Abstract
Puberty and reproduction require proper signaling of the hypothalamic-pituitary-gonadal axis controlled by gonadotropin-releasing hormone (GnRH) neurons, which arise in the olfactory placode region and migrate along olfactory axons to the hypothalamus. Factors adversely affecting GnRH neuron specification, migration, and function lead to delayed puberty and infertility. Nasal embryonic luteinizing hormone-releasing factor (NELF) is a predominantly nuclear protein. NELF mutations have been demonstrated in patients with hypogonadotropic hypogonadism, but biallelic mutations are rare and heterozygous NELF mutations typically co-exist with mutations in another gene. Our previous studies in immortalized GnRH neurons supported a role for NELF in GnRH neuron migration. To better understand the physiology of NELF, a homozygous Nelf knockout (KO) mouse model was generated. Our findings indicate that female Nelf KO mice have delayed vaginal opening but no delay in time to first estrus, decreased uterine weight, and reduced GnRH neuron number. In contrast, male mice were normal at puberty. Both sexes of mice had impaired fertility manifested as reduced mean litter size. These data support that NELF has important reproductive functions. The milder than expected phenotype of KO mice also recapitulates the human phenotype since heterozygous NELF mutations usually require an additional mutation in a second gene to result in hypogonadotropic hypogonadism.
Keywords: NELFnasal embryonic, LHRH factor, NSMFGnRH neuron, migration, infertility
Introduction
Reproductive dysfunction may be manifested as delayed puberty or infertility due to impairment of the hypothalamic-pituitary gonadal (HPG) axis. The HPG axis is regulated by gonadotropin releasing hormone (GnRH). GnRH neurons originate from the olfactory placode/vomeronasal organ and migrate into the hypothalamus where they send projections to the median eminence and release pulsatile GnRH into the hypophyseal portal bloodstream [1,2]. GnRH then interacts with its membrane-bound receptor on pituitary gonadotropes to induce the synthesis and secretion of follicle stimulating hormone (FSH) and luteinizing hormone (LH), which then stimulate the gonads to produce sex steroids and gametes. Sex hormones mediate growth and secondary sex characteristics, which then enables reproduction.
When there is impairment of GnRH neuron specification, migration, secretion, or release of GnRH, hypogonadotropic hypogonadism or Kallmann syndrome (KS) results [3]. Hypogonadotropic hypogonadism is typically an irreversible disorder with low serum sex steroid hormone and gonadotropin levels leading to delayed puberty and infertility [3]. Other etiologies such as pituitary tumors and pituitary dysfunction are absent [4,5]. If olfaction is unaffected, normosmic hypogonadotropic hypogonadism (nHH) is present, but when olfactory impairment accompanies GnRH deficiency, KS results. In addition to infertility, nHH and KS patients present with other nonreproductive anomalies such as renal agenesis, midfacial defects, skeletal anomalies, and a variety of neurological defects [5]. The molecular basis for nHH/KS is now known for approximately 40% of all diagnosed patients [6]. Identified nHH/KS genes with mutations include KAL1, NR0B1, GNRHR, FGFR1, KISS1R, TACR3, TAC3, FGF8, CHD7, PROKR2, PROK2, GNRH1, NELF, WDR11, PCSK1, LEP, LEPR, HS6ST1 and SEMA3A [6–8]. Recently, additional genes—SOX10 [9], FGF17, IL17RD, DUSP6, SPRY4, FLRT3 [10], and FEZF1 [11] have been identified, and digenic disease has been described [12,13]. The inheritance of nHH/KS varies depending upon the gene that is involved [13,14].
Nasal embryonic LHRH factor (NELF, MIM 608137), also known as NMDA receptor synaptonuclear signaling and neuronal migration factor (NSMF), is an nHH/KS gene that was differentially isolated from migratory GnRH neurons and is diffusely expressed in the brain, but most highly in the cortex [15,16]. The NELF gene encodes a predominantly nuclear protein that has putative zinc fingers, suggesting that it is a transcription factor [17]. Nelf knockdown has been shown to impair GnRH neuron migration and distribution in the hypothalamus, but the mechanism is not well understood [16,17]. Jacob, the rat ortholog of NELF, is a highly homologous nuclear protein that binds N-methyl-D-aspartate (NMDA) receptors [18]. NELF mutations have been identified in human KS [12,19], but the mechanisms of NELF’ control of puberty are unknown. To date studies regarding NELF have been done in immortalized GnRH neuronal cell lines [17] and zebra fish [20] as no knockout mouse model was available.
Here we characterize the germline Nelf knockout (KO) mouse. Pubertal development is delayed in female, but not male, Nelf KO mice. Female KO mice had reduced litter frequency, while both sexes had decreased mean litter sizes. In female KO mice, uterine weight was reduced, as was GnRH neuron number, suggesting that NELF actions occur at the hypothalamus.
Results
Construction, Generation and Verification of Nelf KO mouse: Nelf+/+ (WT), Nelf+/− (HET) and Nelf−/− (KO)
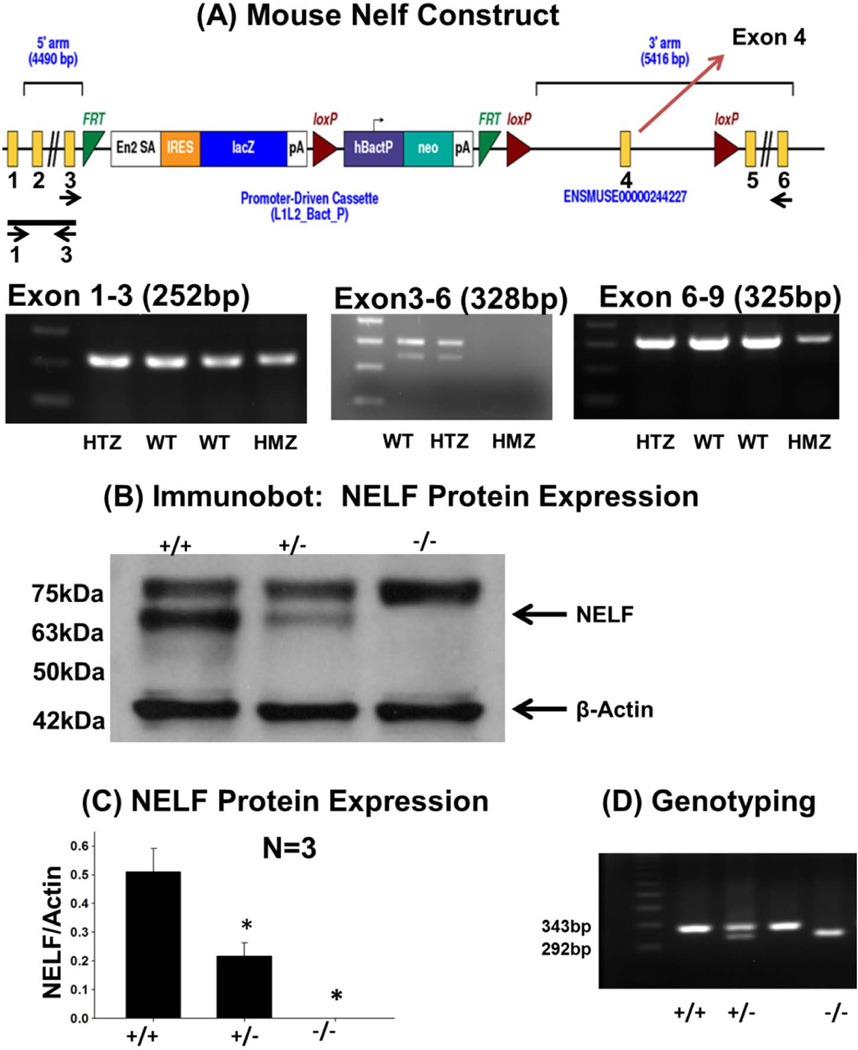
The construct was designed using the knockout first approach [21,22] to target exon 4, which is common to all reported Nelf variants (Figure 1). The germline knockout is accomplished without gene deletion by inserting RNA processing signals (splice acceptor site and polyA signal) into an intron (intron 3, in this case) of the construct, which will interfere with gene transcription downstream of the cassette.[22] Heterozygous mice were provided by the Wellcome Trust, Sanger Institute (UK). Confirmation of KO was demonstrated by RT-PCR and western blot analysis (Figure 1). Protein quantification of the NELF-specific 63kDa showed measurable levels except in the Nelf KO mouse (WT=0.51±0.08, HET=0.22±0.05, KO=0; p=0.002).
Figure 1. Generation and confirmation of the Nelf KO Mouse.
(A) The Nelf 5’ region with the knockout first vector is shown. For germline knockout, this knockout first vector has a Flp-recombinase target (FRT)-flanked selection cassette inserted into the third intron of Nelf, which will trap the transcript through the Engrailed-2 (En2) splice acceptor (En2 SA) element and truncate it through the SV40 polyadenylation signal (pA). LacZ, encoding β-galactosidase, is the reporter gene, which enables tracing of expression. The Neomycin resistance gene (Neo) was used as a marker for ES clone screening which was driven by an autonomous (hBactP) promoter. IRES =internal ribosome entry site. For conditional knockout, the cassette also contains loxP sites flanking exon 4. Primers for RT-PCR of whole brain RNA (Table S2) flanked exons 1–3 (252bp), exons 3–6(328bp) and exons 6–9(325bp). Each exon is labelled (only exons 1–6 are shown); and primer location for RT-PCR is shown below the exons. The PCR product for exons 1–3 is indicated as a line with arrows underneath showing primer location. For the exon 3–6 fragment, primer location is shown by arrows under the corresponding exon (exons 6–9 are not shown). Homozygous KO mice demonstrated the exon 1–3 amplicon, but had decreased intensity of the other two amplicons. (B) Western analysis of whole brain lysate protein from showed the expected 63kDa NELF band which is absent in the Nelf knockout mouse. (C) Quantification of NELF protein in the mouse model of Nelf and β-actin bands as normalized to β-actin, N=3 experiments; and p<0.05. (D) Genotyping of WT, HET, and KO mice was performed using tail DNA.
Female Nelf KO mice have delayed puberty
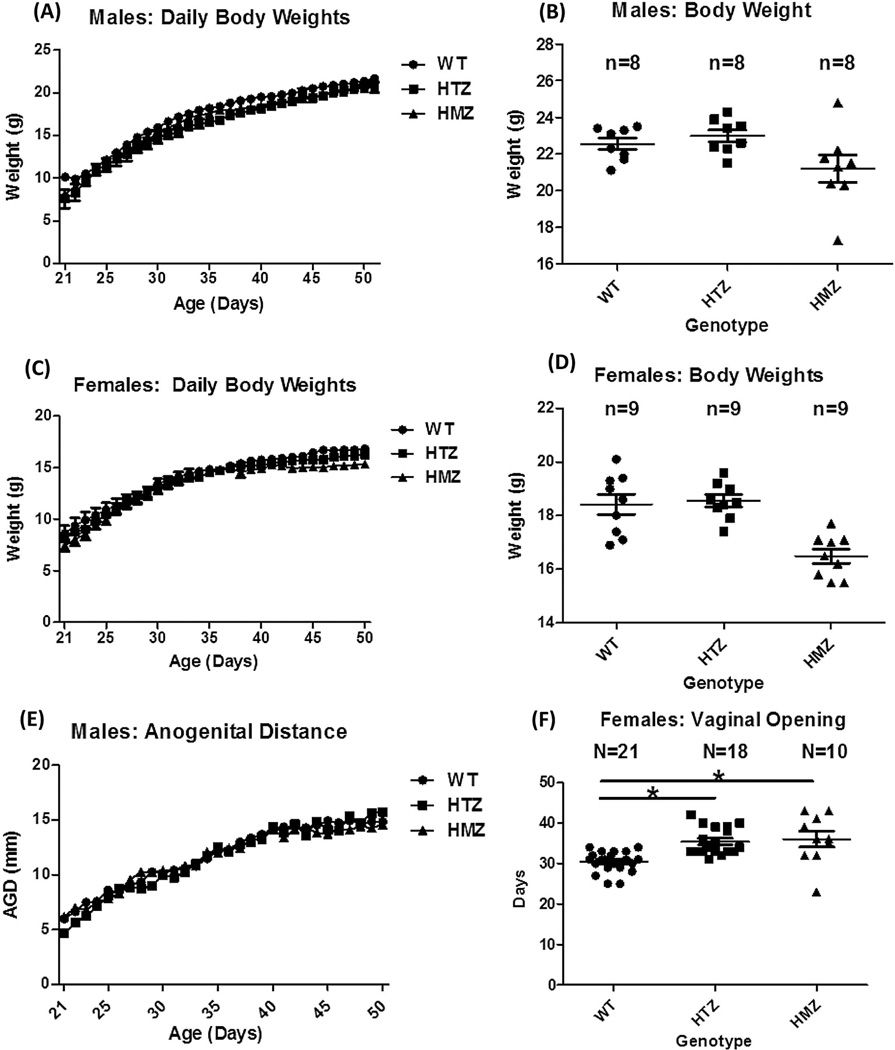
Following weaning, mice were weighed daily from P21-P50, but there were no differences between WT and Nelf KO mice for either sex during this time or at 8 weeks (Figure 2A–D). Anogenital distance in males and vaginal opening in females (Figure 2D,E) are dependent upon testosterone and estradiol, respectively, and reflect activation of the HPG axis. Anogenital distance (Figure 2E) was not different among genotypes. However, vaginal opening in females was significantly delayed by ~4 days in both Nelf HET and Nelf KO vs. WT mice (WT=30.43±0.56 [n=21], HET= 34.2±0.66 [n=18] and KO=34.6±1.6 [n=10]; WT vs. HET and KO p=0.001 (Figure 2F).
Figure 2. Pubertal assessment in Nelf KO mice.
Shown are: (A–D) Body weight and (E) anogenital distance in males and (F) vaginal opening in females. For Figures 2B and 2D, the mice were 8 weeks old. *Vaginal opening in HET and KO mice was delayed by ~4 days vs. WT (ANOVA with Tukey MCP test; P<0.05.) AUC = area under the curve.
Reproductive organs & Sex Steroids
In males, testis and seminal vesicle weights were not different among genotypes (Figure S1A–B). Postpubertal testes of male Nelf KO mice were morphologically normal compared to WT (Figure S1C–E). In the largest testes cross section, testicular length, width, total seminiferous tubules/largest testes cross section, and randomly sampled seminiferous tubule diameters were not different (Figure S1E–I).
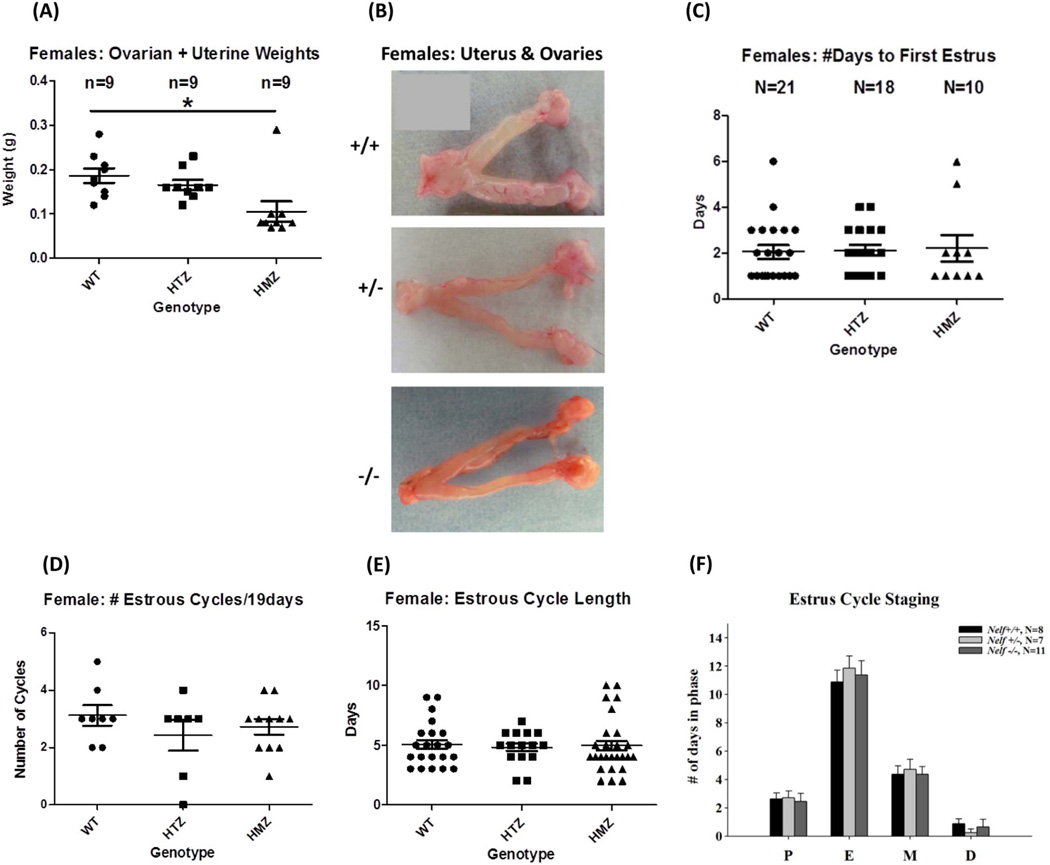
Female Nelf KO mice had significantly reduced uterine weight when measured during proestrus at 8 weeks. Combined uterine/ovarian weight for Nelf KO mice was decreased vs. WT and HET mice (n=8/group, WT=0.19 ± .017, HET=0.17±.011, KO=0.11±.023 g, WT vs KO; p=0.02) (Figure 3A,B). However, ovarian morphology and follicle content did not differ in WT vs. KO female mice. Width, length and ovarian cross sectional area were not different in KO vs. WT mice (Figure S2). Upon histologic analysis, the number of different types of follicles, as classified by Barnett et al [23] and Pedersen et al [24]—primary, secondary, antral, preovulatory, or corpora lutea— were not different for any genotype (Figure S2). One KO female had an ovarian cyst and another demonstrated hyperplasia of the corpus luteum. In summary, all types of follicles, including corpora lutea were seen in Nelf KO and WT mice.
Figure 3. Estrus cycling and fertility in female Nelf KO mice.
(A) Uterine plus ovarian weights were decreased in KO female mice compared to WT (ANOVA with Tukey MCP, P<0.05) when measured during proestrus at 8 weeks of age. (B) Gross structure of the ovaries and uteri is shown. (C) The time to first estrus, (D) the number of estrus cycles/19 days, (E) estrus cycle length, and (F) time spent in each of the four estrous stages were not different in the three genotypes.
Late morning serum samples (1100–1230) were extracted from 8-week postpubertal mice (males: n=8, females at proestrus: n =9) for sex steroids. There were no differences in estradiol in females and testosterone in males for WT vs. KO animals (Figure S3).
Both Sexes of Nelf KO mice manifest subfertility
To analyze ovarian function, several different parameters were evaluated. Estrus cycle length, the number of estrus cycles/19 days, and time spent in each of the four estrus phases were quantitated for WT, HET, and KO groups (Figure 3C–E). Estrus cycle stages were classified according to Caligioni et al [25]. There was no difference in the time to first estrus, estrus cycle length, or the number of cycles/19 day study period for any of the three genotypes. Similarly, there was no difference in the time spent in each phase: proestrus, estrus, metestrus, or diestrus (Figure 3C–F).
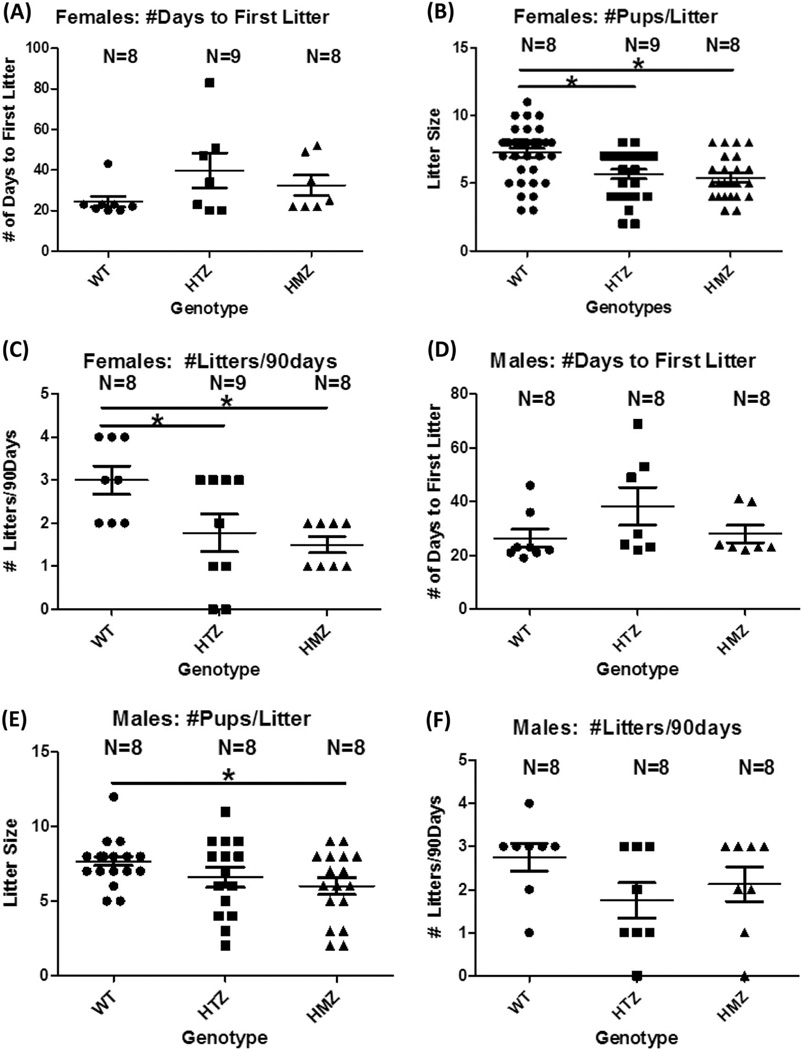
Once mice were 8 weeks of age, individual males of each genotype (WT, HTZ, or KO) were mated with a WT female. Females of each genotype were similarly mated with WT males. Fertility was assessed over a 90-day period per genotype by quantifying the mean litter sizes, the number of litters/90 days, and the number of days before first litter was recorded (Figure 4). As shown in Figure 4A–C, when WT (n=8), HET (n=9), and KO (n=8) females were mated to WT males, time to first litter was not different. However, HET and KO females had significantly smaller mean litter sizes than WT females (WT=7.4±0.41, HET=5.3±0.47 and KO=5.4±0.49, WT vs. HET; p=0.02, WT vs. KO; P =0.01). Similarly, the number of litters/90 days was significantly reduced for HET and KO females compared to WT females (WT=3.0±0.33, HET=1.7±0.41 and KO=1.5±0.12, WT vs. HET; p=0.04, WT vs KO; p=0.02).
Figure 4. Fertility in males (A–C) and females (D–F); GnRH neurons in females (G).
In females, (A) time to first litter was not different (Kruskal-Wallis); (B) mean litter size was significantly reduced in both female HET and KO compared with WT animals (ANOVA with Tukey MCP, P<0.05); (C) number of litters/90 days was reduced in both HET and KO females vs. WT (ANOVA with Tukey MCP, P <0.05). In males, (D) time to first litter was not different (Kruskal-Wallis); (E) mean litter size was significantly reduced in male KO vs. WT animals (ANOVA; Tukey with MCP, P<0.05); and (F) number of litters/90 days was not different in males (ANOVA).
When WT, HET, or KO males were mated with WT females, mean litter size was significantly decreased only when KO males were mated with WT females. Mean litter sizes were WT=7.7±0.30, HET=6.6±0.67, and KO=6.0±0.57; WT vs. KO; P =0.04 (Figure 4D–F). However, there was no significant difference in mean number of litters/90 days or time to first litter. Fertility was affected in both sexes, but was more evident in females.
Nelf KO females, but not males, have decreased GnRH neuron number
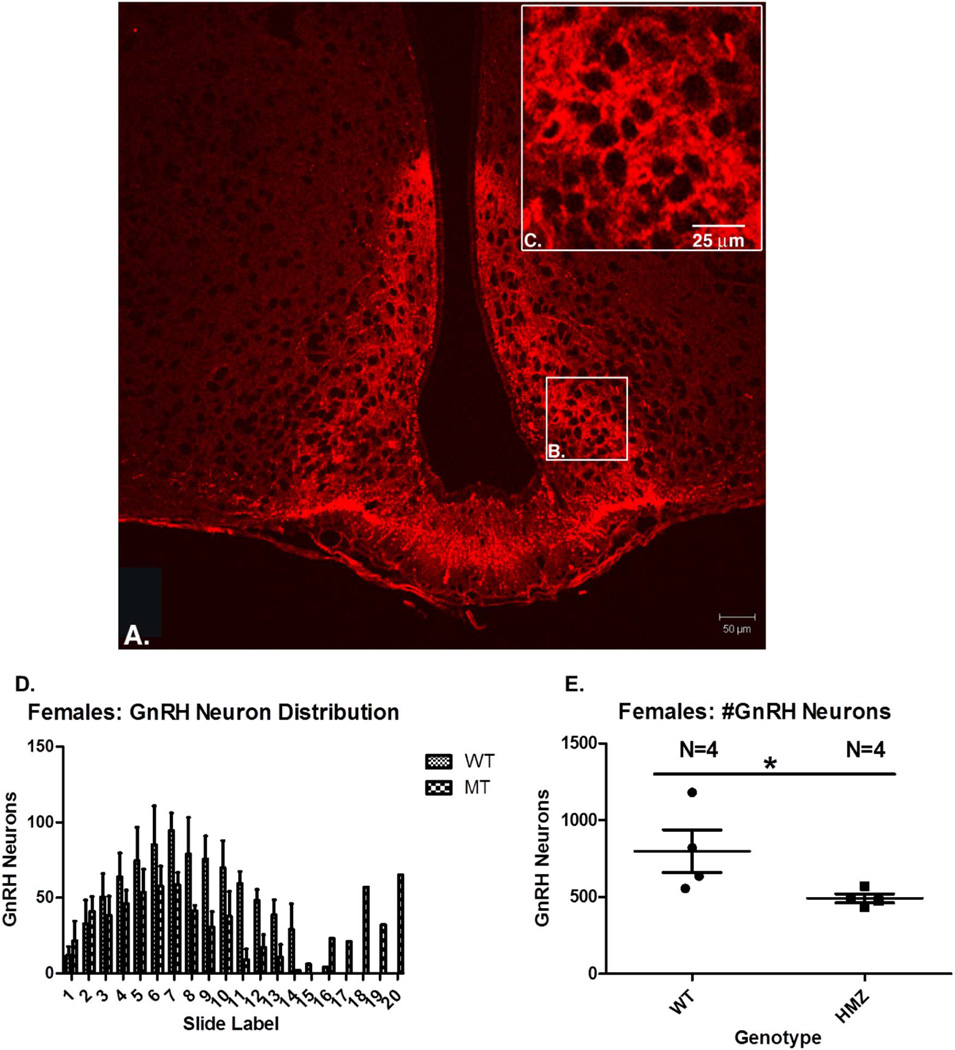
There were no differences in brain weight or brain morphology, including olfactory bulbs, for any genotype of males or females when examined at 8 weeks of age (Figure S4). In addition, brain weight of all groups at 4–6 months of age was not different (Figure S4). Brain coronal sections stained with GnRH were used to count GnRH neurons in the arcuate nucleus and the entire brain (Figure 5A–C). In females, GnRH number was significantly decreased in NELF KO animals: WT =797.8+/−277.9 vs. 491.5+/−57.77 in KO p =0.03 (Figure 5D–E). However, there was no difference in GnRH neuron number in males or GnRH neuron distribution distance in either sex (Figure S5).
Figure 5. GnRH neuron staining in females.
(A) Section of forebrain—GnRH neuron staining in red shows both arcuate nuclei laterally separated by the third ventricle, and the median eminence inferiorly. (B) GnRH staining is shown in the arcuate nucleus, which is shown at higher magnification in (C). (D) Distribution of GnRH neurons in 4WT and 4KO female mice (note one outlier). (E) GnRH neuron number is reduced in KO females vs. WT females (Mann Whitney U; 1 tailed; P=0.03).
Discussion
Understanding the molecular basis of hypogonadotropic hypogonadism has been advanced through studies of knockout and naturally occurring or induced mutant mice. Of the identified genes involved in IHH/KS patients, characterized mouse models include Gnrh1 [26], Gnrhr [27,28], Lepr,[29] Gpr54 (Kiss1r) [30,31], Kiss1[32], Fgfr1 [33,34], Fgf8 [34,35], Chd7 [36–38], Tacr3 [39], Prok2 [40], Prokr2 [41], and Sema3a [42]. These corresponding mouse models manifested variable severity of nHH/KS phenotypes. Although NELF has been studied in WT mice and immortalized GnRH neuronal cells, the Nelf KO mouse has not been described.
Study of the Nelf KO mouse model is important since NELF mutations have been identified in IHH and KS patients. In reported cases, one patient presented with compound heterozygous NELF point mutations [17]; and three others had digenic mutations, including a heterozygous NELF mutation in combination with either a heterozygous FGFR1 [12] or TACR3 [19] mutation or a hemizygous KAL1[19] mutation. Interestingly, all four of these patients were male. Additional phenotypic features in these individuals with NELF mutations included midfacial defects, Duane syndrome (an eye movement disorder), clinodactyly, unilateral renal agenesis, and bilateral cryptorchidism [12,19]. How these mutations actually result in KS with associated anomalies is unknown. It is interesting that most heterozygous human NELF mutations are digenic suggesting that unless they are biallelic, NELF mutations are not sufficient to result in hypogonadism.
Current evidence suggests that NELF is involved in transcription as it is localized in the nucleus and contains zinc finger domains [17]. However, the zinc finger domains are atypical, and there are no sequences to suggest a DNA binding motif.[17] The highly homologous (98%) rat ortholog of Nelf (Jacob protein) shows stimulus dependent nuclear translocation, and by chromatin fractionation has been localized within regions of RNA polymerase 2-containing euchromatin. Moreover, protein complexes immunopurified from euchromatin contain significant levels of DNA implying NELF association, indirectly or directly, with DNA[18,43,44]. Taken together, these findings strongly implicate a role for NELF in transcription, either as a transcription factor or in protein-protein interactions regulating transcription. NELF knockdown in immortalized mouse GnRH neurons impairs migration in vitro [17], while knockdown in zebrafish interferes with normal GnRH neuron migration and misdirects the pathway of GnRH neuron migration [20]. Therefore, understanding the effects of NELF upon GnRH neuron specification, migration, release, and secretion during puberty development and fertility is essential.
In the current study, we describe the generation of germline, homozygous Nelf KO mice. Confirmation of Nelf knockout was accomplished using RT-PCR and western blot analysis demonstrating a marked reduction of Nelf transcript and absent NELF protein. We subsequently characterized the effect of NELF upon pubertal development, gonadal histology, hormone production, fertility, and GnRH neuron number in these mice. Both sexes of Nelf KO animals were viable and healthy, with normal weight gain postnatally. Somewhat surprisingly, the effects of Nelf KO were less severe than would have been predicted, and they appeared to be sex-specific. Female Nelf KO mice presented with delayed puberty, as evidenced by delayed vaginal opening suggesting that NELF is important for the normal timing of puberty in female mice. In contrast, males did not manifest delayed puberty. These milder phenotypes are reminiscent of humans with NELF mutations and perhaps even those 30% of patients with normal puberty and infertility. In addition, mouse KO of Tacr3, a known nHH/KS gene, also causes a much milder than expected phenotype of subfertility affecting females more than males.[39]
Despite pubertal delay in female Nelf KO mice, as adults these mice exhibited normal estrus cycles, as assessed by the time to first estrus, the number of estrus cycles/19 days, mean length of the estrus cycle, as well as the amount of time in any stage of the cycle. However, fertility was impaired in female Nelf KO mice as evidenced by decreased mean litter size when either HET or KO females were mated with WT males. Additionally, the number of litters/90 days was reduced in HET and KO females. Therefore, the subfertility also affected HET females in addition to KO females. Males had somewhat less of an effect upon fertility, as KO (but not HET) males mated with WT females had a significantly reduced mean litter size. There was no difference in the number of litters/90 days for either HET or KO males. In both sexes, there was no difference in the time to achieving first litter.
Reproductive organ weight and histology were analyzed in both male and female KO animals at 8 weeks of age. Uterine plus ovarian weight was decreased by ~40% in KO females vs. WT females. However, the dimensions of the ovary and the number of ovarian follicles at different stages were not different in KO and WT females, indicating that the difference in reproductive weight is due to decreased uterine size in KO females. For males, testicular size and the number and size of seminiferous tubules in cross section were not different for KO mice compared to WT males.
Serum testosterone in adult males and estradiol in adult females did not differ in WT, HET, or KO animals suggesting that adult animals are not hypogonadal. Unfortunately, serum gonadotropins were not obtained, but with sex steroids being normal, their importance is somewhat diminished. However, female mice demonstrated delayed puberty. These findings suggest that NELF’s effect may be more important in females at puberty and that the derangement in Nelf KO occurs early, impairing pubertal onset, but the HPG axis later recovers. This impaired fertility is very comparable to that seen in humans with infertility since most patients are eugonadal, but may have more subtle derangements in the HPG axis [6]. In addition, other investigators who reported KO mice with a phenotype of pubertal delay and subfertility similarly showed a normal hormonal profile in KO mice when they reach adult age, despite having a significant difference at puberty [45]. Hormonal assays were only performed in adulthood at 8 weeks, so it is unknown if these mice were hypogonadal and hypogonadotropic at puberty. A unique feature observed in Nelf KO mice was the presence of corpora lutea hyperplasia in one mouse and an ovarian cyst in another. These findings are similar to that observed in Fgfr1 knockout females suggesting that premature ovarian senescence might contribute to their subfertility [34].
The reason for subfertility in Nelf KO mice is uncertain, but we hypothesized that GnRH neuron migration would be impaired. Nelf knockdown in zebrafish leads to misrouting of GnRH neurons so that less neurons reached the proper location for reproductive function [20]. It is also possible that the rate of GnRH neuron migration could be slower with Nelf knockdown, but with time, a minimal threshold of GnRH neurons is reached and the mice achieve proper activation of the HPG axis, leading to normal estrus cycling and hormonal levels. There is evidence that males require only about 12% and females need 12–34% of the GnRH neuron population for normal activation of the HPG axis [46].
Because of NELF’s role in GnRH neuron migration, GnRH neuronal staining was performed in brains in KO animals vs. WT animals at 4–6 months of age [47]. Our findings demonstrated impaired GnRH neuronal migration only in female Nelf KO mice. The number of GnRH neurons in the brain of female mice was significantly reduced by nearly 40%, but was not reduced in males. A similar 30% reduction in GnRH neurons was identified in HET Fgfr1 KO mice suggesting that our findings likely have biological importance [33]. In contrast, Prok2, Prokr2, and Sema3a knockout mice demonstrate much more profound effects upon the nasal placode/olfactory organ so that GnRH neuron migration is halted [41,42]. In our studies, GnRH neuron number was reduced in females, but no ectopic GnRH neurons were identified. This is in contrast to previous Nelf knockdown studies in zebrafish, in which GnRH neuronal migration was impaired or misrouted to a different anatomical region of the brain [20].
In summary, Nelf KO in mice leads to significantly delayed pubertal development in females, but not in males. Females had decreased uterine weight and reduced GnRH neuron number in the forebrain, while males had normal testicular indices and no reduction in GnRH number. However, sufficient GnRH neuronal function in both sexes is present so that endocrine function is nearly normal by adulthood. Nevertheless, subfertility is present in both sexes, including HET females, which is very similar to the majority of humans with infertility (excluding anatomic defects in females). The Nelf KO mouse phenotype resembles that of the Tacr3 KO mouse in that: 1) the phenotype of Tacr3 KO is less severe than expected given that mutations of the human ortholog cause absent puberty due to hypogonadotropic hypogonadism; 2) Tacr3 KO females have delayed puberty and males do not (although at 60 days, males had smaller testes); and 3) Tacr3 KO females have subfertility.[39] Humans with biallelic TACR3 mutations typically have nHH inherited in an autosomal recessive fashion, with a propensity for spontaneous reversal of their hypogonadism.[39] Nearly all NELF mutations are heterozygous [12,13,19], but are accompanied by heterozygous mutations in a second gene, which may be necessary to manifest the full nHH/KS phenotype. These findings suggest either autosomal recessive disease (biallelic) or a heterozygous NELF mutation requires a second hit to result in hypogonadism in humans.[19]
Methods
Animals
All animal procedures were performed in accordance with a protocol approved by the Georgia Regents University Laboratory Animal Services and Committee Regulations. Four (two male; 2 female) HET Nelf mice were generated and kindly provided by the Wellcome Sanger Trust Institute, Cambridge UK. All mice were on a C57BL/6 background, and were housed under a 12-hour light, 12-hour dark cycle in temperature-controlled conditions with ad libitum chow and water. Heterozygous mice were used for generation of different genotypes.
Genotyping Mice
Following weaning, a piece of tail was cut and incubated in lysis buffer with Proteinase K at 50°C overnight. DNA was extracted the following day using a standard phenol/chloroform protocol. Each mouse was genotyped by PCR using Platinum Taq DNA Polymerase (Invitrogen, Carlsbad, CA). Primer sequence and PCR conditions were obtained from Wellcome Trust Sanger Institute (Table S1). PCR products were run on a 2% agarose gel along with a molecular weight marker for genotype determination.
Confirmation of the Nelf Knockout Mouse
RT-PCR
Total RNA was extracted from mouse brains with TriReagent (Molecular Research Center, Cincinnati, OH) and subjected to Superscript III One-Step RT-PCR with Platinum Taq (Invitrogen) using specific primers with appropriate negative controls. HET Nelf KO mice were generated by the Wellcome Sanger Trust Institute (Cambridge, UK) using the “Knockout First” construct [21,22], in which exon 4 (common to all NELF variants), was targeted with flanking LoxP sites. Deletion of exon 4 creates a frameshift mutation that likely affects transcript stability and alters protein translation [21,22].
To confirm knockout, three primer sets were designed for RT-PCR using RNA extracted from whole mouse brain (Table S2). Primer set 1 amplifies a product from exons 1–3; primer set 2 from exons 3–6; and primer set 3 from exons 6–9. PCR products were run on 1.2% agarose gels to determine amplicon size. For all experiments, unselected mice were used—none were excluded. 8-week old mice were used for experiments unless otherwise states.
Western Blotting
Mice were decapitated following anesthesia and whole brains were removed for protein lysate extraction. Brains were homogenized in cold 250mM sucrose and 1mM MgCl2 supplemented with protease peptide inhibitors (0.4mM PMSF and 10 µg/µl each of pepstatin A and leupeptin) as previously described [48]. Following homogenization the concentration was quantitated using the BCA protein assy. Ten micrograms of protein/well were separated on 10%SDS-polyacrylamide gels at 80V for 1.5 hours and transferred to nitrocellulose membranes. Membranes were blocked using 5% milk and incubated with primary anti-NELF (2ug/ml) and anti-actin (Santa Cruz biotechnology, CA) antibodies, as we published previously [17]. Blotted membranes were washed with TBST, incubated with secondary peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA) at 0.8ug/mL and detected by ECL Detection Reagents (Amersham Biosciences, United Kingdom [17]. Size and amount of protein was determined by densitometry using NIH Image J software.
Vaginal opening, anogenital distance, weight, and estrus cycle phase
Mice were weaned between P21–P23 and genotyped. Following weaning all male and female mice were monitored daily by observers blinded to genotype for anogenital distance and vaginal opening status, respectively. Body weight was recorded daily for both sexes, and mean body weight at 8 weeks was also compared for each genotype. The day of vaginal opening was recorded in females. Vaginal opening and anogenital distance are external parameters used to determine pubertal onset as they are dependent upon the activation of the HPG axis by gonadal estrogen and testosterone [47].
To determine estrous cyclicity, vaginal smears were taken daily from 4–6 month-old females for 17–28 consecutive days. Briefly, vaginal lavage was performed daily between 1100–1200 by flushing the vagina with 0.9% saline. Vaginal fluid was mounted on glass slides for cell type, cycle stage (diestrus, proestrus, estrus, and metestrus), and cycle length [25]. Cells were scored by two blinded observers.
Fertility assessment
At ~8 weeks of age, WT, HET, or homozygous Nelf KO mice were housed singly with a similar-aged C57BL6 WT mouse. The fertility study was a continuous breeding over a 90-day period whereby one male was housed with one female/cage. During this time, mice were checked frequently for litter presence and litter size. The time to first litter, number and frequency of litters were recorded [49,50].
Serum collection and Hormone assays
Intraperitoneal Ketamine/Xylazine was used for anesthesia prior to blood drawing, which was collected from 8-week male and female mice. All females used for hormone analysis were in the proestrus phase of the cycle. Mice were anesthetized with isofluorane (Abbott laboratories, Abbott Park, IL) and trunk blood was collected between 1200–1300 after sacrifice. Following coagulation overnight at 4°C, blood samples were centrifuged at 3000 rpm for 10 minutes and the serum was transferred to a fresh tube and stored at −80°C. All serum was sent together to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (Charlottesville, VA) for estradiol (females) and testosterone (males) by immunoassay.
Weight of Brain, Gonads, and Accessory Organs
Following anesthesia with isofluorane, brains, uteri and ovaries, testes and seminal vesicles were weighed for each genotype. Since ovaries were small and variable in weight, ovarian and uterine weights were taken together.
Gonadal Histology
Eight week-old mice from each genotype were anesthetized with isofluorane and perfused with 30mL of 4% paraformaldehyde. Brains and gonads were removed and fixed in 4% paraformaldehyde overnight before washing in PBS and storing in 70% ethanol. After a series of dehydration steps, the organs were embedded in paraffin and sectioned at 10-micron thickness, followed by staining with hematoxylin and eosin (Histopathology core, GRU, Augusta, GA). For imaging, the largest cross section imaged for each ovary was quantified for primary, secondary, antral, and preovulatory follicles, as well as corpora lutea. The length, width, and area of the ovaries were quantified. The number and diameter of seminiferous tubules, length, width, and area of the testes were quantified. All imaging was done using a Zeiss Axioplan 2 Microscope with AxioCam camera and Olympus DeltaVision Deconvolution Microscope at the Imaging Core (GRU, Augusta, GA).
Adult Brain Morphology and immunofluorescence
Adult (4–6 month) mice were anesthetized with isofluorane and perfused intracardially with 30mL of 4% depolymerized paraformaldehyde (PFA) in 100mM sodium phosphate, pH=7.4. The brain was removed and immersed in 4% PFA overnight. For immunofluorescence, whole brains were washed with 100mM sodium phosphate buffer solution, 12% sucrose, 100mM sodium phosphate pH=7.4, 16% 100mM sodium phosphate pH=7.4, and 18% 100mM sodium phosphate pH=7.4, each for 24hrs at 4°C. Brains were frozen in a 2-methyl butane container placed in liquid nitrogen. All samples were stored at −80C until analysis. Frozen blocks were oriented coronally and cut from the optic chiasm to the posterior cerebrum for sectioning in the Electron Core Microscopy and Histology Core.
Frozen serial sections of 10-microns were coronally sectioned using a cryostat onto GOLD Superfrost Plus slides (Thermo Fisher Scientific) with 4 sections/slide. Approximately 60 slides were processed per mouse and every other slide was stained. On the day of immunolabeling, slides were incubated in 50mM ammonium chloride/50 mM TRIS, pH 7.4 for 30 minutes at room temperature, then in 2 mM sodium phosphate, pH=7.4, and 150mM NaCl, 0.2% TX-100 for 10 min at 4°C. Slides were washed in alternating high (20mM sodium phosphate, pH=7.4 and 500mM NaCl) and low (10mM sodium phosphate, pH=7.4 and 150mM NaCl) PBS solutions. Slides were then blocked in 7.5% goat dilution serum for 1 hour before incubation in 0.2µg/µl mouse monoclonal IgG1 anti-GnRH-1 antibody (Santa Cruz Biotechnology, Dallas TX) and kept at 4°C overnight.
Following incubation with anti-GnRH antibody, slides were washed in alternating high and low PBS before the addition of 2mg/ml Alexa-568 goat anti-mouse (Invitrogen Molecular Probes, Carlsbad CA) antibody for 2 hours at RT in the dark. Slides were mounted in 1mg/ml p-phenylenediamine in 40mM sodium phosphate, pH=7.4, 150mM NaCl, and 70% glycerol following washing. Confocal Microscopy was performed using the Zeiss Inverted Meta 510 imaging software at the Imaging core (GRU, GA). Sequential images of sections 80uM apart were taken at 10× and 20× for assembly to determine the number of GnRH neurons in the brain. GnRH neurons from all 10uM sections 80uM apart were first identified, and then counted by two different blinded observers. A GnRH neuron was defined as a cell with a nucleus surrounded by immunofluorescence.
Statistical Analysis
One-way ANOVA was used to compare the three groups, which included NELF protein expression, weights (body, brain, testes, seminal vesicles, ovaries and uteri), vaginal opening, estrus cycling (length, staging, number of cycles), mean litter sizes, the number of litters per 90days, and follicular stages). A Tukey multiple comparison procedure (MCP) was used to examine post hoc differences if a significant overall test was found in the one-way ANOVA models. Kruskal-Wallis tests were used to compare the three groups when data were not normally distributed, including the number of days before the first litter and hormone levels and the area under the curve for body weight after weaning, anogenital distance, and GnRH distribution. A Kruskal-Wallis multiple comparison procedure was used to examine post hoc differences between groups if the overall test was statistically significant. A two-sample t-test was used to compare two groups and included the length and width of testes and ovaries, seminiferous tubule counts and diameter, and GnRH distribution distance in the brain. All tests were two tailed except for a Mann-Whitney U test used to compare GnRH neuron number in KO vs. WT since a reduction of GnRH neurons was expected. *P <0.05 was considered statistically significant. Statistical analysis was performed using SAS and NCSS software.
Supplementary Material
Highlights.
NELF is an important regulator of puberty and fertility
Male Nelf knockout (KO) mice have normal puberty, but manifest subfertility
Female Nelf KO mice have delayed puberty, small uteri, and lower GnRH neuron number
Female heterozygous KO mice also have delayed puberty and manifest subfertility
NELF disruption more severely affects females, but both sexes manifest subfertility
Acknowledgements
We would like to thank the Wellcome Trust Sanger Institute, Cambridge, UK for generating the Nelf heterozygous mouse. In addition, we thank the University of Virginia, Charlottesville, VA core ligand and assay laboratory, as well as the Electron Core Microscopy and Histology Core, Histopathology core and Imaging Core at Georgia Regents University, Augusta, GA. This work was supported by NIH RO1 HD033004 and NIH RO1HD033004-15 (Diversity Supplement) to LCL. This work is dedicated to the memory and support of Krista Hollabaugh Layman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have nothing to disclose.
References
- 1.Wierman ME, Pawlowski JE, Allen MP, Xu M, Linseman DA, Nielsen-Preiss S. Molecular mechanisms of gonadotropin-releasing hormone neuronal migration. Trends Endocrinol Metab. 2004;15:96–102. doi: 10.1016/j.tem.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Schwanzel-Fukuda M, Crossin KL, Pfaff DW, Bouloux PM, Hardelin JP, Petit C. Migration of luteinizing hormone-releasing hormone (LHRH) neurons in early human embryos. J Comp Neurol. 1996;366:547–557. doi: 10.1002/(SICI)1096-9861(19960311)366:3<547::AID-CNE12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 3.Bhagavath B, Podolsky RH, Ozata M, Bolu E, Bick DP, Kulharya A, Sherins RJ, Layman LC. Clinical and molecular characterization of a large sample of patients with hypogonadotropic hypogonadism. Fertil Steril. 2006;85:706–713. doi: 10.1016/j.fertnstert.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 4.Crowley WF, Jr, Filicori M, Spratt DI, Santoro NF. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res. 1985;41:473–531. doi: 10.1016/b978-0-12-571141-8.50015-9. [DOI] [PubMed] [Google Scholar]
- 5.Layman LC. Genetics of human hypogonadotropic hypogonadism. Am J Med Genet. 1999;89:240–248. doi: 10.1002/(sici)1096-8628(19991229)89:4<240::aid-ajmg8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Layman LC. The genetic basis of female reproductive disorders: Etiology and clinical testing. Mol Cell Endocrinol. 2013;370:138–148. doi: 10.1016/j.mce.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanchate NK, Giacobini P, Lhuillier P, Parkash J, Espy C, Fouveaut C, Leroy C, Baron S, Campagne C, Vanacker C, Collier F, Cruaud C, Meyer V, Garcia-Pinero A, Dewailly D, Cortet-Rudelli C, Gersak K, Metz C, Chabrier G, Pugeat M, Young J, Hardelin JP, Prevot V, Dode C. SEMA3A, a gene involved in axonal pathfinding, is mutated in patients with Kallmann syndrome. PLoS Genet. 2012;8:e1002896. doi: 10.1371/journal.pgen.1002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tornberg J, Sykiotis GP, Keefe K, Plummer L, Hoang X, Hall JE, Quinton R, Seminara SB, Hughes V, Van Vliet G, Van Uum S, Crowley WF, Habuchi H, Kimata K, Pitteloud N, Bulow HE. Heparan sulfate 6-O-sulfotransferase 1, a gene involved in extracellular sugar modifications, is mutated in patients with idiopathic hypogonadotrophic hypogonadism. Proc Natl Acad Sci U S A. 2011;108:11524–11529. doi: 10.1073/pnas.1102284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pingault V, Bodereau V, Baral V, Marcos S, Watanabe Y, Chaoui A, Fouveaut C, Leroy C, Verier-Mine O, Francannet C, Dupin-Deguine D, Archambeaud F, Kurtz FJ, Young J, Bertherat J, Marlin S, Goossens M, Hardelin JP, Dode C, Bondurand N. Loss-of-Function Mutations in SOX10 Cause Kallmann Syndrome with Deafness. Am J Hum Genet. 2013;92:707–724. doi: 10.1016/j.ajhg.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miraoui H, Dwyer AA, Sykiotis GP, Plummer L, Chung W, Feng B, Beenken A, Clarke J, Pers TH, Dworzynski P, Keefe K, Niedziela M, Raivio T, Crowley WF, Jr, Seminara SB, Quinton R, Hughes VA, Kumanov P, Young J, Yialamas MA, Hall JE, Van Vliet G, Chanoine JP, Rubenstein J, Mohammadi M, Tsai PS, Sidis Y, Lage K, Pitteloud N. Mutations in FGF17, IL17RD, DUSP6, SPRY4, and FLRT3 Are Identified in Individuals with Congenital Hypogonadotropic Hypogonadism. Am J Hum Genet. 2013;92:725–743. doi: 10.1016/j.ajhg.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotan LD, Hutchins BI, Ozkan Y, Demirel F, Stoner H, Cheng PJ, Esen I, Gurbuz F, Bicakci YK, Mengen E, Yuksel B, Wray S, Topaloglu AK. Mutations in FEZF1 Cause Kallmann Syndrome. Am J Hum Genet. 2014;95:326–331. doi: 10.1016/j.ajhg.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, Li WP, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest. 2007;117:457–463. doi: 10.1172/JCI29884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quaynor SD, Kim HG, Cappello EM, Williams T, Chorich LP, Bick DP, Sherins RJ, Layman LC. The prevalence of digenic mutations in patients with normosmic hypogonadotropic hypogonadism and Kallmann syndrome. Fertil Steril. 2011;96:1424–1430 e6. doi: 10.1016/j.fertnstert.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sykiotis GP, Plummer L, Hughes VA, Au M, Durrani S, Nayak-Young S, Dwyer AA, Quinton R, Hall JE, Gusella JF, Seminara SB, Crowley WF, Jr, Pitteloud N. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci U S A. 2010;107:15140–15144. doi: 10.1073/pnas.1009622107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer PR, Wray S. Novel gene expressed in nasal region influences outgrowth of olfactory axons and migration of luteinizing hormone-releasing hormone (LHRH) neurons. Genes Dev. 2000;14:1824–1834. [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer PR, Wray S. Nasal embryonic LHRH factor (NELF) expression within the CNS and PNS of the rodent. Brain Res Gene Expr Patterns. 2001;1:23–26. doi: 10.1016/s1567-133x(01)00004-7. [DOI] [PubMed] [Google Scholar]
- 17.Xu N, Bhagavath B, Kim HG, Halvorson L, Podolsky RS, Chorich LP, Prasad P, Xiong WC, Cameron RS, Layman LC. NELF is a nuclear protein involved in hypothalamic GnRH neuronal migration. Mol Cell Endocrinol. 2010;319:47–55. doi: 10.1016/j.mce.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dieterich DC, Karpova A, Mikhaylova M, Zdobnova I, Konig I, Landwehr M, Kreutz M, Smalla KH, Richter K, Landgraf P, Reissner C, Boeckers TM, Zuschratter W, Spilker C, Seidenbecher CI, Garner CC, Gundelfinger ED, Kreutz MR. Caldendrin-Jacob: a protein liaison that couples NMDA receptor signalling to the nucleus. PLoS Biol. 2008;6:e34. doi: 10.1371/journal.pbio.0060034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu N, Kim HG, Bhagavath B, Cho SG, Lee JH, Ha K, Meliciani I, Wenzel W, Podolsky RH, Chorich LP, Stackhouse KA, Grove AM, Odom LN, Ozata M, Bick DP, Sherins RJ, Kim SH, Cameron RS, Layman LC. Nasal embryonic LHRH factor (NELF) mutations in patients with normosmic hypogonadotropic hypogonadism and Kallmann syndrome. Fertil Steril. 2011;95:1613–1620. e1–e7. doi: 10.1016/j.fertnstert.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palevitch O, Abraham E, Borodovsky N, Levkowitz G, Zohar Y, Gothilf Y. Nasal embryonic LHRH factor plays a role in the developmental migration and projection of gonadotropin-releasing hormone 3 neurons in zebrafish. Dev Dyn. 2009;238:66–75. doi: 10.1002/dvdy.21823. [DOI] [PubMed] [Google Scholar]
- 21.Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, Jackson D, Severin J, Biggs P, Fu J, Nefedov M, de Jong PJ, Stewart AF, Bradley A. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Testa G, Schaft J, van der Hoeven F, Glaser S, Anastassiadis K, Zhang Y, Hermann T, Stremmel W, Stewart AF. A reliable lacZ expression reporter cassette for multipurpose, knockout-first alleles. Genesis. 2004;38:151–158. doi: 10.1002/gene.20012. [DOI] [PubMed] [Google Scholar]
- 23.Barnett KR, Schilling C, Greenfeld CR, Tomic D, Flaws JA. Ovarian follicle development and transgenic mouse models. Hum Reprod Update. 2006;12:537–555. doi: 10.1093/humupd/dml022. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 25.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4, Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason AJ, Hayflick JS, Zoeller RT, Young WS, 3rd, Phillips HS, Nikolics K, Seeburg PH. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986;234:1366–1371. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- 27.Wu S, Wilson MD, Busby ER, Isaac ER, Sherwood NM. Disruption of the single copy gonadotropin-releasing hormone receptor in mice by gene trap: severe reduction of reproductive organs and functions in developing and adult mice. Endocrinology. 2010;151:1142–1152. doi: 10.1210/en.2009-0598. [DOI] [PubMed] [Google Scholar]
- 28.Pask AJ, Kanasaki H, Kaiser UB, Conn PM, Janovick JA, Stockton DW, Hess DL, Justice MJ, Behringer RR. A novel mouse model of hypogonadotrophic hypogonadism: N-ethyl-N-nitrosourea-induced gonadotropin-releasing hormone receptor gene mutation. Mol Endocrinol. 2005;19:972–981. doi: 10.1210/me.2004-0192. [DOI] [PubMed] [Google Scholar]
- 29.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 30.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 31.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 32.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Wei Le W, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007 doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 33.Tsai PS, Moenter SM, Postigo HR, El Majdoubi M, Pak TR, Gill JC, Paruthiyil S, Werner S, Weiner RI. Targeted expression of a dominant-negative fibroblast growth factor (FGF) receptor in gonadotropin-releasing hormone (GnRH) neurons reduces FGF responsiveness and the size of GnRH neuronal population. Mol Endocrinol. 2005;19:225–236. doi: 10.1210/me.2004-0330. [DOI] [PubMed] [Google Scholar]
- 34.Tata BK, Chung WC, Brooks LR, Kavanaugh SI, Tsai PS. Fibroblast growth factor signaling deficiencies impact female reproduction and kisspeptin neurons in mice. Biol Reprod. 2012;86:119. doi: 10.1095/biolreprod.111.095992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Pearce SH, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest. 2008;118:2822–2831. doi: 10.1172/JCI34538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bosman EA, Penn AC, Ambrose JC, Kettleborough R, Stemple DL, Steel KP. Multiple mutations in mouse Chd7 provide models for CHARGE syndrome. Hum Mol Genet. 2005;14:3463–3476. doi: 10.1093/hmg/ddi375. [DOI] [PubMed] [Google Scholar]
- 37.Hurd EA, Capers PL, Blauwkamp MN, Adams ME, Raphael Y, Poucher HK, Martin DM. Loss of Chd7 function in gene-trapped reporter mice is embryonic lethal and associated with severe defects in multiple developing tissues. Mamm Genome. 2007;18:94–104. doi: 10.1007/s00335-006-0107-6. [DOI] [PubMed] [Google Scholar]
- 38.Layman WS, McEwen DP, Beyer LA, Lalani SR, Fernbach SD, Oh E, Swaroop A, Hegg CC, Raphael Y, Martens JR, Martin DM. Defects in neural stem cell proliferation and olfaction in Chd7 deficient mice indicate a mechanism for hyposmia in human CHARGE syndrome. Hum Mol Genet. 2009;18:1909–1923. doi: 10.1093/hmg/ddp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang JJ, Caligioni CS, Chan YM, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology. 2012;153:1498–1508. doi: 10.1210/en.2011-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308:1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, Matsuo A, Ishii H, Kobori M, Katoh M, Matsushime H, Furuichi K, Shigeyoshi Y. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci U S A. 2006;103:4140–4145. doi: 10.1073/pnas.0508881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cariboni A, Davidson K, Rakic S, Maggi R, Parnavelas JG, Ruhrberg C. Defective gonadotropin-releasing hormone neuron migration in mice lacking SEMA3A signalling through NRP1 and NRP2: implications for the aetiology of hypogonadotropic hypogonadism. Hum Mol Genet. 2011;20:336–344. doi: 10.1093/hmg/ddq468. [DOI] [PubMed] [Google Scholar]
- 43.Mikhaylova M, Karpova A, Bar J, Bethge P, Yuanxiang P, Chen Y, Zuschratter W, Behnisch T, Kreutz MR. Cellular distribution of the NMDA-receptor activated synapto-nuclear messenger Jacob in the rat brain. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0539-1. [DOI] [PubMed] [Google Scholar]
- 44.Kindler S, Dieterich DC, Schutt J, Sahin J, Karpova A, Mikhaylova M, Schob C, Gundelfinger ED, Kreienkamp HJ, Kreutz MR. Dendritic mRNA targeting of Jacob and N-methyl-d-aspartate-induced nuclear translocation after calpain-mediated proteolysis. J Biol Chem. 2009;284:25431–25440. doi: 10.1074/jbc.M109.022137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colledge WH. Transgenic mouse models to study Gpr54/kisspeptin physiology. Peptides. 2009;30:34–41. doi: 10.1016/j.peptides.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149:597–604. doi: 10.1210/en.2007-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Divall SA, Williams TR, Carver SE, Koch L, Bruning JC, Kahn CR, Wondisford F, Radovick S, Wolfe A. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest. 2010;120:2900–2909. doi: 10.1172/JCI41069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cameron RS, Rakic P. Identification of membrane proteins that comprise the plasmalemmal junction between migrating neurons and radial glial cells. J Neurosci. 1994;14:3139–3155. doi: 10.1523/JNEUROSCI.14-05-03139.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larder R, Clark DD, Miller NL, Mellon PL. Hypothalamic dysregulation and infertility in mice lacking the homeodomain protein Six6. J Neurosci. 2011;31:426–438. doi: 10.1523/JNEUROSCI.1688-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diaczok D, DiVall S, Matsuo I, Wondisford FE, Wolfe AM, Radovick S. Deletion of Otx2 in GnRH neurons results in a mouse model of hypogonadotropic hypogonadism. Mol Endocrinol. 2011;25:833–846. doi: 10.1210/me.2010-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.