Abstract
MicroRNAs (miRNAs) are small non-coding RNAs conserved in metazoans. Depletion of miRNAs results in embryonic lethality, suggesting they are essential for embryogenesis. Similarly, pathways induced by growth factors of the transforming growth factor β (TGF-β) superfamily control cell growth, differentiation, and development. Recently Smad proteins, the signal transducers of the TGF-β pathway, were found to regulate miRNA expression, which, in turn, affects expression of numerous proteins. Smads modulate miRNA expression through both transcriptional and post-transcriptional mechanisms illustrating the complexity of gene regulation by TGF-β. In this chapter we summarize the current knowledge of mechanisms underlying Smad-mediated regulation of miRNA biogenesis.
Introduction
miRNA profiling study suggests that miRNAsare globally reduced in tumor samples as compared to normal tissues [1]. Another study found that miRNA signatures are a better indicatorof tumor source and prognosis that similarly developed mRNA signatures[2]. Deregulation of miRNAs isa criticalcomponent of both developmentaldefects and pathogenesis. Thus, miRNAs are integral to basic physiological functions in metazoanssimilar to the TGF-β super family of growth factors. It is not too surprising, then, that miRNAs are themselves critical components of the TGF-β signaling pathway, nor that the Smad proteins have developed several different mechanisms for regulating miRNA expression and activity.Below, we discuss the recent advances made in understanding the diverse mechanisms of miRNA regulation utilized by the TGF-β signaling pathway and the implications of this regulation to human diseases.
MiRNA Biogenesis
Since the original description of miRNAs in 1993 [3], they have emerged as critical regulators of nearly every aspect of mammalian biology. By one estimation more than 60% of protein-coding regions in the human genome display areas ofdeep conservation in their 3′ UTR indicative of targeting by miRNAs [4]. MiRNAs are important regulatory factors that mediate the expression of large number ofproteins. Profiling studies show thata single miRNA maytune the expression of over 100 different proteins[5]. Partial complementarity between miRNA sequences and their target mRNAsusually occurring in the 3′-UTR,mediatestranslational repression or, more commonly, destabilization of the mRNAs[6, 7]. Interestingly, specific recognition of target mRNAs by a miRNA does not require 100%sequence complementarity [6]. Rather, the 6-8 nucleotides at the 5′ end of the miRNA, known asthe “seed sequence” or miRNA recognition element (MRE), conferthe most critical determinants for targeting mRNAs; though other nucleotidesmay also be important under certain circumstances [8]. Because a relatively small number of bases is required to induce silencing, a large number of transcripts can be targeted by a single miRNA, however, the degree of change for any single transcript is relatively small at ~30-50 % reduction[9].
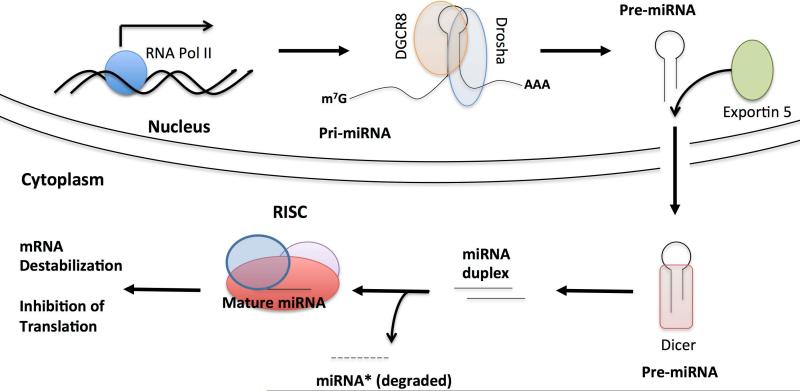
MiRNA biogenesis begins in the nucleus where they are transcribed by RNA polymerase II (RNA Pol II) aslong transcripts known as primary miRNA (pri-miRNA)[10] (Fig. 1). Like mRNAs, pri-miRNAs are poly-adenylated at the 3′ end and bear a 7-methyl-guanosine cap at the 5′ end[11]. Pri-miRNA is cleaved by the RNase III enzyme Drosha into a shorter (<100 bp) transcript known as the precursor miRNA (pre-miRNA)composed of a stem-loopstructure whichencodesthe mature miRNA sequence in the stem [12]. Drosha acts as part of a large processing complexthat consists of multiple proteins,including the DiGeorge critical region 8 (Dgcr8 or Pasha)[13, 14]. Dgcr8 is a double-stranded-RNA-binding protein that stabilizes the association of Drosha with pri-miRNA and determines the precise location of the processing; it is therefore essential for miRNA maturation[13]. Interestingly, Dgcr8 and Drosha cross-regulateexpression of one another presumably to maintain homeostatic control of miRNA biogenesis[15]. DroshadestabilizesDgcr8 mRNA stability through the hairpin structure in the 5′-UTR of the mRNA while Dgcr8stabilizes the Drosha protein [15]. AlthoughDrosha and Dgcr8comprise the minimum components of themicroprocessor complex, many more proteins have been identified that interact with this large structure. Two of these proteins are the DEAD-box helicase proteins p68 (also known as DDX5) and p72 (DDX17)[16]. Deletion of either p68 or p72 leads to embryonic lethality, however neither protein is absolutely essential for miRNA development as deletion of either from mouse embryonic fibroblasts (MEFs) disrupts the expression a distinct, but overlapping, set of miRNAs[16].
Figure 1. MiRNA Biogenesis Pathway.
Biosynthesis of miRNAs begins in the nucleus with the transcription of miRNA genes by RNA Pol II as long transcripts bearing a 7 methyl-guanosine cap at the 5′-end and a poly(A) tail at the 3′-end. The Drosha microprocessor complex, the minimal components of which are DGCR8 and Drosha, cleaves the pri-miRNA into pre-miRNA. Exportin 5 then exports the pre-miRNA into the cytoplasm where it undergoes a secondary processingstep into a short miRNA duplex by Dicer. The miRNA duplex contains both a mature miRNAstrand and a miRNA* strand. The mature miRNAis loaded into Ago proteins and mediates the silencing function as a part of the RISC, while the miRNA* gets degraded.
Following the cleavage of pri-miRNAby the Drosha microprocessor complex, the pre-miRNA is exported from the nucleus throughthe cooperative activity of Ran-GTP, which provides energy and exportin-5 (EXP5), which interacts with the stem-loops structure of pre-miRNAs[17]. Once inthe cytoplasm, the pre-miRNA hairpin associateswith another RNase III enzymecalled Dicer, which further cleaves it into a double-stranded,miRNA duplex of approximately 22 nt, containingthe guide strand (or mature miRNA) and the passenger strand (or miRNA*)[17].The miRNA duplex is then loaded into argonaute (Ago) proteins, which select the guidestrand and present it to the RNA-induced silencing complex (RISC) for targeting and silencing. Mature miRNAs can be generated from either the 5′ or the 3′ arm of the miRNA duplex, but it is rare for both strands to remain at high levels in the cell as the passenger strand isquickly degraded[14].
Smad Signaling
The TGF-βfamily is a pleiotropic group of growth factors that activate an evolutionarily conservedsignal-transduction cascade. TGF-β family ligands include TGF-β itself, integrins and bone morphogenic proteins (BMP).Thisligand variety (46 human open reading frames or ORFs) is mirrored by a large degree of receptor diversity as well (12human TGF-β receptors) [18]. However, this receptor diversity can be separated into two different classes of receptors termed type I and type II receptors. Both receptor classes contain serine-threonine kinase domain and ligand binding induces heterodimerization between the two types of receptor. TGF-β is originally bound by the constitutively active type II receptors which induces binding, phosphorylation and activation of the type I receptor whichpropagates signal transduction into the cell.
Despite its receptor and ligand diversity, TGF-β family signal transduction is carried out by a relatively small number of conserved proteins known as Smads. Smads can be grouped into two distinct sets of proteins, the receptor-specific Smads (R-Smads) and the common Smad (co-Smad/Smad4). R-Smads are only phosphorylated upon receptor dimerization by their specific receptors; with the BMP receptors activating Smads 1, 5 and 8 and the TGF-β receptors activating Smads 2 and 3 [18].Upon phosphorylization, R-Smads dimerize with Smad-4 and translocate into the nucleus as a complex. This interaction with Smad4 is essential for Smad-mediated transcriptional activity [19]. Once in thenucleus the R-Smad-Smad4 complex binds to the Smad-binding element (SBE) canonically identified as the heptamer 5′-CAGAC-3′. Though the SBE is sufficient to modulate Smad binding and activation, additional co-factors are necessary for strong activation of any ORF [20]. In addition to these primary effector molecules a number of other modulatory pathways exist including two inhibitory Smads,Smad6 and Smad7, and the Smad inhibiting protein 1 (SIP1).
Because of their deep conservation it is probably not surprising that Smads pathways are integral for a diverse range of biological processes and are errantly activated or inactivated under variouspathological conditions. Furthermore, deregulation of Smad-controlled miRNAs has been implicated in numerous pathological conditions including cancer [21-24], cardiovascular disease [25]and fibrosis [26, 27]. Understanding how Smad proteins regulate expression of miRNAs and how these unique molecules in turn feed back onto Smad expression are therefore critical elements in unraveling the diverse, context-dependent affect of TGF-β signaling.
That miRNAs comprise critical components of Smad signaling pathways, is illustrated by the large number of miRNAs whose expression change in response to stimulation with TGF-β-family ligands. One recent study found ~20 miRNAs induced and another ~10 miRNAs inhibited in pulmonary arterial smooth muscle cells (PASMC) stimulated with either TGF-β1 or BMP4 [28]. Similarly, in mouse granulosa cells, TGF-β treatment was found to significantly alter the expression of 16 miRNAs [29]. In contrast, interferon (IFN) stimulation of macrophages induced a significant change in only one miRNA [30]. These data illustrate that miRNAs are likely to play an important role in the physiological activity of TGF-β signaling.
Transcriptional regulation of miRNAs by Smads
Chromatin immunoprecipitation (CHIP) analyses reveal that miRNA promoter elements closely resemble those of protein coding regions[31, 32]. Therefore, it is not surprising that Smad proteins controlthe transcription of a variety of miRNA genes. Both in vitro and in vivomiRNA expression-profiling studies confirm that R-Smad protein modulates a unique, but overlapping set of miRNAs [21, 26, 33, 34]. For example, TGF-β induces both miR-216a and miR-217 in glomerular mesengial cells via Smad binding elements (SBEs) in the miR-216 promoter [35]. Conversely,TGF-βinducedSmad3/4complex binding to the miR-24 promoter inhibits the expression of miR-24 in myoblasts [36]. Transcriptional activation of miRNAs has distinct physiological significance in vivo. For instance, transcriptional repression of miR-29 by Smad-binding to the promoter promotes renal fibrosis[33].
Transcriptional activation of miRNA genes by Smad is sensitive to canonical TGF-β signaling cascades. For instance, in mouse kidney epithelial cells, induction of miR-192 by the TGF-β-specific R-Smad,Smad3,can be reduced not only by siRNA againstSmad3 but also by overexpression of the antagonistic Smad protein Smad7 [26]. Furthermore, many miRNAs transcriptionally regulated by Smads areknown to be dependent on Smad4. One recent study of TGF-β stimulated murine mammary gland epithelial cells, indicates that Smad4 knockdown results in deregulation of 28 miRNAs [21]. In these cells, a Smad4 binding site within the miR-155 promoter is critical for induction of this miRNA and subsequent downregulation of its target gene RhoA[21].
In addition to direct association of the Smad complex with the miRNA promoters, Smads can indirectly modulate miRNA levels through activation of transcription factorsthatassociate with their promoters. For instance,the miR-143~145 gene locus, which encodes both miR-143 and miR-145, is transcriptionally regulated by a complex composed ofserum response factor (SRF) and myocardin or myocardin related transcription factors (MRTFs)[37, 38]. TGF-β and BMP signaling increase miR-143 and miR-145 through activation of myocardin and MRTF, respectively[38].Similarly, induction of let-7d and let-7aby TGF-βis mediated bythe binding of Smad3 to a SBE in their promoters[27]. Interestingly, higher let-7d expression is found in the lungs of patients with idiopathic pulmonary fibrosis (IPF) as compared with control individuals [27] and increased expression of let-7d and decreased expression of its target HMGA2 correlate with higher TGF-βactivity [26]. Therefore, deregulation of Smad-controlled miRNA may have serious health consequencesdespite the relatively small effect of a single miRNA:mRNA interaction.
Epigenetic Regulation of miRNAs
Like most transcription factors, Smad activity on the promoters of target genes may be epigenetically modulated. For example, mouse embryonic fibroblasts (MEFs) transfected with the oncogene RasV12become senescent as part of the response to aberrant cell proliferation. Profiling of methylated loci following the expression of RasV12identified multiple targets of the BMP-specific R-Smad Smad1, that are induced or repressed by this process[39]. The promoter/enhancer structure of miRNAs largely reflects that of RNA Pol II transcripts; similarly, the chromatin structure of miRNA promoters reflects that of RNA Pol II transcripts [31]. Thus, it is likely that miRNA expression is susceptible to epigenetic changes similar toprotein coding genes. Epigenetic silencing is documented for several miRNAs,includingmiR-203 [40], miR-9 [41], and miR-124 [42]. Therefore, we postulate it is only a matter of time before epigenetic control of miRNAs by the TGF-βsignaling pathway is identified. Additionally, two TGF-β regulated miRNAs, miR-206 and miR-29, post-transcriptionally repress histone deacetylase 4 (HDAC4) induction and thereby inhibit myogenic differentiation [43]. Thus TGF-β regulated miRNAs can themselves alter epigenetic patterns as a way to mitigate more long-term gene regulation.
Post-transcriptional Regulation of miRNAs by Smads
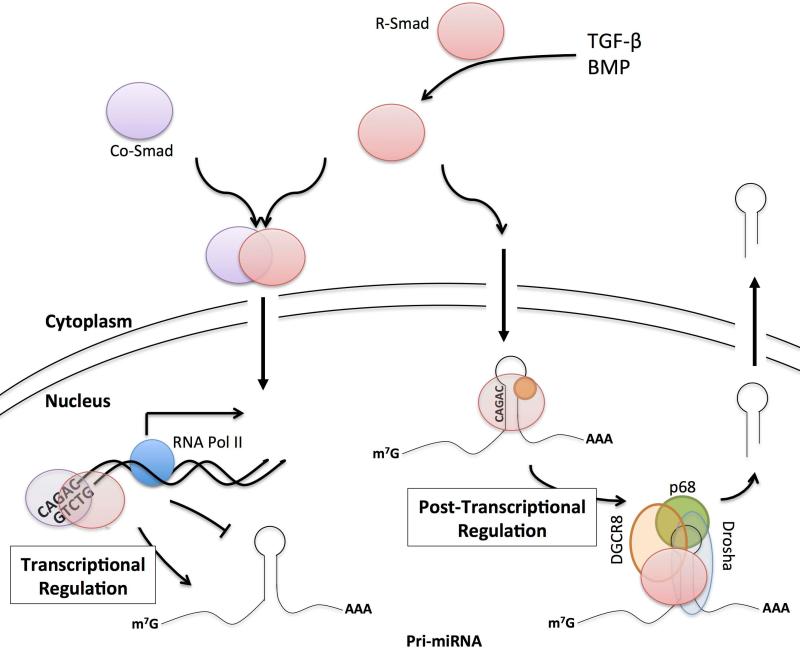
As described above, regulation of miRNA transcription by TGF-β signaling largely conforms to the determinants of normal Smad transcription. Close molecular dissection of TGF-β signalingrecently identified a novelrole of Smads in post-transcriptionally regulating miRNA biogenesis. In vascular smooth muscle cells (VSMCs), TGF-βand BMP4 signaling promotes the maintenance of a synthetic “contractile” phenotype, while lack of TGF-βor BMP4 signaling results in a more proliferative phenotype[44]. This phenotype is maintained, at least partially, due to the induction of miR-21, which leads to downregulation of the miR-21 target programed cell death protein 4 (PDCD4)[45]. Interestingly, theinduction ofmiR-21 by TGF-βor BMP4 occurs without a corresponding increase in pri-miR-21 and is not repressed bythe RNA Pol IIinhibitor α-amanitin[45]. Furthermore, unlike transcriptionally regulated miRNAs, BMP and TGF-β-mediated induction of miR-21 occurs independent of Smad4, though it is completely dependent on the R-Smads[45]. RNA CHIP experiments confirm that RSmads bind directly to the pri-mir-21 hairpins in a ligand-dependent manner [45]. Thus, mature miR-21 expression is induced by R-Smad activity through apost-transcriptional mechanism (Fig. 2).
Figure 2. Regulation of miRNA expression by Smads.
Smad-mediated regulation of miRNAs can be classified into two mechanisms: (i) transcriptional regulation of miRNAs by Smads largely resembles the canonical TGF-β signaling pathway. Signal transduction leads to phosphorylation of an R-Smad, which induces the formation of an R-Smad/co-Smad heterodimer. This complex is imported into the nucleus, binds the SBE and regulates, either positively or negatively, the transcription ofmiRNA genes. The pri-miRNAs then undergoes normal miRNA processing. (ii) Post-transcriptional regulation of miRNA biogenesis acts on pri-miRNA in the nucleus. Phosphorylation of an R-Smad induces nuclear localization. In the nucleus, R-Smad recognizes and binds anSBE-like sequence localized in the stem region of the pri-miRNA, and recruits the Drosha/DGCR8 microprocessor complex to the pri-miRNA. The microprocessor then promotes efficient processing of the pri-miRNA into pre-miRNA.
The mechanism for this process wasactually hinted at by a study performed to identify DNA regulatory proteins that promote Smad3 experiments. Througha yeast two-hybrid system using the carboxyl-terminalMad homology 2 (MH2) domain of Smad3 as bait, the DEAD-box RNA helicase protein p68was identified as a Smad3 interacting protein [46]. As discussed above, p68 is an emerging factor in pri- to pre-miRNA processing[16]. Consistent with this observationthe induction of miR-21 by BMP is dependent on expression of p68 [45]. These data suggest a new mechanism of miRNA regulation by Smads in which ligand stimulation induces translocation of Smads to the nucleus where an association with the Drosha microprocessor complex occurs. Further, it provides a potential mechanism for Smad4-independent gene regulation [47]. Interestingly, while many Smad4 deficient cell types develop normally, they are more prone to tumorigenic transformation [47]. This observation is consistent with the oncogenic function of many TGF-β regulated miRNAs, including miR-21, whichisknown to promote cell growth and migration as well as to inhibit apoptosis in varioustypes of tumors [48]. These results further suggest that Smad-mediated regulation of miRNA processing be important duringdevelopment.
To assess the structural characteristics of pri-miRNA regulated by Smads,miRNA microarray profiling studies wereperformed[28]. These studies found that, approximately 5% (20 of 377) of miRNAs examinedare induced by stimulation with both TGF-β and BMP [28]. Interestingly, a subset of these miRNAs contain an SBE-like RNA sequence (R-SBE) at the 3′ end of their mature miRNAsequence[28].The R-SBE motif (5′-CAGAC-3′) isnecessary for direct binding of Smad proteins topri-miRNA as illustrated by the fact that mutation of this region abrogatesSmad binding[45].When introduced to miRNAsthat do not contain R-SBE, this sequence motifissufficient to confer not only the association with Smadbut alsoTGF-β-dependent recruitment of Drosha and processing[45].
Drosha is a largely non-specific RNase enzyme that requires interactions with other proteins, such asDgcr8, to recruit and allocate the appropriate site for pri-miRNA cleavage[15].Recent evidence suggests that proper RNA binding by microprocessor components is a vital determinant of Drosha cleavage and affects pre-mRNA and subsequently mature miRNA size and stability[15]. Thus the location of the R-SBE within the hairpin structures of the pri-miRNAs may be critical for modulating the final mature miRNA sequence.
Direct association of Smads with the pri-miRNAs is carried out throughthe MH1 domain of Smad proteins [28], the same region responsible for binding the SME to DNA during canonical Smad signaling [20].Althoughthe pri-miRNA binding activity of R-Smads is mediated by a molecular mechanismresembling that of DNA-binding, Smad4 is essential for the association with DNA[49] but not with pri-miRNAs [45].It is also important to note that the phosphorylation of R-Smads at the carboxyl-terminus serine residues by the type I TGF-β receptorsis not essential for the pri-miRNA processing function of R-Smads. It was initially reported that the phosphorylation of R-Smads promotes association of R-Smads with Smad4 and translocation to the nucleus [49]. It is currently unclear whether there is another protein which substitutesfor the role of Smad4 in nuclear importduring processing of pri-miRNAs. A subset of Smad proteins can enter the nucleus in a basal non-phosphorylated setting by a mechanism that does not require importin 7 (Imp7) or Imp8, the canonical Smad nuclear-import factors [50]. Thus R-Smads present in the nucleus in a form not bound to Smad4 might preferentially interact with the Drosha/Dgcr8 microprocessor complex to promote miRNA maturation.However, this hypothesis does not address the TGF-β induciblity of Smad-mediate miRNA maturation. Thus, inducible, Smad4-independent transport of R-Smads into the nucleus remains an interesting open avenue of research. Furthermore, kinases other than the TGF-βreceptors, such asmitogen activated protein kinase (MAPK), glycogen synthase kinase 3 (GSK3) and extracellular signal-regulated kinase 2 (ERK2), can also affect the subcellular localization of R-Smads and thereby alterpri-miRNA processing[51, 52].
p68/p72-interacting transcription factors in addition to Smads
The Drosha microprocessor complex is emerging as a criticalsite for post-transcriptional regulation of miRNA biogenesis. Two additionaltranscription factors, estrogen receptor-α (ERα) and p53, are known to interact with the Droshacomplex similar to R-Smads [53, 54]. In the case of p53, DNA damage induce p53 to interact with the Drosha complex through p68 and induce pri- to pre-miRNA processing of multiple miRNAs, including miR-143, miR-145, and miR-16-1[53]. ERα is also recruited to the Drosha microprocessor complex through p68 in response to estradiol (E2) stimulation, however,the recruitment of ERα with the Drosha complex inhibits the processing ofprimiR-143, pri-miR-145, pri-miR-16-1, pri-miR-195, and pri-miR-125a[54]. Unlike the Smads, the mechanisms of recognition for specific pri-miRNAsbyp53 orERα remain unknown, however, it is plausiblethat furtheranalyses will unveil motifs within thepri-miRNAsequencerecognizedby these transcription factors. Interestingly,miRNA profiling experiments show that BMP4 and TGF-β stimulation in VSMCs also results in the downregulation of a subset of miRNAs [28]. Furthermore, miR-206 is post-transcriptionally downregulated in the C2C12 myoblast cell line following BMP2 treatment [55]. These data suggest that in addition to positive regulation of pri- to pre-miRNA processing, the Smad/Drosha/Dgcr8 complex may also inhibitthe maturation of pri-miRNAs.
The involvement of both p68 and p72 inDrosha processing induced by diverse regulatory pathways and involving divergent transcription factors suggests that these helicase proteins represent a general hubfor the regulation of miRNA biogenesis.However, it remains unclear whether this mechanism is generally applicable to other transcription factors. To date several additional transcription factors, including MyoD[56], Runx2[56], androgen receptor[57] and -catenin[54], have been found to interact with p68 and p72. Thus it is likely that at least some of these proteinsmightcontribute to miRNA biogenesis.Interestingly, ameta-analysis of miRNA profiling foundthat there is no correlation between the levelof pre-miRNA and mature miRNAin cancer cells[58]. This observation suggests thatpost-transcriptional regulatory mechanisms play a pivotal role under pathologicalconditions as well as in normal cells[58].Finally, it is noteworthy thatSmads may not be the only members of the TGF-β signaling pathway that work bypromoting miRNA biogenesis. The protein small nuclear interacting protein 1 (SNIP1), which wasoriginally identified based on its nuclear interaction with Smads, also associates with Drosha [59]. Whether this interaction affects miRNA biogenesis, has not yet been determined.
Reciprocal Regulation: Control of Smad proteins by miRNAs
In addition to controlling miRNA expression,Smad proteins can themselves be targeted by miRNAs. To date, most Smad proteinsare validated targets of at least one miRNA(see Table 1). Smad expression can be modulated by miRNAs under normal physiological conditions. For example, BMP2 signaling promotes osteoblast differentiation at least in part by inhibiting the expression of miR-135 and miR-199* which target Smad5 and Smad1, respectively [21, 60].Smads are also targeted by miRNAs under pathological states; for instance in diffuse large B cell lymphomamiR-155promotes proliferation by downregulating Smad5 expression[23]. Even exogenous signals can alter Smad expression through miRNAs. For example, miR-21 targets theantagonistic Smad; Smad7, thus increasing basal TGF-β signaling in hepatocytes following infection with the hepatitis C virus (HCV)[61]. Recently, Smad3 wasidentified as a target of miR-140 which is regulated only at the protein level [62]. To identify this protein, the authors first identified mRNAs that were indirect targets of miR-140 using mRNA arrays tofindgenes modulated by miR-140, but lacking a seed-sequence [62]. Next, they characterized promoter elements that were underrepresented in the off-target transcripts to identify putative binding sites of the altered transcription factors [62]. Such studies illustrate both the difficulty and the importance of considering the translational effects of miRNA activity. It is likely that many more miRNAs that target Smads remain to be discovered by studies that consider changes occurring onlyat the protein levels.
Table 1.
MiRNAs targeting Smads. MiRNAs have been shown to target numerous Smad proteins under various cellular conditions. Below is a list of miRNAs that have been experimentally validated to target individual Smad proteins and the biological significance of this activity.
| Target | miRNA | Activity | Ref. |
|---|---|---|---|
| miR-18a | Smad2 | Regulates TGF-β signaling in neuroblastoma cells | [69] |
| miR-18a | Smad4 | Regulates TGF-β signaling in neuroblastoma cells | [69] |
| miR-21 | Smad7 | Enhances TGF-β signaling in HCV infected livers | [61] |
| miR-135 | Smad5 | Inhibits osteoblast differentiation | [60] |
| miR-140 | Smad3 | In 3T3 cells, regulates expression only at the protein level | [62] |
| miR-145 | Smad2 | Alters macrophage sensitivity to TGF-β | [70] |
| miR-146-5p | Smad4 | Suppresses TGF-β signaling in thyroid cancer | [71] |
| miR-155 | Smad1 | Induced by Epstein-Bar Virus (EBV) in B cells to inhibit BMP signaling and virus reactivation | [72] |
| miR-155 | Smad2 | Alters macrophage sensitivity to TGF-β | [70] |
| miR-155 | Smad5 | Induced by Epstein-Bar Virus (EBV) in B cells to inhibit BMP signaling and virus reactivation | [72] |
| miR-155 | Smad5 | Promotes tumorigenesis in diffuse large B cell lymphoma | [23] |
| miR-192 | Smad3 | Contributes to collagen production by mouse renal cells and fibrosis in the kidney in vivo. | [26] |
| miR-199a* | Smad1 | Inhibits differentiation into chondrocytes | [73] |
| miR-200 | Smad3 | Promotes epithelial-mesenchymal transition in gastric cancer | [74] |
| miR-224 | Smad4 | Enhances proliferation in mouse granulosa cells | [29] |
| miR-483-3p | Smad4 | Promotes proliferation of pancreatic cancer cells | [24] |
Additional proteins related to the TGF-β-Smad signaling pathways, such as Smad inhibiting protein 1 (SIP1) [63, 64] and TGF-βsuper family receptors, such as ALK4 [65], the type II TGF-β receptor [66] and the type II BMP receptor (BMPRII)[67],as well as TGF-βligand itself [68]are targeted by miRNAs. The case of BMPRII is especially interesting as it is targeted by miR-21 whose expression is elevated by Smad activity[67]. It is currently unclear whether miR-21-dependent regulation of BMPRII plays a role in Smad signalingas BMPRII expression remains high throughout the course of BMP stimulation, at least in VSMCs (A.H., unpublished data). It is possible that miRNA regulation of BMPRII acts as a negative feedback loop to limit the duration of Smadactivity.
Conclusion
In this chapter, we summarized a critical role of Smad proteins in the regulation of miRNA biogenesis. Two primary modes of regulation by Smads are; (i) transcriptional regulation of miRNA genes by nuclear translocation of R-Smad/co-Smad heterodimers, association with the DNA sequence motif known as SBE, and modulation of transcription, and (ii)post-transcriptional regulation of pri- to pre-miRNA processing by R-Smad through therecruitment and activation of the Drosha/Dgcr8/p68microprocessor complex. Additional mechanisms of miRNA regulation by the TGF-β super family of growth factors are likely to be uncoveredin the future. Such mechanisms are an important component of understanding how cells integrate the complex repertoire of miRNAswhose expression levels are fine-tuned by TGF-β signaling pathways and transmit a precise signal in order to control normaldevelopment and maintainhomeostasis.
Acknowledgements
Because of space restrictions and the focus of the article, we deeply apologize to those colleagues whose references we have not had the opportunity to discuss. We thank all members of the Hata for helpful suggestions and critical discussion. This work was supported by grants from the National Institute of Health: HL093154 and HL108317, the American Heart Association: 0940095N and the LeDucq foundation Transatlantic network grant to A.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee EJ, Baek M, Gusev Y, Brackett DJ, Nuovo GJ, Schmittgen TD. Systematic evaluation of microRNA processing patterns in tissues, cell lines, and tumors. RNA. 2008;14:35–42. doi: 10.1261/rna.804508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 3.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 4.Friedman RC, Farh KK-H, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of micro RNAs. Genome Research. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baek D, Viilén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of micro RNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by micro RNAs: are the answers in sight? Nature Reviews Genetics. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 8.Grimson A, Farh KK-H, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA Targeting Specificity in Mammals: Determinants beyond Seed Pairing. Molecular Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in prote in synthes is induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y, Kim M, Han JJ, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–66. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark 0, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 13.Gregory RI, Yan K.-p., Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 14.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 15.Han J, Lee Y, Yeom K-H, Nam J-W, Heo I, Rhee J-K, Sohn SY, Cho Y, Zhang B-T, Kim VN. Molecular Basis for the Recognition of Primary microRNAs by the Drosha-DGCR8 Complex. Cell. 2006;125:887–901. doi: 10.1016/j.cell.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda T, Yamagata K, Fujiyama S, Matsumoto T, Koshida I, Yoshimura K, Mihara M, Naitou M, Endoh H, Nakamura T, Akimoto C, Yamamoto Y, Katagiri T, Foulds C, Takezawa S, Kitagawa H, Takeyama K, 0'Malley BW, Kato S. DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nature cell biology. 2007;9:604–11. doi: 10.1038/ncb1577. [DOI] [PubMed] [Google Scholar]
- 17.Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear Export of MicroRNA Precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, Massagué J. Mechanisms of TGF-β Signaling from Cell Membrane to the Nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 19.Feng XH, Zhang Y, Wu RY, Derynck R. The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for smad3 in TGF-β-induced transcriptional activation. Genes Dev. 1998;12:2153–63. doi: 10.1101/gad.12.14.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes & Development. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 21.Kong W, Yang H, He L, Zhao J.-j., Coppola D, Dalton WS, Cheng JQ. icroRNA-155 Is Regulated by the Transforming Growth Factor β/Smad Pathway and Contributes to Epithelial Cell Plasticity by Targeting RhoA. Molecular and Cellular Biology. 2008;28:6773–6784. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang S, He X, Ding J, Liang L, Zhao Y, Zhang Z, Yao X, Pan Z, Zhang P, Li J, Wan D, Gu J. Upregulation of miR-23a⍰E27a⍰24 decreases transforming growth factor-β-induced tumor-suppressive activities in human hepatocellular carcinoma cells. International Journal of Cancer. 2008;123:972–978. doi: 10.1002/ijc.23580. [DOI] [PubMed] [Google Scholar]
- 23.Rai D, Kim S-W, McKeller MR, Dahia PLM, Aguiar RCT. Targeting of SMAD5 links microRNA-155 to the TGF-β pathway and lymphomagenesis. Proceedings of the National Academy of Sciences. 2010;107:3111–3116. doi: 10.1073/pnas.0910667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao J, Zhang S, Zhou Y, Hu X, Shao C. MicroRNA 483-3p suppresses the expression of DPC4/Smad4 in pancreatic cancer. FEBS Letters. 2011;585:207–213. doi: 10.1016/j.febslet.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Song Y, Zhang Y, Xiao H, Sun Q, Hou N, Guo S, Wang Y, Fan K, Zhan D, Zha L, Cao Y, Li Z, Cheng X, Zhang Y, Yang X. Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell Res. 2011 doi: 10.1038/cr.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung ACK, Huang XR, Meng X, Lan HY. miR-192 Mediates TGF-β/Smad3-Driven Renal Fibrosis. Journal of the American Society of Nephrology. 2010;21:1317–1325. doi: 10.1681/ASN.2010020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandit KV, Corcoran D, Yousef H, Yarlaagadda M, Tzouvelekis A, Gibson KF, Konishi K, Yousem SA, Singh M, Handley D, Richards T, Selman M, Watkins SC, Pardo A, Ben-Yehudah A, Bouros D, Eickelberg O, Ray P, Benos PV, Kaminski N. Inhibition and Role of let-7d in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2010;182:220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell. 2010;39:373–84. doi: 10.1016/j.molcel.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao G, Yin M, Lian H, Liu L, Li X, Sun F. MicroRNA-224 Is Involved in Transforming Growth Factor-β-Mediated Mouse Granulosa Cell Proliferation and Granulosa Cell Function by Targeting Smad4. Molecular Endocrinology. 2010;24:540–551. doi: 10.1210/me.2009-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proceedings of the National Academy of Sciences. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozsolak F, Poling LL, Wang Z, Liu H, Liu XS, Roeder RG, Zhang X, Song JS, Fisher DE. Chromatin structure analyses identify miRNA promoters. Genes & Development. 2008;22:3172–3183. doi: 10.1101/gad.1706508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corcoran DL, Pandit KV, Gordon B, Bhattacharjee A, Kaminski N, Benos PV. Features of Mammalian microRNA Promoters Emerge from Polymerase II Chromatin Immunoprecipitation Data. PLoS ONE. 2009;4:e5279. doi: 10.1371/journal.pone.0005279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin W, Chung ACK, Huang XR, Meng X-M, Hui DSC, Yu C-M, Sung JJY, Lan HY. TGF-β/Smad3 Signaling Promotes Renal Fibrosis by Inhibiting miR-29. Journal of the American Society of Nephrology. 2011;22:1462–1474. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Divakaran V, Adrogue J, Ishiyama M, Entman ML, Haudek S, Sivasubramanian N, Mann DL. Adaptive and Maladptive Effects of SMAD3 Signaling in the Adult Heart After Hemodynamic Pressure Overloading. Circulation: Heart Failure. 2009;2:633–642. doi: 10.1161/CIRCHEARTFAILURE.108.823070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nature cell biology. 2009;11:881–9. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Q, Zhang Y, Yang G, Chen X, Zhang Y, Cao G, Wang J, Sun Y, Zhang P, Fan M, Shao N, Yang X. Transforming growth factor-β-regulated miR-24 promotes skeletal muscle differentiation. Nucleic Acids Research. 2008;36:2690–2699. doi: 10.1093/nar/gkn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long X, Miano JM. Transforming Growth Factor-β1 (TGF-β1) Utilizes Distinct Pathways for the Transcriptional Activation of MicroRNA 143/145 in Human Coronary Artery Smooth Muscle Cells. Journal of Biological Chemistry. 2011;286:30119–30129. doi: 10.1074/jbc.M111.258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis-Dusenbery BN, Chan MC, Reno KE, Weisman AS, Layne MD, Lagna G, Hata A. down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-β and bone morphogenetic protein 4. The Journal of biological chemistry. 2011;286:28097–110. doi: 10.1074/jbc.M111.236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaneda A, Fujita T, Anai M, Yamamoto S, Nagae G, Morikawa M, Tsuji S, Oshima M, Miyazono K, Aburatani H. Activation of Bmp2-Smad1 Signal and Its Regulation by Coordinated Alteration of H3K27 Trimethylation in Ras-Induced Senescence. PLoS Genet. 2011;7:e1002359. doi: 10.1371/journal.pgen.1002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bueno MJ, Pérez de Castro I, Gomez de Cédron M, Santos J, Calin GA, Cigudosa Juan C., Croce CM, Fernández-Piqueras J, Malumbres M. Genetic and Epigenetic Silencing of MicroRNA-203 Enhances ABL1 and BCR-ABL1 Oncogene Expression. Cancer Cell. 2008;13:496–506. doi: 10.1016/j.ccr.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Hsu P-Y, Deatherage DE, Rodriguez BAT, Liyanarachchi S, Weng Y-I, Zuo T, Liu J, Cheng ASL, Huang TH-M. Xenoestrogen-Induced Epigenetic Repression of microRNA-9-3 in Breast Epithelial Cells. Cancer Research. 2009;69:5936–5945. doi: 10.1158/0008-5472.CAN-08-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hackanson B, Bennett KL, Brena RM, Jiang J, Claus R, Chen S-S, Blagitko-Dorfs N, Maharry K, Whitman SP, Schmittgen TD, Liibbert M, Marcucci G, Bloomfield CD, Plass C. Epigenetic Modification of CCAAT/Enhancer Binding Protein a Expression in Acute Myeloid Leukemia. Cancer Research. 2008;68:3142–3151. doi: 10.1158/0008-5472.CAN-08-0483. [DOI] [PubMed] [Google Scholar]
- 43.Winbanks CE, Wang B, Beyer C, Koh P, White L, Kantharidis P, Gregorevic P. TGF-β Regulates miR-206 and miR-29 to Control Myogenic Differentiation through Regulation of HDAC4. Journal of Biological Chemistry. 2011;286:13805–13814. doi: 10.1074/jbc.M110.192625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagna G, Ku MM, Nguyen PH, Neuman NA, Davis BN, Hata A. Control of phenotypic plasticity of smooth muscle cells by bone morphogenetic protein signaling through the myocardin-related transcription factors. The Journal of biological chemistry. 2007;282:37244–55. doi: 10.1074/jbc.M708137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warner DR, Bhattacherjee V, Yin X, Singh S, Mukhopadhyay P, Pisano MM, Greene RM. Functional interaction between Smad, CREB binding protein, and p68 RNA helicase. Biochemical and Biophysical Research Communications. 2004;324:70–76. doi: 10.1016/j.bbrc.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Bardeesy N, Cheng K.-h., Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, DePinho RA. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes & Development. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asangani IA, Rasheed SAK, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2007;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 49.Lagna G, Hata A, Hemmati-Brivanlou A, Massague J. Partnership between DPC4 and SMAD proteins in TGF-beta signalling pathways. Nature. 1996;383:832–6. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- 50.Xu L, Yao X, Chen X, Lu P, Zhang B, Ip YT. Msk is required for nuclear import of TGF-β/BMP-activated Smads. The Journal of cell biology. 2007;178:981–94. doi: 10.1083/jcb.200703106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kretzschmar M, Doody J, Massagu J. Opposing BMP and EGF signalling pathways converge on the TGF-β family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- 52.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating Patterning Signals: Wnt/GSK3 Regulates the Duration of the BMP/Smad1 Signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460:529–33. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 54.Endoh H, Maruyama K, Masuhiro Y, Kobayashi Y, Goto M, Tai H, Yanagisawa J, Metzger D, Hashimoto S, Kato S. Purification and identification of p68 RNA helicase acting as a transcriptional coactivator specific for the activation function 1 of human estrogen receptor alpha. Mol Cell Biol. 1999;19:5363–72. doi: 10.1128/mcb.19.8.5363. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Sato MM, Nashimoto M, Katagiri T, Yawaka Y, Tamura M. Bone morphogenetic protein-2 down-regulates miR-206 expression by blocking its maturation process. Biochemical and Biophysical Research Communications. 2009;383:125–129. doi: 10.1016/j.bbrc.2009.03.142. [DOI] [PubMed] [Google Scholar]
- 56.Fuller-Pace FV, Ali S. The DEAD box RNA helicases p68 (Ddx5) and p72 (Ddx17): novel transcriptional co-regulators. Biochem Soc Trans. 2008;36:609–12. doi: 10.1042/BST0360609. [DOI] [PubMed] [Google Scholar]
- 57.Clark EL, Coulson A, Dalgliesh C, Rajan P, Nicol SM, Fleming S, Heer R, Gaughan L, Leung HY, Elliott DJ, Fuller-Pace FV, Robson CN. The RNA helicase p68 is a novel androgen receptor coactivator involved in splicingand is overexpressed in prostate cancer. Cancer Res. 2008;68:7938–46. doi: 10.1158/0008-5472.CAN-08-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomson JM, Newman M, Parker JS, Morin-Kensicki EM, Wright T, Hammond SM. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006;20:2202–7. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu B, Bi L, Zheng B, Ji L, Chevalier D, Agarwal M, Ramachandran V, Li W, Lagrange T, Walker JC, Chen X. The FHA domain proteins DAWDLE in Arabidopsis and SNIP1 in humans act in small RNA biogenesis. Proceedings of the National Academy of Sciences. 2008;105:10073–10078. doi: 10.1073/pnas.0804218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, Stein GS. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proceedings of the National Academy of Sciences. 2008;105:13906–13911. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marquez RT, Bandyopadhyay S, Wendlandt EB, Keck K, Hoffer BA, Icardi MS, Christensen RN, Schmidt WN, McCaffrey AP. Correlation between microRNA expression levels and clinical parameters associated with chronic hepatitis C viral infection in humans. Lab Invest. 2010;90:1727–1736. doi: 10.1038/labinvest.2010.126. [DOI] [PubMed] [Google Scholar]
- 62.Pais H, Nicolas FE, Soond SM, Swingler TE, Clark IM, Chantry A, Moulton V, Dalmay T. Analyzing mRNA expression identifies Smad3 as a microRNA-140 target regulated only at protein level. RNA. 2010;16:489–494. doi: 10.1261/rna.1701210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-β-induced collagen expression via inhibition of E-box repressors. Proceedings of the National Academy of Sciences. 2007;104:3432–3437. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu M, Xia M, Chen X, Lin Z, Xu Y, Ma Y, Su L. MicroRNA-141 Regulates Smad Interacting Protein 1 (SIP1) and Inhibits Migration and Invasion of Colorectal Cancer Cells. Digestive Diseases and Sciences. 2010;55:2365–2372. doi: 10.1007/s10620-009-1008-9. [DOI] [PubMed] [Google Scholar]
- 65.Wang Q, Huang Z, Xue H, Jin C, Ju X-L, Han J-DJ, Chen Y-G. MicroRNA miR-24 inhibits erythropoiesis by targeting activin type I receptor ALK4. Blood. 2008;111:588–595. doi: 10.1182/blood-2007-05-092718. [DOI] [PubMed] [Google Scholar]
- 66.Kim YJ, Hwang SJ, Bae YC, Jung JS. MiR-21 Regulates Adipogenic Differentiation through the Modulation of TGF-β Signaling in Mesenchymal Stem Cells Derived from Human Adipose Tissue. Stem Cells. 2009;27:3093–3102. doi: 10.1002/stem.235. [DOI] [PubMed] [Google Scholar]
- 67.Qin W, Zhao B, Shi Y, Yao C, Jin L, Jin Y. BMPRII is a direct target of miR-21. Acta Biochimica et Biophysica Sinica. 2009;41:618–623. doi: 10.1093/abbs/gmp049. [DOI] [PubMed] [Google Scholar]
- 68.Wang B, Koh P, Winbanks C, Coughlan MT, McClelland A, Watson A, Jandeleit-Dahm K, Burns WC, Thomas MC, Cooper ME, Kantharidis P. miR-200a Prevents Renal Fibrogenesis Through Repression of TGF-β2 Expression. Diabetes. 2011;60:280–287. doi: 10.2337/db10-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mestdagh P, Boström A-K, Impens F, Fredlund E, Van Peer G, De Antonellis P, von Stedingk K, Ghesquière B, Schulte S, Dews M, Thomas-Tikhonenko A, Schulte JH, Zollo M, Schramm A, Gevaert K, Axelson H, Speleman F, Vandesompele J. The miR-17-92 MicroRNA Cluster Regulates Multiple Components of the TGF-β Pathway in Neuroblastoma. Molecular Cell. 2010;40:762–773. doi: 10.1016/j.molcel.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Louafi F, Martinez-Nunez RT, Sanchez-Elsner T. MicroRNA-155 Targets SMAD2 and Modulates the Response of Macrophages to Transforming Growth Factor-β. Journal of Biological Chemistry. 2010;285:41328–41336. doi: 10.1074/jbc.M110.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geraldo MV, Yamashita AS, Kimura ET. MicroRNA miR-146b-5p regulates signal transduction of TGF-β by repressing SMAD4 in thyroid cancer. Oncogene. 2011 doi: 10.1038/onc.2011.381. [DOI] [PubMed] [Google Scholar]
- 72.Yin Q, Wang X, Fewell C, Cameron J, Zhu H, Baddoo M, Lin Z, Flemington EK. MicroRNA miR-155 Inhibits Bone Morphogenetic Protein (BMP) Signaling and BMP-Mediated Epstein-Barr Virus Reactivation. Journal of Virology. 2010;84:6318–6327. doi: 10.1128/JVI.00635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin EA, Kong L, Bai X-H, Luan Y, Liu C.-j. miR-199a*, a Bone Morphogenic Protein 2-responsive MicroRNA, Regulates Chondrogenesis via Direct Targeting to Smad1. Journal of Biological Chemistry. 2009;284:11326–11335. doi: 10.1074/jbc.M807709200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahn SM, Cha JY, Kim J, Kim D, Trang HTH, Kim YM, Cho YH, Park D, Hong S. Smad3 regulates E-cadherin via miRNA-200 pathway. Oncogene. 2011 doi: 10.1038/onc.2011.484. [DOI] [PubMed] [Google Scholar]