Abstract
TGF-β was originally considered as an immunoregulatory cytokine, but accumulating data demonstrate that it also plays a critical role in development of effector immunity. Since TGF-β has a potent ability to alter immune responses, modulation of the TGF-β pathway for treatment of transplantation patients could be effective if carried out in a target selective manner. This review will focus on the role of TGF-β in T cell differentiation and discuss the prospect of TGF-β as the therapeutic target of lung transplantation acceptance.
Solid-organ transplantation has undergone significant progress in recent years [1]. This is mainly due to the success of controlling short-term graft rejection by immunosuppressive drugs [2]. However, the drugs used to control rejection have been essentially unchanged over the past 20 years. Chronic rejection and the complications of long-term exposure to pharmacologic control of the alloimmune response, continue to severely impact long-term survival. The 10-year median survival of solid-organ transplant patients is at best 60%, with some organ specific survivals much less than 50% [3]. A key problem related to transplant failure is caused by the immunosuppressive regimens. Medications used for immunosuppression currently have multiple side effects such as toxicity to kidneys and bone marrow, and have no effect leading to the induction of tolerance. Therefore, transplant recipients need to take the immunosuppressive therapy for life, which increases the risk of opportunistic infections, cancer and dysfunction of other organs [4]. For example, these medications often directly damage transplanted organs and significantly increase cardiovascular morbidity and mortality [5]. Moreover, immunosuppressive drugs have serious side effects, including carcinogenesis [6]. Cyclosporine A, a prototypic immunosuppressive drug, was demonstrated to increase the risk of cancer due to a TGF-β-dependent cell-intrinsic mechanism [7]. TGF-β is known to augment fibrosis development and promote tumor cell invasiveness [8]. TGF-β transcription is increased with cyclosporine, which raises a concern of cancer recurrence or the emergence of post-transplant lymphoproliferative disorders.
Furthermore, TGF-β has been known to act as a potent immune-regulatory cytokine, which blocks T cell activation [9]. It is considered as a potential target for more specific and less toxic immunosuppression and control of alloimmune responses over the long term. Yet, recent studies have revealed that TGF-β also has pro-inflammatory functions.
Clinically, there have been several observations that indicate that TGF-β is linked to the failure/success of transplantation [10, 11]. For instance, a TGF-β allele was reported to carry a higher risk of renal dysfunctions among heart transplant patients [10]. On the other hand, expression of TGF-β and its receptor was significantly higher in peripheral blood mononuclear cells from transplant patients who maintained graft function after the complete withdrawal of immunosuppressive drugs [12]. Together, these data indicate the significance of TGF-β in both positive and negative outcomes of transplantation. To understand the dichotomy of the effect of TGF-β on the outcomes of transplantation, this article will focus on the mechanism by which TGF-β can modulate immune responses and exploit the potential of TGF-β as the target of immune-regulation in the future.
Current state of lung transplantation
Solid-organ transplantation is a definitive therapy for end-stage disorders of various organs. Among all the solid-organ transplantations, despite advances in surgery and medical management over the past 20 years, the clinical outcomes after lung transplantation remain far below that of other solid-organ transplants [13]. The 5-year survival for lung transplantation is 45%, while the 5-year survival for heart transplantation is 75%. As a primary part of the host defense, the lung has a unique immunologic environment. It must continuously respond to exposure to environmental factors such as infectious agents and air contaminants. Lung disease is the fourth leading cause of death in the USA and a major cause of disability, shortened life expectancy, and social and economic problems worldwide. Lung transplantation is the only definitive therapy for end-stage lung disease, and has therefore become the accepted standard for relieving symptoms and prolonging life.
Bronchiolitis obliterans (clinically called bronchiolitis obliterans syndrome [BOS]) is the major cause of allograft failure, affecting at least 60% of recipients within 5 years of transplant [14]. The histopathology of BOS suggests that both inflammation and response to injury with epithelial and fibroblast proliferation precede small airway obliteration. Acute rejection, primarily triggered by donor HLA proteins, was suggested to predispose recipients to BOS [15]; yet newer therapeutics that have reduced the incidence of acute rejection have not changed the incidence of BOS, suggesting that acute rejection may not be the only high risk factor.
Pharmacologic advances have reduced the frequency of graft failures due to acute rejection but have had no impact on the incidence of BOS or 5 year survival rate. Rejection surveillance after lung transplantation remains essentially the same as it was 15 years ago. Chest x-ray, spirometry and clinical impression remain the first line tools in assessment. Transbronchial biopsy with the inherent risks of bleeding and pneumothorax, limitations of sampling, variability in quality of tissue obtained, as well as interpretation by the pathologist, have lead to continued debate over its value in diagnosing rejection and risk benefit to the patient [16].
An emerging model explaining the mechanism that underlies BOS is that peptide antigens in the transplanted organ normally recognized as ‘self ’, rather than allogeneic MHC molecules, begin to provoke an autoimmune response against the graft by the recipient antigen-presenting cells (APCs), is presented to the immune system as nonself by the recipient APCs [17–20]. In this model, transplantation is considered as the trigger to provoke autoimmunity against antigens that are normally present in self-tissues. This being the current state of clinical lung transplantation, it is critical to develop a novel method to promote tolerance through regulation of immune injury and inflammation after lung transplant.
Regulatory T cells & immune regulation
To address these problems facing transplantation, various therapies are being explored that may allow patients to retain a long-term functioning allograft under no immunosuppression (tolerance), or minimal immunosuppression. The drugs used for immunosuppression have many general toxic effects on all populations of T cells regulating the immune response, and are limited in their ability to produce a specific independent effect. Other, more tailored, strategies are clearly necessary.
A potential tool for the induction of tolerance is the use of regulatory T cells, which have potent long-lasting and antigen-specific immunosuppressive functions [21]. To date there are at least three types of regulatory T cells (Tregs) known. Two of them express the transcription factor Foxp3 and are divided into thymus-derived and periphery-derived Tregs. Another group of Tregs produce IL-10 in response to stimulation but does not express Foxp3.
Thymic-derived Foxp3+ Tregs are called naturally arising regulatory T cells (nTregs) and were originally defined as a group of cells that prevent the onset of autoimmune disease caused by thymectomy of new born mice [22]. Using surface antigens as a marker to identify the cells that confer suppression effect among CD4 T cells, Sakaguchi and colleagues discovered that removal of CD4+CD25+ T cells significantly increased the prevalence and intensity of autoimmune disease [23]. Conversely, adding back these CD4+CD25+ T cells prevented the development of autoimmunity [24, 25]. Thus, CD4+CD25+ T cells were identified as a group of cells that contain immune regulatory functions and were termed Tregs.
Tregs are mostly CD4+CD25+ and constitute 5–10% of peripheral T cells in normal mice [26]. These T cells suppress cytokine production (e.g., IL-2, IL-4, IFN-γ) and proliferation of antigen receptor-stimulated CD4 and CD8 T cells. Although their mode of suppression is not clearly understood, Tregs require direct interactions with the responding T cell/APC complex. IL-2 is a critical growth/survival factor for Tregs and may be required to maintain their function [27]. At the same time, the presence of exogenous IL-2 abrogates the suppressive property of Tregs. Although the surface phenotype of CD4+CD25+ was originally used to isolate these populations, it is not a definitive marker of nTregs since activated effector T cells also express CD25. Currently, the most reliable marker that distinguishes Tregs from other cells is a transcriptional repressor Foxp3 [28–32]. Mutations of the Foxp3 gene result in severe autoimmune disorders both in humans and mice [33–36]. Foxp3 is required for the production and maintenance of Tregs, and the level of Foxp3 expression in effector cells is much lower than that in Tregs [37].
Accumulating evidence suggests that the function of Tregs is not limited to the suppression of autoimmunity but that Tregs also play significant roles toward nonself antigens such as viruses [38]. Other studies documented that the frequency of Tregs increase under tumor bearing conditions [39], as well as during pregnancy [40, 41]. Conversely, decrease of Tregs was reported in cases of autoimmune disease such as multiple sclerosis, Type I diabetes and rheumatoid arthritis [42]. Collectively, the data indicate that the balance between Tregs and non-Tregs may play an important role in controlling the immune responses against nonself antigens, tumor antigens, as well as self-antigens. Manipulation of the balance between regulatory and nonregulatory T cells may be beneficial for tissue transplantation, and prevent anti-allogeneic and potential self-antigen responses provoked by organ transplantation. Indeed, an experimental system that uses murine Tregs that are expanded by stimulation by allogeneic APCs or self-APCs with allogeneic MHCs, successfully induce tolerance and long-term acceptance of an allograft [43].
TGF-β & inducible Tregs
Foxp3+ Tregs generated in the periphery are called inducible Tregs (iTregs). When naive T cells are activated by the antigen in the presence of TGF-β and IL-2, naive CD4 T cells differentiate into Foxp3+ Tregs in an antigen-specific manner [44]. These cells have immunosuppressive functions comparable with nTregs and share the same surface antigen phenotype. Induction of iTregs can be enhanced by retinoic acid, and CD103+ dendritic cells from the intestinal mucosa have been shown as a potent inducer of iTregs [45].
Since iTregs are generated in the periphery in response to antigen stimulation, their repertoire can be controlled by ex vivo or in vivo antigenic stimulation. Most importantly for transplantation, iTregs can be generated toward allogeneic antigens [46]. Induction of these iTregs against transplanted organs can be an effective tool for therapeutic applications in amelioration of graft rejection.
Although these data indicate TGF-β plays a significant role in the induction of Tregs and immune suppression, now it is clear that TGF-β also plays a role in the development of the effector wing of adaptive immunity [47]. For example, when IL-6 is present along with TGF-β, naive CD4 T cells stimulated with antigen preferentially differentiate into pro-inflammatory effector T cells that produce IL-17 (Th17 cells). Other cytokines such as IL-1β and IL-23 are also known to enhance the development of Th17 [48]. IL-17 is a cytokine that promotes the recruitment and proliferation of neutrophils [49]. IL-17 also activates fibroblasts and endothelial cells. Th17 cells are well known as a major causative cell subset for autoimmune disorders [50]. Importantly, Th17 cells develop in response to organ transplantation and these cells are considered to be significant effector cells for tissue rejection [51]. Thus, TGF-β can promote the development of T cells that accelerate the rejection process.
Recently, effector T cells that produce IL-9 were also found to be induced by TGF-β [52]. In this case, the presence of IL-4 was needed for induction. IL-9 plays a critical role in IgE induction, the recruitment and activation of mast cells, the pathogenesis of asthma and other allergic responses. It should be noted that IL-9 has been considered immunoregulatory against transplanted organs via mast cells, which can activate Tregs [53]. Moreover, IL-9 was implicated in preventing fibrosis [54]. Therefore, although Th9 is another group of effector type T cells, they might be helpful for transplantation.
Overall, these data demonstrate that TGF-β plays a role of catalysis in decision making process of naive T cell differentiation but does not dictate the direction by itself. The differentiation of naive T cells into iTregs, Th17 or Th9 is determined by the presence of certain cytokines or molecules in the environment, such as IL-2 or retinoic acid for iTregs, IL-6, IL-1 and/or IL-21 for Th17, and IL- 4 for Th9.
New function of TGF-β in survival of Tregs against p53-induced CD28-dependent T cell apoptosis
It is well established that antigen-activated T cells undergo apoptosis after continuous stimulation (termed activation-induced cell death [AICD]) [55]. In contrast, Tregs must survive continuous stimulation from their antigens since a substantial number of Tregs are reactive to self-antigens. Our recent studies revealed that, in addition to catalyzing differentiation of naive T cells, TGF-β plays a critical role in the survival/maintenance of Tregs against antigen-receptor stimulation [56].
When primary T cells were stimulated by anti-CD3 and anti-CD28 antibodies coated on a solid flat surface, T cells underwent massive apoptosis. However, Tregs were completely resistant to the stimulation. As a consequence, after 2 weeks of ex vivo culture in the plates coated with anti-CD3 and anti-CD28 antibodies, T cells that survived and expanded in the culture were predominantly constituted from Foxp3+ Tregs. These Tregs were functional both in vitro and in vivo for their suppression.
This form of apoptosis is distinctive from classical AICD, which occurs after re-stimulation of T cells in the presence of IL-2. While classical AICD is p53-independent and is blocked by anti-CD28 stimulation, this new form of T cell apoptosis required expression of p53. CD28 stimulation was also required to induce apoptosis. T cells that lacked p53 were completely resistant to the stimulation, underwent robust expansion and outgrew Foxp3 Tregs. Based on genetic evidence, this form of apoptosis was named p53-induced CD28-dependent T cell apoptosis (PICA). Similar to plate-bound antibody simulation, continuous stimulation from allogeneic dendritic cells led to cell death of p53-sufficient primary Foxp3− T cells. Yet, p53-deficicent T cells resisted apoptosis and continued to expand, suggesting that PICA can occur in vivo in response to allogeneic stimuli such as transplant-associated antigens.
Since Tregs are resistant to PICA and selectively expand, PICA can be beneficial for the induction of tolerance against transplanted organs. To understand the mechanism by which Tregs withstand PICA, we have determined the molecular responses that underlie the PICA resistance by Tregs. One of the known characteristics of Tregs are their expression of TGF-β when activated by antigens. Indeed, TGF-β has been implicated for the homeostasis of Tregs [57]. Thus, we examined if TGF-β plays any role in PICA [58]. When TGF-β signaling was inhibited in PICA inducing conditions, nTregs were no longer resistant to PICA. Conversely, when exogenous TGF-β (active form) was added to the culture, effector T cells underwent robust expansion instead of apoptosis. Thus, the data showed that Tregs are resistant to PICA in a TGF-β-dependent manner and that TGF-β can convert PICA-inducing stimuli into effector T cell generating signal. Resistance to apoptosis by T cells was associated with reduced expression of pro-apoptotic molecules such as Bim and FoxO3a, suggesting that TGF-β suppresses apoptosis by controlling the expression of these apoptosis related genes.
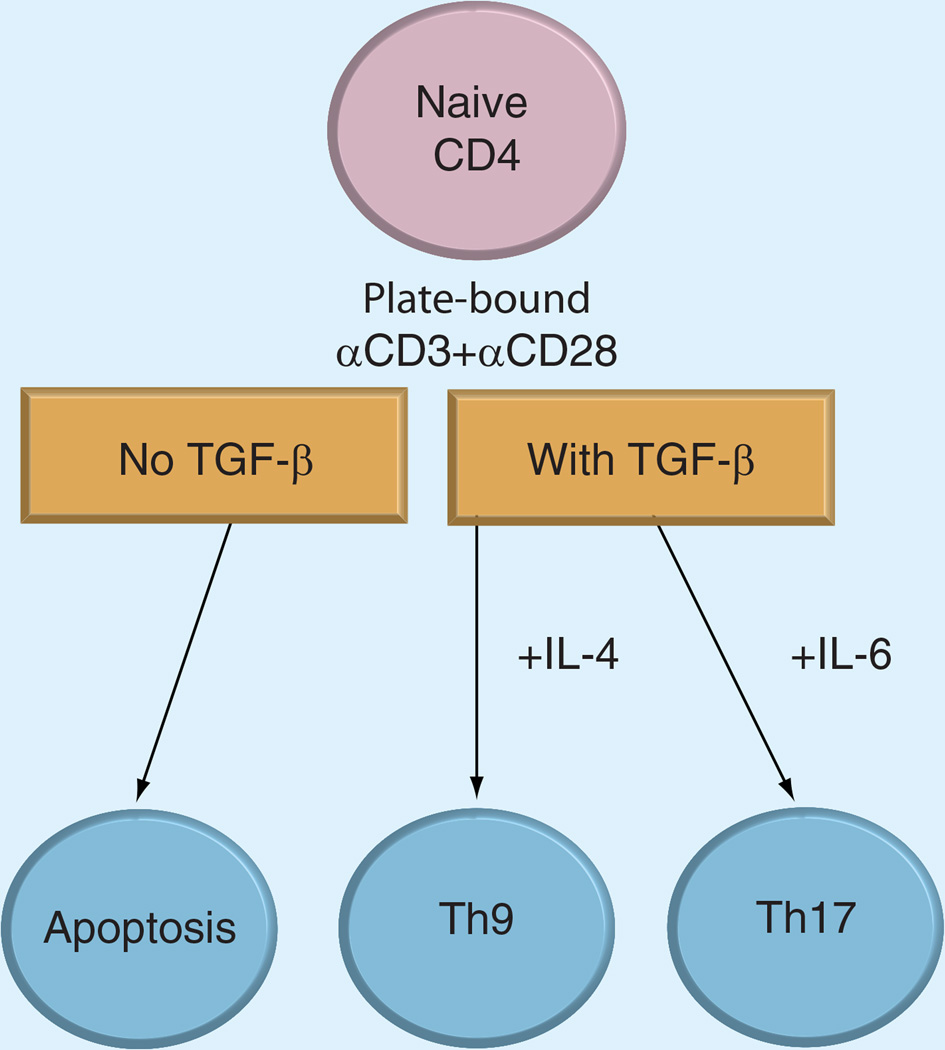
A surprising outcome of the effect of TGF-β was that a significant fraction of cells stimulated with TGF-β differentiated into Th9 cells, instead of Foxp3+ iTregs. Indeed, with PICA-inducing conditions, the Foxp3+ Treg percentage did not increase even in the presence of TGF-β and IL-2. Moreover, the presence of IL-6 induced expansion of Th17 cells. These data suggest that TGF-β signaling plays another role in controlling the numbers of conventional and regulatory CD4+ T cells during antigen stimulation. For induction of Th9 and Th17 cells, anti-CD3 and anti-CD28 antibodies were both coated on the flat surface. When anti-CD28 was provided as a soluble form, T cells differentiated into Foxp3+ iTregs. Therefore, 3D information of how CD28 is engaged appears to dictate the cell fate. The data suggest that TGF-β promotes either regulatory or effector T cell responses depending on the presence of cytokines and the way co-stimulation is provided (Figure 1). It should be noted that though Tregs can activate TGF-β by themselves, mouse effector T cells do not. Therefore, for the induction of effector cells, TGF-β derived from paracrine sources would play a critical role.
Figure 1. Effect of TGF-β on p53-induced CD28-dependent T cell apoptosis.
When naive CD4 T cells are stimulated by plate-bound anti-CD3/anti-CD28 antibodies to induce apoptosis, the presence of TGF-β inhibits apoptosis. If IL-4 is present, naive CD4 T cells differentiate into Th9 cells, while presence of IL-6 promotes development of Th17 cells.
Currently, the molecular mechanism underlying these phenomena is unknown. TGF-β may be simply providing signaling required for the survival of T cells, and IL-4/IL-6 is providing differentiation signaling for TH9/Th17 respectively. Alternatively, TGF-β might be providing signaling required for the initiation/establishment of differentiation.
Crosstalk of TGF-β signaling process with other signaling pathways
Based on the complex effect of TGF-β on T cell responses, it is essential to find the target that is specific for one type of response, such as the induction of Tregs or Th17.
TGF-β exists in three isoforms (β1, β2 and β3) with TGF-β1 being most common in the immune system. TGF-β is secreted in a complex with LAP.
LAP is an inhibitory domain generated when pro-TGF-β is processed intracellularly by proteolysis. LAP forms a noncovalent complex with the active TGF-β [59]. When exported to the extracellular environment, LAP-TGF-β complex is tethered to the plasma membrane. LTBP1~4 is a well-identified family of proteins that bind the LAP/TGF-β complex and anchor the complex to the plasma membrane by interactions with the extra cellular matrix. Recently, glycoprotein A repetitions predominant (GARP) has been identified as another molecule that plays a critical role in membrane attachment of the TGF-β-LAP complex on the cell surface of Tregs [60]. TGF-β complex must be removed from LTBP to become active. LTBP is degraded by a series of proteolytic processes involving metalloproteases such as astacin family members [61]. Precisely how TGF-β is activated and removed from GARP is not understood. After removal of LTBPs, non-covalent binding between LAP and TGF-β is replaced by proteins such as TSP1.
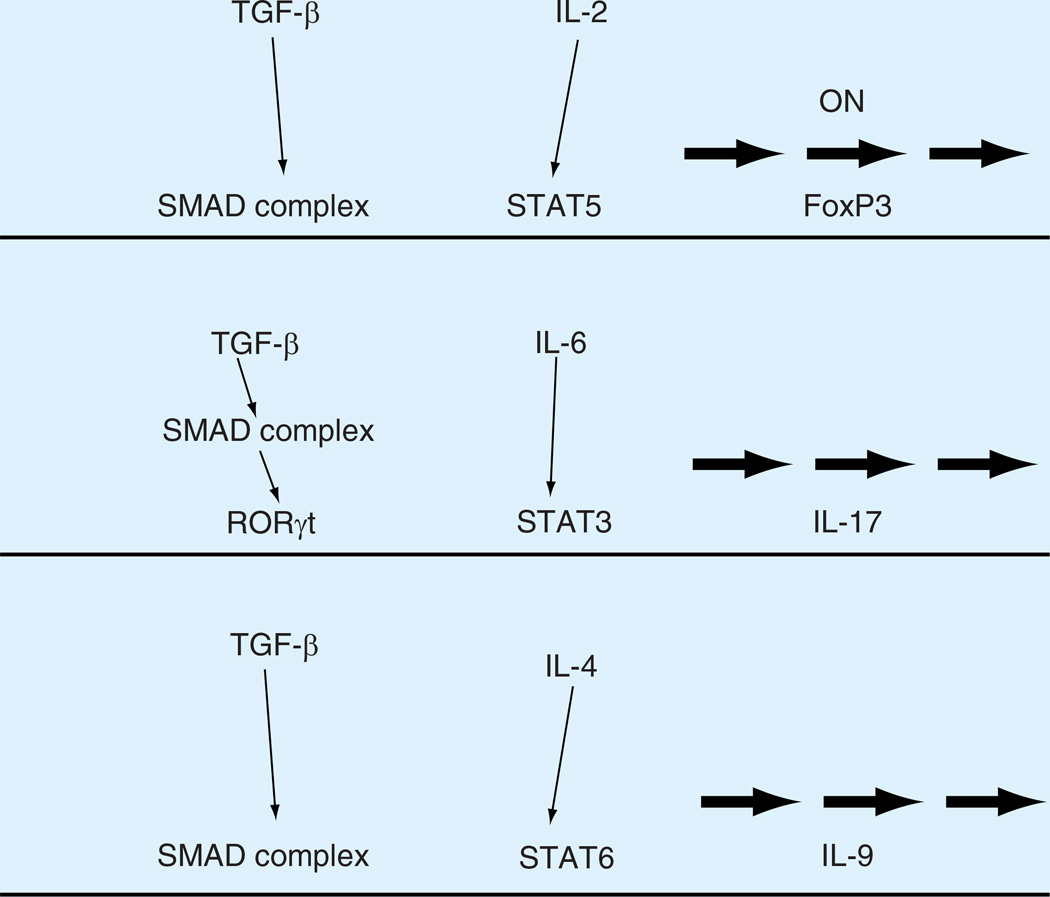
After activation, TGF-β binds its specific receptors. There are three types of receptors for TGF-β, Type 1, 2 and 3. Type 2, expressed as a homodimer on the cell surface [62]. TGF-β binding to Type 2 receptor induces heteromeric complex of Type 1 and Type 2 receptors. Type 2 receptor has a constitutively active kinase domain and association with Type 1 receptor leads to phosphorylation and activation of the Type 1 receptor kinase domain. Activated Type 1 receptor phosphorylates receptor regulated SMAD proteins SMAD2 and SMAD3. Phosphorylated SMAD proteins will heterodimerize with co-SMAD (SMAD 4) and translocate into the nucleus. SMAD heterodimers accumulate in the nucleus due to a decrease in the rate of nuclear export. The SMAD complex has DNA-binding capability in concert with other transcription factors. For example, an enhancer element in the Foxp3 locus has been identified to interact with SMAD3 and NFAT for the induction of Foxp3 expression [63]. For IL-17 activation, it is shown that TGF-β upregulates RORγt, a transcription factor required for Th17 development, which in turn promotes IL-17 expression along with STAT 3 [64]. Similarly, STAT6 appears to function cooperatively with the TGF-β signaling process to induce IL-9 (Figure 2) [65, 66].
Figure 2. Transcriptional control of naive T cell differentiation by TGF-β and other cytokines.
In naive CD4 T cells activated by antigens, TGF-β and IL-2 promote the expression of Foxp3, an essential transcription factor for iTregs development. When IL-6 is present along with TGF-β, pro-inflammatory IL-17 production is induced via activation of RORγt and STAT3. The combination of TGF-β with IL-4 leads to the expression of IL-9 by SMAD complex and STAT6.
Conclusion: application of TGF-β signal alterations
Given the evidence of the function of TGF-β in immune regulation, modification of TGF-β and its signaling process in transplant patients could have various outcomes dependent on the state of the patient (Figure 3). Inhibition of TGF-β could block the generation of pro-inflammatory effector T cells (e.g., Th17) and promote PICA of allo-reactive T cells. On the other hand, inhibition of TGF-β signaling could inhibit the generation of iTregs that can block antigraft rejection and suppress potentially protective Th9 development. Therefore, simple inhibition of the TGF-β axis in a systemic manner is not an ideal approach for the treatment of organ transplant recipients.
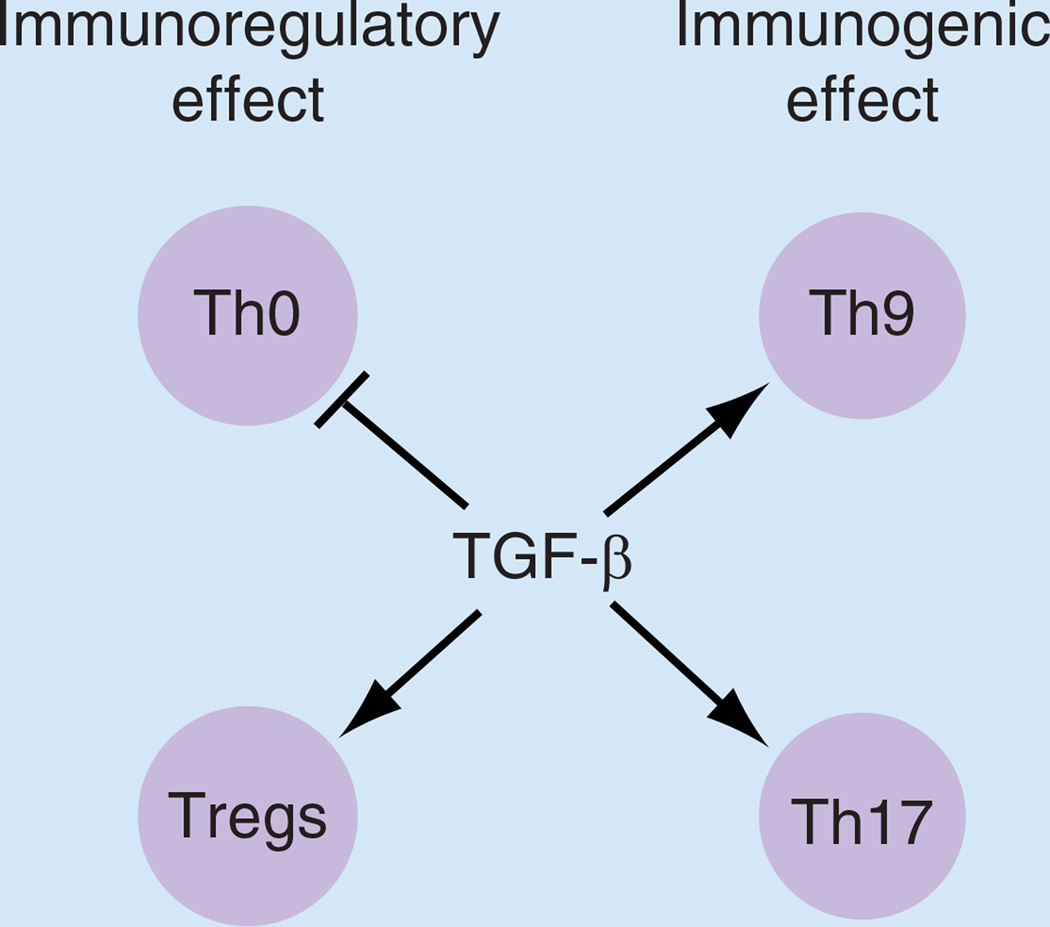
Figure 3. Summary of TGF-β effect on CD4 T cells.
TGF-β has both immunoregulatory and immunogenic effect on CD4 T cells. In the regulatory wing, TGF-β can directly suppress Th0 activation and block cytokine production while promoting the development of inducible Tregs. In the effector wing, TGF-β can promote the differentiation of Th9 and Th17 cells. For transplantation, it is essential to develop the method to enhance the immunoregulatory functions of TGF-β while blocking immunogenic effect. Treg: Regulatory T cell.
These complexities are well depicted by animal models. In experimental allergic encephalomyelitis (EAE), an animal model for multiple sclerosis, systemic administration of TGF-β inhibited the onset of EAE but administration of antibody against TGF-β caused worsening of disease progression [67, 68]. On the other hand, inhibition of TGF-β signaling in T cells prevented Th17 cell generation and promoted resistance to EAE [69]. Local, but not systemic, administration of neutralizing TGF-β1 antibody inhibited Th17 cell generation. Moreover, deletion of the TGFβ1 gene in activated T cells protected mice from EAE and blocked Th17 generation [70].
It is worth noting that, TGF-β is a key factor of inducing fibrosis [71]. Mounting evidence points the pathological role played by TGF-β in pulmonary fibrosis [72]. Overexpression of TGF-β in the lung by adenoviral transduction caused severe fibrosis in a rat model [73]. Transgenic mice that express active TGF-β in airway cells suffered from peribronchial fibrosis with extension to the adjacent lung parenchyma [74]. In a bleomycin-induced lung fibrosis model, TGF-β a nd IL-17 were indicated to operate cooperatively in fibrosis [75].
Recently, regulatory B cells have emerged as another regulatory component of immune responses and have been extensively reviewed by others [76, 77]. One of the known functions provided by regulatory B cells is the production of TGF-β, which helps maintenance and/or generation of Tregs [78, 79]. How TGF-β from B cells affects in vivo T cell responses against transplant is of a critical significance and needs to be analyzed in the future.
Taken together, systemic administration of activators or inhibitors of the TGF-β axis could cause detrimental effect on transplant patients. Instead, manipulation of the axis in a more specific manner with temporal/spatial control will be necessary. For example, to inhibit Th17 development, targeting the molecular processes underlying the crosstalk between TGF-β and other cytokines such as IL-6 would be much more specific and effective. Similarly, to promote PICA and remove graft-reactive T cells, it is necessary to inhibit TGF-β and induce apoptosis of effector T cells. Our data demonstrated that under PICA-inducing conditions, the pro-apoptotic molecules FoxO3a and Bim are suppressed by TGF-β. Hence, inhibition of the specific pathway that connects TGF-β and Bim/FoxO3a will help in maintaining T cells susceptible to PICA and removing graft-reactive effector T cells. It is now necessary to decipher the mechanism by which TGF-β induces these diversified biological processes. After delineation of the signal crosstalk between TGF-β and other cytokines/co-stimulators, pharmacological inhibition/activation of the target will become feasible. For example, TGF-β is known to suppress FoxO3a via interactions with the PI3-K pathway [80]. Would such inhibition also take place in T cells during PICA? Inhibition of the process could render graft-reactive T cells susceptible to PICA. Moreover, TGF-β was shown to induce expression of RORγT but also inhibits its function [64]. What will be effective in maintaining the inhibitory effect of TGF-β on RORγT?
Future perspective
It is thought that TGF-β could act as a potential target for immune modulation of transplant recipients. Yet, because of TGF-β’s multifaced functions, it is essential to determine how each specific biological process is induced by TGF-β signaling. Such information will provide critical molecular interactions that can dictate the cellular responses that lead to immune suppression or enhancement.
Executive summary.
-
▪
The long-term survival rate of some transplanted organs (e.g., lung) is very poor.
-
▪
Current immunosuppressive drugs are not effective enough for the long-term success of transplantation.
-
▪
Regulatory T cells (Tregs) are potent inducers of immunological tolerance and are effective in achieving long-term acceptance of allograft in animal models.
-
▪
Two types of regulatory T cells exist: thymus-derived (natural Tregs) and peripheral-derived (inducible Tregs).
-
▪
TGF-β plays a critical role in the generation of inducible Tregs in the periphery.
-
▪
Under different conditions, TGF-β induces effector T cell (Th9/Th17) differentiation.
-
▪
TGF-β converts the signal that provokes p53-dependent apoptosis into the effector T cell (Th9/Th17) differentiation signals.
-
▪
TGF-β downstream signaling crosstalks with other signaling pathways such as cytokine receptors (IL-6R, IL-4R) and induces distinctive cellular responses.
Acknowledgements
The authors thank J DeMaio for critically reading the manuscript.
This work was supported by the NIH and Van Kampen Caridiopulmonary Research Fund.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
- 1. Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat. Rev. Immunol. 2012;12:417–430. doi: 10.1038/nri3227.. ▪ General review on various regulatory cells and their application in transplantation.
- 2.Taylor AL, Watson CJ, Bradley AJ. Immunosuppressive agents in solid-organ transplantation: mechanisms of action and therapeutic efficacy. Crit. Rev. Oncol. Hematol. 2005;56:23–46. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Meier-Kriesche HU, Schold DJ, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am. J. Transplant. 2004;4:378–383. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 4.Bhorade SM, Stern E. Immunosuppression for lung transplantation. Proc. Am. Thorac. Soc. 2009;6:47–53. doi: 10.1513/pats.200808-096GO. [DOI] [PubMed] [Google Scholar]
- 5.Marcen R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009;69:2227–2243. doi: 10.2165/11319260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Alberu J. Clinical insights for cancer outcomes in renal transplant patients. Transplant. Proc. 2010;42:S36–S40. doi: 10.1016/j.transproceed.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Hojo M, Morimoto T, Maluccio M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 8.Hawinkels LJ, Ten Dijke P. Exploring anti-TGF-beta therapies in cancer and fibrosis. Growth Factors. 2011;29(4):140–152. doi: 10.3109/08977194.2011.595411. [DOI] [PubMed] [Google Scholar]
- 9.Prud’homme GJ, Piccirillo CA. The inhibitory effects of transforming growth factor-beta-1 (TGF-beta1) in autoimmune diseases. J. Autoimmun. 2000;14:23–42. doi: 10.1006/jaut.1999.0339. [DOI] [PubMed] [Google Scholar]
- 10.van de Wetering J, Weimar CH, Balk AH, et al. The impact of transforming growth factor-beta1 gene polymorphism on end-stage renal failure after heart transplantation. Transplantation. 2006;82:1744–1748. doi: 10.1097/01.tp.0000250360.78553.5e. [DOI] [PubMed] [Google Scholar]
- 11.Brunet M. Cytokines as predictive biomarkers of alloreactivity. Clin. Chim. Acta. 2012;413:1354–1358. doi: 10.1016/j.cca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Moraes-Vieira PM, Takenaka MC, Silva HM, et al. GATA3 and a dominant regulatory gene expression profile discriminate operational tolerance in human transplantation. Clin. Immunol. 2012;142:117–126. doi: 10.1016/j.clim.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Krueger T, Berutto C, Aubert DJ. Challenges in lung transplantation. Swiss Med. Wkly. 2011;141:w13292. doi: 10.4414/smw.2011.13292. [DOI] [PubMed] [Google Scholar]
- 14.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J. Heart Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 15.Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J. Heart Transplant. 2002;21:271–281. doi: 10.1016/s1053-2498(01)00360-6. [DOI] [PubMed] [Google Scholar]
- 16.Sandrini A, Glanville AR. The controversial role of surveillance bronchoscopy after lung transplantation. Curr. Opin. Organ Transplant. 2009;14(5):494–498. doi: 10.1097/MOT.0b013e3283300a3b. [DOI] [PubMed] [Google Scholar]
- 17.Dragun D, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N. Engl. J. Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 18.Joosten SA, Sijpkens YW, van Ham V, et al. Antibody response against the glomerular basement membrane protein agrin in patients with transplant glomerulopathy. Am. J. Transplant. 2005;5:383–393. doi: 10.1111/j.1600-6143.2005.00690.x. [DOI] [PubMed] [Google Scholar]
- 19.Rolls HK, Kishimoto K, Dong VM, et al. T-cell response to cardiac myosin persists in the absence of an alloimmune response in recipients with chronic cardiac allograft rejection. Transplantation. 2002;74:1053–1057. doi: 10.1097/00007890-200210150-00028. [DOI] [PubMed] [Google Scholar]
- 20.Duquesnoy RJ, Liu K, Fu XF, et al. Evidence for heat shock protein immunity in a rat cardiac allograft model of chronic rejection. Transplantation. 1999;67:156–164. doi: 10.1097/00007890-199901150-00026. [DOI] [PubMed] [Google Scholar]
- 21. Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010;10:490–500. doi: 10.1038/nri2785.. ▪ Comprehensive review of regulatoy T cells (Tregs) in humans.
- 22.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 23. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164.. ▪ Original article demonstrating the phenotype of naturally arising Tregs.
- 24.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 25.Singh N, Seki Y, Takami M, et al. Enrichment of regulatory CD4(+)CD25(+) T cells by inhibition of phospholipase D signaling. Nat. Methods. 2006;3:629–636. doi: 10.1038/nmeth903. [DOI] [PubMed] [Google Scholar]
- 26.Fehervari Z, Sakaguchi S. Development and function of CD25+CD4+ regulatory T cells. Curr. Opin. Immunol. 2004;16:203–208. doi: 10.1016/j.coi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Fontenot DJ, Rasmussen PJ, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 28. Fontenot DJ, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904.. ▪ Original article that demonstrated the significance of Foxp3 for Tregs.
- 29.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 30.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr. Opin. Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 32.Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J. Biol. Chem. 2001;276:37672–37679. doi: 10.1074/jbc.M104521200. [DOI] [PubMed] [Google Scholar]
- 33.Chatila TA, Blaeser F, Ho N, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 35.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 36.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 37.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat. Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 38.Weiss L, Donkova-Petrini V, Caccavelli L, et al. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249–3256. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 39.Waldmann TA. Effective cancer therapy through immunomodulation. Annu. Rev. Med. 2006;57:65–81. doi: 10.1146/annurev.med.56.082103.104549. [DOI] [PubMed] [Google Scholar]
- 40.Zenclussen AC. CD4(+)CD25+ T regulatory cells in murine pregnancy. J. Reprod. Immunol. 2005;65:101–110. doi: 10.1016/j.jri.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Saito S, Sasaki Y, Sakai M. CD4(+)CD25 high regulatory T cells in human pregnancy. J. Reprod. Immunol. 2005;65:111–120. doi: 10.1016/j.jri.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Lan RY, Ansari AA, Lian XZ, Gershwin ME. Regulatory T cells: development, function and role in autoimmunity. Autoimmun. Rev. 2005;4:351–363. doi: 10.1016/j.autrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Cobbold SP, Adams E, Graca L, et al. Immune privilege induced by regulatory T cells in transplantation tolerance. Immunol. Rev. 2006;213:239–255. doi: 10.1111/j.1600-065X.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 44.DiPaolo RJ, Brinster C, Davidson TS, et al. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J. Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 45. Coombes LJ, Siddiqui KR, Arancibia-Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590.. ▪ Article demonstrating retinoic acid as a co-factor for inducible Treg generation.
- 46. Hippen KL, Riley LJ, June CH, Blazar BR. Clinical perspectives for regulatory T cells in transplantation tolerance. Semin. Immunol. 2011;23:462–468. doi: 10.1016/j.smim.2011.07.008.. ▪ Comprehensive review on the role of Tregs and transplantation.
- 47.Tran DQ. TGF-beta: the sword, the wand, and the shield of FOXP3(+) regulatory T cells. J. Mol. Cell Biol. 2012;4:29–37. doi: 10.1093/jmcb/mjr033. [DOI] [PubMed] [Google Scholar]
- 48.Han G, Li F, Singh TP, Wolf P, Wang XJ. The pro-inflammatory role of TGFbeta1: a paradox? Int. J. Biol. Sci. 2012;8:228–235. doi: 10.7150/ijbs.8.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu S, Cao X. Interleukin-17 and its expanding biological functions. Cell. Mol. Immunol. 2010;7:164–174. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghoreschi K, Laurence A, Yang XP, Hirahara K, O’Shea JJ. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 2011;32:395–401. doi: 10.1016/j.it.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakagiri T, Inoue M, Minami M, Shintani Y, Okumura M. Immunology mini-review: the basics of T(H)17 and interleukin-6 in transplantation. Transplant. Proc. 2012;44:1035–1040. doi: 10.1016/j.transproceed.2011.12.032.. ▪ Current review on the role of TH17 in trasnplantation rejection.
- 52.Stassen M, Schmitt E, Bopp T. From interleukin-9 to T helper 9 cells. Ann. NY Acad. Sci. 2012;1247:56–68. doi: 10.1111/j.1749-6632.2011.06351.x. [DOI] [PubMed] [Google Scholar]
- 53.Eller K, et al. IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J. Immunol. 2011;186:83–91. doi: 10.4049/jimmunol.1001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoyle GW, Brody AR. IL-9 and lung fibrosis: a Th 2 good guy? Am. J. Respir. Cell Mol. Biol. 2001;24:365–367. doi: 10.1165/ajrcmb.24.4.f205. [DOI] [PubMed] [Google Scholar]
- 55.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol. Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 56.Singh N, Yamamoto M, Takami M, et al. CD4(+)CD25(+) regulatory T cells resist a novel form of CD28- and Fas-dependent p53-induced T cell apoptosis. J. Immunol. 2010;184:94–104. doi: 10.4049/jimmunol.0900753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011.. ▪ Role of TGF-β in generation of inducible Tregs.
- 58. Takami M, Love RB, Iwashima M. TGF-beta converts apoptotic stimuli into the signal for Th 9 differentiation. J. Immunol. 2012;188:4369–4375. doi: 10.4049/jimmunol.1102698.. ▪ Effect of TGF-β on effector T cell apoptosis and differentiation.
- 59.Annes PJ, Munger SJ, Rifkin DB. Making sense of latent TGFbeta activation. J. Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 60.Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc. Natl Acad. Sci. USA. 2009;106:13445–13450. doi: 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Webster NL, Crowe SM. Matrix metalloproteinases, their production by monocytes and macrophages and their potential role in HIV-related diseases. J. Leukoc. Biol. 2006;80:1052–1066. doi: 10.1189/jlb.0306152. [DOI] [PubMed] [Google Scholar]
- 62.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 63.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat. Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 64. Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008;9:641–649. doi: 10.1038/ni.1610.. ▪ Role of TGF-β in Th17 differentiation.
- 65.Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J. Autoimmun. 2008;31:252–256. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 67.Racke MK, Cannella B, Albert P, Sporn M, Raine CS, McFarlin DE. Evidence of endogenous regulatory function of transforming growth factor-beta1 in experimental allergic encephalomyelitis. Int. Immunol. 1992;4:615–620. doi: 10.1093/intimm/4.5.615. [DOI] [PubMed] [Google Scholar]
- 68.Racke MK, Layward L, Morris-Downes MM, Dumonde DC, Amor S. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-beta1. J. Immunol. 1991;146:3012–3017. [PubMed] [Google Scholar]
- 69.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat. Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 70.Gutcher I, Donkor MK, Ma Q, Rudensky AY, Flavell RA, Li MO. Autocrine transforming growth factor-beta1 promotes in vivo Th17 cell differentiation. Immunity. 2011;34:396–408. doi: 10.1016/j.immuni.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee CG, Cho SJ, Kang MJ, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J. Exp. Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilson MS, Madala SK, Ramalingam TR, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J. Exp. Med. 2010;207:535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klinker MW, Lundy SK. Multiple mechanisms of immune suppression by B lymphocytes. Mol. Med. 2012;18:123–137. doi: 10.2119/molmed.2011.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat. Rev. Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gros MJ, Naquet P, Guinamard RR. Cell intrinsic TGF-beta1 regulation of B cells. J. Immunol. 2008;180:8153–8158. doi: 10.4049/jimmunol.180.12.8153. [DOI] [PubMed] [Google Scholar]
- 79.Shah S, Qiao L. Resting B cells expand a CD4+CD25+Foxp3+ Treg population via TGF-beta 3. Eur. J. Immunol. 2008;38:2488–2498. doi: 10.1002/eji.200838201. [DOI] [PubMed] [Google Scholar]
- 80.Naka K, Hoshii T, Muraguchi T, et al. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–680. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]