Abstract
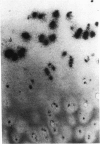
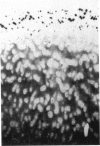
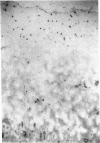
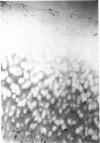
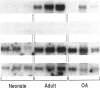
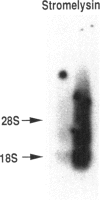
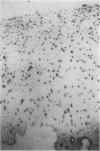
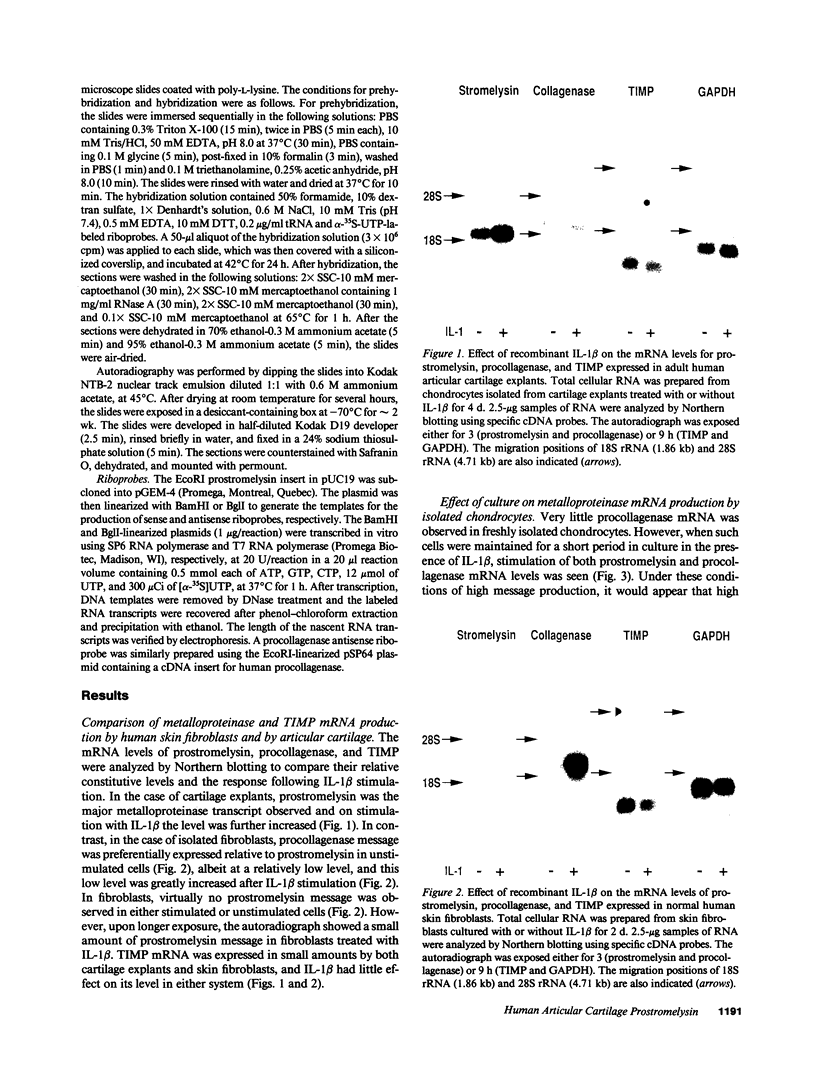
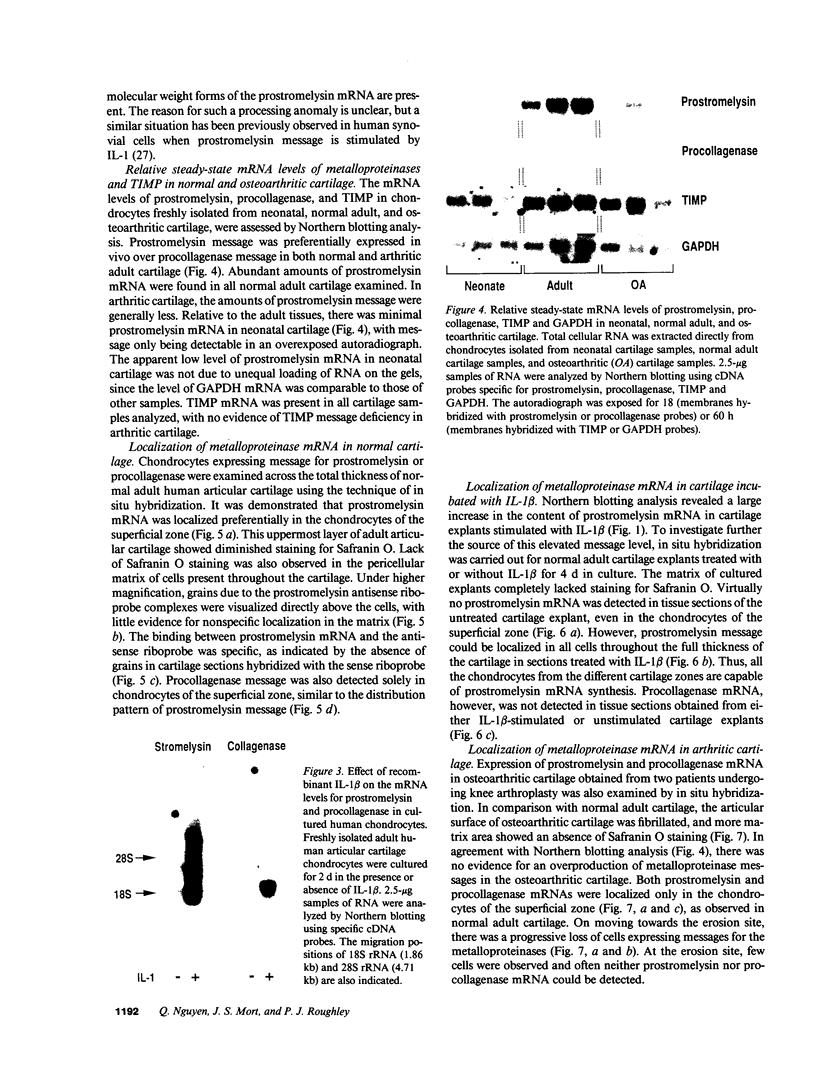
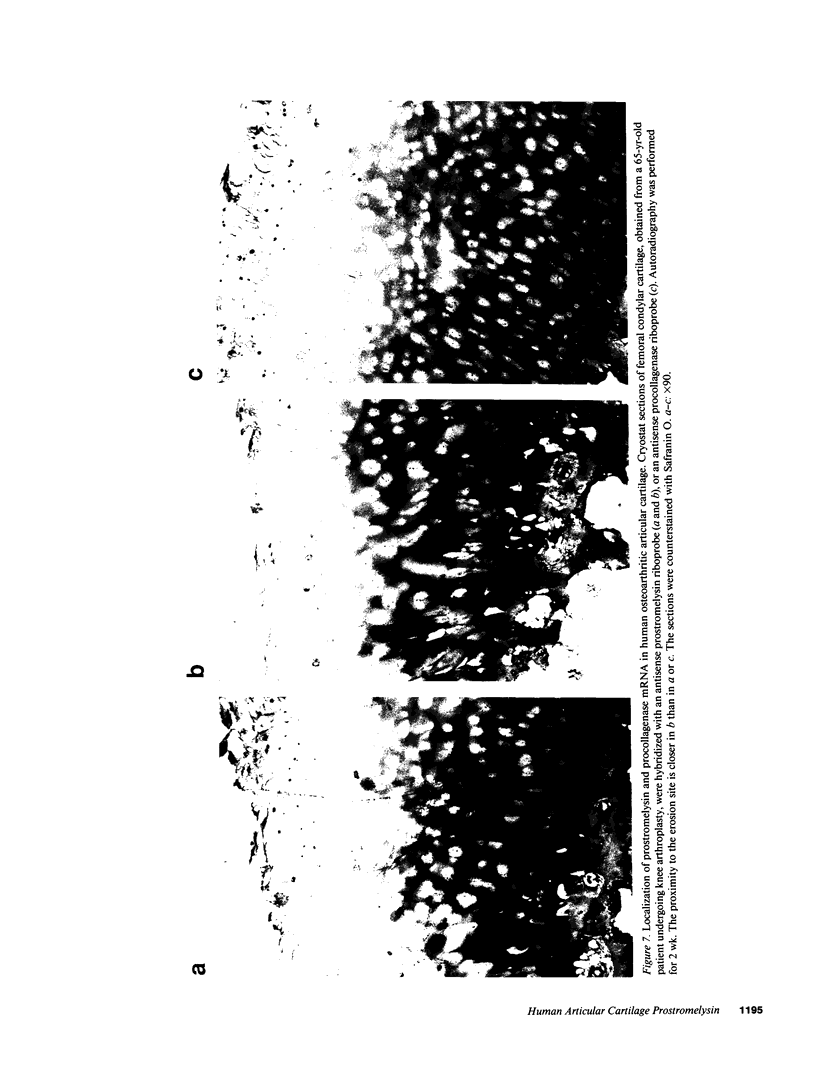
An imbalance between extracellular proteinases and their inhibitors is thought to underlie cartilage degradation. In cultures of adult cartilage, prostromelysin mRNA levels were much higher than those for procollagenase and this differential was increased in cultures stimulated with IL-1 beta. Analysis of mRNA prepared from freshly isolated chondrocytes showed abundant amounts of prostromelysin mRNA in normal adult cartilage but low levels in the neonate. Not all adult cartilage may possess such high levels of prostromelysin mRNA, as the message levels in the cartilage remaining on late-stage osteoarthritic joints were lower than those in normal adult cartilage. Relative to prostromelysin mRNA, little procollagenase and TIMP mRNA were found in the adult cartilage. In situ hybridization revealed that metalloproteinase mRNAs were localized in chondrocytes of the superficial zone in adult cartilage. However, upon IL-1 beta treatment, chondrocytes in all cartilage zones were observed to express prostromelysin mRNA. Relative to the neonate, the normal adult cartilage appears to have a high degradative potential, if one accepts that steady-state mRNA levels reflect prostromelysin production. As the adult cartilage is not apparently undergoing rapid turnover, it would appear that control of prostromelysin activation may be the major regulatory step in stromelysin-induced cartilage degradation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzo W., Woessner J. F., Jr Purification and characterization of an acid metalloproteinase from human articular cartilage. J Biol Chem. 1986 Apr 25;261(12):5434–5441. [PubMed] [Google Scholar]
- Campbell I. K., Guttman A., Golds E. E., Roughley P. J., Mort J. S. Variation in the effects of mononuclear cell products from different individuals on metalloproteinase secretion from human articular cartilage. J Rheumatol. 1989 Dec;16(12):1552–1558. [PubMed] [Google Scholar]
- Campbell I. K., Roughley P. J., Mort J. S. The action of human articular-cartilage metalloproteinase on proteoglycan and link protein. Similarities between products of degradation in situ and in vitro. Biochem J. 1986 Jul 1;237(1):117–122. doi: 10.1042/bj2370117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael D. F., Sommer A., Thompson R. C., Anderson D. C., Smith C. G., Welgus H. G., Stricklin G. P. Primary structure and cDNA cloning of human fibroblast collagenase inhibitor. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2407–2411. doi: 10.1073/pnas.83.8.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Galloway W. A., Mercer E., Murphy G., Reynolds J. J. Purification of rabbit bone inhibitor of collagenase. Biochem J. 1981 Apr 1;195(1):159–165. doi: 10.1042/bj1950159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dean D. D., Martel-Pelletier J., Pelletier J. P., Howell D. S., Woessner J. F., Jr Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989 Aug;84(2):678–685. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring M. B., Birkhead J., Sandell L. J., Kimura T., Krane S. M. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest. 1988 Dec;82(6):2026–2037. doi: 10.1172/JCI113823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunja-Smith Z., Nagase H., Woessner J. F., Jr Purification of the neutral proteoglycan-degrading metalloproteinase from human articular cartilage tissue and its identification as stromelysin matrix metalloproteinase-3. Biochem J. 1989 Feb 15;258(1):115–119. doi: 10.1042/bj2580115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley C. J., Lowther D. A. Extracellular matrix metabolism by chondrocytes. III. Modulation of proteoglycan synthesis by extracellular levels of proteoglycan in cartilage cells in culture. Biochim Biophys Acta. 1977 Nov 7;500(1):132–139. doi: 10.1016/0304-4165(77)90053-8. [DOI] [PubMed] [Google Scholar]
- Khokha R., Denhardt D. T. Matrix metalloproteinases and tissue inhibitor of metalloproteinases: a review of their role in tumorigenesis and tissue invasion. Invasion Metastasis. 1989;9(6):391–405. [PubMed] [Google Scholar]
- Liu J., Roughley P. J., Mort J. S. Identification of human intervertebral disc stromelysin and its involvement in matrix degradation. J Orthop Res. 1991 Jul;9(4):568–575. doi: 10.1002/jor.1100090413. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., Pelletier J. P., Cloutier J. M., Howell D. S., Ghandur-Mnaymneh L., Woessner J. F., Jr Neutral proteases capable of proteoglycan digesting activity in osteoarthritic and normal human articular cartilage. Arthritis Rheum. 1984 Mar;27(3):305–312. doi: 10.1002/art.1780270310. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., Reynolds J. J. Characterization of a specific antiserum to rabbit stromelysin and demonstration of the synthesis of collagenase and stromelysin by stimulated rabbit articular chondrocytes. Coll Relat Res. 1986 Oct;6(4):351–363. doi: 10.1016/s0174-173x(86)80005-x. [DOI] [PubMed] [Google Scholar]
- Murphy G., McGuire M. B., Russell R. G., Reynolds J. J. Characterization of collagenase, other metallo-proteinases and an inhibitor (TIMP) produced by human synovium and cartilage in culture. Clin Sci (Lond) 1981 Dec;61(6):711–716. doi: 10.1042/cs0610711. [DOI] [PubMed] [Google Scholar]
- Murphy G., Nagase H., Brinckerhoff C. E. Relationship of procollagenase activator, stromelysin and matrix metalloproteinase 3. Coll Relat Res. 1988 Jul;8(4):389–391. doi: 10.1016/s0174-173x(88)80009-8. [DOI] [PubMed] [Google Scholar]
- Nahir A. M. Aerobic glycolysis: a study of human articular cartilage. Cell Biochem Funct. 1987 Apr;5(2):109–112. doi: 10.1002/cbf.290050205. [DOI] [PubMed] [Google Scholar]
- Nguyen Q., Murphy G., Roughley P. J., Mort J. S. Degradation of proteoglycan aggregate by a cartilage metalloproteinase. Evidence for the involvement of stromelysin in the generation of link protein heterogeneity in situ. Biochem J. 1989 Apr 1;259(1):61–67. doi: 10.1042/bj2590061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Konomi H., Yada T., Kimata K., Nagase H. Degradation of type IX collagen by matrix metalloproteinase 3 (stromelysin) from human rheumatoid synovial cells. FEBS Lett. 1989 Feb 27;244(2):473–476. doi: 10.1016/0014-5793(89)80586-1. [DOI] [PubMed] [Google Scholar]
- Okada Y., Takeuchi N., Tomita K., Nakanishi I., Nagase H. Immunolocalization of matrix metalloproteinase 3 (stromelysin) in rheumatoid synovioblasts (B cells): correlation with rheumatoid arthritis. Ann Rheum Dis. 1989 Aug;48(8):645–653. doi: 10.1136/ard.48.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J. P., Cloutier J. M., Martel-Pelletier J. In vitro effects of tiaprofenic acid, sodium salicylate and hydrocortisone on the proteoglycan metabolism of human osteoarthritic cartilage. J Rheumatol. 1989 May;16(5):646–655. [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Howell D. S., Ghandur-Mnaymneh L., Enis J. E., Woessner J. F., Jr Collagenase and collagenolytic activity in human osteoarthritic cartilage. Arthritis Rheum. 1983 Jan;26(1):63–68. doi: 10.1002/art.1780260110. [DOI] [PubMed] [Google Scholar]
- Rizzino A. Transforming growth factor-beta: multiple effects on cell differentiation and extracellular matrices. Dev Biol. 1988 Dec;130(2):411–422. doi: 10.1016/0012-1606(88)90337-5. [DOI] [PubMed] [Google Scholar]
- Saus J., Quinones S., Otani Y., Nagase H., Harris E. D., Jr, Kurkinen M. The complete primary structure of human matrix metalloproteinase-3. Identity with stromelysin. J Biol Chem. 1988 May 15;263(14):6742–6745. [PubMed] [Google Scholar]
- Schmid T. M., Mayne R., Jeffrey J. J., Linsenmayer T. F. Type X collagen contains two cleavage sites for a vertebrate collagenase. J Biol Chem. 1986 Mar 25;261(9):4184–4189. [PubMed] [Google Scholar]
- Sirum K. L., Brinckerhoff C. E. Cloning of the genes for human stromelysin and stromelysin 2: differential expression in rheumatoid synovial fibroblasts. Biochemistry. 1989 Oct 31;28(22):8691–8698. doi: 10.1021/bi00448a004. [DOI] [PubMed] [Google Scholar]
- Spencer C. A., Palmer T. N., Mason R. M. Intermediary metabolism in the Swarm rat chondrosarcoma chondrocyte. Biochem J. 1990 Feb 1;265(3):911–914. doi: 10.1042/bj2650911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M. L., Goldring M. B., Birkhead J. R., Krane S. M., Rahmsdorf H. J., Angel P. Stimulation of procollagenase synthesis parallels increases in cellular procollagenase mRNA in human articular chondrocytes exposed to recombinant interleukin 1 beta or phorbol ester. Biochem Biophys Res Commun. 1987 Apr 29;144(2):583–590. doi: 10.1016/s0006-291x(87)80006-2. [DOI] [PubMed] [Google Scholar]
- Treadwell B. V., Neidel J., Pavia M., Towle C. A., Trice M. E., Mankin H. J. Purification and characterization of collagenase activator protein synthesized by articular cartilage. Arch Biochem Biophys. 1986 Dec;251(2):715–723. doi: 10.1016/0003-9861(86)90381-4. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. A. Articular cartilage cultured with catabolin (pig interleukin 1) synthesizes a decreased number of normal proteoglycan molecules. Biochem J. 1985 May 1;227(3):869–878. doi: 10.1042/bj2270869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart H. E., Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham S. E., Murphy G., Angel P., Rahmsdorf H. J., Smith B. J., Lyons A., Harris T. J., Reynolds J. J., Herrlich P., Docherty A. J. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J. 1986 Dec 15;240(3):913–916. doi: 10.1042/bj2400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley D. E., Crossley M. J., Evanson J. M. Collagenase at sites of cartilage erosion in the rheumatoid joint. Arthritis Rheum. 1977 Jul-Aug;20(6):1231–1239. doi: 10.1002/art.1780200612. [DOI] [PubMed] [Google Scholar]