Abstract
Background
Our purpose was to elucidate relative preferences of women with ovarian cancer for symptoms, treatment-related side effects, and progression-free survival (PFS) relevant to choosing a treatment regimen.
Methods
Women with advanced or recurrent ovarian cancer participated in a survey inclusive of three methods to measure patient preferences (ratings, rankings and a discrete-choice experiment [DCE]) for 7 attributes: mode of administration, visit frequency, peripheral neuropathy, nausea and vomiting, fatigue, abdominal discomfort and PFS. Participants were asked to choose between two unlabeled treatment scenarios characterized using the 7 attributes. Each participant completed 12 choice questions wherein attribute levels were assigned according to an experimental design and a fixed choice question representing two chemotherapy regimens for ovarian cancer.
Results
95 women completed the survey. Participants' ratings and rankings revealed greater concern and importance for PFS than for any other attribute (p<0.0001 for all). DCE revealed that the relative odds that a participant would choose a scenario with 18, 21 and 24 months of PFS versus 15 months of PFS were 1.5 (p=0.01), 3.4 (p<0.001) and 7.5 (p<0.001), respectively. However, participants' choices indicated that they were willing to accept lower PFS to avoid severe side effects: 6.7 months to reduce severe nausea and vomiting to mild, 5.0 months to reduce severe neuropathy to mild, and 3.7 months to reduce severe abdominal symptoms to moderate.
Conclusion
PFS is the predominant driver of patient preferences for chemotherapy regimens. However, women in this study were willing to trade significant PFS time for reductions in treatment-related toxicity.
Introduction
While ovarian cancer mortality has dropped slightly over the past 10 years, major therapeutic breakthroughs resulting in overall survival (OS) improvements have been infrequent. Most recently, intraperitoneal chemotherapy1 and dose dense paclitaxel2 have been shown to provide superior OS to comparators in the primary treatment setting. The addition of biologic therapies such as bevacizumab to IV chemotherapy regimens has been shown to improve progression-free survival (PFS), with OS improvement seen only in subgroup analyses3, 4. Neither of these traditional clinical trial endpoints, OS nor PFS, considers how a treatment affects adverse events, quality of life (QOL), and convenience to the patient. Practitioners' understanding of the relative importance of these factors is critical when considering how to best present treatment options to patients.
Patients are likely to have opinions about the relative importance of different types of expected outcomes of their cancer treatment. The preferences of cancer patients concerning quality versus length of life have been reported5, 6, but only rarely in the context of a stated preference study in which patients are asked to jointly consider several aspects of ovarian cancer treatments7, 8. Conjoint analysis represents an approach that systematically examines factors that drive an individual's choice. One type of conjoint analysis, discrete choice experiments, have traditionally been used in marketing, and have more recently been used to examine determinants of patients' preferences for aspects of their healthcare9. In a discrete choice experiment, individuals are asked to consider two or more hypothetical scenarios and choose the one that is preferable to them. Their selections allow researchers to investigate trade offs between positive and negative aspects of the scenarios. In oncology scenarios, this may include tradeoffs between the additional survival afforded by a proposed cancer treatment and the side effects, inconveniences, or costs of the treatment.
We applied a discrete-choice experiment to study the preferences of women with ovarian cancer for disease-related symptoms, side effects, PFS and administration characteristics associated with various treatment regimens.
Methods
A prospective study of 100 women with advanced or recurrent ovarian cancer was approved by the Duke University Institutional Review Board. The sample size was determined by constraints of budget and time. Inclusion criteria were a diagnosis of stage III-IV or recurrent ovarian cancer and signed informed consent. The recruitment period was from May to October 2013. Patients meeting inclusion criteria received a study brochure from their attending gynecologic oncologist by mail or in person during a scheduled appointment, and were approached for informed consent if they expressed interest in participation. Clinical characteristics were abstracted from each participant's medical record.
Survey instrument
Within the survey instrument, participants were asked to report their current age, race, and ethnicity and to complete two validated patient reported outcome (PRO) assessments: the Functional Assessment of Cancer Therapy-Ovarian (FACT-O)10 and the MD Anderson Symptom Inventory (MDASI)11.
Discrete-choice experiment
Discrete-choice experiments (DCE), a specific type of conjoint analysis12-14, are used to quantify preferences for specific attributes or features of health states, medical interventions or health care services. Participants in a DCE are asked to choose between alternative constructed profiles that are characterized by specified levels of each outcome and convenience feature or attribute in the study design. Repeated iterations of this exercise provide data to statistically estimate rates at which respondents are willing to accept tradeoffs among attributes. Guided by best-practice recommendations for conducting conjoint analysis9, 15, we sought to test whether 7 attributes relevant to chemotherapy for ovarian cancer were associated with participants' choices and to evaluate the extent to which women were willing to trade off clinical benefits for reduced toxicity associated with treatment.
Selection of attributes and levels of chemotherapy treatment
Scenarios were developed to represent the key attributes of standard chemotherapy regimens for newly diagnosed, advanced stage ovarian cancer identified by clinical experts and investigators with extensive experience in PROs (Table 1). The seven attributes included symptoms of ovarian cancer (abdominal symptoms), side effects that are relatively common with standard platinum- and taxane-based chemotherapy regimens (neuropathy, nausea and vomiting, fatigue), features of administration that differ between the IV and IP/IV chemotherapy regimens (route of administration and frequency) and a measure of benefit (PFS). We chose to present participants with PFS but not OS information. Inclusion of both would have required restrictions in the DCE study design to disallow overall survival periods that were shorter than PFS periods; this would have led to suboptimal statistical assumptions and would have caused confusion among participants. Further, PFS is now often the primary endpoint in phase III trials in ovarian cancer16. The levels for each attribute were chosen to represent administration modalities, dosing frequency, and the range of severity of toxicities and benefits associated with standard chemotherapy regimens.
Table 1. Attributes and their corresponding levels and descriptions.
| Attribute | Attribute Levels |
|---|---|
| Route of administration | IV treatment only |
| Both IP and IV treatments | |
| Visit frequency | 1 visit every 3 weeks |
| 2 visits every 3 weeks | |
| 3 visits every 3 weeks | |
| Nausea and vomiting | Mild nausea |
| Moderate nausea and vomiting | |
| Severe nausea and vomiting | |
| Neuropathy | Mild neuropathy |
| Moderate neuropathy | |
| Severe neuropathy | |
| Fatigue | Mild fatigue |
| Moderate fatigue | |
| Abdominal symptoms | Mild abdominal symptoms |
| Moderate abdominal symptoms | |
| Severe abdominal symptoms | |
| Progression-free survival | 15 months cancer free |
| 18 months cancer free | |
| 21 months cancer free | |
| 24 months cancer free |
The attributes and corresponding levels were described by a clinician in a video. A transcript is available in the online Appendix
Experimental Design
There are 1296 possible combinations of attribute levels in Table 1. The pairs of profiles chosen for the choice questions were generated using a balanced-overlap experimental design using Sawtooth's Conjoint Value Analysis (CVA) designer (Sawtooth Software, SSI Web, Orem, Utah). A total of 120 pairs of choice questions were generated and blocked into 10 questionnaire versions, each consisting of 12 unique choice questions that were randomly assigned to study participants. Appendix Figure A displays an example choice question.
Educational video
To familiarize participants with the seven attributes and their corresponding levels, we developed a 9-minute video (http://vimeo.com/61893779) that was shown to each participant prior to completing the preference elicitation (video text in Appendix).
Rating and ranking exercises
Following the video, each participant was asked to rate her level of concern for each of the 7 attributes using 5-point Likert scale (1=not concerned to 5=deeply concerned), and then to rank the attributes from most (1) to least (7) important.
Instrument design
In addition to the 12 choice questions, a fixed choice set was embedded within the DCE. In this set, the levels for each of the attributes were set to correspond to an actual therapeutic choice between two standard regimens for treatment of newly diagnosed stage III ovarian cancer: (1) IV paclitaxel, IP cisplatin, and IP paclitaxel (‘both IV and IP treatments’; 2 visits every 3 weeks, moderate nausea/vomiting, moderate neuropathy, moderate fatigue, moderate abdominal symptoms, 24 months PFS) and (2) IV paclitaxel and IV carboplatin (‘IV treatment only’; 1 visits every 3 weeks, mild nausea/vomiting, mild neuropathy, mild fatigue, mild abdominal symptoms, 18 months PFS). Attribute levels for these scenarios were based on administration regimens, general side effect profiles, and PFS estimates from published randomized clinical trials1, 17.
Pre-testing
We pre-tested the full computer-administered survey instrument with 5 patients and used qualitative interviewing methods to gauge their understanding, with a plan to exclude these participants from the final analysis if significant revisions to the survey were necessary.
Statistical analysis
Rating and ranking data
We used descriptive statistics to summarize ratings and rankings for each of the 7 DCE attributes. We used paired t-tests to compare distributions of rankings and ratings between attributes.
Choice-experiment data
We applied mixed-logit regression to model patients' choices as a function of attribute levels and estimated odds ratios controlling for unobserved heterogeneity in preferences. When odds ratios are greater than 1, estimates indicate greater preference for the attribute level relative to the reference level. When odds ratios are less than 1, participants are expressing a smaller preference for the attribute level relative to the reference level. We report results using both the mean attribute effect (effects coding) and an omitted category (dummy coding). We also calculated the implied time in PFS that participants were willing to forgo for an improvement for each attribute from its worst to its best level18. We refer to this as a PFS-time equivalent. We used nonparametric bootstrapping to compute 95% confidence intervals for the PFS-time equivalents.
Exploratory analyses
Exploratory subgroup analyses
We planned to generate separate results for two subgroups: (1) participants currently receiving treatment for ovarian cancer vs. participants not currently receiving treatment; and (2) participants without vs. patients with a history of disease recurrence.
Post-hoc analyses
To account for the possibility that some participants would always choose the alternative with the longest PFS period despite the levels of other attributes, we re-ran the mixed logit models after excluding women who chose the scenario with the longest PFS time in every choice question in which PFS levels differed between alternatives.
Results
Baseline characteristics
Of 259 eligible patients who were initially provided with written recruitment materials concerning the study, 100 participants provided informed consent. Five were excluded from analysis due to early-phase data-capture errors (n=2), withdrawal of consent (n=2) or participant no-show (n=1); 95 remained for final analysis (Figure 1). Participants' characteristics and PRO measures are described in Table 2. Minor technical revisions were made to the survey following pre-testing, and therefore 95 participants were retained for analysis. The study population was predominately Caucasian (85%) with advanced stage at diagnosis (81%). At the time of the survey, 45 (47%) had experienced disease recurrence, 49 (51%) were currently receiving chemotherapy, and 45 (47%) had received at least two prior chemotherapy regimens. The mean Trial Outcome Index (TOI) of the FACT-O was 78.7, which is similar to the TOI of women enrolled in a randomized trial of the addition of bevacizumab to standard chemotherapy for advanced stage ovarian cancer, collected several months after completion of primary chemotherapy19. The mean MDASI symptom severity score was 1.52 (low range).
Figure 1.
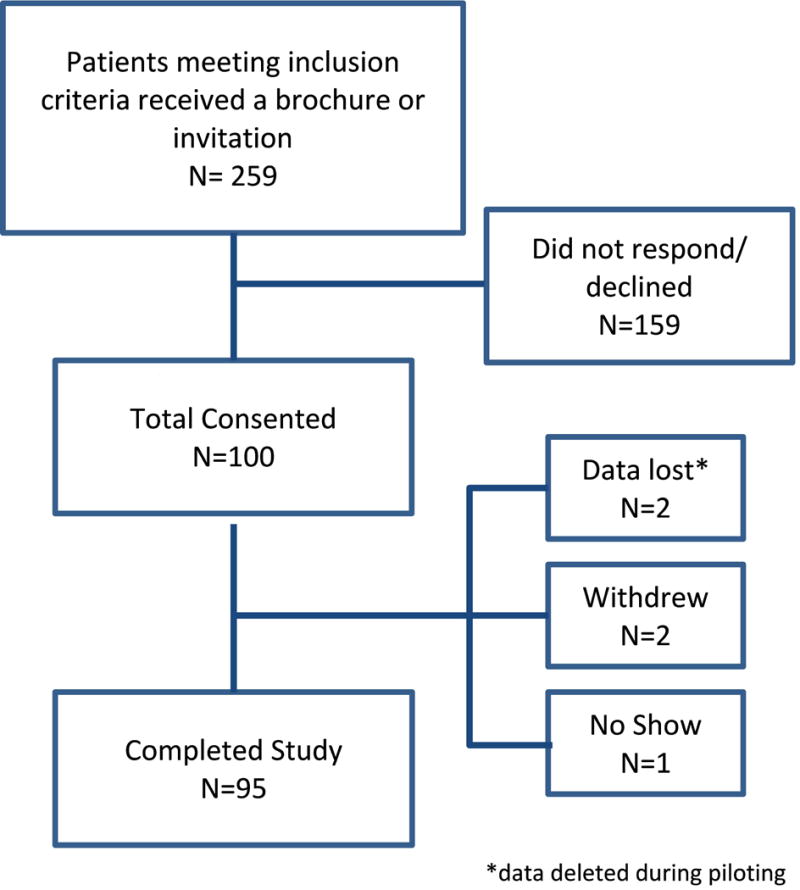
CONSORT Diagram.
Table 2. Participant characteristics.
| Characteristic | N=95 |
|---|---|
|
| |
| Age, mean (SD) | 60.2 (10.1) years |
|
| |
| Age at diagnosis, mean (SD) | 57.8 (10.6) years |
|
| |
| Race, n (%) | |
| White | 81 (85%) |
| Black or African American | 9 (9%) |
| Asian | 2 (2%) |
| Other or not reported | 3 (3%) |
|
| |
| BMI, mean (SD) | 27.5 (5.8) |
|
| |
| Clinical characteristics | |
|
| |
| Stage at diagnosis, n (%) | |
|
| |
| 1 | 2 (2%) |
|
| |
| 2 | 10 (11%) |
|
| |
| 3 | 59 (62%) |
|
| |
| 4 | 18 (19%) |
|
| |
| Not available | 6 (6%) |
|
| |
| Has experienced disease recurrence, n (%) | 45 (47%) |
|
| |
| Currently receiving chemotherapy, n (%) | 49 (52%) |
|
| |
| Receipt of prior treatment, n (%) | |
|
| |
| Neoadjuvant chemotherapy | 17 (18%) |
|
| |
| IV chemotherapy | 95 (100%) |
|
| |
| IP chemotherapy | 21 (22%) |
|
| |
| 2 or more prior chemotherapy regimens | 45 (47%) |
|
| |
| Radiotherapy | 6 (6%) |
|
| |
| GOG Performance status, n (%) | |
| 0 | 37 (39%) |
| 1 | 13 (14%) |
| 2 | 2 (2%) |
| 3 | 1 (1%) |
| Not reported | 42 (44%) |
|
| |
| Prior documentation of CTCAE grade 2 or greater chemotherapy toxicities, n (%) | |
| Peripheral neuropathy | 10 (10%) |
| Nausea/vomiting | 8 (8%) |
| Fatigue | 17 (18%) |
|
| |
| PRO Measures | |
|
| |
| TOI of FACT-O (range 0-100), mean (SD) | 78.7 (14.1) |
|
| |
| MDASI symptom severity score, mean (SD) | 1.52 (1.49) |
| MDASI symptom interference score, mean (SD) | 1.50 (2.04) |
SD, standard deviation
BMI, body mass index
PRO, patient-reported outcomes
GOG, Gynecologic Oncology Group
CTCAE, Common Terminology Criteria for Adverse Events
TOI of FACT-O, Trial Outcome Index of the Functional Assessment of Cancer Therapy-Ovarian
MDASI, MD Anderson Symptom Index
Ratings and rakings of attributes
Table 3 describes the relative level of concern and ranking for each attribute. PFS received the highest level of concern (mean 4.0, ‘very concerned’); just over 50% of participants were deeply concerned about PFS, while 11% were deeply concerned about abdominal symptoms and fewer than 10% were deeply concerned about any other attribute. All of the remaining attributes receiving mean scores between 2 (‘a little bit concerned’) and 3 (‘moderately concerned’). The findings from the ranking exercise further emphasized importance of PFS with nearly 76% of respondents assigning to this attribute the top ranking (1, most important). Treatment modality and treatment schedule were of least concern and importance.
Table 3. Ratings and rankings for DCE attributes.
| Attribute | Ratings* | Rankings** | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Mode | Percent rated as “Deeply concerned” | Mean (SD) | Mode | Percent with #1 ranking | |
| Treatment modality | 2.5 (1.2) | 2 | 8.4% | 4.6 (1.9) | 7 | 3.2% |
| Treatment schedule | 2.2 (1.2) | 1 | 2.1% | 5.1 (1.9) | 7 | 4.2% |
| Abdominal symptoms | 2.9 (1.2) | 3 | 11.6% | 4.1 (1.7) | 3 | 4.2% |
| Peripheral neuropathy | 2.8 (1.2) | 2 | 7.4% | 4.3 (1.7) | 4 | 2.1% |
| Fatigue | 2.6 (1.0) | 3 | 4.2% | 3.9 (1.6) | 3 | 4.2% |
| Nausea and vomiting | 2.5 (1.2) | 3 | 3.2% | 4.2 (1.8) | 4 | 6.3% |
| Progression-free survival | 4.0 (1.3)*** | 5 | 50.5% | 1.9 (1.8)*** | 1 | 75.8% |
Ratings correspond to 1=‘Not concerned’ 2=“A little bit concerned” 3=“Moderately concerned” 4=“Very concerned” 5=“Deeply concerned”.
Rankings correspond to 1=most important to 7=least important.
p<0.0001 representing comparisons between PFS and each other attribute.
Discrete-choice experiments
Odds ratios indicating greater (i.e. >1) or lower (i.e. <1) preference for each of the attribute levels relative to the mean effect (effects coding) or relative to the reference level (dummy coding) of each attribute are presented in Table 4. Figure 2 graphically depicts the relative impact of attribute levels on treatment choices. In regard to the treatment regimen, participants were significantly less likely (OR=0.81, p<0.01) to prefer the IP/IV route of administration compared to IV administration. They did not significantly differ with regard to a preference between one visit every three weeks or two visits every three weeks, but a weekly visit was significantly less preferred (OR=0.68, p<0.01). As expected, side-effect preferences for nausea and vomiting and for fatigue were consistent with the natural ordering—less-severe were preferred to more-severe levels. Participants discriminated between moderate versus mild levels of severity (OR=0.60, p<0.01) for nausea and vomiting. However, participants' choices were not significantly different for scenarios with moderate versus mild levels of peripheral neuropathy (p=0.57) or fatigue (p=0.69).
Table 4. Results from mixed-logit models (full cohort, n=95).
| Effects Coding* | Dummy Coding** | |||||
|---|---|---|---|---|---|---|
| Attribute | Levels | Odds ratios (95% CI) | p-value | Odds ratios (95% CI) | p-value | PFS time-equivalent in months± (95% CI) |
| Administration | IV treatment only | 1.11 (1.02 – 1.21) | 0.01 | 1.00 | 1.0 (0.2 - 1.8) | |
| Both IP and IV treatments | 0.90 (0.82 – 0.98) | 0.01 | 0.81 (0.68 – 0.96) | 0.01 | ||
| Visit frequency | One visit every 3 weeks | 1.19 (1.04 – 1.35) | 0.01 | 1.00 | 1.8 (1.0 - 2.6) | |
| Two visits every 3 weeks | 1.05 (0.91 – 1.20) | 0.52 | 0.88 (0.70 – 1.11) | 0.29 | ||
| Three visits every 3 weeks | 0.80 (0.71 – 0.91) | <0.01 | 0.68 (0.54 – 0.84) | <0.01 | ||
| Nausea and vomiting | Mild | 1.95 (1.69 – 2.24) | <0.01 | 1.00 | 6.7 (5.4 - 8.3) | |
| Moderate | 1.16 (1.02 – 1.33) | 0.02 | 0.60 (0.47 – 0.75) | <0.01 | ||
| Severe | 0.44 (0.38 – 0.51) | <0.01 | 0.23 (0.18 – 0.29) | <0.01 | ||
| Peripheral neuropathy | Mild | 1.48 (1.30 – 1.69) | <0.01 | 1.00 | 5.0 (3.9 - 6.2) | |
| Moderate | 1.39 (1.22 – 1.58) | <0.01 | 0.94 (0.75 – 1.17) | 0.57 | ||
| Severe | 0.49 (0.42 – 0.56) | <0.01 | 0.33 (0.26 – 0.41) | <0.01 | ||
| Fatigue | Mild | 0.98 (0.90 – 1.07) | 0.69 | 1.00 | 0.15 # | |
| Moderate | 1.02 (0.94 – 1.11) | 0.69 | 1.03 (0.88 – 1.22) | 0.69 | ||
| Abdominal symptoms | Mild | 1.04 (0.91 – 1.18) | 0.59 | 1.00 | 3.7 (2.6 - 5.0) | |
| Moderate | 1.48 (1.29 – 1.71) | <0.01 | 1.43 (1.13 – 1.81) | <0.01 | ||
| Severe | 0.65 (0.57 – 0.75) | <0.01 | 0.63 (0.50 – 0.79) | <0.01 | ||
| Progression-free survival | 15 months | 0.40 (0.33 – 0.49) | <0.01 | 1.00 | 9 months | |
| 18 months | 0.59 (0.49 – 0.72) | <0.01 | 1.47 (1.09 – 1.98) | 0.01 | ||
| 21 months | 1.38 (1.16 – 1.65) | <0.01 | 3.41 (2.49 – 4.68) | <0.01 | ||
| 24 months | 3.01 (2.52 -3.61) | <0.01 | 7.45 (5.48 – 10.12) | <0.01 | ||
with effects coding, the odds ratios are interpreted as the relative odds for an attribute level compared to the ‘average’ such that all attribute levels are centered around 1.
with dummy coding, the odds ratios are interpreted as the relative odds for an attribute level compared to the ‘reference’ level for which the odds ratio is equal to 1.
from least preferred to most preferred level
not computed due to nonsignificant difference between most extreme levels
Figure 2.

Relative impact of attribute levels on treatment choice (n=95). 1VQ3W, one visit every 3 weeks; 2VQ3W, 2 visits every 3 weeks; 3VQ3W, 3 visits every 3 weeks.
Findings pertaining to severity of abdominal symptoms were unexpected. While participants were less likely to prefer scenarios with severe abdominal symptoms compared to mild abdominal symptoms (OR=0.63, p<0.0001), they were significantly more likely to prefer scenarios with moderate relative to mild abdominal symptoms (OR=1.43, p=0.003). In comparison to 15 months of PFS, women progressively preferred longer periods of PFS.
Given the 9-month difference between the highest and lowest levels of PFS, participants' choices indicated that they would accept a reduction of 6.7 (95%CI, 5.4 – 8.3) months of PFS to move from a health state characterized by severe nausea and vomiting during treatment to a health state characterized by mild nausea and vomiting (Table 4). For an improvement from severe peripheral neuropathy to mild neuropathy, participants were willing to give up 5.0 (95%CI, 3.9 – 6.2) months of PFS. An improvement from severe to moderate abdominal symptoms was equivalent to 3.7 (95%CI, 2.6 – 5.0) months of PFS.
Group comparisons
DCE findings were similar between women currently receiving chemotherapy and those who were not and between women with disease recurrence and those without (Appendix Figure B).
Post-hoc analysis
Twenty participants (21%) always selected the alternative describing longer PFS despite other attribute levels shown. When excluding these respondents, findings from the mixed logit models revealed very similar findings with regard to direction of model parameters and statistical significance, but greater willingness to trade off time in PFS (Appendix, Table A).
Fixed-choice scenarios
In the realistic fixed-choice scenario comparing ‘IV treatment only’ to ‘both IP and IV treatments’ (Table 2), 52% of all respondents chose IP/IV treatment. Among patients who had not experienced disease recurrence, 60% opted for the IP/IV treatment option compared to 42% of patients who had experienced disease progression (Appendix Figure C, p= 0.08). Among patients who had previously received IP administration, 86% chose the IP/IV alternative compared to 42% who had not previously received IP chemotherapy (p<0.01).
Discussion
In the current clinical-trial environment, a statistically significant difference in PFS as a primary endpoint may be considered a success, regardless of the number of months gained. However, other factors such as side effects and convenience of a treatment regimen could influence both clinicians' and patients' preferences and also should be presented when discussing treatment options. Across the three sets of questions designed to investigate patients' preferences for treatment outcome and convenience features relevant to treatment of ovarian cancer, we found that PFS is the most important factor that women consider. However, in the discrete-choice experiment, 79% of women in our sample accepted reductions in PFS in return for improvements in side effects or convenience. These findings indicate that most women in our study were willing to trade off PFS time for improvements in health-related quality of life. Most strikingly, women's choices revealed that they were willing to give up nearly 7 months of PFS to reduce nausea and vomiting during treatment from a severe to mild level and 5 months of PFS to reduce peripheral neuropathy from severe to mild. These data are similar to the findings of Herzog et al., who conducted an online survey of ovarian cancer survivors and reported that women were only willing to accept higher toxicity if treatment were potentially curative. Furthermore, survivors who completed the survey expected a minimum of 5 additional months of PFS or OS from any new therapy8.
Because discrete-choice experiments require an individual to consider tradeoffs among several factors simultaneously, they arguably better represent real-world preferences than rating and rankings exercises. Although choices made throughout the design and conduct of complex survey research can impart potential biases, the consistencies we found with regard to hypothesized and observed relationships between factors and across the preference exercises are reassuring. Women consistently expressed aversion to scenarios characterized by severe levels of peripheral neuropathy, abdominal symptoms, and nausea and vomiting. However, the greater preference for moderate vs. mild abdominal symptoms was perplexing. Upon review of our descriptions of mild and moderate abdominal symptoms, our description of the mild level included uncertain events (e.g. “may have occasional discomfort”) as opposed to our use of certain events for the moderate level (e.g. “has abdominal fullness and tightness”). Other researchers with experience in conjoint analysis suggest that describing events as uncertain increases decision-making complexity 20.
When taken in the context of the 2-6 month PFS advantage afforded by recently reported novel therapies for ovarian cancer such as bevacizumab, our data suggest the need to incorporate women's preferences into treatment discussions. The clinical benefit that might be derived from a novel therapy is likely not defined by PFS alone; composite endpoints have been developed and put into practice for a variety of cancers21, 22. Endpoints such as “clinical benefit response” often are defined by functional status and specific symptoms, but no formal scheme for weighting components of a composite has been proposed23. The current data can help inform development of more principled approaches to developing patient-centered composite endpoints for ovarian cancer. One limitation to any weighting of a composite endpoint is that the preferred balance between PFS, QOL and treatment toxicity will differ among individuals. Heterogeneity in preferences was evident as about one in four women ranked an attribute other than PFS as being most important. Given the variation in preferences, generating a patient-specific composite endpoint may be the ultimate goal to guide decision-making.
Our fixed-choice analysis gives us some insight into patient preferences regarding IP/IV chemotherapy, an approach that has been demonstrated to significantly improve survival outcomes among women with optimally debulked stage III ovarian cancer1. In the current study, participants were fairly evenly divided in regard to their preference for IP/IV versus IV chemotherapy. Interestingly, those who had personally been treated with IP/IV chemotherapy were significantly more likely to choose it over IV treatment in the fixed choice question. To put this finding into clinical context, 27% of the women who were previously treated with IP/IV chemotherapy had experienced grade 3 or 4 (severe or life-threatening) adverse events with that treatment, while 48% had experienced grade 1 adverse events or less. Patients who had received IP/IV had a median PFS of 11 months following that treatment. Despite their personal experience with the toxicities of IP/IV treatment, this cohort may have been more familiar with the OS data from randomized trials favoring IP/IV chemotherapy, and their prior personal choice of this modality may have strengthened their subsequent stated preferences for IP/IV. It is also likely that had all participants been presented with the large (> 1 year) OS advantage of IV/IP over IV chemotherapy that was observed in the GOG 172 randomized clinical trial1 rather than the smaller PFS advantage presented in this study, the IP/IV regimen would have been chosen more frequently.
Our study has several limitations. First, we did not investigate patients' understanding of the separate concepts of OS and PFS. For the methodological reasons described in Methods as well as the increasingly common use of PFS, but not OS, as a primary endpoint in phase III clinical trials, we chose to present PFS as the only survival outcome for this analysis. The relative importance of both of these endpoints to patients is worthy of further study. Our sample size and heterogeneous study population limit the generalizability of our findings. Sample size estimation for conjoint analysis is challenging in health care applications9; our sample of 95 patients was sufficient to demonstrate statistically significant differences in utility weights between levels in 6 of the 7 attributes. However, a larger sample of women with similar preferences would have provided tighter confidence intervals and have provided additional statistical power to make comparisons between groups. There is also the potential bias introduced by the hypothetical nature of a preference elicitation study completed electronically; this method may reduce the participant's emotional connection to each choice. Finally, while use of a video to provide education about each attribute has the advantage of standardizing this portion of the exercise, its effectiveness in comparison to a more interactive approach is unknown.
There have been no reported studies testing whether a physician's receipt of preference information has an impact on treatment decisions or satisfaction among women with ovarian cancer. Elit et al. developed a Decision Board visual aid to assist in presenting the side effects and survival outcomes of two available IV chemotherapy options for advanced ovarian cancer to patients. They found that use of the Decision Board resulted in a higher probability that the physician presented survival data to women with newly diagnosed cancer7. However the effect on patient satisfaction or choice was not assessed. Data from patient preference studies may be useful to providers who are framing treatment discussions. Likewise, women who are making difficult treatment decisions may benefit from studies demonstrating that women like them are willing to make tradeoffs between benefits and toxicities. This type of research is applicable to the future development of shared decision tools to guide both patients and providers in their treatment choices and recommendations.
In conclusion, our results indicate that while PFS is the most important determinant of the choice between chemotherapy regimens, women may be willing to give up significant PFS time to avoid specific severe toxicities of a proposed treatment. Patients' preferences should be considered and discussed when making shared cancer treatment decisions.
Supplementary Material
Acknowledgments
We would like to express our gratitude to F. Reed Johnson for his careful review and suggestions to improve our reporting of this study.
Funding: This study was supported by the National Cancer Institute of the National Institutes of Health under award number KM1CA156687
Footnotes
Prior presentation: Presented as a poster at the American Society of Clinical Oncology Annual Meeting, Chicago Illinois, May 31, 2014
Financial disclosures: Drs. Havrilesky, Reed, Weinfurt, Abernethy, and Ms Ehrisman report grant funds from The National Cancer Institute, during the conduct of the study (see Funding above). Drs. Alvarez Secord, Berchuck, Lee, Gaillard, Samsa, and Cella have nothing to disclose.
Dr. Valea reports personal fees from Genentech outside the submitted work.
Dr. Abernathy reports grants from the National Institute of Nursing Research, Agency for Healthcare Research and Quality, DARA Biosciences, GlaxoSmithKline, Dendreon, Celgene, Helsinn, Pfizer, and KangLaiTe outside the submitted work; grants and personal fees from Bristol-Myers Squibb; and personal fees from ACORN research, Athena Health, Advoset, and Orange Leaf Associates, outside the submitted work.
References
- 1.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. New England Journal of Medicine. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 2.Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncology. 2013;14:1020–1026. doi: 10.1016/S1470-2045(13)70363-2. [DOI] [PubMed] [Google Scholar]
- 3.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. New England Journal of Medicine. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 4.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. New England Journal of Medicine. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 5.Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113:3459–3466. doi: 10.1002/cncr.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zafar SY, Alexander SC, Weinfurt KP, Schulman KA, Abernethy AP. Decision making and quality of life in the treatment of cancer: a review. Supportive Care in Cancer. 2009;17:117–127. doi: 10.1007/s00520-008-0505-2. [DOI] [PubMed] [Google Scholar]
- 7.Elit LM, Levine MN, Gafni A, et al. Patients' preferences for therapy in advanced epithelial ovarian cancer: development, testing, and application of a bedside decision instrument. Gynecologic Oncology. 1996;62:329–335. doi: 10.1006/gyno.1996.0244. [DOI] [PubMed] [Google Scholar]
- 8.Herzog TJ, Armstrong DK, Brady MF, et al. Ovarian cancer clinical trial endpoints: Society of Gynecologic Oncology white paper. Gynecologic Oncology. 2014;132:8–17. doi: 10.1016/j.ygyno.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health--a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value in Health. 2011;14:403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. Journal of Clinical Oncology. 2001;19:1809–1817. doi: 10.1200/JCO.2001.19.6.1809. [DOI] [PubMed] [Google Scholar]
- 11.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 12.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ (Clinical Research Ed) 2000;320:1530–1533. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients' preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77:224–231. doi: 10.1016/j.lungcan.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 14.de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Economics. 2012;21:145–172. doi: 10.1002/hec.1697. [DOI] [PubMed] [Google Scholar]
- 15.Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value in Health. 2013;16:3–13. doi: 10.1016/j.jval.2012.08.2223. [DOI] [PubMed] [Google Scholar]
- 16.Stuart GC, Kitchener H, Bacon M, et al. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. International Journal of Gynecological Cancer. 2011;21:750–755. doi: 10.1097/IGC.0b013e31821b2568. [DOI] [PubMed] [Google Scholar]
- 17.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. Journal of Clinical Oncology. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 18.Johnson FR, Hauber AB, Ozdemir S. Using conjoint analysis to estimate healthy-year equivalents for acute conditions: an application to vasomotor symptoms. Value in Health. 2009;12:146–152. doi: 10.1111/j.1524-4733.2008.00391.x. [DOI] [PubMed] [Google Scholar]
- 19.Monk BJ, Huang HQ, Burger RA, et al. Patient reported outcomes of a randomized, placebo-controlled trial of bevacizumab in the front-line treatment of ovarian cancer: a Gynecologic Oncology Group Study. Gynecologic Oncology. 2013;128:573–578. doi: 10.1016/j.ygyno.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bewtra M, Kilambi V, Fairchild AO, Siegel CA, Lewis JD, Johnson FR. Patient preferences for surgical versus medical therapy for ulcerative colitis. Inflammatory Bowel Diseases. 2014;20:103–114. doi: 10.1097/01.MIB.0000437498.14804.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole BF, Gelber RD, Gelber S, Mukhopadhyay P. A quality-adjusted survival (Q-TWiST) model for evaluating treatments for advanced stage cancer. Journal of Biopharmaceutical Statistics. 2004;14:111–124. doi: 10.1081/BIP-120028509. [DOI] [PubMed] [Google Scholar]
- 22.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. Journal of Clinical Oncology. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 23.Ohorodnyk P, Eisenhauer EA, Booth CM. Clinical benefit in oncology trials: is this a patient-centred or tumour-centred end-point? European Journal of Cancer. 2009;45:2249–2252. doi: 10.1016/j.ejca.2009.05.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


