Abstract
Objectives
To assess if streptozotocin (STZ) induced diabetic rats develop iodinated contrast induced acute kidney injury (CIAKI). The intra-renal R2* (= 1/ T2*) was evaluated continuously before-during-after contrast administration. Renal injury was confirmed by urinary neutrophil gelatinase-associated lipocalin (uNGAL) measurements.
Materials and Methods
Six Sprague-Dawley rats were administered STZ to induce diabetes (Group 1). R2* was measured before, during, and after administration of iodixanol. R2* readings were sampled from four renal regions: inner medulla, inner stripe of outer medulla (ISOM), outer stripe of outer medulla, and cortex. Peak R2* and initial up-slope of R2* increase following iodinated contrast were calculated. Data from 12 non-diabetic rats pre-treated with nitric oxide synthase and prostaglandin inhibitors to induce susceptibility to CIAKI (pre-treatment model) from a previous study were re-analyzed for peak R2* and initial up-slope of R2* increase following contrast. Six of these animals received saline (Group 2) and the other six received furosemide (Group 3) prior to idoxianol.
Results
Peak R2* and initial up-slope of R2* increase were used as BOLD response parameters. R2* in ISOM were comparable in all three groups prior to administration of furosemide. Except for the furosemide group, ISOM showed a rapid increase in R2* immediately following contrast administration. Unlike the L-NAME & indomethacin treated groups, the diabetic group showed a quick reversal of R2* towards baseline measurements after contrast administration. Urinary NGAL indicated significant increase in diabetic rats 4 hours following contrast administration. The observed trends with peak R2* and initial up-slope of R2* increase in renal ISOM were in agreement with those of uNGAL.
Conclusion
The STZ induced diabetic rat may be suitable for studying the effects of iodinated contrast on renal oxygenation status and may mimic human condition closer than the pre-treatment model described before. The peak R2* value and initial up-slope of R2* in ISOM appear to be effective MRI markers to predict renal injury following administration of an iodinated contrast agent.
Keywords: Streptozotocin, diabetes, BOLD MRI, NGAL, iodinated contrast, rats
INTRODUCTION
Iodinated contrast induced acute kidney injury (CIAKI) is one of the leading causes of hospital-acquired acute kidney injury (AKI) 1. The exact underlying mechanisms of CIAKI have yet to be fully elucidated but are likely to involve the interplay of decreased blood flow, renal medullary ischemia and hypoxia 2, 3. Clinically, the definition of CIAKI is based on serum creatinine (sCr) levels 4. While the limitations of using this marker are well recognized, the relative simplicity makes it attractive for routine use. A major limitation is that the increase in sCr takes 48 to 72 hours post contrast exposure. This limits implementing any preventive strategies to avoid developing AKI because the optimal treatment window is believed to be within 24 hours after patient exposure to iodinated contrast 5. Recent studies have shown urinary neutrophil gelatinase-associated lipocalin (uNGAL) rise much before sCr levels increased 6. Studies have shown uNGAL can detect changes as early as 8 hours post-contrast in human 7 and as early as 4 hours post-contrast in rats 8, 9. Urinary NGAL has a wider dynamic range than serum NGAL to indicate AKI 10. Recent studies by blood oxygenation level dependent (BOLD) MRI have shown near-real-time changes following contrast administration 8, 9, 11.
Clinical studies have shown diabetes mellitus as an independent risk factor for CIAKI 12 due to enhanced renal medullary hypoxia and impaired endothelium-derived vasorelaxation. Previous pre-clinical studies have used a functional CIAKI model by simulating endothelial dysfunction by pre-treating rats with L-NAME (nitric oxide synthase inhibitor) and indomethacin (prostaglandin inhibitor). While the model has shown higher susceptibility to CIAKI and has been demonstrated to be useful in the evaluation of iodinated contrast media induced changes in intra-renal oxygenation using BOLD MRI 8, 9, 11, 13, the clinical relevance to prevalent diseases in human is not obvious to general readers. Streptozotocin (STZ)-induced diabetes is the most common animal model of human diabetes that mimics many complications observed in the diabetic human, such as nephropathy 14, 15. In this study, we tested whether the STZ induced diabetic rats develop CIAKI and if the observed changes in renal oxygenation status as evaluated by BOLD MRI are comparable to those in the functional CIAKI model 8, 9. R2* (=1/T2*) relaxation rate has been used as BOLD MRI parameter based on our previous reports 8, 9. Others have also concluded that R2* changes post-iodinated contrast are reflective of renal oxygenation 16. The renal injury after iodinated contrast was confirmed using uNGAL. Peak R2* (indication of magnitude of change) and initial up-slope (rate of increase) were evaluated as response parameters. The responses observed in the diabetic rats were compared to those rats from a previous study using functional CIAKI model 9.
MATERIALS AND METHODS
Animal and Drugs
Under the guidelines of our Institutional Animal Care and Use Committees approved protocol, six Sprague-Dawley (SD) rats (Harlan Laboratories, Madison, WI, USA) were treated with STZ (Sigma-Aldrich, St. Louis, MO, USA, 50–55 mg/kg body weight) via the lateral tail vein under light anesthesia (isoflurane 2%) to induce diabetes mellitus (Group 1). Approximately 14 days (13.8 ± 1.2 days) after STZ administration, all rats had assessed blood glucose level (BGL) values over 500 mg/dL (549.8 ± 22.3 mg/dL) when MRI was performed. For comparison, data from 12 rats from a previous study 9 were used and re-analyzed using different parameters for BOLD MRI response. These 12 rats received intravenous injection of L-NAME (10mg/kg, Sigma-Aldrich, St. Louis, MO, USA) and indomethacin (10 mg/kg, Sigma-Aldrich, St. Louis, MO, USA) to induce susceptibility to CIAKI. Group 2 (n=6) received saline while Group 3 (n=6) received furosemide (10mg/kg) following pretreatment with L-NAME and indomethacin, as shown in Table 1. On the day of the MRI, the rats were anesthetized using inactin (100 mg/kg i.p., Sigma-Aldrich, St. Louis, MO, USA) and the femoral vein was catheterized for contrast administration. All rats received iodinated contrast iodixanol (1600 mg of organic iodine per kilogram body weight). This iodine load is comparable to what a patient with a normal GFR would receive during a contrast enhanced CT (0.5 gmI/1ml/min GFR). The relative time sequence of the image data acquisition and group information is illustrated in Table 1.
Table 1.
Group, chemical treatment and scan timeline
| # of Rats | BOLD R2* Scan Timeline | |||||||
|---|---|---|---|---|---|---|---|---|
| Group Number |
Group Name | MRI | NGAL | Phase 1 | Phase 2 | Phase 3 | Saline/ Furosemide |
Iodinated Contrast |
| 1 | Diabetes | 6 | 5 | baseline | saline | iodixanol | ||
| 2 | pre-treatment | 6 | 4 | baseline | L-NAME | indomethacin | saline | iodixanol |
| 3 | pre-treatment + Fur | 6 | 3 | baseline | L-NAME | indomethacin | Furosemide | iodixanol |
| scanning time | 15' | 15' | 15' | 15' | 60' | |||
| R2* map # | 1-2-3-4-5 | 6-7-8-9-10 | 11-12-13-14-15 | 16-17-18-19-20 | 21-22-…-39-40 | |||
Note: Five BOLD scans over 15 minutes were performed at baseline, post L-NAME, indomethacin, saline or furosemide. Twenty BOLD scans spanning 60 minutes were performed after iodixanol.
Urinary NGAL
Urine (200 µl) was collected in the diabetic rats prior to the MRI, and 4 hours after contrast media (CM) administration. The urine samples were centrifuged immediately after collection, and the supernatant was transferred to cryovials and preserved at −80°C until assessment. At the time of assessment, the aliquot samples were thawed to room temperature. uNGAL was examined according to the instructions of rat NGAL ELISA Kit (046, BioPorto Diagnostics, Gentofte, Denmark). The average values were derived in duplicate for all of the samples. The standard curve and the absorbance of the samples were measured with a SpectraMax M2e micro-plate reader from Molecular Devices, LLC (Sunnyvale, CA) at a wavelength of 450 nm. Urinary creatinine levels were also measured in order to minimize any confounding effects of urine flow rate 17.
R2* Mapping and Data Analysis
Imaging was performed on a 3.0T scanner (Siemens Magnetom Verio) using a multiple gradient 18, 19 recalled echo sequence (TE = 3.6–41.3 ms with 3.4ms increment; FOV = 12 × 6 cm; TR = 69 ms; phase FOV = 50%; bandwidth = 320Hz / pixel; FA = 30°; NEX = 20; matrix: 256 × 128; slice thickness = 2 mm) to acquire 12 T2* weighted images. The rat kidneys were positioned in the middle of a 8 channel commercial knee coil (Invivo, Gainesville, USA). The receive coil has a tapered shape to reflect the shape of the leg with inner diameter varying approximately from 16 to 13 cm. One transverse slice was selected in the middle of the kidney. BOLD MR images were acquired every 3 min continually as described in Table 1. R2* maps were generated inline immediately following data acquisition on the scanner. Quantitative regional R2* measurements were performed using manually defined region-of-interest (ROI) measurements. ROIs were placed in the inner and outer stripe of the outer medulla (ISOM, OSOM), inner medulla (IM) and cortex (CO) as shown in reference 8. Circular ROIs were used in renal IM and ISOM (>20 pixels). Freehand ROIs (>30 pixels) were used in renal OSOM and CO area as shown in Figure 1. Higher R2* values indicate lower oxygenation or higher hypoxia.
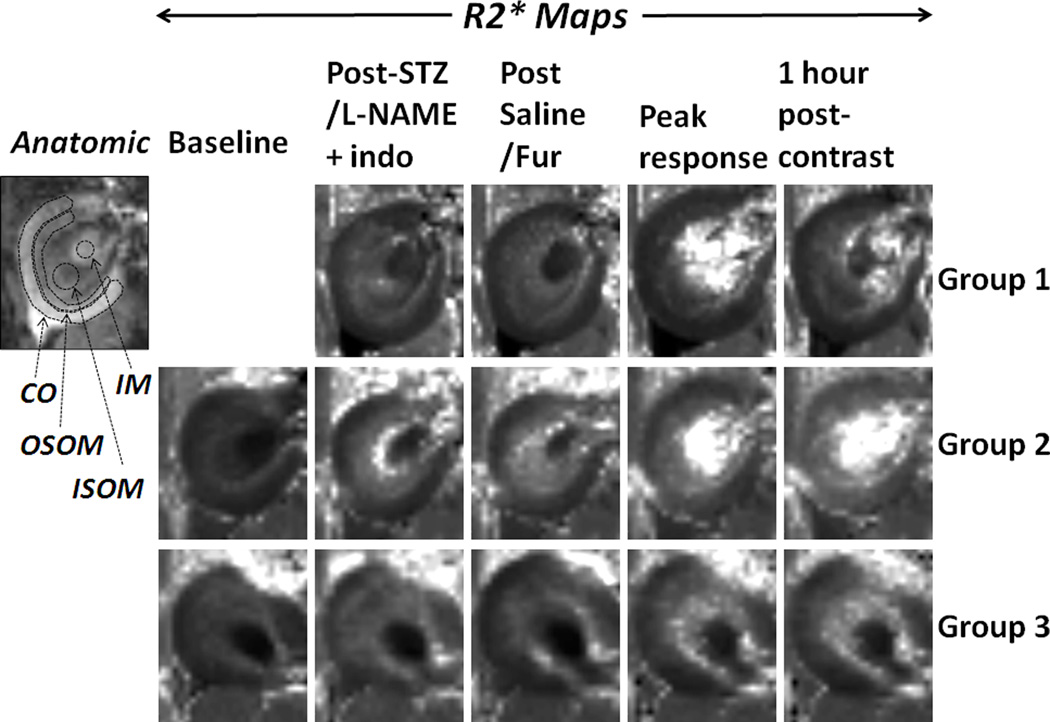
Figure 1.
R2* maps from one representative rat in each group obtained at representative time points. Also included is an anatomical image from the animal in Group 1 with typical ROIs positions and sizes used. Renal regions are defined in anatomic image. IM: inner medulla; ISOM: inner stripe of outer medulla; OSOM: outer stripe of outer medulla; CO: cortex. All R2* maps are displayed with the same window and level settings. The baseline: the R2* map before any treatments in Groups 2 and 3. Indo: indomethacin; Fur: furosemide. Peak response: the response at inflection point following contrast administration.
Statistical Analysis
Based on prior experience 8, 9, ISOM is associated with most sensitive responses to iodinated contrast administration. The R2* values in ISOM following contrast administration in the diabetic rats showed a fast rise followed by a return to baseline (Figures 1 and 2). We tested the linearity of the observed response in ISOM for the diabetes rats. Unlike our previous reports 9, there was a significant quadratic trend in R2*changes over time (p = 0.0133). Therefore, a linear model using all data points as used in the previous reports is not appropriate for the data in this study. For that reason, two new parameters were chosen to evaluate BOLD MRI response, namely peak R2* and the initial up-slope. Peak R2* was defined as the inflection point following administration of iodixanol. Initial slope was defined from one time-point before contrast to the time-point of inflection when R2* stopped to increase (see Figure 2.b). The data of two pre-treatment groups from the previous study 9 were included in the analysis. Since the previous report utilized overall slope from the entire R2* time course, these data were re-analyzed using peak R2* and initial up-slope as response parameters for comparison purposes.
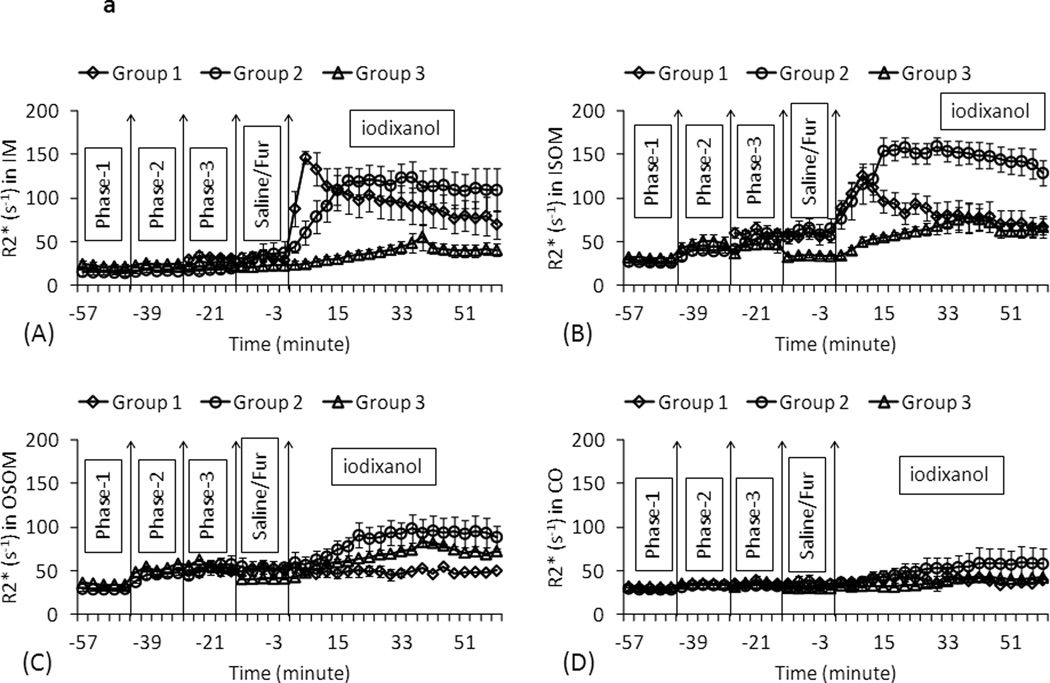
Figure 2.
a. The summary of the temporal changes in R2* measurements in four renal regions in three animal groups. Each data point represents the mean R2* over all the animals within the group and the error bars represent the standard error in the measurements. Phase-1: baseline for pre-treatment groups; Phase-2: L-NAME for pre-treatment groups; Phase-3: indomethacin for pre-treatment groups / R2* baseline for diabetic group; Fur: furosemide. IM: inner medulla; ISOM: inner stripe of outer medulla; OSOM: outer stripe of outer medulla; CO: cortex. Each data point is the average of R2* measurements in 6 rats in the same group at one scan time. Time course consisted of 40 time points in pre-treatment rats (Groups 2 and3) and 30 time points in diabetic rats (Group1). The vertical lines show the time of administration of pre-treatments (L-NAME or indomethacin), intervention (furosemide or placebo) and iodinated contrast iodixanol. Note the key difference between the diabetic animals vs. pre-treatment rats in terms of the response following contrast administration in ISOM and IM. While the pre-treatment group shows a rise and reaching an asymptotic value, the diabetic animals show a rise followed by a quick fall back towards baseline values.
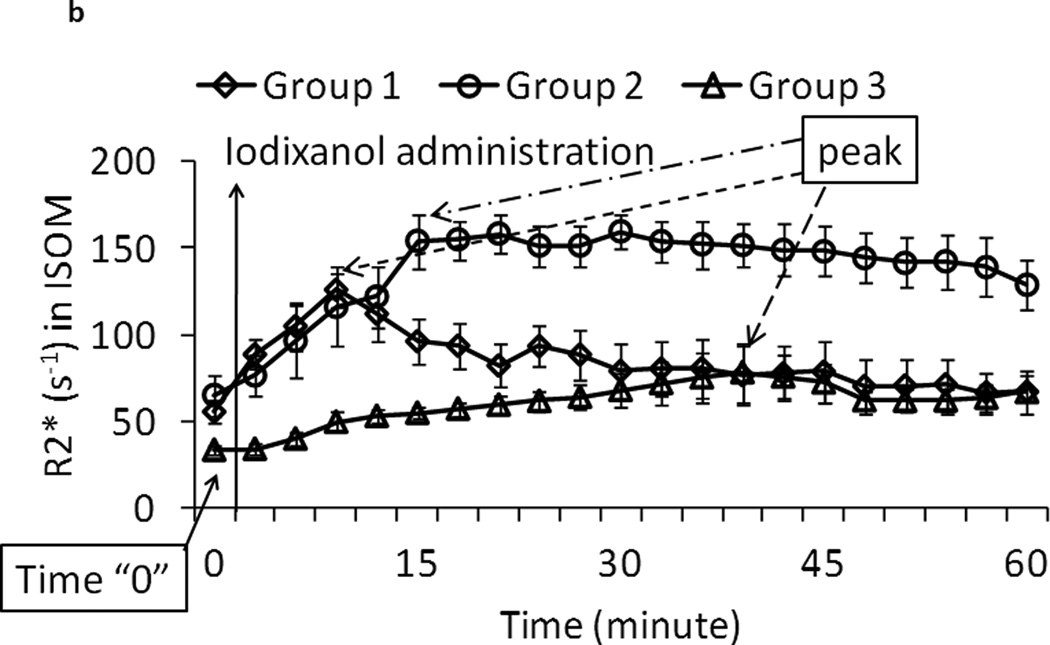
b. Zoomed-in version of plot of ISOM from figure 2.a. Each data point represents the mean R2* over all the animals within the group and the error bars represent the standard error in the measurements. Peak R2* is defined as the inflection point following administration of iodixanol (marked with arrows). The slope was determined using the time-points between Time “0” and the time corresponding to the peak R2*.
To compare the initial-up slopes among three groups, a mixed effect regression model was used to assess R2* measurements in terms of changes over time (slope) from baseline to time-point of inflection. Fixed effects in the model include group, time (continuous) and group by time interactions. The first order auto-regressive variance–covariance structure was specified for the model and random effects accounted for the variation in individual rats.
Urinary NGAL at 4 hours from baseline, peak R2*, and initial up-slope values were compared in different groups using repeated measure ANOVA. The multiple comparisons among groups were adjusted by the Hochberg step-up method. Statistical analyses were carried out by SAS 9.2 (SAS, Cary, NC, USA), and p<0.05 was regarded as statistically significant.
RESULTS
Figure 1 shows R2* maps from representative animals from each group and at representative time points. Figure 2.a shows the summary of R2* time course in the three groups in four renal regions. R2* values were generally higher in all renal regions after iodixanol. The renal cortex had the least response to iodixanol. R2* in IM and ISOM increased quickly followed by a “washout” phase after contrast administration in the diabetic rats. The quick “washout” might be related to the known persistent polyuria in diabetic rats (up to 10 times higher urine flow rates compared to controls 15). Note the comparable R2* values in all regions during phase 3 between all three groups in renal ISOM.
Figure 2.b illustrates the selection of time points for the calculation of initial up-slope of R2* change. The start of R2* time course in ISOM was defined as the time point immediately before contrast administration as illustrated in Figure 2.b. The point of inflection (also the peak) was defined based on group mean R2* value. A linear fit was performed to estimate the slope. Note that there is no significant difference in R2* level at the time point immediately before contrast administration in renal IM, OSOM and CO in all three groups. However, the R2* in ISOM at the time point immediately before contrast administration in Group 3 is lower than the other two groups (p <0.05 vs. Group 1) and (p = 0.06 vs. Group 2), suggesting the reduced hypoxia by furosemide in this group. No difference was observed between Groups 1 and 2 in ISOM at the same time point.
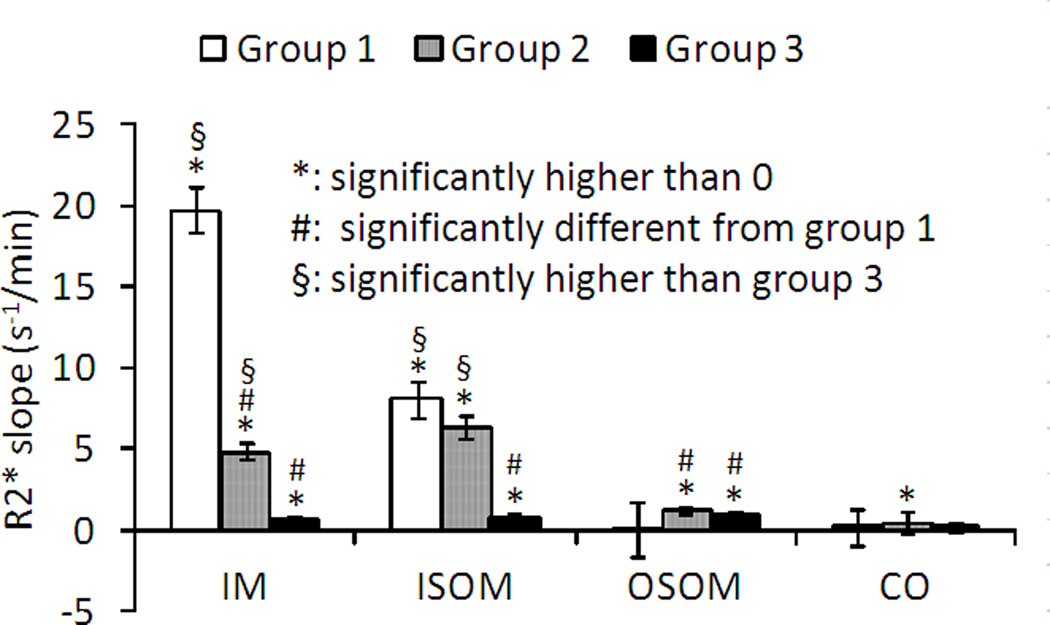
Figure 3 is the summary of the initial up-slopes from the three groups and the four renal regions. Following ANOVA analysis, post-hoc pair-wise comparisons were performed between all three pairs of groups. In the renal IM, R2* in the diabetic group increased significantly faster than the other two groups. In the renal ISOM, R2* in the Groups 1 and 2 increased faster than the Group 3. The R2* values in the renal cortex and OSOM showed slower increase following iodixanol compared to renal IM and ISOM. Also note the initial up-slope is not significantly different between Groups 1 and 2 in ISOM, which is the region that is most sensitive to hemodynamic changes during contrast administration 18, 19.
Figure 3.
Summary of R2* initial up-slope (mean ± SE) post contrast administration in three groups and 4 renal regions. The initial up-slope was defined as the slope from pre-contrast baseline (see Figure 2.b) to the point of inflection. IM: inner medulla; ISOM: inner stripe of outer medulla; OSOM: outer stripe of outer medulla; CO: cortex.
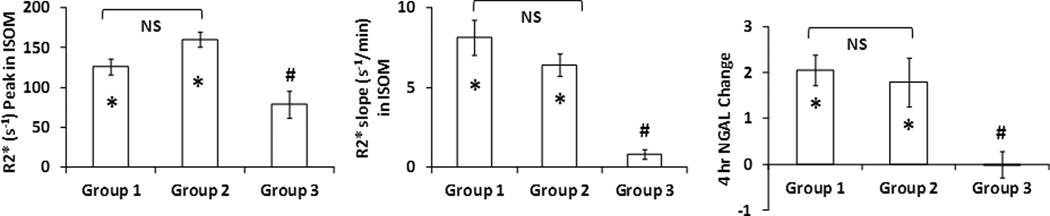
The peak R2* values in ISOM, the initial up-slope of R2* in ISOM, and uNGAL are summarized in Figure 4. There was a clear agreement in the trends observed with peak R2* values, R2* initial up-slope and the changes in 4-hr uNGAL levels among the three groups. Rats receiving furosemide as intervention showed significantly lower peak R2* and R2* initial up-slope in ISOM compared to other two groups, and no significant change in uNGAL (suggesting no renal injury). On the other hand, rats in the other two groups showed significant R2* increase (both peak and initial up-slope) and a corresponding increase in uNGAL at 4 hr post-contrast. There was no difference between the two groups susceptible to CIAKI (Groups 1 and 2) in any of the three measurements.
Figure 4.
Summary of peak R2*, initial up-slope of R2* in renal ISOM and change in uNGAL post contrast administration in three groups and 4 renal regions. Data shown as mean ± standard error. IM: inner medulla; ISOM: inner stripe of outer medulla; OSOM: outer stripe of outer medulla; CO: cortex. The initial up-slope was defined as the slope from pre-contrast baseline (see Figure 2.b) to the time-point of inflection when R2* stopped to increase. To account for any variations in urine flow, uNGAL concentrations were normalized to urine creatinine concentrations. The units are mg NGAL/mg Cr.
DISCUSSION
The results from this study support the feasibility of using STZ induced diabetic rats to study iodinated contrast induced AKI in concert with BOLD MRI and uNGAL. The R2* level in phase-3 in STZ induced diabetic rats was similar to those treated with L-NAME and indomethacin (Figure 2.a), suggesting a similar level of renal oxygenation before intervention. The changes in peak R2* and the initial up-slope in R2* (ISOM) were consistent with those in uNGAL measurements at 4 hour post iodixanol. Further, the responses observed in the diabetic rats (Group 1) were similar to those observed in rats pretreated with L-NAME + indomethacin (Group 2). The comparable results from R2* and uNGAL indicate both models are suitable as a CIAKI susceptible models. The key difference in response between the diabetic rats and the pretreatment group is the relatively fast “washout” associated with the diabetic rats probably related to the polyuria in the early stages of progression 15. Polyuria in diabetic rats may help kidney to remove nephrotoxins faster, however, as our results show that even the initial “contact” of iodinated contrast media with the kidney apparently is sufficient to result in injury as confirmed by NGAL. This strongly supports the need for continuous monitoring of responses to appreciate the early changes in renal hypoxia. In other words, obtaining pre-contrast and a delayed time point post-contrast may not show a response on BOLD MRI and could lead to mis-interpretation. This has practical implications for translation to humans. Based on the logistics, BOLD MRI may be only available before and maybe one hour post-contrast in clinical settings. It is possible that such measurements may not reflect the true degree of changes in renal oxygenation during and immediately after contrast administration. Our data suggest that to specifically monitor renal medullary oxygenation, an alternate method that can be performed simultaneously with the contrast administration may be necessary. Unfortunately, there are no known methods currently available. Monitoring pelvic urine pO2 may be a possible surrogate measure of renal medullary oxygenation 20. There is a single report in literature showing that pelvic urine pO2 closely reflects renal medullary pO2 in humans 21. While measuring pelvic pO2 will necessitate invasive measurements, data exists suggesting that freshly voided urine could be used to monitor relative changes in urine pO2 22. Continuous monitoring of bladder urine pO2 has been shown to be feasible in patients undergoing cardiac bypass procedures 23.
Changes in R2* observed using BOLD MRI in both animal models and humans to furosemide administration parallel those observed with microelectrodes in rat kidneys 24–26. Similarly, changes in BOLD MRI parallel those with microprobes in diabetic rats 27 and in animals treated with nitrix oxide synthase inhibitors (NOSi) 28, 29. While BOLD MRI responses show qualitative agreement with changes in renal oxygenation, quantitative agreement has been shown not to be robust 30, 31. This is due to the multi-factorial dependence of R2* measurement (R2, blood volume, changes in hemoglobin oxygen-desaturation, changes in hematocrit etc.) and limitation of the inherent assumption that blood oxygenation is a representation of surrounding tissue oxygenation 31. However, given the lack of any other non-invasive measurement to evaluate relative oxygenation status, BOLD MRI has received a lot of attention in the recent years. A previous article 8 includes extensive discussion on how the observed increase in R2* may be related to increase hypoxia following iodinated contrast administration.
In conclusion, the results of this study support the use of STZ induced diabetic rat for studying the effects of iodinated contrast to predict development of AKI. Future studies should include larger number of animals to compare different contrast media and interventional strategies, and be performed in a random order and blinded fashion. It may also be interesting to add continuous monitoring of urine pO2 in this model to further validate the feasibility of using urine pO2 as a surrogate for renal medullary oxygenation that may be more practical for human translation.
Acknowledgments
Work supported in part by a grant from the National Institutes of Health, RO1- DK-053221.
REFERENCES
- 1.O'Sullivan S, Healy DA, Moloney MC, et al. The role of N--acetylcysteine in the prevention of contrast-induced nephropathy in patients undergoing peripheral angiography: a structured review and meta-analysis. Angiology. 2013;64(8):576–582. doi: 10.1177/0003319712467223. [DOI] [PubMed] [Google Scholar]
- 2.Heyman SN, Rosenberger C, Rosen S, Khamaisi M. Why is diabetes mellitus a risk factor for contrast-induced nephropathy? Biomed Res Int. 2013;2013:123589. doi: 10.1155/2013/123589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brezis M, Rosen S. Hypoxia of the renal medulla--its implications for disease. N Engl J Med. 1995;332(10):647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 4.Dennen P, Parikh CR. Biomarkers of acute kidney injury: can we replace serum creatinine? Clin Nephrol. 2007;68(5):269–278. doi: 10.5414/cnp68269. [DOI] [PubMed] [Google Scholar]
- 5.Malyszko J, Bachorzewska-Gajewska H, Poniatowski B, et al. Urinary and serum biomarkers after cardiac catheterization in diabetic patients with stable angina and without severe chronic kidney disease. Ren Fail. 2009;31(10):910–919. doi: 10.3109/08860220903216113. [DOI] [PubMed] [Google Scholar]
- 6.Buelow MW, Dall A, Regner K, et al. Urinary interleukin-18 and urinary neutrophil gelatinase-associated lipocalin predict acute kidney injury following pulmonary valve replacement prior to serum creatinine. Congenit Heart Dis. 2012;7(5):441–447. doi: 10.1111/j.1747-0803.2012.00662.x. [DOI] [PubMed] [Google Scholar]
- 7.Lacquaniti A, Buemi F, Lupica R, et al. Can neutrophil gelatinase-associated lipocalin help depict early contrast material-induced nephropathy? Radiology. 2013;267(1):86–93. doi: 10.1148/radiol.12120578. [DOI] [PubMed] [Google Scholar]
- 8.Li LP, Lu J, Zhou Y, et al. Evaluation of intrarenal oxygenation in iodinated contrast-induced acute kidney injury-susceptible rats by blood oxygen level-dependent magnetic resonance imaging. Invest Radiol. 2014;49(6):403–410. doi: 10.1097/RLI.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li LP, Thacker J, Lu J, et al. Efficacy of preventive interventions for iodinated contrast-induced acute kidney injury evaluated by intrarenal oxygenation as an early marker. Invest Radiol. 2014;49(10):647–652. doi: 10.1097/RLI.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippi G, Aloe R, Storelli A, et al. Evaluation of NGAL Test, a fully-automated neutrophil gelatinase-associated lipocalin (NGAL) immunoassay on Beckman Coulter AU 5822. Clin Chem Lab Med. 2012;50(9):1581–1584. doi: 10.1515/cclm.2011.839. [DOI] [PubMed] [Google Scholar]
- 11.Li LP, Franklin T, Du H, et al. Intrarenal oxygenation by blood oxygenation level-dependent MRI in contrast nephropathy model: effect of the viscosity and dose. J Magn Reson Imaging. 2012;36(5):1162–1167. doi: 10.1002/jmri.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berwanger O, Cavalcanti AB, Sousa AM, et al. Acetylcysteine for the prevention of renal outcomes in patients with diabetes mellitus undergoing coronary and peripheral vascular angiography: a substudy of the acetylcysteine for contrast-induced nephropathy trial. Circ Cardiovasc Interv. 2013;6(2):139–145. doi: 10.1161/CIRCINTERVENTIONS.112.000149. [DOI] [PubMed] [Google Scholar]
- 13.Prasad PV, Priatna A, Spokes K, Epstein FH. Changes in intrarenal oxygenation as evaluated by BOLD MRI in a rat kidney model for radiocontrast nephropathy. J Magn Reson Imaging. 2001;13(5):744–747. doi: 10.1002/jmri.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen MP, Klein CV. Glomerulopathy in rats with streptozotocin diabetes. Accumulation of glomerular basement membrane analogous to human diabetic nephropathy. J Exp Med. 1979;149(3):623–631. doi: 10.1084/jem.149.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palm F, Ortsater H, Hansell P, et al. Differentiating between effects of streptozotocin per se and subsequent hyperglycemia on renal function and metabolism in the streptozotocin-diabetic rat model. Diabetes Metab Res Rev. 2004;20(6):452–459. doi: 10.1002/dmrr.472. [DOI] [PubMed] [Google Scholar]
- 16.Arakelyan K, Cantow K, Hentschel J, et al. Early effects of an x-ray contrast medium on renal T(2) */T(2) MRI as compared to short-term hyperoxia, hypoxia and aortic occlusion in rats. Acta Physiol (Oxf) 2013;208(2):202–213. doi: 10.1111/apha.12094. [DOI] [PubMed] [Google Scholar]
- 17.Xin C, Yulong X, Yu C, et al. Urine neutrophil gelatinase-associated lipocalin and interleukin-18 predict acute kidney injury after cardiac surgery. Ren Fail. 2008;30(9):904–913. doi: 10.1080/08860220802359089. [DOI] [PubMed] [Google Scholar]
- 18.De Greef KE, Ysebaert DK, Persy V, et al. ICAM-1 expression and leukocyte accumulation in inner stripe of outer medulla in early phase of ischemic compared to HgCl2-induced ARF. Kidney Int. 2003;63(5):1697–1707. doi: 10.1046/j.1523-1755.2003.00909.x. [DOI] [PubMed] [Google Scholar]
- 19.Heyman SN, Brezis M, Reubinoff CA, et al. Acute renal failure with selective medullary injury in the rat. J Clin Invest. 1988;82(2):401–412. doi: 10.1172/JCI113612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans RG, Smith JA, Wright C, et al. Urinary oxygen tension: a clinical window on the health of the renal medulla? Am J Physiol Regul Integr Comp Physiol. 2014;306(1):R45–R50. doi: 10.1152/ajpregu.00437.2013. [DOI] [PubMed] [Google Scholar]
- 21.Leonhardt KO, Landes RR, McCauley RT. Anatomy and Physiology of Intrarenal Oxygen Tension: Preliminary Study of the Effects of Anesthetics. Anesthesiology. 1965;26:648–658. doi: 10.1097/00000542-196509000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Giannakopoulos X, Evangelou A, Kalfakakou V, et al. Human bladder urine oxygen content: implications for urinary tract diseases. Int Urol Nephrol. 1997;29(4):393–401. doi: 10.1007/BF02551103. [DOI] [PubMed] [Google Scholar]
- 23.Kainuma M, Yamada M, Miyake T. Continuous urine oxygen tension monitoring in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 1996;10(5):603–608. doi: 10.1016/s1053-0770(96)80137-6. [DOI] [PubMed] [Google Scholar]
- 24.Brezis M, Heyman SN, Epstein FH. Determinants of intrarenal oxygenation. II. Hemodynamic effects. Am J Physiol. 1994;267(6 Pt 2):F1063–F1068. doi: 10.1152/ajprenal.1994.267.6.F1063. [DOI] [PubMed] [Google Scholar]
- 25.Li LP, Vu AT, Li BS, et al. Evaluation of intrarenal oxygenation by BOLD MRI at 3.0 T. J Magn Reson Imaging. 2004;20(5):901–904. doi: 10.1002/jmri.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Priatna A, Epstein FH, Spokes K, Prasad PV. Evaluation of changes in intrarenal oxygenation in rats using multiple gradient-recalled echo (mGRE) sequence. J Magn Reson Imaging. 1999;9(6):842–846. doi: 10.1002/(sici)1522-2586(199906)9:6<842::aid-jmri12>3.0.co;2-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.dos Santos EA, Li LP, Ji L, Prasad PV. Early changes with diabetes in renal medullary hemodynamics as evaluated by fiberoptic probes and BOLD magnetic resonance imaging. Invest Radiol. 2007;42(3):157–162. doi: 10.1097/01.rli.0000252492.96709.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li LP, Ji L, Santos E, et al. Effect of nitric oxide synthase inhibition on intrarenal oxygenation as evaluated by blood oxygenation level-dependent magnetic resonance imaging. Invest Radiol. 2009;44(2):67–73. doi: 10.1097/RLI.0b013e3181900975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agmon Y, Peleg H, Greenfeld Z, et al. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J Clin Invest. 1994;94(3):1069–1075. doi: 10.1172/JCI117421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niendorf T, Pohlmann A, Arakelyan K, et al. How bold is blood oxygenation level-dependent (BOLD) magnetic resonance imaging of the kidney? Opportunities, challenges and future directions. Acta Physiol (Oxf) 2014 doi: 10.1111/apha.12393. [DOI] [PubMed] [Google Scholar]
- 31.Pohlmann A, Arakelyan K, Hentschel J, et al. Detailing the relation between renal T2* and renal tissue pO2 using an integrated approach of parametric magnetic resonance imaging and invasive physiological measurements. Invest Radiol. 2014;49(8):547–560. doi: 10.1097/RLI.0000000000000054. [DOI] [PubMed] [Google Scholar]