moderate, with no difference found in IDUAc-L animals comparing to untreated MPS I mice (P = 0.85). The MPS/WT animals exhibited moderate improvement on grooming behavior (0.8% increase in activity) but were still significantly abnormal comparing to IDUAe-M group (43% increase). These observations suggested that a complete normalization of the short-term memory deficits shown in repeated open-field test could be achieved in MPS I mice by CNS delivery of IDUAe at plasma IDUA levels of at least 55 U/ml (above mH levels) and that correction of abnormal exploratory behavior could be obtained by peripheral IDUAe at normal physiological levels (i.e., IDUAe-M group).
Figure 6.
Supraphysiological plasma levels of IDUAe, not IDUAc, lead to behavioral normalization of MPS I mice in repeated open-field test. Age-matched MPS I, normal heterozygote (HET) or gene therapy treated mice were evaluated 6–7 months after transplantation for changes in (a) horizontal activities and (b) grooming activities. Data are presented as mean ± SEM. *P < 0.05. IDUA, α-l-iduronidase; MPS I, mucopolysaccharidosis type I.
Discussion
Studies described here demonstrate long-term brain metabolic correction and normalization of exploratory behavioral deficits in a mouse model of MPS I by peripheral delivery of physiological levels (between normal carrier and WT) of BBB-targeted IDUAe fusion protein. These levels of enzyme were derived from LV-mediated HSC gene therapy at low transduction efficiency (0.1 VCN/genome) using an erythroid/megakaryocyte-specific hybrid promoter. Compared to nontargeted control IDUAc, moderate amounts of engineered IDUAe in the circulation (of IDUAe-M group) achieved better CNS benefits than those observed in the highest dosage group treated by IDUAc, suggesting more effective CNS efficacy of IDUAe than IDUAc with log-fold less amounts of enzyme (less than 7%). These physiological levels of IDUAe (in IDUAe-M group) could lead to not only similar or better brain GAG reduction than those in MPS I mice fully engrafted with WT HSC (i.e., MPS/WT) but also significantly more improvement of cerebral pathology and better restoration toward normal behavior, even though similar or significantly less enzymatic activities were detected in cerebrum or cerebellum of these mice than in those of MPS/WT mice. In general, the elevated IDUA activities in both cerebrum and cerebellum of IDUAe-treated mice were directly correlated with plasma IDUAe levels, yet their correlation with transgene dosages was poor and obscured by high variation of expression levels among transductants most likely due to variable promoter strength affected by microenvironment at insertion sites.
Several therapeutic strategies have been explored using viral vectors to treat neurological lysosomal storage disorders in adult animal models, including direct in vivo injection of adeno-associated virus or LV by routes of intracranial,25,26,27 intrathecal,28,29 and/or systemic delivery,30,31,32 and LV-mediated HSC gene therapy using either myeloid33 or erythroid/megakaryocyte15 restricted or ubiquitous overexpression with strong viral promoter.9 Correction of CNS abnormalities is commonly associated with high levels of enzyme activities in the brain (17–250% of normal) because the main enzyme-generating source is either within the CNS or on the BBB-forming brain endothelial cells, or it requires 1.2–12 VCN/genome in cases of HSC-mediated approaches. In this study, normalization of CNS GAG and correction of habituation deficits were achieved by BBB-targeted IDUAe when the enzyme-producing source is confined in the peripheral blood. Moreover, this robust CNS efficacy was accomplished when minimal amounts of brain enzyme activities (up to 1% of normal levels) were detected with relatively low levels (0.1 VCN) of HSC gene transfer (as in IDUA-M group). Several factors may have contributed to this phenomenon. First, delivery via the circulation can provide rapid and wide distribution throughout the whole brain because the microvasculature is so dense that all neurons or glial cells are within 20 µm from the nearest capillary.34 This view is supported by widespread distribution of IDUAe in both cerebrum and cerebellum by immunofluorescent analysis. Our notion of LRP-1-mediated transcytosis across the BBB via this receptor-binding peptide has also been confirmed recently when tested in a fusion protein setting with the lysosomal enzyme arylsulfatase A.35 Secondly, unlike the mannose-6-phosphate receptor that is mostly expressed only in neurons within an adult brain, LRP-1 and very low-density lipoprotein receptor are abundantly expressed on neurons, astrocytes, and pericytes.21,22 This adopted delivery pathway of IDUAe via LPR1 would facilitate secondary distribution of IDUAe after its arrival into the CNS, thus leads to rapid “on-target” delivery of IDUAe (not IDUAc) to affected cells, such as perivascular cells with abnormal lysosomal storage, activated astrocytes and neurons (especially Purkinje neurons), resulting in significant neurological efficacy. Finally, constant consumption/inactivation of IDUAe along the delivery path may have caused underestimation of the amounts of IDUAe arriving in CNS.
Allogeneic HSC transplantation performed before 2 years of age has presented the only circumstances of clear CNS benefits in some MPS I patients, most likely due to donor-derived cells with normal enzyme levels that can still migrate to the CNS and prevent the cognitive decline.5,6,36 In such cases, besides gradual cell replacement in brain, it is not clear if IDUA expressed at wild-type levels can be secreted in sufficient amounts and be internalized/used by neighboring deficient cells. It is known that peripheral blood mononuclear cells from normal individuals release minimum levels of IDUA in vitro,37 although IDUA from enzyme-overexpressing brain macrophages was critical to achieving complete correction of CNS disease manifestations in MPS I mice after HSC-mediated gene therapy.9 Compared to enzyme-producing donor cells migrated inside the CNS of MPS/WT group, BBB-targeted IDUAe protein delivered at moderate levels in the circulation (in MPS/IDUAe-M) successfully introduced comparable levels of IDUA activities in cerebrum. Moreover, significantly less CNS pathology and healthier neurological behavior were observed by sustained levels of plasma enzyme therapy in MPS/IDUAe-M mice than MPS/WT group, suggesting better correction of CNS deficits. These observations implicate that, in addition to the amounts of enzyme reaching within CNS, efficient enzyme distribution to affected cells is equally critical to CNS treatment efficacy.
Cerebrum histopathology scored by vacuolated, perivascular cells appeared to be biphasically associated with peripheral IDUAe levels. The threshold to significant improvement was low and detected in the lowest enzyme dosage group with IDUAe exhibiting subphysiological IDUA levels in plasma (3 U/ml) and barely detectable levels in brain. However, further correction (but not normalization) quickly reached a plateau in MPS I mice with physiological plasma enzyme levels found in IDUAe-M (12 U/ml) and beyond (up to 160 U/ml in IDUAe-H). The easy-to-be-cured perivascularly located cells may represent “newly” migrated monocyte/macrophages that have been “precharged” with IDUAe or IDUAc by endocytosis from constant and high levels of enzyme in the circulation and thus displayed normal cellular morphology. Moreover, CNS pathology in MPS I contains an inflammatory component which encourages more diapedesis of mononuclear cells than that occurs under healthy condition,38 and this may facilitate the initial phase of reduction in brain histopathology. This notion is also consistent with the observations that similar levels of improvements were found in IDUAe-L and higher dosage groups of IDUAc-treated mice in pathology and that significant reduction of brain GAG levels and behavioral improvement were detected in MPS I mice treated with IDUAc at the highest levels. On the other hand, it is also known that a subset of brain-resident macrophages located in perivascular space is derived from bone marrow precursors that populate the brain in early postnatal life and turn over slowly throughout adulthood.39,40 These cells are constitutively phagocytic41 and play time-dependent roles at the blood–brain interface in CNS responses to inflammatory insults.42 Although it is not designed to be answered directly in this study, those vacuolated perivascular cells responsive particularly to BBB-penetrating IDUAe (but not to IDUAc) may represent the preexisting, hard-to-treat brain macrophage population. Others have also reported that some perivascular cells with storage appeared to be more resistant to treatment than neurons or glial cells.28
In recent years, the cerebellum, a region principally involved in motor control and coordination, has gained much attention in its association with cognitive function such as learning and memory, attention-deficit/hyperactivity disorder, autism spectrum disorders, and age-related cognitive decline.43,44 In this study, the abnormal habituation and hyperactivity in MPS I mice were normalized in the repeated open-field test by moderate amounts of peripheral IDUAe (in IDUAe-M group). This observation is consistent with the correction of metabolic GAG accumulation in cerebellum of these animals, although residual amounts of enzyme activities were detected (~1% of normal). The notion of trans-BBB delivery of IDUAe into the cerebellum is also supported by wide distribution of IDUAe (not IDUAc) in Purkinje neurons (most likely due to the abundance of both LRP-1 and MPR on this type of cells) as well as in molecular and granule layers shown by immune-fluorescent analysis. Pathological improvement in the cerebellum scored by Purkinje cells with storage vacuoles did not occur in MPS I mice until plasma IDUAe reached supraphysiological levels (in IDUAe-mH), suggesting higher threshold of enzyme dosage may be required to diminish storage in these cells. This observation is in line with a previous report that correction of storage material in Purkinje cells was associated with high dosages of adeno-associated virus injected intrathecally and required cerebellar enzyme activities exceeding normal levels.28
This study demonstrates that complete normalization of several CNS deficits can be achieved by long-term peripheral delivery of physiological levels of BBB-targeted IDUAe protein. The resulting CNS benefits from clinically attainable levels of HSC gene transfer (0.1 VCN/genome) seem to be superior to those obtained in mice after conventional treatment with HSC transplantation. It is still to be determined whether similarly low levels of IDUAe would lead to comparable CNS benefits in larger animals and humans. The erythroid/megakaryocyte lineage-restricted expression of IDUAe tested here can be readily combined with expression from other blood lineages, such as in monocytes/macrophages, for better CNS efficacy. Taken together, this preclinical evaluation in MPS I mouse model documents that the genetically engineered IDUAe protein is more than log-fold efficacious than the nontargeted control protein, and the application of IDUAe with gene therapy or enzyme replacement therapy would await for further clinical translation.
Materials and Methods
LV vector construction and generation. The IDUAc and IDUAe (i.e., IDUAe1) fusion cassettes were generated by PCR-cloning technique as described previously.20 Lineage-restricted LVs were constructed by insertion of IDUAc or IDUAe into a third-generation LV backbone LV-TW at the HpaI site, which is downstream of an erythroid/MK-specific hybrid promoter and upstream of an IRES-GFP expression cassette.15,16 LV vectors were generated by 4-plasmid transfection system and concentrated by ultracentrifugation as previously described.45 The potency of viral stocks (typical 108–109 TU/ml) was determined in murine erythroid leukemia cells that were exposed to serial LV dilutions by fluorescence-activated cell sorting analysis for GFP+ percentage.
Isolation, transduction, and transplantation of Lin-cells expressing IDUAe and IDUAc. To enrich HSC, lineage-depleted low-density bone marrow cells (Lin−) were stained with a set of biotinylated antibodies including anti-CD3e, B220, CD4, CD8, CD11b, Gr-1, and Ter119 and followed by magnetic cell sorting using antibiotin microbead-mediated MACS LS column (Miltenyi Biotec, San Diego, CA). Ex vivo transduction of Lin− cells was conducted by culturing the cells for 12 hours of prestimulation period in serum-free StemSpan medium (StemCell Technologies, Vancouver, British Columbia, Canada) that was supplemented with 40 µg/ml LDL, 50 ng/ml stem cell factor, 20 ng/ml thrombopoietin, 10 ng/ml IL3, and 50 ng/ml IL6. Cells were then transduced twice with a total multiplicity of infection at 2, 10, or 50 within 24 hours in the presence of 8 µg/ml protamine sulfate, followed by transplantation into lethally irradiated MPS I mice. To increase the number of animals in each enzyme dosage group (especially the L-groups), 2° transplantation was conducted using low-density bone marrow cells harvested from gene therapy MPS I mice >5 months after primary transduction/transplantation. To obtain MPS/WT treatment control mice, Lin− cells from WT animals were transplanted into MPS I animals. In all animals, transplantation was conducted by injecting Lin− cells (1.2–1.5 × 105 cells/mouse) or low-density bone marrow cells (3–4 × 106 cells/mouse) into lethally irradiated (split dosage of 700 and 475 cGy) MPS I mice.
Animal's transcardial perfusion. All animal procedures were approved by the Cincinnati Children's Hospital Medical center Animal Care and Use Committee. After anesthetizing the mouse with sodium pentobarbital (50–90 mg/kg) by intraperitoneal injection, the thoracic cavity was opened and an incision in the right atrium of the heart was made to allow outflow of the perfusate. A 25-gauge needle which is connected directly to a peristaltic pump was inserted into the right ventricle of the beating heart. Mice was perfused with an ice-cold phosphate-buffered saline (PBS) (flow rate: 5 ml/minute) to remove blood from the vasculature. The success of this procedure was confirmed by a loss of color in the liver and the blood vessels that flank the midline of the rib cage.
Quantification of IDUA transgene frequency by qPCR. To quantify transgene frequency, genomic DNA isolated from bone marrows was analyzed by real-time qPCR for IDUA cDNA and murine apoB DNA with TaqMan primer/probes. The primers and probe for IDUA cDNA were designed by Primer Expression 3.0 software (Applied Biosystems, Foster City, CA), and the sequences were as follows: 5′ CTGGTCTGGTCGGATGAACA-3′ (sense), 5′-CCGTCCTGAGAGAACTGGATCT -3′ (antisense), and 5′-FAM-TCCAAGTGCCTGTGGAC-3′ (probe). Amplification of endogenous apoB with VIC-labeled probe served as an internal control for normalization of DNA as described previously.46 The standard curve for IDUA cDNA frequency (ranging from 0.001 to 1 VCN/genome) was established from (Ct) values using a set of standard samples generated from a mixture of NIH3T3 and NIH3T3-IDUA cells (IDUA overexpressing cells containing approximately one copy of IDUA determined by western blot). Each real-time reaction contained 100 ng of genomic DNA, 300 nmol/l of IDUA or apoB primers, 200 nmol/l TaqMan IDUA or apoB probe, and TaqMan 2× Universal Master Mix (Applied Biosystems). Amplification conditions were 2 minutes at 50 °C and 10 minutes at 95 °C for the initial cycle, followed by 40 cycles at 95 °C for 15 seconds and 60 °C for 1 minute. Each sample was analyzed in two separate qPCR assays with duplicate amplifications.
Tissues and plasma activity level assay for IDUA. The catalytic activity of IDUA was determined using a fluorometric enzyme assay as previously described.20 Brain or cerebellar tissues were homogenized in a lysis buffer (150 mmol/l NaCl and 50 mmol/l Tris–HCl with 1% Triton X-100) using an ultrasonic processor. Aliquots of cleared lysates were incubated at 37 °C with 2.5 mmol/l fluorogenic substrate (4-methylumbelliferyl (4MU)-α-l-idopyranosiduronic acid sodium salt (Toronto Research Chemicals, Toronto, Ontario, Canada) in 0.4 mol/l of sodium formate buffer (pH 3.2)) followed by addition of glycine carbonate buffer (0.1 mol/l (pH 10.5)) to stop the reaction. The fluorescent product released from each reaction was analyzed with an excitation wavelength of 365 nm and an emission wavelength of 450 nm using a SpectraMax M2 fluorometer (MDS Analytical Technologies, Sunnyvale, CA). Samples were assayed in duplicate and quantified in duplicate wells, together with buffer controls in parallel. Protein was determined using PierceBCA Protein Assay kit (Thermo Fisher Scientific, Waltham, MA). One unit of enzyme activity is defined by the release of 1 nmol of 4MU in a 1-hour reaction at 37 °C. IDUA activity was calculated as units per milligram of protein of tissues. Plasma IDUA levels were expressed as nmol/ml/hour. Two to four brain specimens were assayed for each animal.
β-Hexosaminidase activity. Cerebellar tissues were homogenized in 0.9% sodium chloride solution containing 1% Triton X-100, followed by dilutions (1:250 and 1:500) in distilled water. The diluted homogenates were incubated with 1.2 mmol/l 4MU-β-N-acetylglucosaminide (Sigma-Aldrich, St Louis, MO) in 10 mmol/l citrate/20 mmol/l phosphate buffer, pH 4.5, for 1 hour at 37 °C. The reaction was stopped by the addition of glycine carbonate buffer (pH 10.5). The fluorescent product released from each reaction was analyzed with an excitation wavelength of 365 nm and an emission wavelength of 450 nm by using a SpectraMax M2 fluorometer (MDS Analytical Technologies). The β-hexosaminidase activity was calculated as units per milligram of protein of tissues.
Immunofluorescent staining of tissues. Half the brain and cerebellar tissues were removed from ice-cold PBS perfused animals followed by postfixation in 4% paraformaldehyde for 24 hours, and 4% paraformaldehyde containing 30% sucrose overnight at 4 °C. Frozen sections (10 µm) were obtained from fixed tissues and permeabilized with 1× PBS containing 0.1% Triton X-100 for 1 hour at room temperature. After permeablization, tissue sections were treated with blocking solution (PBS containing 5% horse serum and 0.05% Tween-20). Sections were then incubated overnight at 4 °C with the following primary antibodies: mouse an-c-Myc antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA) to detect IDUA, rabbit anti-NeuN (1:200; EnCor Biotechnology, Gainesville, FL), rat anti- mouse CD31 (1:200; BD Pharmingen, San Jose, CA), and rabbit anti-GFAP (1:500; Dako North America, Carpinteria, CA). To get rid of excess antibodies, sections were washed three times with PBS and then incubated for 1 hour with a secondary antibody of the appropriate species: Alexa 488 goat anti-mouse (1:500; Life Technologies, Grand Island, NY) and Alexa 568 goat anti-rabbit or anti-rat (1:500; Life Technologies). To eliminate autofluorscence, sections were treated with autofluorscence eliminator reagents (EMD Millipore, Billerica, MA) according to the manufacturer instruction, followed by mounting with Vectashield mounting medium containing 4, 6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA) and analyzed using a Leica DMI6000 B microscope system (Leica Microsystems, Wetzlar, Germany).
Tissue GAG quantitation. Frozen pieces of brain cortex and cerebellar samples were homogenized with a minimum volume of water (10% vol/weight). The total protein in brain homogenates was measured by using the BCA Protein Assay kit (Thermo Fisher Scientific). Then, 1 mg of protein from each brain homogenate was defatted by treatment with chloroform:methanol (1:2) mixture for 3 hours at room temperature. Collected defatted pellets were washed with 100% ethanol. Thereafter, defatted pellets were dried for 10 minutes at 37 °C followed by digestion for 3 hours at 65 °C with papain reagent (0.25 mg papain in 0.12 ml of 100 mmol/l sodium acetate buffer, pH 5.5, containing 5 mmol/l cysteine and 5 mmol/l ethylenediaminetetraacetic acid). After papain digestion, samples were treated with DNase (1 U/µl) for 30 minutes at 37 °C. Soluble GAG was precipitated from the papain/DNase treated samples and assessed using 1,9-dimethylmethylene blue chloride dye as previously described.47 Heparan sulfate was used as standard to calculate the GAG values. GAG values were normalized to their respective amounts of total protein.
Urine GAG quantitation. Urine samples were diluted to 1:25 to 1:100 fold in 0.2 mol/l sodium format buffer (pH 3.2), followed by GAG precipitation after mixing with 1,9-dimethylmethylene blue chloride dye. Urine GAG values were normalized to creatinine levels in urine samples.
Chemical staining and pathology evaluation. Forebrain and cerebellum samples were fixed by 2% (weight/volume) glutaraldehyde in 0.175 mol/l sodium cacodylate buffer (pH 7.4) at 4 °C. The tissues were then treated with 1% osmium tetroxide, washed in 0.175 mol/l sodium cacodylate buffer, dehydrated by a graded ethanol series, and embedded in LX112 resin (Ladd Research, Williston, VT). Sections (0.5–1µm) were prepared and stained with 1% toluidine blue in 1% sodium borate, followed by examination for the presence of pathological storage vacuoles. For brain pathology scoring, the number and percentage of brain vasculatures that were associated with vacuolated perivascular cells were determined in forebrain sections, with more than 500 vasculatures scored for each animal. For cerebellum pathology scoring, cytoplasmic vacuoles in Purkinje cells were evaluated with more than 400 cells per mouse. Cells containing no vacuoles were considered normal, whereas cells containing ≥1 vacuole were considered a positive or diseased cell. A minimum of two animals per group were analyzed with six sections randomly selected from at least two slides for each tissue, and the percentages of all sections from two to four mice contributed to statistical analysis.
Open-field behavior. The repeated open-field test was performed after bone marrow transplantation at the age of 7 months, as described previously.24 The test was performed using an open-field test apparatus (60 × 60 cm) consisting of a white Plexiglas box with 25 squares (12 × 12 cm) painted on the floor (16 outer and 9 inner). Mice were placed in one of the four corners of the apparatus and allowed to explore the whole field freely for 5 minutes. Activity was monitored and quantified for ambulation (number of inner and outer squares crossed) and time spent grooming by two observers who did not know the genotype or treatment of the animal during testing. Each mouse was tested for three repeated trials with a 30-minute intertrial interval.
Statistical analysis. All quantitative assays were performed in duplicate or triplicate from at least two individual experiments. Pathology quantitation was obtained from at least two animals per group. Data are presented as mean ± SEM unless otherwise specified. Comparisons between two groups were performed using two-tailed, paired Student's t-tests. P values of ≥ 0.05 were considered as statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Long-term expression of IDUAe or IDUAc in plasma of MPS I chimeras at various dosage groups over 5 months after LV-mediated HSC gene therapy. Table S1. Summarized results of complete blood cell count in mice expressing IDUAe (MPS I/IDUAe-H), IDUAc (MPS I/IDUAc-H), and un-manipulated IDUA (MPSI/WT) as well as in age-matched MPS I or normal controls mice after 5- months of transplantation. Table S2. Summarized results of plasma IDUA levels 20 weeks after transplantation, transgene frequencies (VCN/genome) and MOI of transduction for each MPS I animal treated with IDUAe or IDUAc.
Acknowledgments
We are grateful for the technical assistance of Phuong Cao, Meghan Bromwell, John Strickley, and the Comprehensive Mouse Core. This work was supported by grants from the National Institutes of Health (NS064330 and NS086134).
Supplementary Material
Long-term expression of IDUAe or IDUAc in plasma of MPS I chimeras at various dosage groups over 5 months after LV-mediated HSC gene therapy.
Summarized results of complete blood cell count in mice expressing IDUAe (MPS I/IDUAe-H), IDUAc (MPS I/IDUAc-H), and un-manipulated IDUA (MPSI/WT) as well as in age-matched MPS I or normal controls mice after 5- months of transplantation.
Summarized results of plasma IDUA levels 20 weeks after transplantation, transgene frequencies (VCN/genome) and MOI of transduction for each MPS I animal treated with IDUAe or IDUAc.
References
- Neufeld ES, Muenzer J.2007The mucopolysaccharidoses. Scriver CR.et al. (eds). The Online Metabolic and Molecular Bases of Inherited DiseasePart 16, Chapter 136. McGraw-Hill; Medical. DOI: 10.1036/ommbid.165. [Google Scholar]
- Campos D, Monaga M. Mucopolysaccharidosis type I: current knowledge on its pathophysiological mechanisms. Metab Brain Dis. 2012;27:121–129. doi: 10.1007/s11011-012-9302-1. [DOI] [PubMed] [Google Scholar]
- Pan D. Cell- and gene-based therapeutic approaches for neurological deficits in mucopolysaccharidoses. Curr Pharm Biotechnol. 2011;12:884–896. doi: 10.2174/138920111795542679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DW, Abkowitz JL. Mature monocytic cells enter tissues and engraft. Proc Natl Acad Sci USA. 1998;95:14944–14949. doi: 10.1073/pnas.95.25.14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, et al. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N Engl J Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- de Ru MH, Boelens JJ, Das AM, Jones SA, van der Lee JH, Mahlaoui N, et al. Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure. Orphanet J Rare Dis. 2011;6:55. doi: 10.1186/1750-1172-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratko TA, Marbella A, Godfrey S, Aronson N. Enzyme-Replacement Therapies for Lysosomal Storage Diseases. Agency for Healthcare Research and Quality; Rockville, MD; 2013. [PubMed] [Google Scholar]
- Baum C, Modlich U, Göhring G, Schlegelberger B. Concise review: managing genotoxicity in the therapeutic modification of stem cells. Stem Cells. 2011;29:1479–1484. doi: 10.1002/stem.716. [DOI] [PubMed] [Google Scholar]
- Visigalli I, Delai S, Politi LS, Di Domenico C, Cerri F, Mrak E, et al. Gene therapy augments the efficacy of hematopoietic cell transplantation and fully corrects mucopolysaccharidosis type I phenotype in the mouse model. Blood. 2010;116:5130–5139. doi: 10.1182/blood-2010-04-278234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- Kaufmann KB, Büning H, Galy A, Schambach A, Grez M. Gene therapy on the move. EMBO Mol Med. 2013;5:1642–1661. doi: 10.1002/emmm.201202287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière I, Dunbar CE, Sadelain M. Hematopoietic stem cell engineering at a crossroads. Blood. 2012;119:1107–1116. doi: 10.1182/blood-2011-09-349993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zhang W, Kalfa TA, Grabowski G, Davies S, Malik P, et al. Reprogramming erythroid cells for lysosomal enzyme production leads to visceral and CNS cross-correction in mice with Hurler syndrome. Proc Natl Acad Sci USA. 2009;106:19958–19963. doi: 10.1073/pnas.0908528106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M, Han J, El-Amouri SS, Brady RO, Pan D. Platelets are efficient and protective depots for storage, distribution, and delivery of lysosomal enzyme in mice with Hurler syndrome. Proc Natl Acad Sci USA. 2014;111:2680–2685. doi: 10.1073/pnas.1323155111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Nakagawa T, Wu B, Onodera M, Huang CL, Kusaka T, et al. Transporters in the brain endothelial barrier. Curr Med Chem. 2010;17:1125–1138. doi: 10.2174/092986710790827816. [DOI] [PubMed] [Google Scholar]
- Wang D, El-Amouri SS, Dai M, Kuan CY, Hui DY, Brady RO, et al. Engineering a lysosomal enzyme with a derivative of receptor-binding domain of apoE enables delivery across the blood-brain barrier. Proc Natl Acad Sci USA. 2013;110:2999–3004. doi: 10.1073/pnas.1222742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Sakai J, Fujino T, Hattori H, Zenimaru Y, Suzuki J, et al. The very low-density lipoprotein (VLDL) receptor: characterization and functions as a peripheral lipoprotein receptor. J Atheroscler Thromb. 2004;11:200–208. doi: 10.5551/jat.11.200. [DOI] [PubMed] [Google Scholar]
- Polavarapu R, Gongora MC, Yi H, Ranganthan S, Lawrence DA, Strickland D, et al. Tissue-type plasminogen activator-mediated shedding of astrocytic low-density lipoprotein receptor-related protein increases the permeability of the neurovascular unit. Blood. 2007;109:3270–3278. doi: 10.1182/blood-2006-08-043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annu Rev Biochem. 2002;71:405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- Pan D, Sciascia A, 2nd, Vorhees CV, Williams MT. Progression of multiple behavioral deficits with various ages of onset in a murine model of Hurler syndrome. Brain Res. 2008;1188:241–253. doi: 10.1016/j.brainres.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch A, Perret E, Desmaris N, Heard JM. Long-term and significant correction of brain lesions in adult mucopolysaccharidosis type VII mice using recombinant AAV vectors. Mol Ther. 2000;1:63–70. doi: 10.1006/mthe.1999.0005. [DOI] [PubMed] [Google Scholar]
- Lattanzi A, Neri M, Maderna C, di Girolamo I, Martino S, Orlacchio A, et al. Widespread enzymatic correction of CNS tissues by a single intracerebral injection of therapeutic lentiviral vector in leukodystrophy mouse models. Hum Mol Genet. 2010;19:2208–2227. doi: 10.1093/hmg/ddq099. [DOI] [PubMed] [Google Scholar]
- Bu J, Ashe KM, Bringas J, Marshall J, Dodge JC, Cabrera-Salazar MA, et al. Merits of combination cortical, subcortical, and cerebellar injections for the treatment of Niemann-Pick disease type A. Mol Ther. 2012;20:1893–1901. doi: 10.1038/mt.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson G, Bastacky J, Belichenko P, Buddhikot M, Jungles S, Vellard M, et al. Intrathecal administration of AAV vectors for the treatment of lysosomal storage in the brains of MPS I mice. Gene Ther. 2006;13:917–925. doi: 10.1038/sj.gt.3302735. [DOI] [PubMed] [Google Scholar]
- Gray SJ, Nagabhushan Kalburgi S, McCown TJ, Jude Samulski R. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 2013;20:450–459. doi: 10.1038/gt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Kang L, Jennings JS, Moy SS, Perez A, Dirosario J, et al. Significantly increased lifespan and improved behavioral performances by rAAV gene delivery in adult mucopolysaccharidosis IIIB mice. Gene Ther. 2007;14:1065–1077. doi: 10.1038/sj.gt.3302961. [DOI] [PubMed] [Google Scholar]
- Chen YH, Claflin K, Geoghegan JC, Davidson BL. Sialic acid deposition impairs the utility of AAV9, but not peptide-modified AAVs for brain gene therapy in a mouse model of lysosomal storage disease. Mol Ther. 2012;20:1393–1399. doi: 10.1038/mt.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzo A, Marcó S, García M, Villacampa P, Ribera A, Ayuso E, et al. Correction of pathological accumulation of glycosaminoglycans in central nervous system and peripheral tissues of MPSIIIA mice through systemic AAV9 gene transfer. Hum Gene Ther. 2012;23:1237–1246. doi: 10.1089/hum.2012.029. [DOI] [PubMed] [Google Scholar]
- Sergijenko A, Langford-Smith A, Liao AY, Pickford CE, McDermott J, Nowinski G, et al. Myeloid/Microglial driven autologous hematopoietic stem cell gene therapy corrects a neuronopathic lysosomal disease. Mol Ther. 2013;21:1938–1949. doi: 10.1038/mt.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär T. The vascular system of the cerebral cortex. Adv Anat Embryol Cell Biol. 1980;59:I–VI,1. doi: 10.1007/978-3-642-67432-7. [DOI] [PubMed] [Google Scholar]
- Böckenhoff A, Cramer S, Wölte P, Knieling S, Wohlenberg C, Gieselmann V, et al. Comparison of five peptide vectors for improved brain delivery of the lysosomal enzyme arylsulfatase A. J Neurosci. 2014;34:3122–3129. doi: 10.1523/JNEUROSCI.4785-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad VK, Kurtzberg J. Transplant outcomes in mucopolysaccharidoses. Semin Hematol. 2010;47:59–69. doi: 10.1053/j.seminhematol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Pan D, Aronovich E, McIvor RS, Whitley CB. Retroviral vector design studies toward hematopoietic stem cell gene therapy for mucopolysaccharidosis type I. Gene Ther. 2000;7:1875–1883. doi: 10.1038/sj.gt.3301298. [DOI] [PubMed] [Google Scholar]
- Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci USA. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechmann I, Kwidzinski E, Kovac AD, Simbürger E, Horvath T, Gimsa U, et al. Turnover of rat brain perivascular cells. Exp Neurol. 2001;168:242–249. doi: 10.1006/exnr.2000.7618. [DOI] [PubMed] [Google Scholar]
- Vallières L, Sawchenko PE. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J Neurosci. 2003;23:5197–5207. doi: 10.1523/JNEUROSCI.23-12-05197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabriek BO, Polfliet MM, Vloet RP, van der Schors RC, Ligtenberg AJ, Weaver LK, et al. The macrophage CD163 surface glycoprotein is an erythroblast adhesion receptor. Blood. 2007;109:5223–5229. doi: 10.1182/blood-2006-08-036467. [DOI] [PubMed] [Google Scholar]
- Serrats J, Schiltz JC, García-Bueno B, van Rooijen N, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci. 2012;13:619–635. doi: 10.1038/nrn3312. [DOI] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD. Moving forward: age effects on the cerebellum underlie cognitive and motor declines. Neurosci Biobehav Rev. 2014;42:193–207. doi: 10.1016/j.neubiorev.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Worsham DN, Pan D. Co-expression of MGMT(P140K) and alpha-L-iduronidase in primary hepatocytes from mucopolysaccharidosis type I mice enables efficient selection with metabolic correction. J Gene Med. 2008;10:249–259. doi: 10.1002/jgm.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsham DN, Schuesler T, von Kalle C, Pan D. In vivo gene transfer into adult stem cells in unconditioned mice by in situ delivery of a lentiviral vector. Mol Ther. 2006;14:514–524. doi: 10.1016/j.ymthe.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa I, Garcia S, Barbier-Chassefière V, Caruelle JP, Martelly I, Papy-García D. Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology. 2003;13:647–653. doi: 10.1093/glycob/cwg082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Long-term expression of IDUAe or IDUAc in plasma of MPS I chimeras at various dosage groups over 5 months after LV-mediated HSC gene therapy.
Summarized results of complete blood cell count in mice expressing IDUAe (MPS I/IDUAe-H), IDUAc (MPS I/IDUAc-H), and un-manipulated IDUA (MPSI/WT) as well as in age-matched MPS I or normal controls mice after 5- months of transplantation.
Summarized results of plasma IDUA levels 20 weeks after transplantation, transgene frequencies (VCN/genome) and MOI of transduction for each MPS I animal treated with IDUAe or IDUAc.