Abstract
Exposure to carcinogenic metals, such as trivalent arsenic [As(III)] and hexavalent chromium [Cr(VI)], through drinking water is a major global public health problem and is associated with various cancers. However, the mechanism of their carcinogenicity remains unclear. In this study, we used azoxymethane/dextran sodium sulfate (AOM/DSS)-induced mouse colitis-associated colorectal cancer model to investigate their tumorigenesis. Our results demonstrate that exposure to As(III) or Cr(VI), alone or in combination, together with AOM/DSS pretreatment has a promotion effect, increasing the colorectal tumor incidence, multiplicity, size, and grade, as well as cell inflammatory response. Two-dimensional differential gel electrophoresis coupled with mass spectrometry revealed that As(III) or Cr(VI) treatment alone significantly changed the density of proteins. The expression of β-catenin and phospho-GSK was increased by treatment of carcinogenic metals alone. Concomitantly, the expression of NADPH oxidase1 (NOX1) and the level of 8-OHdG were also increased by treatment of carcinogenic metals alone. Antioxidant enzymes, such as superoxide dismutase (SOD) and catalase, were decreased. Similarly, in an in vitro system, exposure of CRL-1807 to carcinogenic metals increased reactive oxygen species (ROS) generation, the expression of β-catenin, phospho-GSK, and NOX1. Inhibition of ROS generation by addition of SOD or catalase inhibited β-catenin expression and activity. Our study provides a new animal model to study the carcinogenicity of As(III) and Cr(VI) and suggest that As(III) and Cr(VI) promote colorectal cancer tumorigenesis, at least partly, through ROS-mediated Wnt/β-catenin signaling pathway.
Keywords: As(III), Cr(VI), tumorigenesis, colorectal cancer, ROS, Wnt/β-catenin pathway
Introduction
Trivalent arsenic [As(III)] and hexavalent chromium [Cr(VI)] are human carcinogens. Both are classified as Group I carcinogens by the International Agency for Research on Cancer (IARC, 1990). Exposure to these metals occurs in both occupational and environmental settings. Acute and chronic exposure to carcinogenic metals via drinking water has been reported in many countries of the world. Drinking water contamination by carcinogenic metals remains a major public health concern and is associated with an enhanced risk of development of various cancers. Chronic As(III) exposure causes tumors of the skin, bladder, lung, liver, prostate, and colon (Tchounwou et al., 2003). Chronic Cr(VI) exposure causes tumors of the lung, gastrointestinal, and central nervous systems (Gatto et al., 2010; Stout et al., 2009).
Although epidemiological studies have documented the global impact of carcinogenic metal contamination, the mechanisms of their carcinogenicity remain unclear. One problem in establishing their carcinogenic activity is that the animal models are very limited. It is difficult to provide experimental evidence of the carcinogenicity of As(III) or Cr(VI) in laboratory animals (Tokar et al., 2010). A recent National Toxicology Program study showed an increased rates of oral-cavity tumors in rate and small intestine tumors in mice administrated Cr(VI) in drinking water for 2 years (Stout et al., 2009). More recent work with oral sodium arsenate in the drinking waster for 18 months showed an increase of lung tumor multiplicity and size in male stain A/J mice (Cui et al., 2006; Ding et al., 2009; Tokar et al., 2010). Although As(III) and Cr(VI) are believed to act through very different mechanisms (Hamilton et al., 1998; Huff et al., 2000; Zhang et al., 2011), they share several properties in regard to their carcinogenicity. Both can activate NADPH oxidase, which is a major source of cellular reactive oxygen species (ROS) and is able to induce oxidative stress (Qian et al., 2005; Wang et al., 2011; Zhang et al., 2011). Oxidative stress plays an important role in both the initiation and the progression of various types of cancer.
Colorectal cancer is one of the most common neoplasias in western countries and the second leading cause of cancer-related death (Jemal et al., 2010). The most current five years of data from the United States Cancer Statistics show that Kentucky has the second highest colorectal cancer incidence in the U.S. compared to other states, especially in the Appalachian region (USCS, 2011). In this area, the concentrations of carcinogenic metal reported in drinking water are relatively high compared with EPA standards (Johnson et al., 2011). These ecological studies suggest that there is a correlation between metals level in the environment and colorectal cancer incidence. However, the exact molecular mechanism is still unknown.
The Wnt/β-catenin signal pathway has a critical role in carcinogensis (Polakis 2000, 2007). Abnormal subcellular localization and aberrant accumulation of β-catenin are often observed in human cancers, including colorectal cancer (Polakis 2000). The cellular levels of β-catenin protein are regulated by the ubiquitin-proteasome system (Peifer and Polakis 2000; Polakis 2000, 2007). Phosphorylation of β-catenin by GSK3β is essential for the ubiquitination of β-catenin. Initiation of Wnt signaling leads to inhibition of GSK3β-dependent phosphorylation and degradation of β-catenin, activating the β-catenin transcriptional pathway (Peifer and Polakis 2000; Polakis 2000, 2007). Aberrant activation of Wnt signaling is common in colorectal cancer (Barker and Clevers 2006; Segditsas and Tomlinson 2006). A Recent study has shown that NADPH oxidase 1 (NOX1) modulates Wnt and NOTCH1 signaling to control the fate of proliferative cells in the colon (Coant et al., 2010).
We hypothesized that carcinogenic metals play an important role in colorectal tumor development. We used the azoxymethane/dextran sodium sulfate (AOM/DSS) murine colitis-associated colorectal cancer model, which is a well established model for studying colon carcinogenesis. Animals treated with AOM and DSS developed colitis-associated colorectal tumors (De Robertis et al., 2011; Greten et al., 2004). We found that the carcinogenic metals As(III) or Cr(VI), alone or in combination, in drinking water promote tumorigenesis in the murine AOM/DSS colitis-associated colorectal cancer model. ROS-mediated β-catenin activation may play an important role in this promotion effect.
Material and methods
Animal and experimental design
Eighty five-week old C57BL/6J mice were obtained from Charles River Laboratories, Inc. They were maintained at University of Kentucky Animal Facility according to the Institutional Animal Care Guidelines. Animals were allowed to acclimatize to their new environments for 1 week prior to use. Animals were housed in plastic cages (5 mice/ cage) with free access to food and drinking water, under controlled conditions of humidity (50 ± 10%), light (12/12 h light/dark cycle) and temperature (23 ± 2 °C). Mice were randomized by body weight into 16 groups: control, As(III), Cr(VI), As(III)+Cr(VI); AOM, AOM+As(III), AOM+Cr(VI), AOM+As(III)+Cr(VI); DSS, DSS+As(III), DSS+Cr(VI), DSS+As(III)+Cr(VI); AOM+DSS, AOM+DSS+As(III), AOM+DSS+Cr(VI), and AOM+DSS+As(III)+Cr(VI). Metals containing drinking water was prepared by dissolving sodium arsenite (Sigma, USA) or sodium dichromate dehydrate (Sigma, USA), alone or in combination, in water at a concentration of 58 mg/L and 167 mg/L, respectively. Mice in the groups receiving AOM (Sigma, USA) were injected a single intraperitoneal dose of AOM (12.5 mg/kg of body weight) at the age of 6 weeks. After 3-day recovery period, mice in groups receiving DSS were received single cycle of 2% DSS in the drinking water for 7 days. After DSS treatment, the mice were exposed to As(III), Cr(VI) or As(III)+Cr(VI) in drinking water for following 20 weeks. Control group was injected with saline and free access to basal drinking water. Mice were weighted twice weekly, and the signs of weight loss were monitored during the experiment. All mice were sacrificed at the age of 27 weeks using CO2 asphyxiation and the whole colorectal tissues were collected. Tissue samples were fixed immediately in 10% formalin or frozen in liquid nitrogen. Tumor number and size were examined in the entire large bowel using a dissecting microscope.
Cell line and cell culture
CRL-1807 human coloncytes were purchased from the American Type Culture Collection (Manassas, VA). CRL-1807 cells are derived from normal fetal human coloncyte CRL-1790 immortalized by a temperature-sensitive mutant of SV40 virus which functions at 33 °C. CRL-1807 cells are non-tumorigenic and undergo contact inhibition in cell culture. CRL-1807 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and 5% penicillin/strepyomycin at 33 °C in a humidified atmosphere with 5% CO2 in air. For treatment, cells were grown to 80–90% confluent, then the medium was replaced with DMEM medium containing 0.1% FBS for overnight before indicated treatment with carcinogenic metals. In some experiments, cells were first pretreated with PEG-SOD (Sigma, USA) or catalase (Sigma, USA) for 2 h, and then exposed to carcinogenic metals for indicated time.
Two-dimensional differential gel electrophoresis (2D-DIGE) and protein identification
Sample labeling was performed by Applied Biomics, Inc (San Fancisco, USA). Briefly, tissue proteins were precipitated, resuspended in labeling buffer and aliquots stained by different fluorescent cyanine dyes. Samples were pooled, mixed with rehydration solution and applied to a rehydration tray. Separation was in a two-dimensional gel, using isoelectric focusing (IEF) in the first dimension and SDS polyacrylamide gel electrophoresis (SDS-PAGE) in the second dimension. After electrophoresis, the gel was scanned using a Typhoon image scanner. Each scan revealed one of the CyDye signaling (Cy3 and Cy5). ImageQuant software was used to generate the image presentation data. Differentially expressed proteins were identified using the following parameters: expression ratio lower than 0.75 or higher than 1.35 and a p-value of p<0.05, as obtained by using DeCyder 2-D Differential Analysis Software v7.0 (GE Healthcare). Proteins of interest were cut from dried gel and identified by mass spectrometry. Tryptic digestion was performed by addition of 10% of formic acid/50% acetonitrile followed by three-fold lyophilisation in a SpeedVac (Thermo Savant). Identification of the extracted peptides was done by using a MALDL-TOF/TOF mass spectrometer (4800 Proteomics Analyzer, Applied Biosystems, Europe). Database searches were carried out using the MS/MS ion search (MASCOT, http://www.matrixscience.com) against all entries of the Swiss-Prot database (http://www.expasy.org). Proteins were defined as unambiguously identified, if the Mowse score was higher than 100, and at least two different peptides (P<0.05) were used for identification. Molecular weight and pI of the identified protein were cross-checked with the gel position of the excised spot.
Histopathology and immunohistochemistry
All tumors and mucosa specimens were subjected to Hematoxylin & Eosin (H&E) staining for histopathology assessment. Intraepithelial dysplasia was defined as low- or high- grade. High grade dysplasia in the colonic adenomas represents biologically “carcinoma is situ” which does not show invasive growth or metastatic potential. High grade dysplasia is histologically defined by marked architectural changes such as back-to-back gland configuration and cribriform patter as well as marked cytologic atypia (WHO 2010). Immunohistochemical staining was performed on formalin-fixed paraffin-embedded tissues. Sections were cut at 5 micrometer on positively charged slides, and heated at 60 °C for 1 hour. They were deparaffinized and hydrated stepwise. Endogenous peroxidase activity was quenched and slides were incubated in anti-Iba1 (Cell Signaling, 1:200), or 8-OHdG (Santa Cruz, 1:200) for 1 hour at room temperature or overnight at 4 °C. Slides were washed and then incubated in corresponding secondary antibodies (Dako Envision Flex) for 30 minutes and the reaction was visualized using DAB. Slides were counterstained with hematoxylin and blued in ammonia water, followed by dehydration, clearing in xylene and mounting. For immunofluorescence, slides were counterstained with DAPI (Invitrogen) and mounted with Prolong Gold antifade (Invitrogen).
Cell viability assay
Cell viability was determined using 3-(4,5-dime-thylthiazol-2yl-2,5-diphenyl tetrazolium bromide (MTT) method as described previously (Wang et al., 2007).
Measurement of intracellular ROS
Detection of ROS was performed using carboxy-H2DCFDA (sensitive to oxidation; Invitrogen) and oxidized carboxy-DCFDA (insensitive to oxidation; Invitrogen) as described previously (Sun et al., 2010). The fluorescence in cells preloaded with carboxy-H2DCFDA was normalized to that in cells preloaded with carboxy-DCFDA (ratio of H2DCFDA/DCFDA) to control for the cell number, dye uptake, and ester cleavage differences between different treatment groups. The cells were plated in 96-well plates and treated with As(III), Cr(VI), or their combination for the indicated time. After incubation with 10 µM carboxy-H2DCFDA or carboxy-DCFDA in serum-free medium for 30 min at 37 °C, the cells were washed with PBS twice and analyzed by using a SPECTRA max Gemini XPS plate reader. The fluorescence intensity of DCF was measured at an excitation wavelength of 492 nm and an emission wavelength of 517 nm.
Western blot analysis
Western blot analysis was performed as previously described (Wang et al., 2007) using corresponding antibodies against β-catenin (1:1000, Cell Signaling) and phospho-GSK3β (1:1000, Cell Signaling), actin (1:3000, Santa Cruz), SOD1 (1:1000, Santa Cruz), SOD2 (1:1000, Santa Cruz), catalase (1:1000, Novus Biologicals), NOX1 (1:1000, Abcam).
Luciferase reporter assays
β-catenin activity was measured using luciferase reporter assay. The luciferase reporter contains eight optimal copies of the LEF/TCF binding site upstream of a minimal thymidine kinase promoter directing transcription of a luciferase gene. The LEF/TCF reporter is designed to monitor the activity of Wnt signal transduction in cultured cells. CRL-1807 cells were transfected with firefly luciferase construct and renilla luciferase control using lipofectmine 2000 (Invitrogen) according to the manufacturer’s instructions and treated 24 h after transfection. Luciferase assays were performed with the Dual-Luciferase®-reporter assay system (Promega).
Statistical analysis
Differences among treatment groups were tested using ANOVA. Differences in which p was < 0.05 were considered statistically significant. In cases where significant differences were detected, specific post hoc comparisons between treatment groups were examined with Student- Newman-Keuls tests. The analyses were performed using SPSS software (SPSS, Chicago, IL, USA).
Results
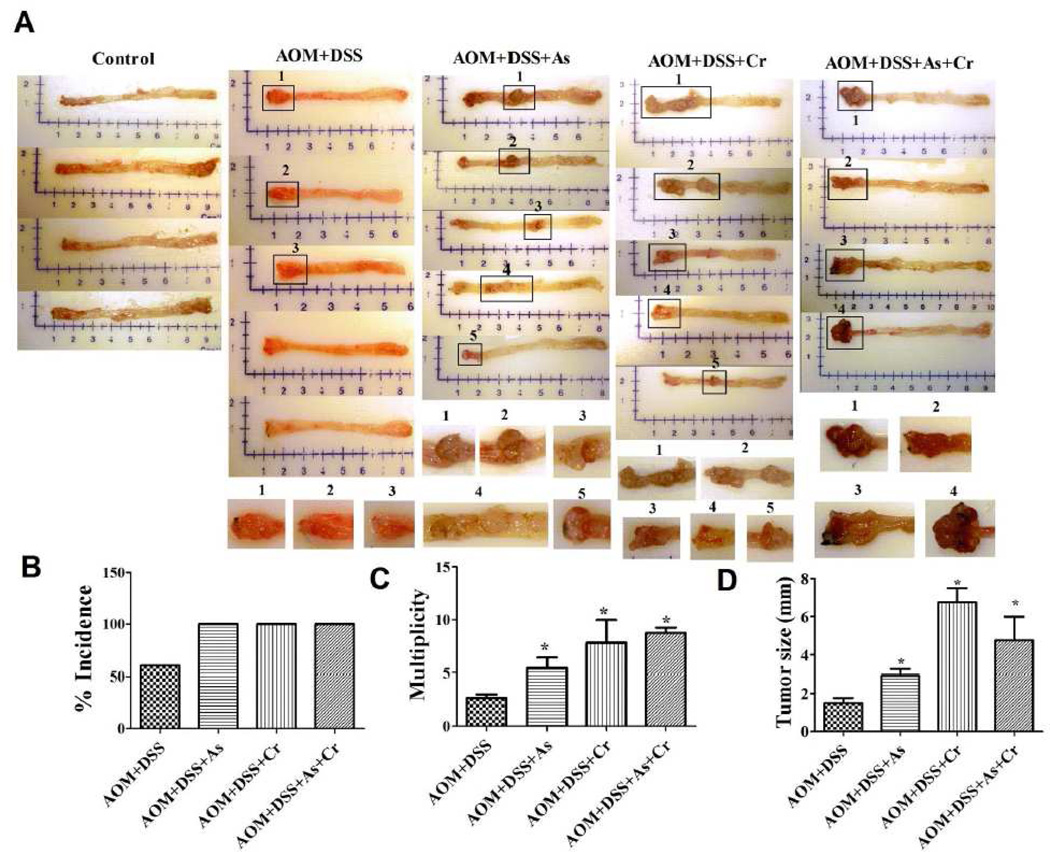
As(III) or Cr(VI), alone or in combination, in drinking water enhanced the incidence and multiplicity of colorectal tumor in AOM/DSS pretreated mice
Body weight of mice in various treatments at the age of 27 weeks was not significantly different compared with that of control groups. Treatment with carcinogenic metals alone or combination with either AOM or DSS did not produce colorectal tumors in the observation period. However, carcinogenic metals in combination with AOM/DSS pretreatment markedly increased the incidence and multiplicity of colorectal tumors (Fig.1A). AOM/DSS-treated mice formed colorectal tumors at an incidence (percentage of mice with colon adenomas) of 60% and multiplicity (number of adenomas/colon) of 2.667 ± 0.577 (mean ± SE; n= 5). In contrast, the combination of AOM/DSS with As(III) enhanced colon adenomas incidence by 67% and multiplicity by 87%; the corresponding values for combination of AOM/DSS with Cr(VI) were 67% and 300%; for combination of AOM/DSS with As(III)+Cr(VI) were 67% and 237% (Figure 1B and 1C). Accompanying the increase in colorectal tumor incidence and multiplicity, tumor size in mice treated with a combination of AOM/DSS with metals were also increased compared with AOM/DSS alone treatment. 2-fold, 4.6-fold, and 3.1-fold increases in tumor volume occurred in the combination treatment of AOM/DSS with As(III), Cr(VI) and As(III)+Cr(VI), respectively (Figure 1D). Taken together, these results demonstrate that the carcinogenic metals As(III) and Cr(VI) in drinking water enhanced the colorectal tumor development in mice colitis-associated colon cancer model.
Figure 1.
Carcinogenic metals in drinking water increased the incidence and multiplicity of colorectal tumors in AOM/DSS-induced mouse colitis-associated colorectal cancer model. (A) Representative gross specimens from indicated treatments with longitudinally opened colons. Incidence (B), multiplicity (C), and average tumor size (D) at weeks 27 in carcinogenic metals combination with AOM/DSS pretreated mice. Treatment with carcinogenic metals alone or combination with either AOM or DSS did not produce colorectal tumors in the observation period. For graph (B–D), the data are expressed as the mean ± S.E. (n= 4 or 5). *p < 0.05, statistically significant difference from AOM/DSS pretreated alone group.
Adenomas Pathology
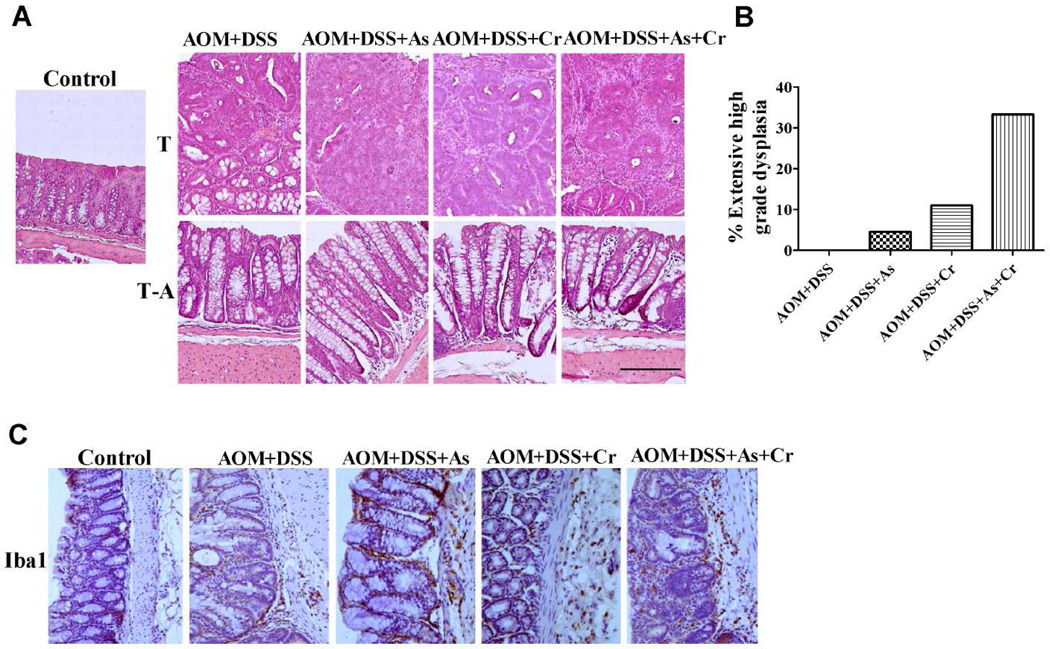
As shown in Figure 2A, As(III), Cr(VI) or As(III)+Cr(VI), in combination with AOM/DSS pretreatment increased the grade of adenomas. AOM/DSS treatment did not cause extensive high grade dysplasia in colorectal tissue. In combination with As(III), Cr(VI), or As(III)+Cr(VI), the extensive high grade dysplasia increased to 4.5%, 11%, and 33.3%, respectively (Figure 2B). Overexpression of inflammation markers is a hallmark of colorectal tumors. As shown in Figure 2C, there was an increase of the expression of Iba1, which is a macrophage/microglia-specific calcium-binding protein, in the groups treated with carcinogenic metals together with AOM/DSS, compared with those from the control group and AOM/DSS alone group. Taken together, our results indicated that carcinogenic metals enhanced the grade of colorectal tumor.
Figure 2.
Carcinogenic metals combination with AOM/DSS pretreatment increased the grade of the adenomas. (A) Representative H&E staining demonstrating tumors from indicated treatments. The polyp samples and adjacent sections were stained by H&E. Normal tissue from control groups were used as the control. Representative photographs are shown. Bar =1 mm. T: Tumor tissue; T-A: Tumor-adjacent tissue. (B) The percentage of mice with extensive high grade dysplasia in indicated treatment mice. The data are expressed as the mean ± S.E. (n= 4 or 5). (C) Immunohistochemistry staining of Iba1 in indicated treatment. Magnification was 200×.
2D-DIGE analysis and mass spectrometry
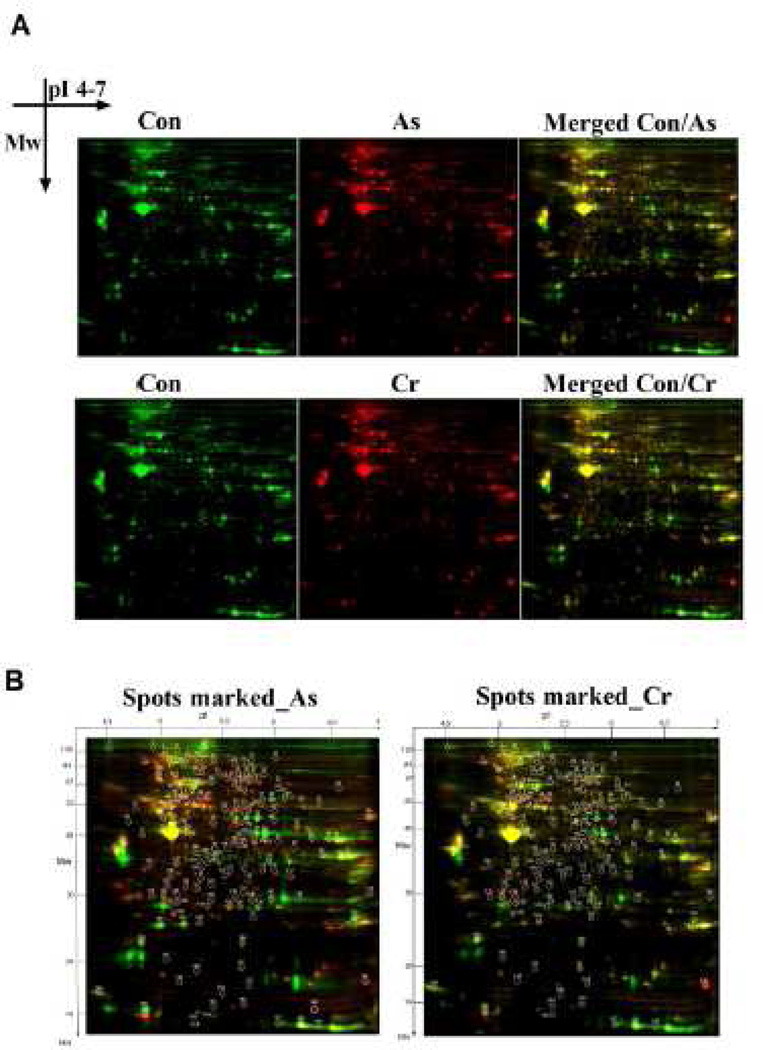
To identify proteins differentially expressed in colon of carcinogenic metals-treated mice, we performed 2D-DIGE analysis with the pI range of 4.0–7.0 and molecular weight range between 10 kDa and 125 kDa. After 2D-DIGE separation, images of As(III)- or Cr(VI)-induced protein expression profile were acquired from the same gel with untreated control groups under different wavelengths (Figure 3A). Using the DeCyder software version 7.0, 169 differentially expressed protein spots (p< 0.05) were detected in the As(III)-treated group and 159 spots in the Cr(VI)-treated group, compared with control group (Figure 3A). These results showed that As(III) or Cr(VI) alone in drinking water modified the colorectal proteome of C57BL/6J mice. Thirty six protein spots with significant changes in their levels (p < 0.05; fold change ≥ 1.5) compared with control group were excised from the gels as candidates for MALDI-TOF-TOF/MS (Figure 3B). As shown in Table 1, the latter analysis successfully identified 31 sports from As(III) and 16 sports from Cr(VI) treatment. The identified proteins from As(III) treatment included 17 up-regulated proteins and 14 down-regulated proteins. For Cr(VI) treatment, the identified proteins included 6 up-regulated proteins and 10 down-regulated proteins. These differentially expressed proteins were implicated in four functional groups, including carbohydrate/energy metabolism, protein metabolism and modification, cytoskeleton dynamics, and oxidant-reduction response. Among them, there are many proteins are reported to link to beta-catenin in cancer, such as myosin-1 (Geisbrecht and Montell 2002), protein disulfide-isomerase A6 (Verras et al., 2008), heat shock 70kD protein 5 (Tao et al., 2009), myosin light-chain protein (Zhou et al., 2008). These cellular/metabolic process-related proteins were either up-regulated or down-regulated, suggesting the functional importance of these processes in carcinogenic metal-induced carcinogenesis.
Figure 3.
Carcinogenic metals As(III) and Cr(VI) in drinking water changed the colorectal proteome expression profile. (A) 2D-DIGE of colorectal proteins. Each individual sample (As, Cr, and control) and a pooled reference sample were labeled with Cy5 and Cy3, respectively, mixed, and separated on a 2D-PAGE gel. Gels were scanned and a set of Cy5 and Cy3 images were obtained from each gel. An overlay of two dye scan-images was obtained. (B) Spots with a student’s t-test p value less than 0.05 in As(III)- or Cr(VI)- treated group, are shown in circles and number according to pI and Mw. Thirty six protein spots with significant change in their levels (p < 0.05; fold change ≥ ±1.5) were cut out of this gel and subjected to tryptic digestion followed MS analysis.
Table 1.
Altered proteins in colorectal tissue from As(III) or Cr(VI) treated groups identified by MALDI-TOF MS
| Spot No. |
Genebank Aceessoin NO. |
protein description | Average ratio for As group |
Average ratio for Cr group |
Sequence coverage |
Number of matched peptied |
Function3 |
|---|---|---|---|---|---|---|---|
| 6 | gi|82524274 | Myosin-1 | −∞ | −∞ | 8.5 | 13 | Motor protein |
| 13 | gi|54037163 | Neutral alpha-glucosidase AB | 1.75 | 10 | 6 | Glucosidase | |
| 14 | gi|31543942 | Vinculin | 2.48 | 2.88 | 17.2 | 10 | Cell adhesion |
| 16 | BAE257731 | G protein-regulated inducer of neurite outgrowth 1 | 1.94 | 18.8 | 13 | Phosphoprotein binding | |
| 20 | gi|50511175 | mKIAA1905 protein | 2.07 | 9.4 | 6 | Actin binding | |
| 26 | gi|74220835 | Lamin A2 | 1.62 | 2.46 | 9.2 | 4 | Nuclear assembly, chromatin organization, nuclear membrane and telomere dynamics |
| 27 | gi|74186081 | Moesin2 | 2.11 | 2.87 | 12.8 | 8 | Cytoskeletal structual constituent |
| 29 | gi|8567336 | Calcium activated chloride channel regulator 1 precursor | 2.29 | 3.36 | 9.3 | 8 | Ion channel |
| 30 | gi|20330802 | Serotransferrin precursor | −1.66 | −1.91 | 17.9 | 15 | Ferric iron binding |
| 38 | gi|730933 | Glutamine gamma-glutamytransferase E | −1.74 | −2.01 | 27.8 | 8 | Transferase |
| 40 | gi|74137565 | Albumin 12 | −2.72 | 34.7 | 12 | DNA binding, antioxidant activity, and drugs and ions binding | |
| 47 | gi|38503465 | Keratin 17n | 1.98 | 10.2 | 3 | Structual constituent | |
| 58 | gi|71059931 | Lap3 | 1.94 | 18.1 | 8 | Aminopeptidase | |
| 70 | gi|74192607 | T-cell specific GTpase2 | 4.12 | 3.6 | 2 | Transferase | |
| 77 | gi|71059715 | Eno3 | −13.34 | −13.88 | 27 | 8 | Lyase |
| 79 | gi|6679651 | Beta-enolase isoform1 | −44.71 | 41 | 14 | Lyase | |
| 81 | gi|6753428 | Creatine kinase U-type, mitochondrial precursor | 2.65 | 34.9 | 15 | Transferase | |
| 120 | gi|20137004 | Proteasome activator complex subunit 2 isoform 1 | 2.1 | 24.3 | 6 | Protein metabolism | |
| 121 | gi|6755212 | Proteasome activator complex subunit 1 | 2.68 | 12 | 3 | Protein metabolism | |
| 124 | gi|33563264 | Myosin light chain 3 | 1.75 | 2.41 | 11.8 | 2 | Structual constituent |
| 125 | gi|13384888 | Mucosal pentraxin precursor | 11.84 | 12.3 | 2 | Metal ion binding | |
| 126 | gi|8202239 | Calpain small subunit 1, Capns1 | 3.59 | 21.6 | 3 | Protease | |
| 128 | gi|74203337 | Apolipoprotein A-I2 | −1.81 | 15.2 | 5 | Lipoprotein metabolism | |
| 137 | gi|3114387 | Glutathion S-transferase A4 | −2.72 | 22.1 | 5 | Transferase | |
| 138 | gi|31982861 | Carbonic anhydrase 3 | −13.25 | 15.5 | 4 | Anhydrase | |
| 157 | gi|4760586 | Beta-1-globin | −3.42 | −7.03 | 43.2 | 5 | Oxygen transport |
| 158 | Q9CRZ2_MOUSE1 | Hemoglobin, beta adult major chain | −3.37 | −8.01 | 89.8 | 16 | Oxygen transport |
| 159 | gi|12846616 | Hemoglobin, beta chain2 | −3.06 | −6.85 | 29.9 | 16 | Oxygen transport |
| 160 | Q5U410_MOUSE1 | Similar to glyceraldehyde-3-phosphate | −∞ | 41.7 | 17 | Dehydrogenase | |
| 160 | Q5U410_MOUSE1 | Similar to glyceraldehyde-3-phosphate dehydrogenase | −∞ | 41.7 | 17 | Dehydrogenase | |
| 162 | gi|31982861 | Carbonic anhydrase 3 | −∞ | 41.2 | 8 | Anhydrase | |
| 163 | gi|37589525 | Myosin light-chain protein | −∞ | 23.5 | 4 | Structual constituent | |
| 164 | gi|3959400 | Adenylate kinase isoenzyme 1 | −∞ | 45.4 | 9 | Isoenzyme | |
| 166 | Q9DC41_MOUSE1 | Heat shock 70kD protein 5 | −∞ | 28.3 | 13 | ER overload response | |
| 167 | PDIA6_MOUSE1 | Protein disulfide-isomerase A6 | ∞ | 25.2 | 8 | Isomerase | |
| 168 | gi|817939 | Histone H2A | ∞ | 11.7 | 2 | DNA binding |
Data present average ratio (“+” or “−“ sign, respectively, indicates n-fold increase or decrease of protein in indicated treated groups as compared to control), GenBank accession number, protein name, number of matched peptides, protein sequence coverage (SC, %), and protein function. The numbering corresponds to that of Figure 3B.
p < 0.05, statistically significant difference from control group. Average volume ratio > 1.5 or < −1.5 were accepted.
: identified by MSDB;
: identified by CDD Result;
: function by UniProtKB.
As(III) or Cr(VI), alone or in combination, in drinking water increased the expression of β- catenin in colon tissue
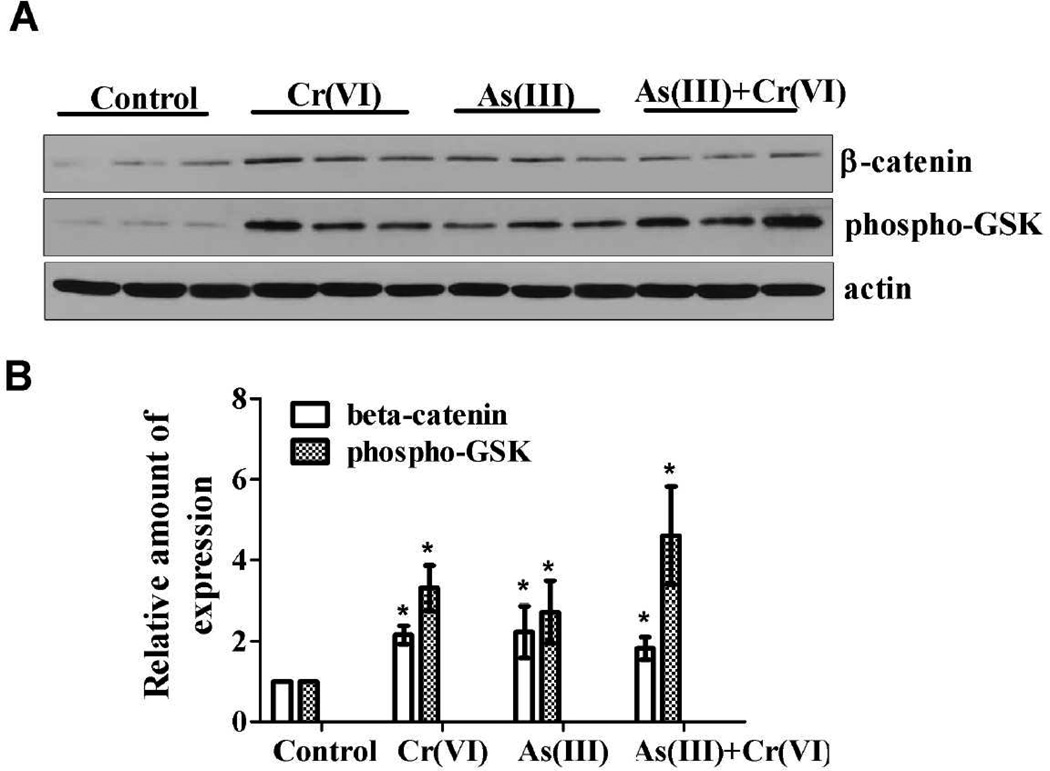
Aberrant expression of β-catenin can be regarded as a key event during colorectal tumorigenesis. The level of β-catenin is tightly regulated by GSK-3β. The activity of GSK-3β can be inhibited through phosphorylation at Ser-9, resulting in more nonphosphorylated β-catenin moving into the nucleus. In the nucleus, β-catenin acts as a transcriptional co-activator and activates genes involved in cell proliferation and survival. As shown in Figure 4A and 4B, As(III) or Cr(VI), alone or in combination, without AOS/DSS treatment, increased the expression level of β-catenin, compared with control group. Concomitantly, the expression level of Ser-9 phosphorylation of GSK3β was also increased in carcinogenic metals treatment. These results indicate that the stimulation effect of As(III) and Cr(VI) might through the Wnt/β-catenin signaling pathway.
Figure 4.
Carcinogenic metals alone increased the expression of β-catenin and phospho-GSK3β in the colon tissue. (A) The mice received As(III), Cr(VI), or As(III)+Cr(VI) in drinking water alone for 20 weeks. The expression of β-catenin and phospho-GSK3β in indicated treatment were determined with immunoblotting. The expression of actin served as an internal control. (B) The relative amounts of β-catenin and phospho-GSK3β was quantified microdensitometrically and normalized to the expression of actin. Each data point was the mean ± SEM of three independent experiments. *p < 0.05, statistically significant difference from control group.
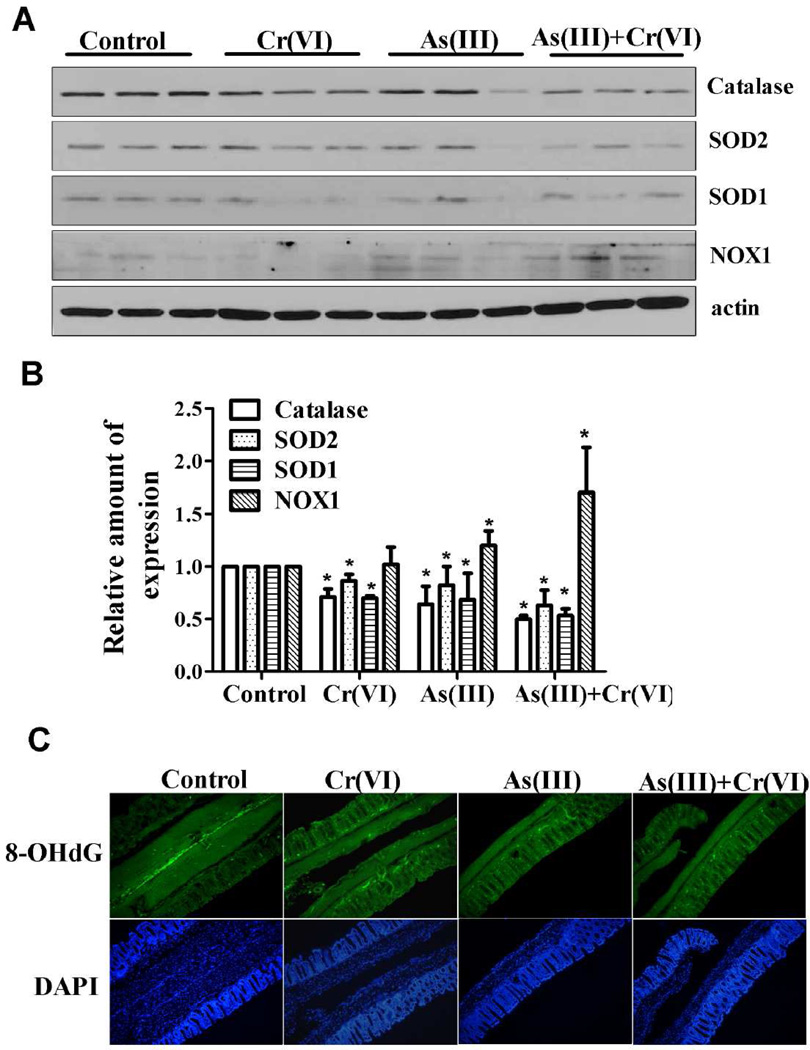
As(III) or Cr(VI), alone or in combination, in drinking water increased the expression of NOX1 and the level of 8-OHdG, a DNA oxidative damage maker, and decreased antioxidant enzymes expression in colon
Both As(III) and Cr(VI) are well-known ROS inducers. NOX1, a member of ROS-generating NADPH oxidase family, is highly expressed in colorectal cancers. As shown in Figure 5A and 5B, the expression of NOX1 was upregulated by As(III) or Cr(VI), alone or in combination, in drinking water for 20 weeks in mice without AOM/DSS treatment. As(III) or Cr(VI) decreased cellular antioxidant capacity by decreasing the expression of SOD1, SOD2, and catalase. 8-Hydroxydeoxyguanosine (8-OHdG) has been frequently used as a biomarker for oxidative DNA damage caused by oxidative stress. As shown in Figure 5C, a very low level of 8-OHdG immunoreactivity was observed in the control colon mucosa, whereas an obvious increase in immunoreactivity was observed in mice treated with carcinogenic metals. Thus, it appears that As(III) or Cr(VI), alone or in combination, can induce oxidative stress, which, at least partly, was mediated by upregulation of NOX1 and impaired antioxidant defense capacity.
Figure 5.
Oxidative stress was induced in carcinogenic metals alone treated mice. (A) Carcinogenic metals alone increased the expression level of NOX1, and decreased the expression level of antioxidant enzymes, such as SOD and catalase, in colon tissue. Exposure to As(III), Cr(VI), or their combination in drinking water for 20 weeks. The expression of NOX1, SOD1, SOD2, and catalase were determined by immunoblotting. The experiment was replicated three times. (B) The relative amounts of NOX1, SOD1, SOD2, and catalase were measured microdensitometrically and normalized to the expression of actin. Each data point was the mean ± SEM of three independent experiments. *p < 0.05, statistically significant difference from control group. (C) Immunofluorescence staining for 8-OHdG in indicated treatments. Green: 8-OHdG; Blue: DAPI. Magnification was 200×.
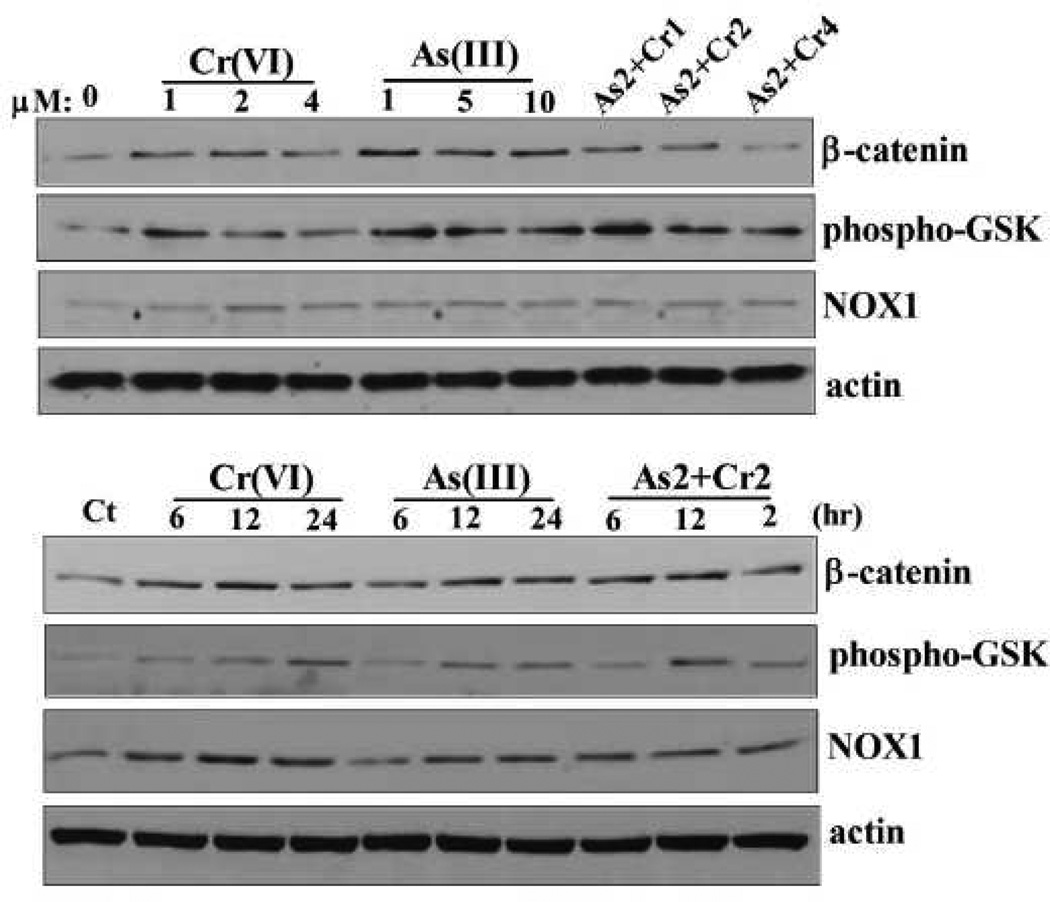
As(III) or Cr(VI), alone or in combination, increased the expression of β-catenin in CRL-1807 cells
To determine the effect of carcinogenic metals on β-catenin expression in vitro system, CRL-1807 human coloncytes cells were used in the following studies. First, we evaluated the effect of As(III) or Cr(VI), alone or in combination, on cell viability. MTT results revealed that As(III) or Cr(VI) exposure, alone or in combination, decreased cell viability in a time- and dose- dependent manner (Supplemental data). Exposure to 10 µM of As(III), 4 µM of Cr(VI), or a combination of 2 µM As(III) with 2 µM Cr(VI) for 48 h decreased cell viability to 30%, 58%, and 43%, respectively (Supplemental data). We selected 10 µM As(III), 4 µM Cr(VI), and a combination of 2 µM As(III) with 2 µM Cr(VI), for our following short-term experiment. As shown in Figure 6, As(III) or Cr(VI), alone or in combination, increased the expression level of β-catenin in a dose- and time- dependent manner, compared with untreated cells. Concomitantly, the expression level of Ser-9 phosphorylation of GSK3β was also increased in metals-treated cells. These results indicated that As(III) or Cr(VI), alone or in combination, increased the expression of β-catenin in CRL-1807 cells and phosphorylation of its upstream inhibitor, GSK3β.
Figure 6.
Carcinogenic metals increased the expression of β-catenin and phospho-GSK3β in CRL-1807 cells in does- and time- dependent manner. CRL-1807 cells were treated with Cr(VI) (0, 1, 2, 4 µM), As(III) (0, 1, 5, 10 µM), or their combination for 24 h, or treated with 4 µM Cr(VI), 10 µM As(III), or 2 µM of As(III) combination with 2 µM of Cr(VI) for indicated time points. The expression of β-catenin and phospho-GSK3β were determined with immunoblotting. The expression of actin served as an internal control. As2+Cr1, As2+Cr2, As2+Cr4: 2 µM of As(III) combination with 1, 2, 4 µM of Cr(VI), respectively.
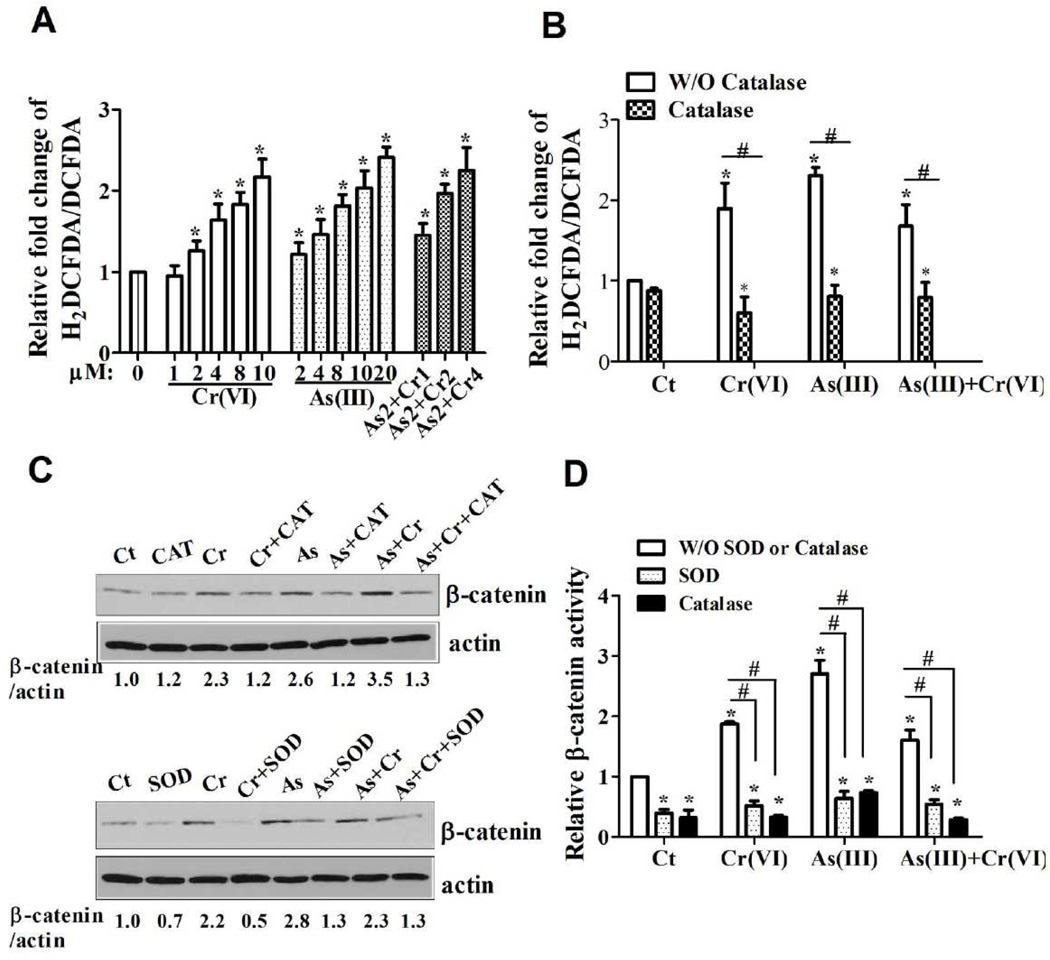
Inhibition of ROS generation decreased carcinogenic metals induced β-catenin activation in CRL-1807 cells
To further determine the role of ROS in β-catenin activation induced by As(III) or Cr(VI), we examined the ROS generation induced by carcinogenic metals in CRL-1807 cells. The fluorescence intensity produced by DCFDA was significantly higher in metals-treated group, compared with control group (Figure 7A). DCF signal was inhibited by catalase (H2O2 scavenger), which verified the ROS generation induced by As(III) or Cr(VI), alone or in combination (Figure 7B). We further examined the role of ROS in β-catenin activation induced by As(III) or Cr(VI) in CRL-1807 cells. As shown in Figure 7C, cotreatment of As(III) Cr(VI) or As(III)+Cr(VI) with either PEG-SOD or CAT reduced β-catenin expression level. Similar results were observed in the β-catenin luciferase activities. As shown in Figure 7D, β-catenin activity was significantly increased in metals-treated groups compared with the control group. Addition of SOD or catalase caused a strong inhibition. Taken together, these results suggest that inhibition of ROS generation decreased carcinogenic metals-induced β-catenin activation in CRL-1807 cells.
Figure 7.
ROS generation induced by carcinogenic heavy metal mediated β-catenin activation. (A) ROS generation induced by carcinogenic metals in CRL-1807 cells was determined by DCF assay. CRL-1807 cells were treated with As(III) or Cr(VI), alone or in combination, for 24 h; the ratio of carboxy-H2DCFDA (oxidation sentive) to carboxy-DCFDA (Oxidation insensitive) was compared. Each data point was the mean ± SEM of three independent experiments. *p < 0.05, statistically significant difference from untreated control cells. As2+Cr1, As2+Cr2, As2+Cr4: 2 µM of As(III) combination with 1, 2, 4 µM of Cr(VI), respectively. (B) Addition of catalase inhibited carcinogenic metals-induced ROS generation. CRL-1807 cells were treated with Cr(VI) (0 or 4 µM), As(III) (0 or 10 µM), As(III) combination with Cr(VI) [As: 2 µM; Cr(VI): 2 µM], or pre-treated with catalase (1000 Unit/ml) for 2 h followed by carcinogenic metals exposure for 24 h. After exposure, cells were labeled with H2DCFDA (10 µM) as described under the Material and Method. (C) Addition of PEG-SOD or catalase inhibited the β-catenin expression level induced by carcinogenic metals in CRL-1807 cells. CRL-1807 cells were treated with Cr(VI) (0 or 4 µM), As(III) (0 or 10 µM), As(III) combination with Cr(VI) [As: 2 µM, Cr(VI): 2 µM], or pre-treated with SOD (1000 Unit/ml) or catalase (1000 Unit/ml) for 2 h followed by carcinogenic metals exposure for 24 h. The expression of β-catenin was determined with immunoblotting. The expression of actin served as an internal control. (D) Addition of PEG-SOD or catalase inhibited the β-catenin activity induced by carcinogenic metals in CRL-1807 cells. The cells were transfected with super TOP flash luciferase reporter and renilla reporter followed by treatment with carcinogenic metals treatment. The cells were harvested for the measurement of luciferase activity after 24 h. Values are expressed as relative units after internal normalization with renilla luciferase. *p < 0.05, statistically significant difference from control cells. The data are expressed as the mean ± S.E. of three independent experiments. #p < 0.05, statistically significant difference from carcinogenic metals-treated cells.
Discussion
In this study, we investigated the tumorigenesis of orally-administrated carcinogenic metals As(III) or Cr(VI), alone or in combination, in drinking water in a mouse colitis-associated colorectal cancer model. Our results showed that As(III) or Cr(VI), alone or in combination, with either AOM or DSS, exhibited no tumorgenesis in the observation period. But when combined with AOM/DSS, all of these treatments developed colorectal cancer. One of the most intriguing observations was a high increase in tumor incidence, multiplicity, size and grade. Histological analysis of Iba1 indicated that As(III) or Cr(VI), alone or in combination, greatly aggravates cell inflammatory response. 2D-DIGE coupled with mass spectrometry revealed that carcinogenic metals significantly changed the expression profile of colon proteins. The expression of β-catenin and phospho-GSK3β were increased in the treatment of carcinogenic metals alone. Concomitantly, the expression of NOX1 and the level of 8-OHdG were also increased. Antioxidant enzymes, SOD and catalase, were decreased. Similarly, in an in vitro system, exposure of CRL-1807 cells to carcinogenic metals increased ROS generation and the expression of β-catenin, phospho-GSK and NOX1. Inhibition of ROS generation by SOD or catalase decreased the β-catenin expression and activity. These results indicate that the stimulation effect of As(III) and Cr(VI) in drinking water on colorectal tumorigenesis may involve ROS-mediated Wnt/β-catenin signaling pathway.
As(III) and Cr(VI) are considered class I human carcinogens (IARC, 1990). There is an increasing concern about human health effects from exposure to carcinogenic metals via drinking water. Epidemiological studies have demonstrated a significant increase in the risk of lung, skin, liver, bladder, and other cancers associated with high levels of As(III) in drinking water. Cr(VI) is linked to the high incidence of respiratory cancers through inhalation. However, its carcinogenic potential when orally ingested remains controversial. In 2008, the National Toxicology Program (NTP) completed a 2-year cancer bioassay for Cr(VI) in drinking water. This study, investigators found that Cr(VI) caused tumors in the small intestines of mice and the oral mucosa of rats at exposure of 20–180 mg/L Cr(VI) in the form of sodium dichromate dehydrate (SDD) (NTP 2008b; (Stout et al., 2009). An earlier study also found an increased incidence of benign and malignant combined forestomach neoplasms in mice orally exposed to Cr(VI) (Borneff et al., 1968).
Colorectal cancer is the third most common malignancy and the fourth most common cause of cancer mortality worldwide (Jemal et al., 2010). More than 1 million new cases of colorectal cancer are diagnosed worldwide each year (Tenesa and Dunlop 2009). Colorectal cancer is also the second most common cause of cancer deaths in the Unites States and other developed counties (Jemal et al., 2010; Tenesa and Dunlop 2009). The most current five years of data from the United States Cancer Statistics show that Kentucky has the second highest colorectal cancer incidence in the U.S. compared to other states (USCS, 2011). The colorectal cancer incidence rate for Kentucky (58.0 per 100,000 population) is significantly higher than the rate for the U.S. (48.9/100,000, p < 0.05) (USCS, 2011). The very highest rate (60.0/100,000) occurs in the Appalachian region of Kentucky (USCS, 2011). In this area, the carcinogenic metals concentrations in drinking water are higher compared to EPA standard (Johnson et al., 2011). These ecological studies indicate that there is a correlation between carcinogenic metals level in the environment and colorectal cancer incidence. However, the exact molecular mechanism is still unknown.
Although As(III) and Cr(VI) are established carcinogens in human, the mechanisms of their carcinogenesis in humans remains to be investigated. In contrast to most other human carcinogens, it has been difficult to investigate the carcinogenicity of these metals in experimental animals. Xenoplant model, which is straightforward and easily achievable, is most widely used in study of carcinogenicity of metals in vivo. The ability of tumor cells to develop tumors after subcutaneous or intravenous injection in immunodeficient mouse stains (nude, bg/nu/xid, or SCID mice) allowed the analysis of tumors induced by carcinogenic metals in vivo (Garofalo et al., 1993). Although the xenoplant models represents an important step towards a pathobiologically relevant model of tumor metastasis, subcutaneous or intravenous injection of tumor cells establish a model that neglects the complexity of tumor growth and metastasis and the interactions between tumor and microenvironment, which largely controls the biological characteristics of tumors.
In this study, we used AOM/DSS-induced colitis-associated colon cancer model to study tumorigenesis of carcinogenic metals alone, a combination of metals with either AOM or DSS, and with AOM/DSS. Different murine strains have different susceptibilities to AOM/DSS treatment (De Robertis et al., 2011). It is reported that there was 100% tumor frequency in C57BL/6J mice, when mice were injected a single i.p. injection of AOM (10 mg/kg body weight), followed by 4 cycles of 4% DSS treatment (each cycle = 4 d of 4% DSS followed by untreated water for 21 d; total 21d) (Clapper et al., 2007). The AOM/DSS protocol used in this study included a single i.p. injection of AOM (10 mg/kg body weight), followed by 2% DSS in drinking water for 7 days. Then the indicated mice were exposed to carcinogenic metals for following 20 weeks. Mice treated with metals alone, or combination with either AOM or DSS, did not induce tumors during the observation period. It is possible that after longer exposure time these metals, alone or in combination with AOM or DSS, in drinking water could induce colon tumors. AOM/DSS treatment showed 60% tumor formation, with no extensive high grade dysplasia. After given AOM/DSS and administration of As(III) or Cr(VI) in drinking water, alone or in combination, for 20 weeks, the incidence of colorectal tumors was 100% and the tumor were easily detectable, graded by histological examination as extensive high grade dysplasia compared to AOM/DSS alone treatment. Carcinogenic metals markedly enhanced the severity of colitis-associated colonic tumors, as evidenced by increased tumor grade, size, incidence and multiplicity.
It’s reported that arsenic and chromium could have co-carcinogenic and tumor co-promoting activities in several kinds of cell types, such as epidermal keratinocytes and, as a result of increasing levels of β-catenin and phosphorylated (inactive) GSK3β (Ali et al., 2011; Patterson et al., 2005). To determine the mechanism of promotion effect of carcinogenic metals on colorectal tumor formation, it’s important to clarify the effect of carcinogenic metals alone on colon tissue. We performed experiments to address this issue. Beta-catenin, a central molecule of the Wnt-signaling pathway is known to be involved in the tumorigenesis of various gastronintestinal cancers such as gastric cancer and colon cancer. In the absence of Wnt stimulation, this pathway is regulated at least in part by a multiprotein complex consisting of axin, GSK3β, and the tumor suppressor protein APC (Peifer and Polakis 2000; Polakis 2000, 2007). Formation of this complex facilitates the phosphorylation of critical residues by GSK3β in the NH2 terminus of β-catenin, targeting it for degradation by the ubiquitin/proteasome pathway. Wnt stimulation shuts off β-catenin degradation by inhibiting GSK3β in the axin complex (Bienz and Clevers 2003). This inhibition is believed to be the key event in the activation of the Wnt/β-catenin signaling pathway (Tamai et al., 2004). To investigate the effect of carcinogenic metals alone on the protein expression profile, we performed 2D-DIGE analysis and mass spectrometry. The results showed As(III) or Cr(VI) alone treatment significantly changed the density of proteins. Among them, there are many proteins are linked to beta-catenin in cancer, such as myosin-1 (Geisbrecht and Montell 2002), protein disulfide-isomerase A6 (Verras et al., 2008), heat shock 70kD protein 5 (Tao et al., 2009), myosin light-chain protein (Zhou et al., 2008). Our results show that As(III) or Cr(VI), alone or in combination, in vivo or in vitro, increased β-catenin expression level. Phosphorylation of GSK3β (Ser9), which is the inactive form of GSK3β, was also increased after carcinogenic metals alone treatment in vivo and in vitro. The results suggest that carcinogenic metals induced activation of β-catenin is, at least partly, through the GSK3β-dependent pathway. However, it is noted that there is no duplicate effect on the expression of β-catenin in As(III) and Cr(VI) combination treatment both in vivo and in vitro. One of the possible explanations is that the synergistic or independent toxicity induced by mixtures of As(III) and Cr(VI) may depend on the end point investigated and the experimental protocol employed.
Carcinogenic metals are well known ROS inducer. It is believed that ROS play an important role in cancer development, both in the initiation and promotion stages of carcinogenesis (Huang et al. 2004). In a multi-step process of colon carcinogenesis, ROS were found to enhance colon carcinogenesis at all stages: initiation, promotion, and progression (Erdelyi et al., 2009). NADPH oxidase is one of the major sources of cellular ROS. NOX1, a member of NADPH oxidase family that is highly expressed in colonic epithelial cells, plays a pivotal role in cell signaling, cell growth, angiogenesis, and motility that integrates Wnt/β-catenin signals (Coant et al., 2010). It has been reported that NOX1 overexpression in colon tumors could contribute to the development of colorectal cancer (Laurent et al., 2008). The present study shows that As(III) or Cr(VI), alone or in combination, increased NOX1 expression in vivo. In addition, the expression of several important antioxidant enzymes, such as SOD and catalase, were decreased in mice exposure to carcinogenic metals in drinking water. The level of 8-OHdG, which is an oxidative DNA damage marker, was also increased, indicating that oxidative stress was involved in carcinogenic metals- induced tumor promotion effect. The expression of β-catenin and phospho-GSK3β, as well as the level of ROS generation was also increased by As(III) or Cr(VI), alone or in combination in CRL-1807 cells. SOD or catalase decreased metals-induced β-catenin expression and activity, suggesting that ROS is important in casuing β-catenin activation induced by carcinogenic metals.
In conclusion, the present study demonstrated that As(III) or Cr(VI), alone or in combination, in drinking water promote tumorigenesis in murine AOM/DSS colitis-associated colorectal cancer model. ROS-mediated β-catenin activation may play an important role in this promotion effect.
Supplementary Material
Highlights.
Carcinogenic metals in drinking water promote colorectal tumor formation in vivo
Carcinogenic metals induce β-catenin activation in vivo and in vitro
ROS generation induced by carcinogenic metals mediated β-catenin activation
Acknowledgments
Funding
This work was supported by National Institutes of Health grants (1R01CA119028, 1R01CA116697, R01 ES015375, and 1R01ES015518).
Abbreviations
- AOM
Azoxymethane
- DSS
Dextran sodium sulfate
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
Fetal bovine serum
- H&E
Hematoxylin & Eosin
- Cr(VI)
Hexavalent chromium
- 8-OHdG
8-Hydroxydeoxyguanosine
- IARC
International Agency for Research on Cancer
- IEF
Isoelectric focusing
- NOX1
NADPH oxidase 1
- NTP
National Toxicology Program
- MTT
3-(4,5-dime-thylthiazol-2yl-2,5-diphenyl tetrazolium bromide
- ROS
Reactive oxygen species
- SDS-PAGE
SDS polyacrylamide gel electrophoresis
- SOD
Sodium dichromate dehydrate
- As(III)
Trivalent arsenic
- 2D-DIGE
Two-dimensional differential gel electrophoresis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None of the listed authors has any financial or other interests that could be of conflict.
References
- Ali AH, Kondo K, Namura T, Senba Y, Takizawa H, Nakagawa Y, Toba H, Kenzaki K, Sakiyama S, Tangoku A. Aberrent DNA methylation of some tumor suppressor genes in lung cancers from workers with chromate exposure. Mol. Cacinog. 2011;50:89–99. doi: 10.1002/mc.20697. [DOI] [PubMed] [Google Scholar]
- Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- Bienz M, Clevers H. Armadillo/beta-catenin signals in the nucleus--proof beyond a reasonable doubt? Nat. Cell Biol. 2003;5:179–182. doi: 10.1038/ncb0303-179. [DOI] [PubMed] [Google Scholar]
- Borneff J, Engelhardt K, Griem W, Kunte H, Reichert J. Carcinogens in water and soil. XXII. Experiment with 3,4-benzopyrene and potassium chromate in mice drink. Arch. Hyg. Bakteriol. 1968;152:45–53. [PubMed] [Google Scholar]
- Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta. pharmacol. Sin. 2007;28:1450–1459. doi: 10.1111/j.1745-7254.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- Coant N, Ben Mkaddem S, Pedruzzi E, Guichard C, Treton X, Ducroc R, Freund JN, Cazals-Hatem D, Bouhnik Y, Woerther PL, Skurnik D, Grodet A, Fay M, Biard D, Lesuffleur T, Deffert C, Moreau R, Groyer A, Krause KH, Daniel F, Ogier-Denis E. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol. Cell Biol. 2010;30:2636–2650. doi: 10.1128/MCB.01194-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Wakai T, Shirai Y, Hatakeyama K, Hirano S. Chronic oral exposure to inorganic arsenate interferes with methylation status of p16INK4a and RASSF1A and induces lung cancer in A/J mice. Toxicol. Sci. 2006;91:372–381. doi: 10.1093/toxsci/kfj159. [DOI] [PubMed] [Google Scholar]
- De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, Signori E, Fazio VM. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Liu W, Cooper KL, Qin XJ, de Souza Bergo PL, Hudson LG, Liu KJ. Inhibition of poly(ADP-ribose) polymerase-1 by arsenite interferes with repair of oxidative DNA damage. J. Biol. Chem. 2009;284:6809–6817. doi: 10.1074/jbc.M805566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo A, Chirivi RG, Scanziani E, Mayo JG, Vecchi A, Giavazzi R. Comparative study on the metastatic behavior of human tumors in nude, beige/nude/xid and severe combined immunodeficient mice. Invasion Metastasis. 1993;13:82–91. [PubMed] [Google Scholar]
- Gatto NM, Kelsh MA, Mai DH, Suh M, Proctor DM. Occupational exposure to hexavalent chromium and cancers of the gastrointestinal tract: a meta-analysis. Cancer Epidemiol. 2010;34:388–399. doi: 10.1016/j.canep.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Geisbrecht ER, Montell DJ. Myosin VI is required for E-cadherin-mediated border cell migration. Nat. Cell Biol. 2002;4:616–620. doi: 10.1038/ncb830. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hamilton SR, Aaltonen LA. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC press; 2010. World Health Organization Classification of Tumours. 2010. [Google Scholar]
- Hamilton JW, Kaltreider RC, Bajenova OV, Ihnat MA, McCaffrey J, Turpie BW, Rowell EE, Oh J, Nemeth MJ, Pesce CA, Lariviere JP. Molecular basis for effects of carcinogenic heavy metals on inducible gene expression. Environ. Health Perspect. 1998;106(Suppl 4):1005–1015. doi: 10.1289/ehp.98106s41005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Ke Q, Costa M, Shi X. Molecular mechanisms of arsenic carcinogenesis. Mol. Cell Biochem. 2004;255:57–66. doi: 10.1023/b:mcbi.0000007261.04684.78. [DOI] [PubMed] [Google Scholar]
- Huff J, Chan P, Nyska A. Is the human carcinogen arsenic carcinogenic to laboratory animals? Toxicol. Sci. 2000;55:17–23. doi: 10.1093/toxsci/55.1.17. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer. J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Laurent E, McCoy JW, 3rd, Macina RA, Liu W, Cheng G, Robine S, Papkoff J, Lambeth JD. Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int. J. Cancer. 2008;123:100–107. doi: 10.1002/ijc.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TJ, Reznikova TV, Phillips MA, Rice RH. Arsenic maintains germinative state in cultured human epidermal cells. Toxicol. Appl. Pharmacol. 2005;207:69–77. doi: 10.1016/j.taap.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes. Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Polakis P. The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Qian Y, Liu KJ, Chen Y, Flynn DC, Castranova V, Shi X. Cdc42 regulates arsenic-induced NADPH oxidase activation and cell migration through actin filament reorganization. J. Biol. Chem. 2005;280:3875–3884. doi: 10.1074/jbc.M403788200. [DOI] [PubMed] [Google Scholar]
- Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- Stout MD, Nyska A, Collins BJ, Witt KL, Kissling GE, Malarkey DE, Hooth MJ. Chronic toxicity and carcinogenicity studies of chromium picolinate monohydrate administered in feed to F344/N rats and B6C3F1 mice for 2 years. Food Chem. Toxicol. 2009;47:729–733. doi: 10.1016/j.fct.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, St Clair DK, Xu Y, Crooks PA, St Clair WH. A NADPH oxidase-dependent redox signaling pathway mediates the selective radiosensitization effect of parthenolide in prostate cancer cells. Cancer Res. 2010;70:2880–2890. doi: 10.1158/0008-5472.CAN-09-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol. Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Tao Y, Hart J, Lichtenstein L, Joseph LJ, Ciancio MJ, Hu S, Chang EB, Bissonnette M. Inducible heat shock protein 70 prevents multifocal flat dysplastic lesions and invasive tumors in an inflammatory model of colon cancer. Carcinogenesis. 2009;30:175–182. doi: 10.1093/carcin/bgn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchounwou PB, Patlolla AK, Centeno JA. Carcinogenic and systemic health effects associated with arsenic exposure--a critical review. Toxicol. Pathol. 2003;31:575–588. doi: 10.1080/01926230390242007. [DOI] [PubMed] [Google Scholar]
- Tenesa A, Dunlop MG. New insights into the aetiology of colorectal cancer from genome-wide association studies. Nat. Rev. Genet. 2009;10:353–358. doi: 10.1038/nrg2574. [DOI] [PubMed] [Google Scholar]
- Tokar EJ, Benbrahim-Tallaa L, Ward JM, Lunn R, Sams RL, Waalkes MP., 2nd Cancer in experimental animals exposed to arsenic and arsenic compounds. Crit. Rev. Toxicol. 2010;40:912–927. doi: 10.3109/10408444.2010.506641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verras M, Papandreou I, Lim AL, Denko NC. Tumor hypoxia blocks Wnt processing and secretion through the induction of endoplasmic reticulum stress. Mol Cell Biol. 2008;28:7212–7224. doi: 10.1128/MCB.00947-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fan Z, Wang B, Luo J, Ke ZJ. Activation of double-stranded RNA-activated protein kinase by mild impairment of oxidative metabolism in neurons. J. Neurochem. 2007;103:2380–2390. doi: 10.1111/j.1471-4159.2007.04978.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Son YO, Chang Q, Sun L, Hitron JA, Budhraja A, Zhang Z, Ke Z, Chen F, Luo J, Shi X. NADPH oxidase activation is required in reactive oxygen species generation and cell transformation induced by hexavalent chromium. Toxicol. Sci. 2011;123:399–410. doi: 10.1093/toxsci/kfr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wang X, Cheng S, Sun L, Son YO, Yao H, Li W, Budhraja A, Li L, Shelton BJ, Tucker T, Arnold SM, Shi X. Reactive oxygen species mediate arsenic induced cell transformation and tumorigenesis through Wnt/beta-catenin pathway in human colorectal adenocarcinoma DLD1 cells. Toxicol. Appl. Pharmacol. 2011;256:114–121. doi: 10.1016/j.taap.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Zhou X, Liu Y, You J, Zhang H, Zhang X, Ye L. Myosin light-chain kinase contributes to the proliferation and migration of breast cancer cells through cross-talk with activated ERK1/2. Cancer Lett. 2008;270:312–327. doi: 10.1016/j.canlet.2008.05.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.