Abstract
In this study, we examined the effects of a short mindfulness meditation induction (MMI) on the performance of a P300-based brain-computer interface (BCI) task. We expected that MMI would harness present moment attentional resources, resulting in two positive consequences for P300-based BCI use. Specifically, we believed MMI would facilitate increases in task accuracy and promote the production of robust P300 amplitudes. Sixteen-channel electroencephalographic data were recorded from 18 subjects using a row/column speller task paradigm. Nine subjects participated in a 6-min MMI and an additional nine subjects served as a control group. Subjects were presented with a 6×6 matrix of alphanumeric characters on a computer monitor. Stimuli were flashed at an SOA of 125ms. Calibration data were collected on 21 items without providing feedback. These data were used to derive a stepwise linear discriminate analysis classifier that was applied to an additional 14 items to evaluate accuracy. Offline performance analyses revealed that MMI subjects were significantly more accurate than control subjects. Likewise, MMI subjects produced significantly larger P300 amplitudes than control subjects at Cz and PO7. Discussion focuses on the potential attentional benefits of MMI for P300-based BCI performance.
Keywords: Mindfulness, Brain-Computer Interface, EEG, P300, Event-Related Potential
1. Introduction
Brain-Computer Interfaces (BCIs) are communication and control devices that allow users to convey intent and execute computer commands via variations in brain signals [1]. Users are thereby capable of communicating by non-traditional means (e.g., spoken words). Many BCIs utilize non-invasively recorded brain signals from a subject’s electroencephalogram (EEG, [2]), and implement classification of various brain signals into external outputs. As noted by Farwell and Donchin [2], one means of recording EEG non-invasively targets the P300 component of the ERP. The P300 is a positive deflection in the EEG that peaks approximately 300 milliseconds after the presentation of a specific target stimulus that is presented with a low probability. The P300 manifests a distinct amplitude, post-stimulus latency, and scalp distribution of time-locked and averaged responses; typically, the response is largest at posterior scalp locations (electrode Pz), and decreases at more anterior electrode locations along the midline (e.g., electrode Cz and Fz) e.g., [3–6]. Farwell and Donchin used the P300 to create the first P300-based spelling program, with the idea of providing a viable communication option for people with severe neuromuscular disabilities. A number of studies now report that people with amyotrophic lateral sclerosis (ALS), who have lost all muscle control (i.e., they have become “locked-in”), can communicate via P300-based BCI with minimal system training because of response’s robust nature [7–10]. However, P300-based BCIs are not without their problems. In fact, users need to be at least 70% accurate for successful BCI use, yet some individuals cannot meet this threshold [11, 12]. Accordingly, recent studies have targeted optimizing electrode montages [13], stimulus properties [10, 14], signal processing methods [15], and presentation paradigms [16–21] in efforts to optimize users’ speller performance. Nonetheless, attentional errors, including general lapses of attention, mind wandering, and lack of focused attention can all undermine performance in non-BCI tasks [22]. Similarly, task-demands and distractions reduce P300 amplitudes [23–26] and may contribute to BCI errors [16, 21, 27]. Thus, inducing a state that would heighten attentional resources and reduce distractibility may improve BCI performance. In this research, we examined the possibility that inducing mindfulness (via meditative mindfulness induction or MMI) may be a means of increasing attentional focus and continuity, and thereby improving P300-based BCI performance.
Mindfulness is an attentional construct that entails purposeful and focused attention to what is happening in the present moment. Mindfulness is both a state of consciousness as well as a dispositional trait that varies in the degree to which people are purposefully cognizant of their immediate experience [28]. In both forms, mindfulness can be brought to bear in a therapeutic or meditative context; indeed, a basic tenet of meditative practice is that situationally inducing heightened state levels of mindfulness facilitate increases in short-term attentional resources at the state level as well as enduring increases in trait mindfulness [29, 30]. Several lines of converging behavioral and neurological evidence point to mechanisms that may explain these increases. PET scan and fMRI data, for example, demonstrate that MMI activates neural systems such as the dorsolateral prefrontal cortex, fronto-parietal pathways, cingulate cortex, and striatum, which combine to facilitate orienting of attention and concentration, organization, behavioral regulation and inhibition, and other executive functioning and control processes [31–34]. Relative to controls, those who have undergone MMI training are better able to detect subtle visual differences in the immediate environment (e.g., flashes of light) due to enhanced processing of visual stimuli [35]. MMI training can even reduce the duration of the attentional blink, which occurs when subjects have trouble detecting a stimulus 200–500ms after a prior stimulus [36]. When conscious attention is directed toward a target, those with MMI are more vigilant because they process information more efficiently than those without MMI [31, 37–39]. Interference on Stroop tasks is also reduced through MMI training [40]. Moreover, EEG-based studies attest to differences in spatiotemporal characteristics of EEG spectra for those with MMI relative to controls, [36, 41, 42] especially in the theta and alpha bandwidths [35, 41, 43–45]. After minimal MMI training, these differences also include significantly higher P300 amplitude [36, 37].
In light of this evidence, which strongly suggests that increases in MMI improve several aspects of attention, including cue sensitivity [33–36], distraction inhibition [40], and vigilance [31–33, 37–39], we hypothesized that MMI would provide two important, albeit somewhat interrelated, benefits for P300-based BCI users. Specifically, because of their attunement to target items, which decreases the likelihood of distraction by non-target items, we believed that those undergoing MMI would be more accurate than controls. We also believed that individuals undergoing MMI would produce significantly higher amplitude P300 responses, which would provide a proxy measure of attentional focus.
2. Methods
2.1. Subjects
Eighteen able-bodied adults (11 female, 7 male; age range 18–33) were recruited from the East Tennessee State University undergraduate psychology subject pool. All were naïve to BCI use and all reported no prior experience with meditative practice. All subjects had normal or corrected-to-normal vision and no known cognitive deficits. The study was approved by the East Tennessee State University Institutional Review Board.
2.2. Data Acquisition
EEG was recorded with a 16-channel tin electrode cap (Electro-Cap International, Inc.). All channels were referenced to the right mastoid and grounded to the left mastoid. Impedance was reduced to below 10.0 kΩ before recording. A g.tec (Guger Technologies) 16-channel USB biosignal amplifier was used to record EEG data, which were digitized at 256 Hz, and bandpass filtered from 0.5 Hz to 30 Hz. Only electrodes Fz, Cz, P3, Pz, P4, PO7, PO8, and Oz [46] were used for BCI operation [15]. BCI2000 [47] controlled stimulus presentation, data collection, and online processing.
2.3. Procedure
Following provision of informed consent, subjects were seated in a comfortable chair approximately 1.5 meters from a computer monitor. The monitor displayed the stimulus presentation of a 6×6 matrix comprised of alphanumeric characters. Nine subjects underwent a meditative mindfulness induction (MMI), while an additional 9 subjects comprised a non-MMI control group (STD). All subjects were instructed to focus their attention to a specific item in the matrix and mentally note each time this item flashed. Stimuli were flashed in the form of an entire row or column (12 flashes, 6 row flashes and 6 column flashes comprised a sequence) at an SOA of 125ms (62.5ms inter-stimulus interval and 62.5ms stimulus duration), and each item was flashed 26 times (13 sequences containing two flashes of each item). We collected data on 21 items without providing the subject feedback. These data were used to derive a stepwise linear discriminate analysis (SWLDA) classification algorithm. Subjects then completed an additional 14 items for which they received online performance feedback. Thirteen sequences of flashes were also used in the online portion of the study.
We employed a mindfulness induction technique that has been successfully used by a number of researchers to increase mindfulness, thereby harnessing state-level attentional resources [33, 48–51]. Over a period of 6-min, the induction introduces attentional control via concentrative meditation techniques. The goal was to promote concentrative attention, such that conscious attunement to an immediate situation, task, or target occurs without attentional disruptions that are due to external or internal distractions. After hearing the task instructions, MMI subjects were asked to close their eyes, take a few deep breaths, and turn their attention to their thoughts, though they were instructed to just “observe them and let them go.” The experimenter guided subjects through a series of attentional alterations, moving from subjects’ thoughts, to the breath, to the abdomen, to bodily sensations that occur with each breath, and ultimately to the computer screen and the target. During this time, the experimenter reiterated instructions about the task, including directions to note mentally each time the target flashed, told subjects to close their eyes and turn their attention back to their breath during the 15-sec break between trial blocks and to focus their full attention to the computer screen and the target when they reopened their eyes. The experimenter mentioned that it was “okay” if they found their minds wandering; they should just note the thought and return their full attention to the target. At the end of this induction, the experimenter told MMI subjects the word and first target letter, and instructed them to open their eyes, find the target in the matrix, pay full attention to the letter, and note each time it flashes.
After hearing instructions, STD subjects in the control condition were told to relax, find a comfortable position, and take a few moments to breathe. During this time of relaxation, the experimenter reiterated the task instructions, including directions to mentally note each time the target flashed. Subjects in the control group were told to just relax during the 15-sec break between trial blocks, and to focus on the next letter when it began again. They were told that if they found their minds wandering, they should try harder to focus on the task. Once STD subjects indicated their readiness to begin the task, the experimenter told them the word and first target letter, and instructed them to concentrate on the target and note each time it flashes.
2.4. Dependent Measures
Accuracy was analyzed offline and performance was estimated after each of the 13 sequences of flashes for each target. Performance was analyzed between experimental conditions (MMI and STD). Waveform morphology differences were analyzed by examining peak amplitude and latency between 100–400ms for the positive peak, and between 400–650ms for the negative peak at electrodes Cz, Pz, PO7, and PO8. A power spectrum density (PSD) examined between-group differences using the mean values for three electrode clusters (Frontal [F3, Fz, P4], Central [C3, Cz, C4], Posterior [P3, Pz, P4]) and four spectra bandwidths (delta [2–4Hz], theta [4–7Hz], alpha [8–12Hz], beta [13–24Hz]). Prior to statistical analyses, volume conducted blink correction was performed using an independent component analysis (ICA) to extract independent signals from the EEG signals. Fast Fourier transform was applied to the independent components and the features were extracted. One-second epochs, with 50% overlap (1.004Hz resolution, range 128Hz, 256 points), were removed from the continuous recording of the experimental tasks. Thereafter, data were high-pass filtered (at 1Hz, 24dB) and artifacts were rejected on a +/− 100µV criteria. All epochs were baseline corrected and cosign tapered over the entire epoch, and a natural log was applied to the grand mean bands.
3. Results
3.1. Offline Accuracy
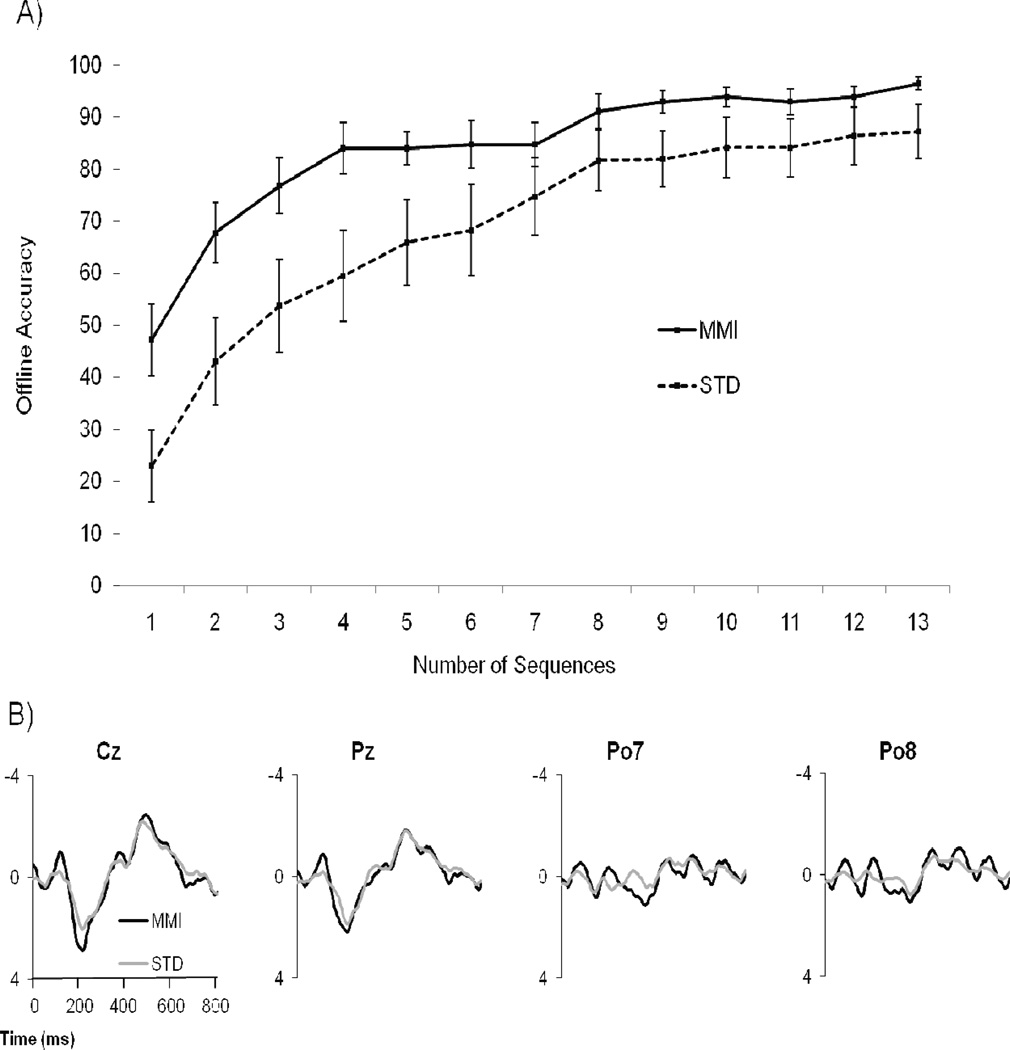
We analyzed accuracy using a 2 (Attentional Condition, MMI & STD) X 13 (Number of Sequences, 1–13) repeated measures mixed factorial ANOVA, with Greenhouse-Geisser corrections for within-subjects effects. Statistically significant main effects emerged for both Attentional Condition and Number of Sequences. See Figure 1 (A). Overall, MMI subjects were significantly more accurate than controls (F(1,16)=5.47, p=.03, η2=.26) and accuracy increased as subjects were presented more stimuli (F(4.2,67.5)=65.71, p<.01, η2=.80).
Fig 1.
A) Offline accuracy was analyzed by taking the grand average of all subjects compared between groups for every sequence from 1–13. Each stimulus sequence represents two item flashes. B) Grand mean waveforms for all 18 participants at electrode locations Cz, Pz, PO7, and PO8. The top row compares target responses for both conditions (n = 9 in each condition).
Most importantly, we also found a statistically significant interaction (F(4.2,67.5)=2.74, p<.03, η2=.15, 1–β =.50). Compared to the STD condition, MMI accuracy improved rapidly. Indeed, MMI subjects were only slightly below (67.8%) the minimum accuracy threshold for BCI use (70%) in the second sequence, and they surpassed this threshold in the third sequence (76.8%) [e.g., 12]; STD subjects did not surpass this minimum performance standard until the seventh sequence (74.6%). For the sake of completeness, we conducted t-tests at each sequence, and found that MMI subjects evidenced significantly greater accuracy (all ps < .05) than their control group counterparts in all but three sequences (7, 8, & 12).
At first glance of Figure 1(A), it appears that each group met a relative plateau around the eighth sequence and that they performed relatively congruently over the remaining sequences. However, the difference in average accuracy across this plateau (from sequence 8 to sequence 13) was 9.4%; MMI subjects averaged 93.6% accuracy while STD averaged 84.2%. A visual inspection of Figure 1(A) also suggests that MMI subjects’ responses were less variable (i.e., more reliable) than those in the control, especially among later sequences. To examine this possibility, we conducted a t-test of the standard deviations for each group across all sequences; MMI average responses were significantly more consistent (p < .001) than those of control subjects. Collectively, these results support the contention that MMI’s attentional benefits are not just immediate (though these benefits are particularly strong); rather, MMI also seems to provide enduring performance benefits for P300-based BCI use.
3.2. Waveform Morphologies
In Figure 1 (B), individual and grand mean waveforms indicate that MMI subjects produced significantly higher positive amplitude peaks at Cz (M=3.37 µV; SD=1.17) than the STD subjects (M=2.39µV; SD=1.04, t(16)=2.20,p<.04, d=1.10, 1-β =.59). Also, MMI subjects produced significantly higher positive amplitude peaks at PO7 (M=1.89µV; SD=1.55) than the STD subjects (M=.08µV; SD=.67, t(16)=2.10, p<.05, d=1.05, 1-β =.55). These results provide a proxy measure of task devoted concentration. No differences in non-target amplitudes were observed on the electrodes of interest.
3.3 Power Spectrum Density Analysis
A 2 (Attentional Condition, MMI & STD) X 3 (Electrode Cluster Location, Frontal, Central, & Posterior) X 4 (Spectral Bandwidth, Delta, Theta, Alpha, & Beta) repeated measures mixed factorial ANOVA was used to analyze PSD. No differences in power were observed between the two experimental conditions.
4. Discussion
4.1. General Discussion
BCIs utilize non-invasively recorded brain signals, such as the P300, from subjects’ EEGs and convert these into external outputs. BCI technology allows many with severe neuromuscular disabilities to communicate [7–10]; however, the technology is still in a nascent stage. Because these systems require sustained focused attention, problems such as general attentional lapses and mind wandering can undermine user performance. With this in mind, we examined if increasing attentional resources via a short MMI could improve P300 speller performance. A number of important findings emerged. Subjects who underwent MMI performed significantly better than their control group counterparts. Specifically, MMI subjects’ accuracy increased relatively immediately, they were consistently more accurate overall, and their responses evidenced significantly less variability across the course of trial sequences. Differences also emerged in the waveform morphologies of the two groups. Relative to controls, MMI subjects produced significantly higher amplitude P300 responses at electrode locations Cz and Po7. We expand on the importance of these findings below.
4.2. Implications of MMI for BCI Use
Significantly higher accuracy in the MMI condition supports the contention that increasing user mindfulness provides increases to concentrative resources. These increases seemed to facilitate immediate attunement to the BCI task at hand, and helped to overcome distractions that prevent optimal task performance. The reliability of MMI subjects’ responses also speaks to its potential to mitigate the effects of task-specific or external distractions. Previous neurophysiological research likewise demonstrates that MMI serves to increase attentional focus, inhibit mind wandering, and decrease the extent to which irrelevant stimuli lead to distraction [e.g., 35, 40]. These attentional benefits may be attributable to a number of different neurological systems, including those directly involved in attention regulation [e.g., 31, 33] as well as those that may indirectly influence attention. For example, one of the cortical benefits of MMI is that of effective amygdala modulation in the face of negative stimuli (52,53). Given its role in limbic system emotional processing, modulating the amygdala presumably underlies why MMI relates to decreases in cortisol, a sympathetic nervous system marker of the stress response, and improvements to vagal tone, a marker of parasympathetic nervous system activation and relaxation [54, 55]. To the extent that relaxation promotes attentional focus, MMI may indirectly affect attention via alterations in emotion-relevant, biological stress responses.1
Perhaps most importantly, we found evidence that MMI subjects surpassed minimal accuracy thresholds more quickly than controls and sustained higher accuracy over the course of the task. This finding may be of particular relevance to BCI users who have been diagnosed with diseases like ALS. Indeed, over an extended period, users could incur significant timesavings by minimizing the number of flashes necessary for accurate BCI use. Moreover, the significant differences in waveform morphology (i.e., increased P300 amplitude for MMI subjects) not only cohere with previous research and theory [e.g., 36], but they also suggest that MMI training could improve disabled users’ likelihood of successful BCI performance even with minimal training. Stated differently, these findings offer further support that state-level mindfulness, and the concentrative focus it entails, can be induced with relatively minimal effort to increase performance on attention-relevant tasks, and they suggest that MMI may be a means to allow BCI use for those who might otherwise be unable to do so.
More broadly, these findings highlight the fact that BCI researchers must be cognizant of factors that may promote user focus on, or divert user attention from, BCI tasks (whether in the laboratory or in a real-world setting). For instance, it is possible that forceful appeals for subjects to pay attention may actually undermine user performance. That is, even if these appeals cause subjects to concentrate (which might create short-term benefits), they will also likely increase subjects’ negative emotional states, which would thereby diminish long-term BCI performance even further. Our results suggest that a more adaptive approach might be to incorporate a brief MMI into research protocol, which may bolster subjects’ performance by increasing their concentration and task engagement and by preventing or attenuating general lapses of attention.
4.3. Limitations and Future Directions
Though we found that MMI improved P300-based BCI performance, there are a number of limitations to our conclusions and important avenues for future research. For example, the spectral analysis results did not replicate those reported in the mindfulness literature [35, 41, 43–45]; however, this study utilized a much shorter (6-min) mindfulness induction technique than typically used and the P300 speller paradigm employs a task that is quite different from studies that conduct spectral analyses. Also, because we employed a between-groups design and used a MMI that entailed a single, short induction, we cannot draw conclusions about any long-term attentional benefits beyond the time necessary for completion of the BCI speller task we employed. Accordingly, it will be useful to conduct long-term (longitudinal), within-group studies with larger samples to examine the enduring benefits MMI training might afford for BCI use. We are limited in drawing firm conclusions about the benefits for BCI use among individuals with severe neuromuscular difficulties, who may differ in response to MMI relative to the able-bodied participants used in this study. Thus, future longitudinal research should aim to include clinical populations as subjects, hopefully within a randomized control trial (RCT) framework. Relative to the current study, a RCT would afford greater experimenter control and power to detect significant performance differences. In concert with other evidence of MMI’s enduring attentional benefits [31, 37–39], as well as evidence that long-term MMI training increases [56] or at least staves off the degradation of gray matter [37], we suspect that the effects of longitudinal MMI for BCI use will be even more robust than the effects found in short-term MMI, especially among those with clinical issues like ALS whose attentional capabilities wane with disease progression. Finally, even though the row/column paradigm currently represents the gold standard of P300 speller presentation, recent advances in other presentation paradigms surpass the row/column paradigm in usability [e.g., 21]. Thus, it will be important to pair MMI training with these other presentation paradigms to examine benefits for user performance. Collectively, these next steps will allow researchers to understand and maximize fully the benefits of MMI for BCI use.
Acknowledgements
This work has been supported by: NIH/NIBIB & NINDS (EB00856; EWS); NIH/NIDCD (R21 DC010470-01; EWS); NIH/NIDCD (R15 DC011002-01; CEL & EWS)
We thank Gerald Frye, David Ryan, James Bailey, and Mallory McGhee for their data collection efforts, Dr. Kirk Warren Brown for advice with the mindfulness induction, and Dr. Nathan Gates and the anonymous reviewers for insightful comments on a previous version of this manuscript.
Footnotes
We do not mean to suggest that being relaxed and being mindful are synonymous states (or that the experience of stress occurs only in the absence of mindfulness). Consider, for instance, the moments immediately prior to falling asleep; the body is physically relaxed but not mentally aware of and attentive to the present moment. Mindfulness, on the other hand, seems to entail a state of mental acuity coupled with physical relaxation. This distinction aligns with previous research and theory [e.g., 32, 49] as well as the present results. Accordingly, we are not asserting that physical relaxation would fully explain MMI’s attentional benefits; only that relaxation due to limbic system modulation may be an indirect means of attentional improvement.
References
- 1.Wolpaw JR, Birbaumer N. Brain Communication Interfaces for Communication and Control. 2006:602–614. [Google Scholar]
- 2.Farwell LA, Donchin E. Talking off the top of your head: toward a mental prosthesis utilizing event-related brain potentials. Electroencephalogr Clin Neurophysiol. 1988;70(6):510–523. doi: 10.1016/0013-4694(88)90149-6. [DOI] [PubMed] [Google Scholar]
- 3.Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150(700):1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- 4.Pritchard WS. Psychophysiology of P300. Psychol Bull. 1981;89(3):506–540. [PubMed] [Google Scholar]
- 5.Donchin E. Presidential address, 1980. Surprise!…Surprise? Psychophysiology. 1981;18(5):493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- 6.Fabiani M, Gratton G, Karis D, Donchin E. Definition, Identification, and Reliability of Measurement of the P300 Component of the Event Related Potential. Advances in Psychophysiology. 1987;2:1–78. [Google Scholar]
- 7.Kubler A, et al. Patients with ALS can use sensorimotor rhythms to operate a brain-computer interface. Neurology. 2005;64(10):1775–1777. doi: 10.1212/01.WNL.0000158616.43002.6D. [DOI] [PubMed] [Google Scholar]
- 8.Sellers EW, Vaughan TM, Wolpaw JR. A brain-computer interface for long-term independent home use. Amyotroph Lateral Scler. doi: 10.3109/17482961003777470. (in press). [DOI] [PubMed] [Google Scholar]
- 9.Nijboer F, et al. A P300-based brain-computer interface for people with amyotrophic lateral sclerosis. Clin Neurophysiol. 2008;119(8):1909–1916. doi: 10.1016/j.clinph.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sellers EW, Krusienski DJ, McFarland DJ, Vaughan TM, Wolpaw JR. A P300 event-related potential brain-computer interface (BCI): the effects of matrix size and inter stimulus interval on performance. Biol Psychol. 2006;73(3):242–252. doi: 10.1016/j.biopsycho.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Kubler A, et al. A brain-computer interface controlled auditory event-related potential (p300) spelling system for locked-in patients. Ann N Y Acad Sci. 2009;1157:90–100. doi: 10.1111/j.1749-6632.2008.04122.x. [DOI] [PubMed] [Google Scholar]
- 12.Sellers EW, Donchin E. A P300-based brain-computer interface: initial tests by ALS patients. Clin Neurophysiol. 2006;117(3):538–548. doi: 10.1016/j.clinph.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Krusienski DJ, et al. A comparison of classification techniques for the P300 Speller. J Neural Eng. 2006;3(4):299–305. doi: 10.1088/1741-2560/3/4/007. [DOI] [PubMed] [Google Scholar]
- 14.Serby H, Yom-Tov E, Inbar GF. An improved P300-based brain-computer interface. IEEE Trans Neural Syst Rehabil Eng. 2005;13(1):89–98. doi: 10.1109/TNSRE.2004.841878. [DOI] [PubMed] [Google Scholar]
- 15.Krusienski DJ, Sellers EW, McFarland DJ, Vaughan TM, Wolpaw JR. Toward enhanced P300 speller performance. J Neurosci Methods. 2008;167(1):15–21. doi: 10.1016/j.jneumeth.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martens SM, Hill NJ, Farquhar J, Scholkopf B. Overlap and refractory effects in a brain-computer interface speller based on the visual P300 event-related potential. J Neural Eng. 2009;6(2):026003. doi: 10.1088/1741-2560/6/2/026003. [DOI] [PubMed] [Google Scholar]
- 17.Hong B, Guo F, Liu T, Gao X, Gao S. N200-speller using motion-onset visual response. Clin Neurophysiol. 2009 doi: 10.1016/j.clinph.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 18.Takano K, Komatsu T, Hata N, Nakajima Y, Kansaku K. Visual stimuli for the P300 brain-computer interface: A comparison of white/gray and green/blue flicker matrices. Clin Neurophysiol. 2009 doi: 10.1016/j.clinph.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Salvaris M, Sepulveda F. Visual modifications on the P300 speller BCI paradigm. J Neural Eng. 2009;6(4):046011. doi: 10.1088/1741-2560/6/4/046011. [DOI] [PubMed] [Google Scholar]
- 20.Guger C, et al. How many people are able to control a P300-based brain-computer interface (BCI)? Neurosci Lett. 2009;462(1):94–98. doi: 10.1016/j.neulet.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 21.Townsend G, et al. A novel P300-based brain-computer interface stimulus presentation paradigm: Moving beyond rows and columns. Clin Neurophysiol. 2010 doi: 10.1016/j.clinph.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smallwood J, Schooler JW. The restless mind. Psychol Bull. 2006;132(6):946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- 23.Wickens C, Kramer A, Vanasse L, Donchin E. Performance of concurrent tasks: a psychophysiological analysis of the reciprocity of information-processing resources. Science. 1983;221(4615):1080–1082. doi: 10.1126/science.6879207. [DOI] [PubMed] [Google Scholar]
- 24.Kramer AF, Wickens CD, Donchin E. An analysis of the processing requirements of a complex perceptual-motor task. Hum Factors. 1983;25(6):597–621. doi: 10.1177/001872088302500601. [DOI] [PubMed] [Google Scholar]
- 25.Kramer AF, Wickens CD, Donchin E. Processing of stimulus properties: evidence for dual-task integrality. J Exp Psychol Hum Percept Perform. 1985;11(4):393–408. doi: 10.1037//0096-1523.11.4.393. [DOI] [PubMed] [Google Scholar]
- 26.Wickens CD, Kramer AF, Donchin E. The event-related potential as an index of the processing demands of a complex target acquisition task. Ann N Y Acad Sci. 1984;425:295–299. doi: 10.1111/j.1749-6632.1984.tb23550.x. [DOI] [PubMed] [Google Scholar]
- 27.Fazel-Rezai R. Human error in P300 speller paradigm for brain-computer interface. Conference Preceedings IEEE Engineering in Medicine and Biology Society. 2007:2516–2519. doi: 10.1109/IEMBS.2007.4352840. [DOI] [PubMed] [Google Scholar]
- 28.Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology. 2003 Apr;84(4):24. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- 29.Kabat-Zinn J. Full catastrophe living. New York: Dell; 1990. [Google Scholar]
- 30.Kabat-Zinn J. Mindfulness-based interventions in context: Past, present, and future. Clinical Psychology: Science and Practice. 2003 May;10(2):12. [Google Scholar]
- 31.Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci U S A. 2007;104(27):11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazar SW, et al. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11(7):1581–1585. [PubMed] [Google Scholar]
- 33.Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cogn Affect Behav Neurosci. 2007;7(2):109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- 34.Farb NA, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci. 2007;2(4):313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan N, Baijal S. Concentrative meditation enhances preattentive processing: a mismatch negativity study. Neuroreport. 2007;18(16):1709–1712. doi: 10.1097/WNR.0b013e3282f0d2d8. [DOI] [PubMed] [Google Scholar]
- 36.Slagter HA, et al. Mental training affects distribution of limited brain resources. PLoS Biol. 2007;5(6):e138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pagnoni G, Cekic M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiol Aging. 2007;28(10):1623–1627. doi: 10.1016/j.neurobiolaging.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Linden W. Practicing of meditation by school children and their levels of field dependence-independence, test anxiety, and reading achievement. J Consult Clin Psychol. 1973;41(1):139–143. doi: 10.1037/h0035638. [DOI] [PubMed] [Google Scholar]
- 39.Rani NJ, Rao PV. Meditation and attention regulation. Journal of Indian Psychology. 1996 Jan-Jul;14(1–2):4. [Google Scholar]
- 40.Chan D, Woollacott M. Effects of level of meditation experience on attentional focus: is the efficiency of executive or orientation networks improved? J Altern Complement Med. 2007;13(6):651–657. doi: 10.1089/acm.2007.7022. [DOI] [PubMed] [Google Scholar]
- 41.Dunn BR, Hartigan JA, Mikulas WL. Concentration and mindfulness meditations: unique forms of consciousness? Appl Psychophysiol Biofeedback. 1999;24(3):147–165. doi: 10.1023/a:1023498629385. [DOI] [PubMed] [Google Scholar]
- 42.Liao HC, Lo PC. Investigation on spatiotemporal characteristics of Zen-meditation EEG rhytms. Journal of International Society of Life Information Science. 2007;25(1):63–71. [Google Scholar]
- 43.Dietl T, Dirlich G, Vogl L, Lechner C, Strian F. Orienting response and frontal midline theta activity: a somatosensory spectral perturbation study. Clin Neurophysiol. 1999;110(7):1204–1209. doi: 10.1016/s1388-2457(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 44.Sarang SP, Telles S. Changes in p300 following two yoga-based relaxation techniques. Int J Neurosci. 2006;116(12):1419–1430. doi: 10.1080/00207450500514193. [DOI] [PubMed] [Google Scholar]
- 45.Murata T, et al. Individual trait anxiety levels characterizing the properties of zen meditation. Neuropsychobiology. 2004;50(2):189–194. doi: 10.1159/000079113. [DOI] [PubMed] [Google Scholar]
- 46.Sharbrough FW. Advances in epilepsy surgery offer patients new hope. Minn Med. 1991;74(10):9–12. [PubMed] [Google Scholar]
- 47.Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004;51(6):1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 48.Heppner WL, Kernis MH, Lakey CE, Campbell WK, Goldman BM, Davis PJ, Cascio EV. Mindfulness as a means of reducing aggressive behvaior: Dispositional and situational evidence. Aggressive Behavior. 2008;34(5):486–496. doi: 10.1002/ab.20258. [DOI] [PubMed] [Google Scholar]
- 49.Jain S, et al. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Ann Behav Med. 2007;33(1):11–21. doi: 10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- 50.McHugh L, Simpson A, Reed P. Mindfulness as a potential intervention for stimulus over-selectivity in adults. Research in Developmental Disabilites. 2010;31(1):178–184. doi: 10.1016/j.ridd.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Arch JJ, Craske MG. Mechanisms of mindfulness: Emotion regulation following a focused breathing induction. Behaviour Research and Therapy. 2006;44(12):1849–1858. doi: 10.1016/j.brat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosom Med. 2007;69:560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- 53.Way BM, Creswell JD, Eisenberger NI. Dispositional mindfulness and depressive symptomatology: Correlations with limbic and self-referential neural activity during rest. Emotion. 2010;10:12–24. doi: 10.1037/a0018312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli F, Urbanowski F, Harrington A, Bonnus K, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosom. Med. 2003;65:564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- 55.Ditto B, Eclache M, Goldman N. Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Ann Behv Med. 2006;32(3):227–234. doi: 10.1207/s15324796abm3203_9. [DOI] [PubMed] [Google Scholar]
- 56.Holzel BK, et al. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc Cogn Affect Neurosci. 2008;3(1):55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]