Abstract
Critical limb ischemia (CLI) is often poorly treatable by conventional management and alternatives such as autologous cell therapy are increasingly investigated. Whereas previous studies showed a substantial impairment of neovascularization capacity in primary bone-marrow (BM) isolates from patients, little is known about dysfunction in patient-derived BM mesenchymal stromal cells (MSCs). In this study, we have compared CLI-MSCs to healthy controls using gene expression profiling and functional assays for differentiation, senescence and in vitro and in vivo pro-angiogenic ability. Whereas no differentially expressed genes were found and adipogenic and osteogenic differentiation did not significantly differ between groups, chondrogenic differentiation was impaired in CLI-MSCs, potentially as a consequence of increased senescence. Migration experiments showed no differences in growth factor sensitivity and secretion between CLI- and control MSCs. In a murine hind-limb ischemia model, recovery of perfusion was enhanced in MSC-treated mice compared to vehicle controls (71 ± 24% versus 44 ± 11%; P < 1 × 10−6). CLI-MSC- and control-MSC–treated animals showed nearly identical amounts of reperfusion (ratio CLI:Control = 0.98, 95% CI = 0.82–1.14), meeting our criteria for statistical equivalence. The neovascularization capacity of MSCs derived from CLI-patients is not compromised and equivalent to that of control MSCs, suggesting that autologous MSCs are suitable for cell therapy in CLI patients.
Introduction
Peripheral arterial disease (PAD) is a manifestation of atherosclerosis that is highly prevalent in aging western populations.1 When arterial occlusion reaches the point where metabolic demands of resting muscle can no longer be met, PAD progresses to critical limb ischemia (CLI), a condition associated with a very poor prognosis with respect to both life and limb1 and quality of life.2 In a substantial proportion (ca. 40%) of patients, conventional and surgical treatment options will be exhausted during the course of disease and amputation of the affected limb is inevitable.1 Consequently, new treatment modalities have been explored in the form of autologous progenitor cell transplantation.3 Initially, bone-marrow (BM) mononuclear cells (MNCs) were used in the assumption that this mixture of cells contains pro-angiogenic progenitor cells. Although initial results seemed promising,3,4 restrictions to the clinical utilization of this BM cell therapy for PAD patients arise from the impaired angiogenic activity of patient derived BM progenitor cells.4,5,6 A promising alternative are BM-derived mesenchymal stromal cells (MSCs).7,8 MSCs make up the stromal part of the BM stem cell niche9 and constitute a multipotent cell population that can differentiate into several mesenchymal tissue lines.10 Because MSCs are exceedingly rare in BM,11 therapeutic applications generally involve expansion in culture. Culture expanded MSCs possess a pronounced pro-angiogenic capacity, that is likely to be superior to that of BM-MNCs.7,12
It is thought that MSCs possess strong immunomodulatory properties13 and low intrinsic antigenicity,14 allowing allogeneic therapeutic strategies. However, differentiation of MSCs after application in vivo has been shown to upregulate expression of MHC complexes, leading to delayed immunization against the injected cells.15,16 This notwithstanding, allogeneic MSCs have been proven safe as single-dose therapy for ischemic cardiomyopathy.17,18
There is significant uncertainty whether neovascularization capacity of MSCs from patients with cardiovascular disease is impaired, which would limit autologous clinical application in CLI. Previous studies have shown that MSCs from patients with auto-immune diseases, such as systemic lupus erythematosus19 or systemic sclerosis20 show alterations in growth factor secretion when compared to healthy donors. In addition, aging has been shown to reduce the efficacy of human MSCs in a murine model of myocardial infarction.21 On the other hand, studies investigating angiogenic cytokine secretion in MSCs obtained from patients with end stage renal disease found no alterations.22,23 Studies involving MSCs from human donors with cardiovascular disease showed that MSCs yields and numbers are not impaired24,25 compared to age-matched controls.
In this study, we investigated whether cultured BM-MSCs obtained from CLI patients are dysfunctional when compared to BM-MSCs from a healthy control population. We have started by global gene expression profiling to identify persistent differences after culture, but found no significantly differentially expressed genes in MSCs obtained from patients versus control MSCs. In a series of functional experiments, we quantified MSC response to stimuli inducing differentiation or promoting angiogenesis in vitro an in vivo. As our starting hypothesis was that there are no differences between CLI- and control MSCs, we used Bayesian inference to give credible intervals of the difference between groups and show statistical equivalence, where present.
Results
Patient characteristics
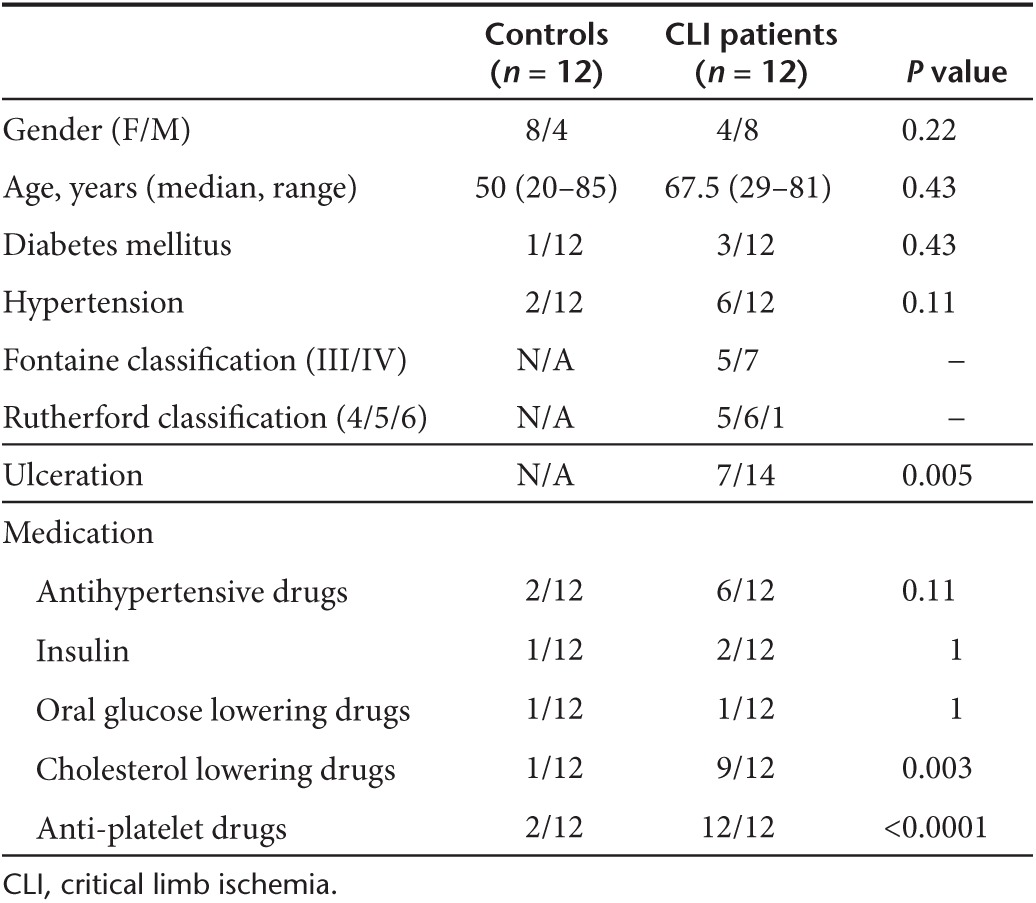
Patient characteristics are shown in Table 1.
Table 1. Patient characteristics.
Isolation and phenotype
BM-MSCs from 12 patients and 12 healthy controls were successfully expanded to a tissue culture surface of 300 cm2. Cell yields were between 1.8 × 106 and 4 × 106 (data not shown) and time to expansion was similar between groups (19.25 ± 1.39 and 18.44 ± 0.83 days for healthy- and CLI-MSCs respectively). Immunophenotyping showed that the cells were positive for CD90, CD105, CD140b, and CD73, but negative for CD14, CD45, CD19, and CD34 (Supplementary Figure S1d).
Gene expression profiling
Gene expression profiling was performed on MSCs from six CLI and six control donors.
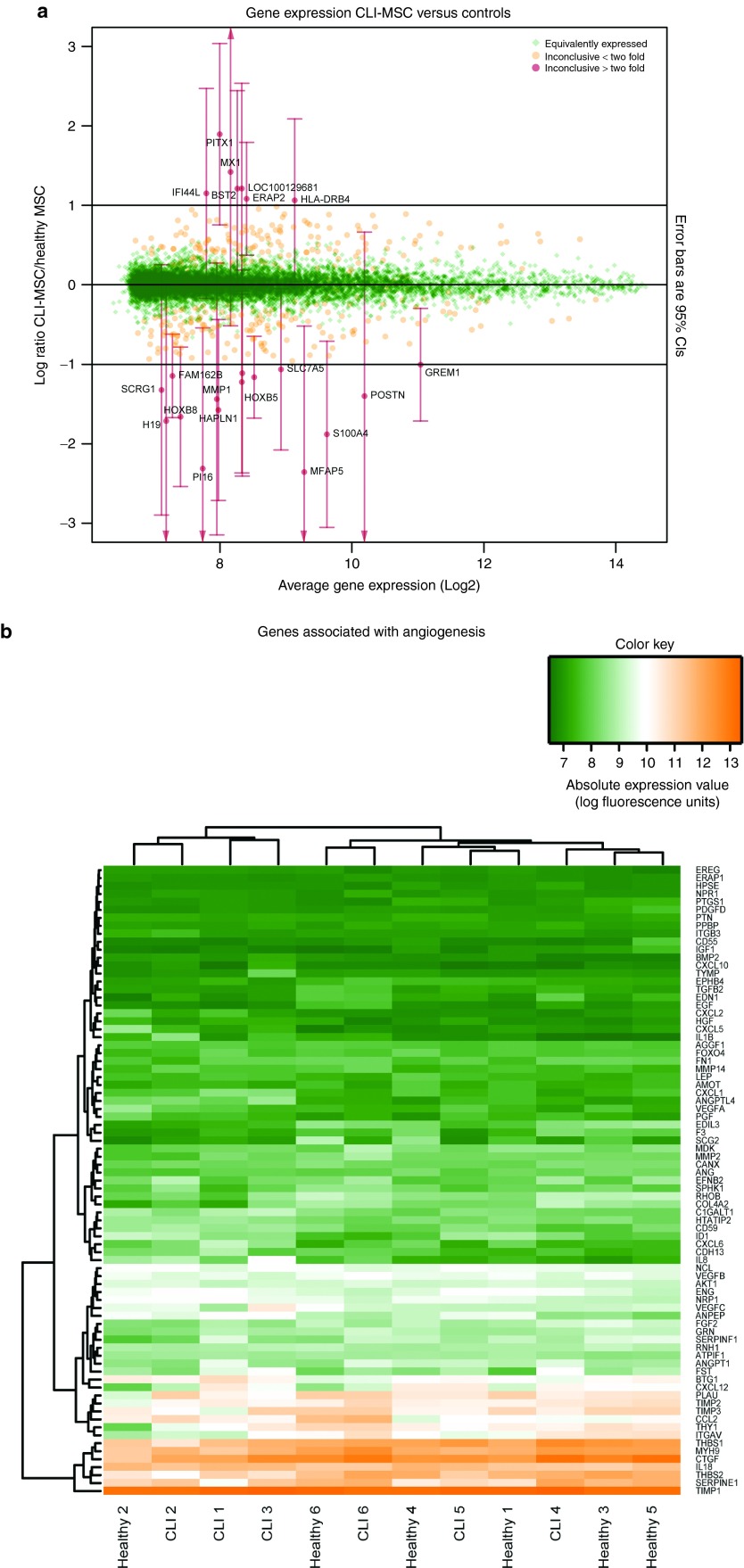
Not a single significantly differentially expressed gene was observed between MSCs derived from CLI patients and healthy donors (Supplementary Table S1). Only 22 genes showed an on average greater than twofold change in expression (Figure 1a); namely MFAP5, PI16, S100A4, H19, HOXB8, HAPLN1, MMP1, POSTN, SCRG1, TRIB3, HOXB5, FAM162B, DDIT4, SLC7A5, GREM1, HLA-DRB4, ERAP2, IFI44L, BST2, MX1, and PITX1, although with substantial variation (Supplementary Figure S2a). Genes associated with angiogenesis were stably and equally expressed in donors from both groups (Figure 1b).
Figure 1.
Gene expression. (a) Relative expression of genes in CLI-MSCs/Control MSCs. On this M/A plot, the x-axis denotes the average expression (in units of fluorescence intensity), the y-axis denotes the ratio of expression in CLI- MSCs/Control MSCs. Expression of genes shown in green is with 95% confidence intervals less than a factor differentially expressed. The remaining genes are inconclusive, genes with an average fold change of >2 are indicated in red and 95% confidence intervals are given. (b) Heatmap showing genes associated with angiogenesis, clustering on columns shows no clear separation between CLI- and Control MSCs.
Analyses on similarity show that 98.7% of detected genes are with 95% confidence less than a factor 2 differentially expressed between CLI-MSCs and control MSCs. The remaining 332 genes (inconclusive, IC) were analyzed for association with known biological processes, in order to find areas of variability or trends towards a difference. This analysis is demonstrated in Figure 1a, which depicts the ratio of gene expression (log2) on the y-axis and the average expression level on the x-axis (low to high from left to right respectively). Comparison of the IC gene list with other studies shows that genes associated with chondrogenic differentiation of MSCs26 (P < 7 × 10−12) and aging27 (P < 2 × 10−17) are overrepresented (Supplementary Figure S2b,c).
Differentiation
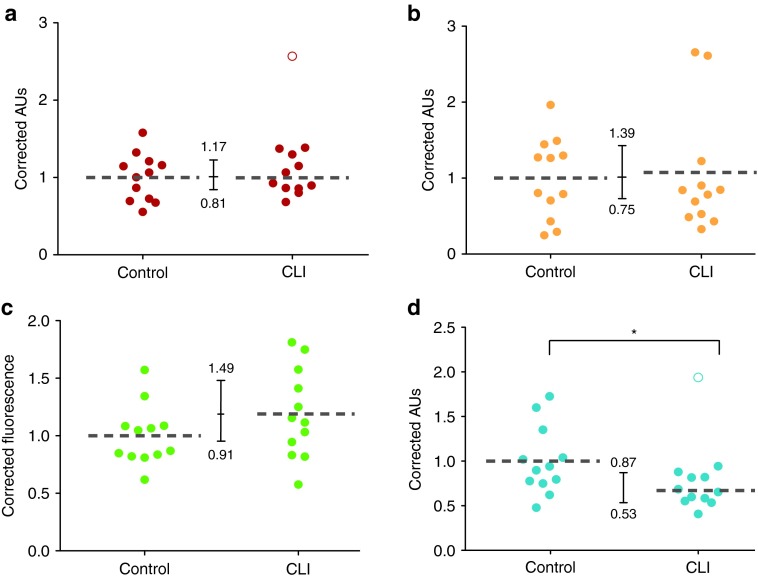
Alizarin red S staining showed aggregates of hydroxyapatite-mineralized matrices that stained red after retention of the dye (Supplementary Figure S1a). Quantification of dye retention by acidic extraction showed equivalent amounts of dye retention (ratio CLI:Control = 0.99, 95% CI = 0.81–1.17) in MSCs obtained from CLI patients and controls (Figure 2a). Alkaline Phosphatase activity was similar in both groups, but not equivalent (CLI:Control = 1.07, 95% CI = 0.75–1.39; Figure 2b). MSCs treated with adipogenic medium displayed lipid droplets that are characteristic for differentiation into pre-adipocytes (Supplementary Figure S1b,c). Quantification of the amount of Lipidtox Green uptake by fluorescence measurement showed a trend towards increased adipogenic differentiation in CLI donors (CLI:Control = 1.2, 95% CI = 0.71–1.49; Figure 2c). Chondrogenic differentiation of MSCs induced the formation of glycosaminoglycans (GAGs). Soluble GAG production was about a 33% lower in CLI-MSC compared to controls (P = 0.02, Figure 2d).
Figure 2.
Differentiation. (a) Quantification of alizarin red retention in osteogenically differentiated MSCs was found to be equivalent; CLI:Control = 0.99 (95% CI = 0.81–1.17), the open circle indicates and outlier that has been left out of analysis. (b) ALP activity in MSCs after osteogenic differentiation; CLI:Control = 1.07, 95% CI = 0.75–1.39. (c) Adipogenic Differentiation measured by Lipidtox Green staining, CLI:Control = 1.2, 95% CI = 0.71–1.49. (d) Chondrogenic differentiation measured by sGAG production. sGAGs levels were significantly lower in CLI-MSCs.
Senescence
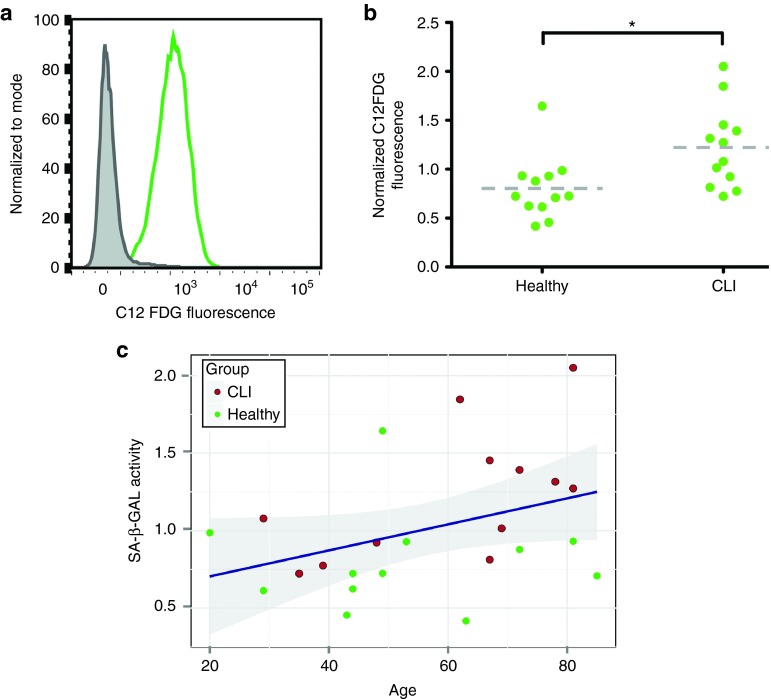
Senescence was assessed by the conversion of C12FDG, a fluorescently labeled β-galactosidase substrate, and subsequently measured by flow cytometry (Figure 3a). There was a significant increase in senescence associated β-galactosidase activity in MSCs obtained from CLI donors (Figure 3b). In a model that includes donor age, age is shown to be a significant explanatory variable (Figure 3c, R = 0.37). After inclusion of donor age in the model, donor group (i.e., healthy or CLI) ceases to be significantly different, with a trend towards a larger age associated increase in senescence in CLI-MSCs (P = 0.07 for interaction age*group). As an additional marker of senescence the number of spontaneous DNA double strand break damage foci was measured by nuclear staining for gH2AX. CLI-MSCs showed a minor increase in gH2AX signal and number of damage foci (Supplementary Figure S3a–d), but this difference was not significant. gH2AX fluorescence in donors of both groups was associated with both age and CD12FDG signal (Supplementary Figure S3e,f). Both donor age and senescence were associated with differentiation potential (Supplementary Figure S4), leading to a shift from chondrogenic differentiation towards adipogenic differentiation.
Figure 3.
Senescence. (a) Representative example of SA-β-galactosidase activity as measured by flow cytometry. (b) Senescence is increased in CLI MSCs compared to Healthy controls (P = 0.01). (c) MSC senescence is significantly associated with donor age (P = 0.02), shaded band indicates 95% confidence interval.
Migration and conditioned medium
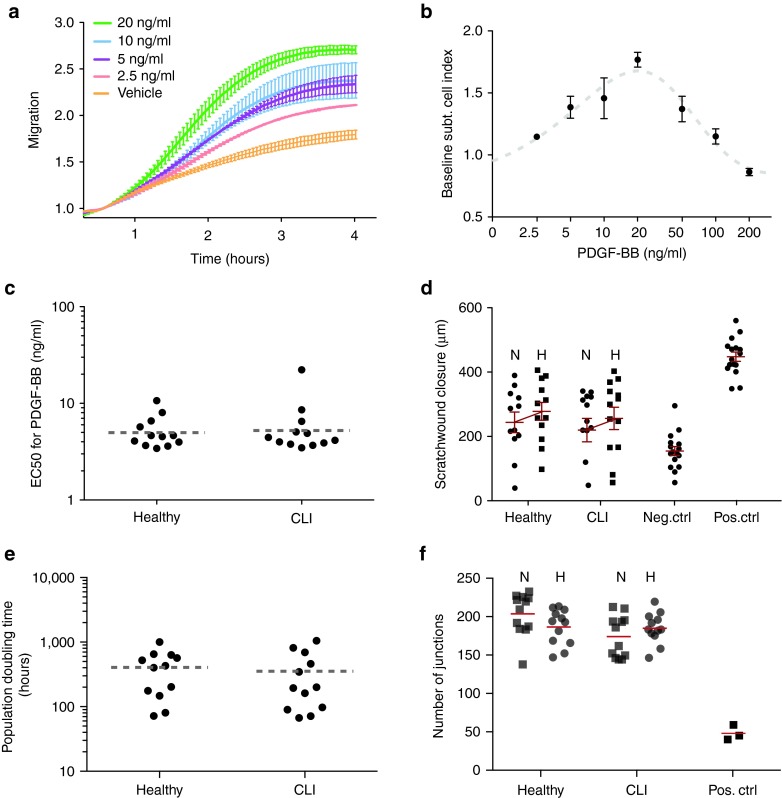
We observed clear time- and dose-dependent migration of BM-MSCs towards PDGF-BB (see Figure 4a for a representative readout, Figure 4b for a concentration response curve). The concentration-dependent response for each donor, as summarized by the half-maximum effective concentration (EC50), was determined as final readout (Figure 4c). MSCs from healthy donors migrated to PDGF-BB with an average EC50 of 5.35 ng/ml whereas the average EC50 for CLI-MSCs was not different at 5.62 ng/ml (CLI:Control = 1.05, 95% CI = 0.66–1.62)
Figure 4.
Migration. (a) Real-time migration of MSCs towards different concentrations of PDGF-BB (lines for higher concentrations have been omitted for clarity). (b) Dose-response curve of MSCs to PDGF-BB. (c) EC50 values of dose-response curves of CLI- and control MSCs (CLI:Control = 1.05, 95% CI = 0.66–1.62). (d) Scratch wound closure of microvascular endothelial cells after exposure to MSC-conditioned medium. N and H indicate CM collected under normoxic and hypoxic culturing conditions, respectively. There is a significant effect of hypoxia (P = 0.02), but not of donor group (P = 0.33) The Negative control consists of empty medium, the positive control of medium containing 10% FCS. Error bars indicate SEM. (e) Population doubling times of HMEC-1 endothelial cells grown in MSC-CM (CLI: Control = 0.96, 95% CI = 0.47–2.12). (f) Number of junctions per image in tubule formation assay of HMEC-1 cells on matrigel, in the presence of MSC CM. No differences was observed between CLI and Control MSC CM or between CM collected under hypoxic conditions. N stands for normoxic, H for hypoxic CM.
The ability of MSC-conditioned medium (CM) to induce endothelial repair was assessed using a scratch-wound closure assay. In addition, we examined whether MSC CM was affected equally by hypoxia in either group. A significant effect of oxygen tension was observed, as hypoxic CM significantly increased wound closure by ~20% (P = 0.02) compared to normoxic CM (Figure 4d). CLI-MSC-conditioned medium did not differ significantly from control CM in wound closure (CLI:Control = 1.01, 95% CI = 0.77–1.25) or the increase thereof in response to hypoxia. In examining the effect of MSC CM on population doubling times (PDT) of endothelial cells, we did not observe differences between CM of CLI MSCs and Healthy MSCs (CLI:Control = 0.96, 95% CI = 0.47–2.12), (Figure 4e). Tubule formation assays of endothelial cells in the presence of MSC CM similarly did not show any differences between CM obtained from CLI MSC and controls (CLI:Control = 0.99, 95% CI = 0.88–1.10), nor was there an effect of hypoxia (Figure 4f).
In analogy to the effects of MSC senescence on differentiation ability, we examined whether MSC senescence is associated with pro-angiogenic effects, but no relationship was found (Supplementary Figure S5).
Hind-limb ischemia
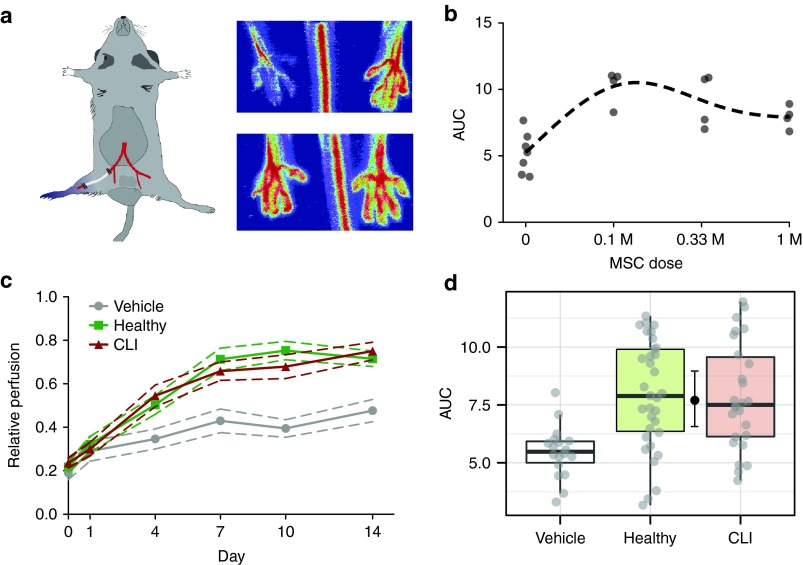
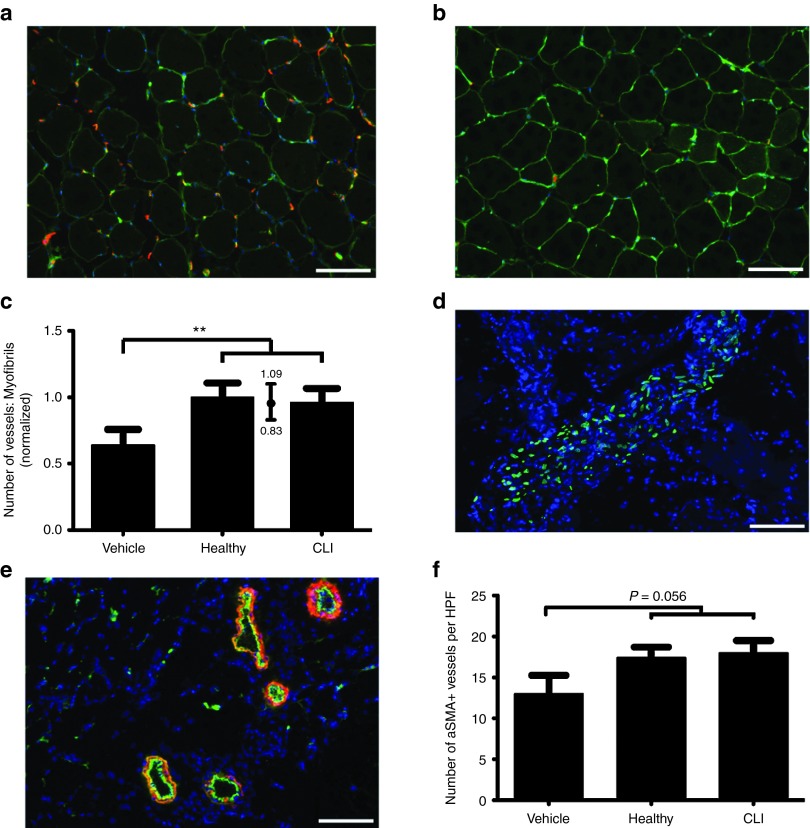
In order to assess the ability of MSCs to promote neovascularization in vivo, we performed hind-limb ischemia experiments on NMRInu/nu mice (Figure 5a). In the initial phase of the experiment, we performed a dose response curve of intra-muscularly injected MSCs obtained from a healthy donor. Animals that were treated with MSCs showed a significant increase in perfusion compared to controls. We observed a maximum effect at the lowest investigated dose of 105 cells, with a trend towards reduced effects at higher doses (Figure 5b). In the final experiment, we proceeded with the lowest dose of 105 cells and compared MSCs from 10 CLI donors and 10 control donors, testing cells from each donor in three mice. Animals treated with MSCs showed increased blood flowrecovery compared to vehicle controls after 2 weeks (71 ± 24%; SD) in MSC-treated mice and 44 ± 11% in vehicle controls (Figure 5c, pMCMC < 1 × 10−6). Time-dependent increase in perfusion did not differ between animals treated with CLI-MSC and healthy MSC. Areas under curve (AUCs) of relative perfusion over time for animals treated with CLI-MSCs were equivalent to animals treated with healthy MSCs (Figure 5d, ratio CLI:Control 0.98, 95% CI = 0.82–1.14). Staining for capillaries in the adductor muscles similarly showed an increased number of vessels in MSC-treated animals (Figure 6a,b), but did not differ between CLI-MSC- and control-MSC–treated animals (Figure 6c). aSMA staining for the formation of arterioles (Figure 6e) showed an insignificant increase in the number of arterioles in MSC-treated animals (Figure 6f), as well as a trend towards an increased aSMA+ surface area (Supplementary Figure S6a) and an enrichment of smaller vessels (Supplementary Figure S6b).
Figure 5.
Hind-limb ischemia model. (a) Hind-limb ischemia model and representative graphical readout. (b) Dose-response curve in of intramuscularly injected MSCs in the HLI model. (c) Relative perfusion over time of ischemic hind limbs over time in vehicle- and MSC-treated animals, MSC-treated animals show significantly higher perfusion than vehicle-treated animals (P < 1·10−6). Error lines indicate SEM. (d) AUCs of relative perfusion over time as a summary estimate for equivalence (CLI:Control 0.98, 95% CI = 0.82–1.14).
Figure 6.
Histology. CD31 staining (red) in (a) CLI-MSC–treated and (b) vehicle-treated animals, perimysia are stained in green (scale bar = 100 µmol/l). (c) Number of vessels as ratio vessels:myofibrils; vehicle-treated animals show a significant reduction in the number of vessels compared to MSC-treated animals (P < 0.001). The number of vessels in CLI- and healthy MSC–treated animals is equivalent (CLI:Control 0.96, 95% CI = 0.83–1.09). (d) Staining with human specific anti A/C Lamin shows that injected MSCs are predominantly retained in localized clusters, presumably the injection site, after 14 days (scale bar = 100 µmol/l). (e) Representative image of aSMA (red) staining, CD31 staining is shown in green (scale bar = 100 µmol/l). (f) The number of aSMA+ vessels did not differ per group.
In accordance with previous studies,28 only very small numbers of MSCs could be traced back 14 days after administration. Although isolated MSCs were found in the muscle stroma, the majority of MSCs were found in localized clusters in between larger muscle fibers, presumably the injection site (Figure 6d). Double-staining for nuclei of proliferating cells with Ki67 and human specific A/C Lamin showed that MSCs did not proliferate after implantation in vivo, although clusters of Ki67 positive nuclei were found in the vicinity of injection sites (Supplementary Figure S6d). While MSCs stained weakly for aSMA, they did not appear to participate in the formation of new aSMA+ vessels (Supplementary Figure S6e) nor did they appear to integrate into larger existing vessels (Supplementary Figure S6f).
In order to identify possible predictors for in vivo angiogenic ability, we performed correlations between the various assays performed in this paper (Supplementary Figure S7a). Paracrine stimulation of endothelial proliferation was most closely associated with neovascularization in vivo (Supplementary Figure S7b).
Discussion
In this study, we show that MSCs obtained from CLI patients are equivalent to MSCs obtained from healthy controls in their ability to restore perfusion in murine ischemic hind limbs. These results are consistent with our initial findings using genome-wide expression profiling, which showed that there were no differentially expressed genes between CLI- and control MSCs. CLI-MSCs show slightly increased senescence compared to controls; while this appears to affect differentiation ability, it does not affect migration, in vitro angiogenic effects of conditioned medium or pro-angiogenic ability in vivo.
Studies investigating BM-MNCs, which contain a small MSC subfraction (<0.01%),11 found that BM-MNCs from patients with cardiovascular disease have a reduced neovascularization capacity.5,6 Previous results from our group also show that in vitro paracrine angiogenic activity of ex vivo cultured early endothelial progenitor cells (EPCs) from the same CLI patient cohort as used in this study is markedly impaired.29 Similar impairment in in vivo models has also been observed in EPCs from diabetic patients.30 Moreover, a study by Yan et al.31 showed that BM-MSCs from diabetic db/db mice are impaired in their ability to promote neovascularization, although it must be noted that in that particular study the effects of diabetes were due to a gene defect, which persists in cell culture.
The results of this study suggest that selective expansion of human MSCs by ex vivo culture yields a cell population that is unaffected by disease with regard to its neovascularization capacity. Gene expression profiling performed at the outset of this study showed strikingly similar gene expression profiles for CLI- and control MSCs, with not a single gene being significantly differentially expressed between both groups. As the number of MSCs is very low in collected BM aspirates, the cells in this study were cultured for 12–15 population doublings before gene expression profiling was performed. Ex vivo culture of primary cells rapidly causes changes in gene expression, usually resulting in dedifferentiation of the cells.32,33 Cells can also gain useful properties through culturing, as in the case of early EPCs, which acquire their angiogenic phenotype only after selection and incubation in culture.34 In contrast to the finding in MSCs in this study, disease-mediated dysfunction in early EPCs is not reversed by ex vivo culture.35 It may be that unexpanded MSCs show only little susceptibility to disease-mediated dysfunction. Alternatively MSCs may be more amenable to modifying effects of cell culture, perhaps due to their replicative potential.
A report investigating abnormalities in fibroblasts obtained from dogs with congestive heart failure, for instance, showed that phenotypic abnormalities could be reversed after as little as 48 hours in culture.36
We did observe subtle differences in MSC differentiation capacity, i.e., decreased chondrogenic differentiation and a trend towards increased adipogenic differentiation in CLI-MSCs. These differences were associated with increased cellular senescence, and may in part be attributed to an overrepresentation of older donors in the CLI group. Donor age has previously been associated with increased MSC senescence and reduced differentiation potential37,38,39 with a shift towards adipogenic differentiation.38
As the primary objective of this paper was to identify differences in the ability to promote neovascularization between CLI- and control MSCs, we performed a series of in vitro experiments to study pro-angiogenic activity. In none of these experiments did we observe any differences between MSCs from the CLI- and control group. Interestingly, the results also seemed to be unrelated to MSC senescence.
Migration was assessed using PDGF-BB as a chemoattractant, as previous studies identified PDGF as the most potent chemotactic stimulus for MSCs.40 Furthermore, PDGF has been shown to be important in the interplay between endothelium and MSCs during angiogenesis.41 We observed a typical bell-shaped dose-response relationship in migration of MSCs towards PDGF-BB, with an optimum dose of 20 ng/ml. If there would be differences in migration ability between CLI and control MSCs, we would expect a shift in sensitivity towards PDGF-BB. We did not observe such a shift but found that CLI- and control MSCs showed very similar sensitivity towards PDGF-BB.
The currently prevailing theory on the mechanism behind MSC-induced angiogenesis is that it is mediated by paracrine secretion of growth factors.42 We found no differences in migration stimulatory capacity between MSC-CM under basal conditions. We examined whether hypoxia further promotes pro-angiogenic activity,43 as this will be a stress condition that MSCs are likely to be subjected to after implantation in vivo. While we did see a significant effect of hypoxic stress on the ability of MSC-CM to promote scratch-wound closure, we did not observe differences between CM derived from CLI- and control MSCs. Similarly we did not find differences in the ability of CLI- and control MSCs to promote endothelial proliferation and tubule formation.
In the in vivo hind-limb ischemia experiment, we observed a very pronounced effect of MSC-treatment over vehicle controls. We observed a bell-shaped dose-response curve with regard to cell dose, as was previously reported in intra-coronary MSC injections in animals44 and humans,17 potentially due to increased local competition for nutrients and oxygen at higher cell doses in the injection site. The CLI- and control-MSC–treated groups showed identical reperfusion patterns and were statistically equivalent, within the pre-defined thresholds. Although we observed an association between senescence and differentiation capacity, in accordance with previous studies,37,39 no such associations were found with senescence and assays for pro-angiogenic activity. Others have shown that senescence induces a senescence-associated secretory phenotype (SASP)45 in fibroblasts. This SASP enhances senescent cells' ability to induce angiogenesis in a mouse xenograft tumor model.46 The differences in senescence observed in this study are comparatively minor due to the restricted donor age range and strict emphasis on keeping passage numbers equal, which prevents conclusions on the effect of a SASP on MSC-mediated neovascularization. While the primary objective of this study was not to identify a mechanism behind MSC-mediated neovascularization, the differential effects of senescence on differentiation capacity and pro-angiogenic effects as well as the tracing studies in Figure 6 and Supplementary Figure S6 point towards paracrine affects, unrelated to stemness.47
Similarly, paracrine stimulation of endothelial proliferation was most closely associated with efficacy of a given MSC donor in vivo.
The findings of this study suggest that cell therapy in CLI patients using autologous BM-MSCs will not be impaired by disease-mediated cell dysfunction. As undifferentiated MSCs have very low inherent antigenicity14 and immunomodulatory properties,13 this cell type potentially offers an opportunity for allogeneic cell therapy without the need for immunosuppression. The question therefore arises whether there is a need for autologous therapy. Allogeneic administration of MSCs allows “off the shelf” applications for acute ischemic events and does not involve an invasive harvesting procedure in fragile patients, as is often the case in CLI. A recent study by Schwarz et al.28 showed that MSCs are cleared relatively soon after injection and that only a small number of MSCs remains after more than 3 weeks, which do not contribute to neovascularization. It has been shown, however, that eventual differentiation of remaining MSCs upregulates expression of MHC complexes, leading to delayed immunization against the injected cells.15,16 In the POSEIDON trial,17 it was shown that 8 out of 27 patients were sensitized to allogeneic HLAs at baseline of the trial, and an additional subject developed alloantibodies after a single MSC administration. In the case of CLI, the wide-spread extent of atherosclerosis, the relative ease of cell administration and the limited effective cell dose per injection and the progressive nature of disease all call for repeated cell administration.48 In addition, the findings of the PROVASA study49 suggest that there may an additional benefit of more than one injection. As repeated exposure will likely aggravate alloimmunization, autologous MSC therapy may be preferable for this indication.
This study demonstrates equivalent neovascularization potential of MSCs from CLI patients as compared to healthy controls in a murine hind-limb ischemia model. These findings suggest that autologous MSC therapy could be a promising therapeutic strategy, particularly in CLI patients.
Materials and Methods
CLI patients and healthy controls. Patient BM was harvested during the JUVENTAS trial, which investigates the efficacy of repeated intra-arterial BM-MNCs injections in patients with CLI48 (Trial identifier: NCT00371371). Inclusion criteria for the trial consisted of severe infra-popliteal arterial occlusive disease and ineligibility for surgical or endovascular revascularization procedures. For this study, we cultured BM-MSCs from 12 sequentially included patients from March to August 2011. Control BM of 12 donors without PAD was collected during elective orthopedic interventions. Procedures were approved by the central commission for human research in the Netherlands (The Hague, NLD), and are in accordance with the Declaration of Helsinki.
MSC isolation and culture. BM from all donors was obtained from the iliac crest by needle aspiration. For each donor, 10 × 106 MNCs were suspended in MEM alpha (Gibco, Grand Island, NY), 10% fetal calf serum (FCS; Lonza, Breda, NLD) and were left to adhere for 24 hours in a 10 cm2 tissue culture well. Cells were expanded for three passages until they covered four 75 cm2 tissue culture flasks and were then cryopreserved. All further experiments were conducted with cells in passage 3+1 and were started 48 hours after thawing and seeding the cryopreserved cells.
Whole-genome gene expression profiling. RNA from each cell sample was amplified and labeled cRNA was hybridized to an Illumina (San Diego, CA) WT-12 v4.0 Expression Beadchip. Samples included RNA from MSCs of six patients and six controls.
Differentiation. MSCs were differentiated towards adipogenic and osteogenic lineages in confluent monolayers, using defined differentiation media (see Supplementary Materials and Methods); chondrogenic differentiation was performed in a three-dimensional pellet culture system. Osteogenic differentiation was assessed by Alkaline Phosphatase (ALP) activity using p-Nitrophenyl Phosphate (pNPP; Sigma-Aldrich, St Louis, MO) and by Alizarin red retention. Adipogenic differentiation as assessed by lipidtox green (Molecular Probes/Invitrogen, Eugene, OR) staining and chondrogenic differentiation by production of soluble glycosaminoglycans (sGAGs)
Fluorescent staining for senescence with C12 FDG. 8 × 104 cells were incubated with 100 nmol/l bafilomycin A1 (Sigma) for 1 hour to induce lysosomal alkalinization. Next 5-dodecanoylaminofluorescein di-β-D-galactopyranoside (C12FDG, Molecular Probes) was added to a final concentration of 30 µmol/l and cells were incubated for an additional hour. Cells were trypsinized and median cellular fluorescence was quantified using flow cytometry.
Migration assays. Real-time measurement of cell migration was performed using the xCELLigence RTCA DP device from Roche Diagnostics (Mannheim, DEU). This system is a variant of the two-chamber trans-well in which vertical cell migration through a microporous membrane is recorded by measuring impedance on the underside of the membrane. In the lower wells of CIM-Plates 16 with 8 µm pore-size, a short serial dilution of platelet-derived growth factor subunit B homodimer (PDGF-BB) was prepared in serum-free medium, for each donor. In the upper wells, 40,000 MSCs were seeded in serum-free medium and allowed to settle for 15 minutes prior to the beginning of the experiment. All conditions were conducted in duplicate. Data acquisition and analysis was performed with the RTCA software (version 1.2, Roche Diagnostics).
For each well, the end-point migration was calculated by subtracting the baseline value from the point of maximum impedance. A migration index was calculated by subtracting the cumulative migration from the chemo-attractant-free control. MSCs from each donor were allowed to migrate towards a dose-range of 0, 5, 10, and 20 ng/ml PDGF-BB. The half-maximal effective dose was calculated for each donor by fitting a four-parameter log-logistic function.
Collection of conditioned medium and assays for pro-angiogenic activity. Conditioned Medium (CM) was collected in serum-free aMEM for 24 hours under normoxic (21% O2) and Hypoxic (2% O2) culturing conditions. Ability to promote endothelial repair was assessed using a scratch-wound assay. In this assay, a scratch is made in a confluent monolayer of immortalized endothelial cells (HMEC-1) using a pipette-tip and CM or control stimuli were added. Scratch-wound closure was quantified by comparing the widths of the scratch on t = 0 and t = 6 hours on photomicrographs. Proliferation of HMEC-1 cells was assessed using real-time Cell Impedance Measurement with an xCelligence machine. 2,000 HMEC-1 cells were seeded per well and cell impedance signal was recorded every 15 minutes for 72 hours, Population Doubling Time (PDT) was calculated from the linear part of the proliferation curve.
In order to assess the angiogenic effect of MSC-CM in vitro, tubule formation assays using HMEC-1 cells were performed.50 HMEC-1 cells suspended in MSC-CM obtained under hypoxic and normoxic conditions, laid upon gels of Growth Factor-Reduced Matrigel (Becton Dickinson, Breda, NLD) cast in IBIDI µ-wells (Martinsried, DEU), and allowed to form tubules for 24 hours. Tubule network characteristics were quantified using automated image analysis.
Hind-limb ischemia model. Hind-limb ischemia was induced in 8–10 week old male nude NMRI FoxN1nu/nu mice (Harlan, Horst, NLD). The femoral artery was occluded with the use of an electrocauterization device (Bovie, Clearwater, FL) directly as it emerged from under the femoral ligament, closing off both superficial and deep branches. The femoral vein and nerve were left intact. The segment of the superficial femoral artery distal to the occlusion was then carefully stripped away from the adjacent vein until the popliteal bifurcation was reached.
Cell injections were performed 24 hours after femoral ligation. For the dose ranging 1, 3.3, and 10 × 105 MSCs were injected intramuscularly divided over five different sites in the adductor muscle (10 µl/injection). In subsequent experiments using patient derived cells, 1 × 105 MSCs were used. MSCs from 10 donors in each group were injected in three mice per donor; in addition, a total of 21 vehicle controls was included. Relative perfusion of the ischemic limb compared to the control limb was measured using Laser Doppler Perfusion Imaging, with a moorLDI2-HR (Moor Instruments, Devon, UK) imager with an 830 nm Helium-Neon laser. Relative perfusion was followed over time by measurements on day 0, 1, 4, 7, 10, and 14.
Histology. Vessels and muscle fibers in the adductor muscles were stained using anti-Mouse CD31 (Santa Cruz, Dallas, TX) and Triticum vulgaris lectin (Sigma). Human nuclei were visualized with anti-human nuclear A/C lamin (Vector Labs, Burlingame, CA; see Supplementary Figure S6c). Staining for arterioles was done using anti-Mouse CD31 and anti-alpha Smooth Muscle Actin (aSMA; Sigma).
Design and statistics. Values are presented as relative to the Healthy MSC donor group to allow a comparison between CLI and control MSCs in the various assays performed in this study. Data were analyzed by using generalized linear mixed models, using a restricted maximum likelihood (REML) approach to estimate model parameters. P values and (highest posterior density, HPD) credibility intervals were estimated by means of Markov Chain Monte Carlo (MCMC) sampling from the posterior distribution of parameter values (10,000 iterations). In the analysis of the HLI experiments, a hierarchical linear mixed model was used, in which mice were nested within MSC donors. To create a meaningful summary measure for equivalency, we calculated areas under curve (AUCs) of relative perfusion over time. Analyses were conducted in ‘R' software (version 2.15.3), using the lmer function in the lme4 package.
Groups were considered equivalent when 95% of the posterior distribution was within a pre-defined threshold. As threshold a range of 0.8–1.25, taken from bioequivalency guidelines set forth by drug regulatory agencies such as the FDA or EMA, was used. In the microarray experiment, a moderated t-test approach as employed in the “limma” package was used to test for differences between groups. As a lack of a statistically significant difference may for some genes be attributable to a lack of power in the experiment, we took a reverse approach to analysis by excluding genes that with 95% confidence did not differ by more than a factor 2 in expression and are thus considered equivalently expressed.
SUPPLEMENTARY MATERIAL Figure S1. Alizarin red staining in MSCs after osteogenic differentiation. Figure S2. Modified volcano plot showing intergroup versus intragroup variance for CLI-MSCs and control MSCs. Figure S3. Representative nucleus showing gH2AX damage foci. Figure S4. Associations of differentiation capacity with donor senescence. Figure S5. Associations of measures of proangiogenic capacity with senescence. Figure S6. Supplemental histology. Figure S7. Correlations between assays. Table S1. Top 50 of most differentially expressed genes. Materials and Methods.
Acknowledgments
This project was funded by a ZonMW TASO grant (#116001026). This work described in this manuscript was done exclusively in the University Medical Center Utrecht, Utrecht, The Netherlands. The authors declare no conflict of interest.
Supplementary Material
References
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. TASC II Working Group Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45 Suppl S:S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Sprengers RW, Teraa M, Moll FL, de Wit GA, van der Graaf Y, Verhaar MC. JUVENTAS Study Group; SMART Study Group Quality of life in patients with no-option critical limb ischemia underlines the need for new effective treatment. J Vasc Surg. 2010;52:843–9, 849.e1. doi: 10.1016/j.jvs.2010.04.057. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Kimura M, Hara K, Noma K, Jitsuiki D, Nakagawa K, et al. Autologous bone-marrow mononuclear cell implantation improves endothelium-dependent vasodilation in patients with limb ischemia. Circulation. 2004;109:1215–1218. doi: 10.1161/01.CIR.0000121427.53291.78. [DOI] [PubMed] [Google Scholar]
- Teraa M, Sprengers RW, van der Graaf Y, Peters CE, Moll FL, Verhaar MC. Autologous bone marrow-derived cell therapy in patients with critical limb ischemia: a meta-analysis of randomized controlled clinical trials. Ann Surg. 2013;258:922–929. doi: 10.1097/SLA.0b013e3182854cf1. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- Li TS, Kubo M, Ueda K, Murakami M, Ohshima M, Kobayashi T, et al. Identification of risk factors related to poor angiogenic potency of bone marrow cells from different patients. Circulation. 2009;120 11 Suppl:S255–S261. doi: 10.1161/CIRCULATIONAHA.108.837039. [DOI] [PubMed] [Google Scholar]
- Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, et al. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res. 2005;66:543–551. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lasala GP, Silva JA, Kusnick BA, Minguell JJ. Combination stem cell therapy for the treatment of medically refractory coronary ischemia: a Phase I study. Cardiovasc Revasc Med. 2011;12:29–34. doi: 10.1016/j.carrev.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremmels H, Fledderus JO, Teraa M, Verhaar MC. Mesenchymal stromal cells for the treatment of critical limb ischemia: context and perspective. Stem Cell Res Ther. 2013;4:140. doi: 10.1186/scrt351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- Huang XP, Sun Z, Miyagi Y, McDonald Kinkaid H, Zhang L, Weisel RD, et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LY, Zhang HY, Feng XB, Hou YY, Lu LW, Fan LM. Abnormality of bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus. 2007;16:121–128. doi: 10.1177/0961203306075793. [DOI] [PubMed] [Google Scholar]
- Guiducci S, Manetti M, Romano E, Mazzanti B, Ceccarelli C, Dal Pozzo S, et al. Bone marrow-derived mesenchymal stem cells from early diffuse systemic sclerosis exhibit a paracrine machinery and stimulate angiogenesis in vitro. Ann Rheum Dis. 2011;70:2011–2021. doi: 10.1136/ard.2011.150607. [DOI] [PubMed] [Google Scholar]
- Fan M, Chen W, Liu W, Du GQ, Jiang SL, Tian WC, et al. The effect of age on the efficacy of human mesenchymal stem cell transplantation after a myocardial infarction. Rejuvenation Res. 2010;13:429–438. doi: 10.1089/rej.2009.0986. [DOI] [PubMed] [Google Scholar]
- Roemeling-van Rhijn M, Reinders ME, de Klein A, Douben H, Korevaar SS, Mensah FK, et al. Mesenchymal stem cells derived from adipose tissue are not affected by renal disease. Kidney Int. 2012;82:748–758. doi: 10.1038/ki.2012.187. [DOI] [PubMed] [Google Scholar]
- Reinders ME, Roemeling-van Rhijn M, Khairoun M, Lievers E, de Vries DK, Schaapherder AF, et al. Bone marrow-derived mesenchymal stromal cells from patients with end-stage renal disease are suitable for autologous therapy. Cytotherapy. 2013;15:663–672. doi: 10.1016/j.jcyt.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Friis T, Haack-Sørensen M, Hansen SK, Hansen L, Bindslev L, Kastrup J. Comparison of mesenchymal stromal cells from young healthy donors and patients with severe chronic coronary artery disease. Scand J Clin Lab Invest. 2011;71:193–202. doi: 10.3109/00365513.2010.550310. [DOI] [PubMed] [Google Scholar]
- Neef K, Choi YH, Weichel A, Rahmanian PB, Liakopoulos OJ, Stamm C, et al. The influence of cardiovascular risk factors on bone marrow mesenchymal stromal cell fitness. Cytotherapy. 2012;14:670–678. doi: 10.3109/14653249.2012.663483. [DOI] [PubMed] [Google Scholar]
- Boeuf S, Kunz P, Hennig T, Lehner B, Hogendoorn P, Bovée J, et al. A chondrogenic gene expression signature in mesenchymal stem cells is a classifier of conventional central chondrosarcoma. J Pathol. 2008;216:158–166. doi: 10.1002/path.2389. [DOI] [PubMed] [Google Scholar]
- Alves H, van Ginkel J, Groen N, Hulsman M, Mentink A, Reinders M, et al. A mesenchymal stromal cell gene signature for donor age. PLoS ONE. 2012;7:e42908. doi: 10.1371/journal.pone.0042908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz TM, Leicht SF, Radic T, Rodriguez-Araboalaza I, Hermann PC, Berger F, et al. Vascular incorporation of endothelial colony-forming cells is essential for functional recovery of murine ischemic tissue following cell therapy. Arterioscler Thromb Vasc Biol. 2012;32:e13–e21. doi: 10.1161/ATVBAHA.111.239822. [DOI] [PubMed] [Google Scholar]
- Teraa M, Sprengers RW, Westerweel PE, Gremmels H, Goumans MJ, Teerlink T, et al. JUVENTAS study group Bone marrow alterations and lower endothelial progenitor cell numbers in critical limb ischemia patients. PLoS ONE. 2013;8:e55592. doi: 10.1371/journal.pone.0055592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino SA, Bahlmann FH, Besler C, Müller M, Schulz S, Kirchhoff N, et al. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- Yan J, Tie G, Wang S, Messina KE, DiDato S, Guo S, et al. Type 2 diabetes restricts multipotency of mesenchymal stem cells and impairs their capacity to augment postischemic neovascularization in db/db mice. J Am Heart Assoc. 2012;1:e002238. doi: 10.1161/JAHA.112.002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson BL, Ylöstalo J, Prockop DJ. Human multipotent stromal cells undergo sharp transition from division to development in culture. Stem Cells. 2008;26:193–201. doi: 10.1634/stemcells.2007-0524. [DOI] [PubMed] [Google Scholar]
- Boquest AC, Shahdadfar A, Frønsdal K, Sigurjonsson O, Tunheim SH, Collas P, et al. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16:1131–1141. doi: 10.1091/mbc.E04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- Dawson K, Wu CT, Qi XY, Nattel S. Congestive heart failure effects on atrial fibroblast phenotype: differences between freshly-isolated and cultured cells. PLoS ONE. 2012;7:e52032. doi: 10.1371/journal.pone.0052032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- Kim M, Kim C, Choi YS, Kim M, Park C, Suh Y. Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: implication to age-associated bone diseases and defects. Mech Ageing Dev. 2012;133:215–225. doi: 10.1016/j.mad.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- Ding W, Knox TR, Tschumper RC, Wu W, Schwager SM, Boysen JC, et al. Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood. 2010;116:2984–2993. doi: 10.1182/blood-2010-02-269894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- Annabi B, Lee YT, Turcotte S, Naud E, Desrosiers RR, Champagne M, et al. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells. 2003;21:337–347. doi: 10.1634/stemcells.21-3-337. [DOI] [PubMed] [Google Scholar]
- Houtgraaf JH, de Jong R, Kazemi K, de Groot D, van der Spoel TI, Arslan F, et al. Intracoronary infusion of allogeneic mesenchymal precursor cells directly after experimental acute myocardial infarction reduces infarct size, abrogates adverse remodeling, and improves cardiac function. Circ Res. 2013;113:153–166. doi: 10.1161/CIRCRESAHA.112.300730. [DOI] [PubMed] [Google Scholar]
- Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clin Pharmacol Ther. 2007;82:241–243. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- Sprengers RW, Moll FL, Teraa M, Verhaar MC. JUVENTAS Study Group Rationale and design of the JUVENTAS trial for repeated intra-arterial infusion of autologous bone marrow-derived mononuclear cells in patients with critical limb ischemia. J Vasc Surg. 2010;51:1564–1568. doi: 10.1016/j.jvs.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Walter DH, Krankenberg H, Balzer JO, Kalka C, Baumgartner I, Schlüter M, et al. PROVASA Investigators Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA) Circ Cardiovasc Interv. 2011;4:26–37. doi: 10.1161/CIRCINTERVENTIONS.110.958348. [DOI] [PubMed] [Google Scholar]
- Poldervaart MT, Gremmels H, van Deventer K, Fledderus JO, Oner FC, Verhaar MC, et al. Prolonged presence of VEGF promotes vascularization in 3D bioprinted scaffolds with defined architecture. J Control Release. 2014;184:58–66. doi: 10.1016/j.jconrel.2014.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.