Abstract
Objectives
Substantial evidence associates persistent organic pollutants (POP) with metabolic disturbances related to diabetes, but longitudinal studies with repeated measures are scarce. We aimed to characterize the association between background exposures to POPs with repeated measures of glucose homeostasis over 23-years.
Methods
Within the Coronary Artery Risk Development in Young Adults study (year 0 ages: 18–30 years), we measured POPs in serum obtained in 1987–88 (follow-up year 2) in 90 non-diabetic controls and 90 cases diabetes-free at year 2 who became diabetic by year 20. We analyzed 32 POPs detectable in ≥75% of participants and created summary scores for 32 POPs, 23 polychlorinated biphenyls (PCB), and 8 organochlorine pesticides (OCP). Dependent variables were measures of glucose homeostasis at years 0–25 (up to 8 examinations). We explored associations using repeated measures regression adjusted for race, sex, concurrent body mass index (BMI), examination center and period, separately for cases and controls.
Results
The associations between the three summary scores and measures of glucose homeostasis were present for observations at ages 40–55 years, and particularly between 48–55 years: the 23 PCB summary was associated with HbA1c (never-diabetics: slope [value per unit of summary score], β=0.008, p=0.02; diabetics: β=0.03, p=0.07), fasting glucose (never-diabetics: β=0.24, p=0.003; diabetics: β=1.10, p=0.03), and insulin sensitivity% (never-diabetics: β=−2.82, p < 0.001, diabetics: β=−0.31, p=0.30). No associations were observed at younger ages.
Conclusions
Glucose homeostasis may worsen after decades of exposure to PCBs and OCPs at background environmental levels, independent of BMI and after participants reached the 5th decade of life.
Keywords: POPs, Glucose, HbA1c, PCB, Organochlorine
1. Introduction
There is substantial in-vitro and in-vivo evidence that persistent organic pollutants (POPs) can act as endocrine disruptors and promote metabolic dysregulation (Lee et al., 2014). Recent studies showed that rats fed high-fat diets with POPs-contaminated salmon oils (compared to decontaminated salmon oil) (Ruzzin et al., 2010), and mice fed commercially farmed salmon filets contaminated with background POPs (compared to salmon specially raised to avoid POPs exposure) (Ibrahim et al., 2011) developed greater insulin resistance, abdominal obesity, and hepatosteatosis. In various population-based cross-sectional studies worldwide, exposure to several POPs at background concentrations (particularly polychlorinated biphenyls (PCB) and organochlorine pesticides) has been found to have strong positive associations with insulin resistance, pre-diabetes and diabetes after adjustment for confounders (Codru et al., 2007; Gasull et al., 2012; Lee et al., 2006, 2007a, 2007b; Uemura et al., 2008). Longitudinal studies in the USA, Sweden, Taiwan and Italy have also reported significant associations of organochlorine pesticides and PCBs with incidence of diabetes (Lee et al., 2011a; Turyk et al., 2009; Vasiliu et al., 2006; Wang et al., 2008) and diabetes-related deaths (Bertazzi et al., 2001). Nevertheless, longitudinal studies with repeated measures are scarce. Such studies could greatly contribute to establish the temporal sequence of metabolic events following POPs exposure.
We previously described non-monotonic (specifically, inverted U-shaped) associations of organochlorine pesticide and PCB blood concentrations with diabetes in a case (diabetes)-control study nested within the Coronary Artery Risk Development in Young Adults (CARDIA) study (n=180) (Lee et al., 2010). Among controls, organochlorine pesticides and PCBs also had positive quadratic associations with BMI, triglycerides and insulin resistance, and negative associations with HDL-cholesterol 18 years after the measurement of POPs (Lee et al., 2011b). The reported inverted U-shaped associations of organochlorine pesticides and PCBs with diabetes support the hypothesis that high body burdens of endocrine disrupting chemicals can exert inhibitory effects on processes that are stimulated at much lower doses.
The present investigation further characterizes the associations between blood concentrations of POPs from background exposures in 1987–1988 and glucose dysregulation over the following 23 years among participants with and without diabetes of the CARDIA nested case-control study. Given the possibility of varying associations between POP exposures and glucose dysregulation at different stages of life (Lee et al., 2014), and considering increasing accumulation of POPs in tissues and higher prevalence of cardiovascular and endocrine alterations with age (particularly, after age 40) (National Center for Health Statistics, 2013), we tested the hypothesis that the associations between POPs and glucose dysregulation would be strongest among older individuals.
2. Materials and methods
2.1. Participant selection
In 1985–1986 (CARDIA year 0), CARDIA examined 5115 black and white participants 18–30 years of age, recruited from the general populations of Birmingham, AL, Minneapolis, MN, and Chicago, IL and from the Kaiser Permanente Medical Care Plan in Oakland, CA (Friedman et al., 1988; Hughes et al., 1987). Since year 0, there have been 7 follow-up examinations at years 2, 5, 7, 10, 15, 20 and 25 (2010–2011). The study was approved by the institutional review boards of the University of Minnesota, University of Alabama at Birmingham, Northwestern University, and the Division of Research at Kaiser Permanente Health Care Plan. Participants signed informed consent at every examination.
We included participants in the CARDIA nested case-control study (Lee et al., 2010). Ninety cases were randomly selected from among 116 people having taken anti-diabetic medications or having fasting glucose ≥126 mg/dl at two or more examinations after POPs exposure measurement in year 2 (CARDIA years 7, 10, 15 and 20), but having had no diagnosis of diabetes at years 0 and 2. Ninety controls were selected from those who had fasting glucose below 100 mg/dL at follow-up years 0, 7, 10, 15, and 20. Controls were frequency matched to cases, selected at random within several year 0 body mass index (BMI) categories (< 20, 20–24.9, 25–29.9, 30–39.9, and 40 + kg/m2).
Because the definition of controls required attendance at years 0, 7, 10, 15, and 20, they had little missing data in the current analyzes: only 6% (n=5) of controls did not attend the year 25 examination. Cases could be identified earlier than year 20, and missing follow-up examinations occurred in 11% (n=10) at year 7,10% (n=9) at year 10, 17% (n=15) at year 15, 12% (n=11) at year 20 and 20% (n=18) at year 25.
2.2. Measures
Information regarding demographics, health behaviors and anthropometrics was obtained at baseline and follow-up examinations. Participants with diabetes were allowed to follow their usual diabetes treatment before and during the examination. Blood samples were collected after an overnight fast of at least 8 h. Hemoglobin-A1c (HbA1c), expressed as percent, was measured at the University of Minnesota using ion-exchange high-performance liquid chromatography at years 20 and 25. Fasting plasma glucose concentrations were determined using a hexokinase-ultraviolet method at Linco Research Inc. (now Millipore Inc., Billerica, MA, USA) at years 0, 7, 10, 15 and 20, and at the Molecular Epidemiology and Biomarker Research Laboratory (University of Minnesota) at year 25. Fasting insulin concentrations were measured at Linco Research at years 0, 7, 10, 15 and 20 by radioimmunoassay with an overnight equilibrium incubation using a high-specificity antibody (< 0.2% cross-reactivity with human proinsulin and Des 31,32 proinsulin) (Haffner et al., 1994). Insulin was measured at the University of Minnesota at year 25 on a Roche Elecsys 2010 Analyzer (Roche Diagnostics, Indianapolis, IN) using a sandwich immunoassay method (Roche Diagnostics). Based on re-assays of glucose in December 2007 in approximately 200 samples stored since year 7, 10, 15, and 20, glucose concentrations were recalibrated to values provided by the National Institute of Standards and Technology. The insulin assay at year 20 was similarly recalibrated based on re-assay of year 15 samples; then all insulin values from years 0, 7, 10, 15, and 20 were further multiplied by 0.7049 to recalibrate to the values of the very precise immunoassay performed at year 25.
Percent insulin sensitivity and β-cell function were estimated using the updated Homeostasis Model Assessment (HOMA-2) calculator (University of Oxford, Oxford, UK, http://www.dtu.ox.ac.uk/homacalculator/). HOMA-2 is a steady-state prediction of insulin resistance and β-cell function for many plausible combinations of fasting glucose and insulin concentrations, which accounts for renal glucose losses and assumes reduced suppression of hepatic glucose production and increased insulin secretion at high glucose concentrations (Muniyappa et al., 2008). We have smaller sample sizes for most exam years when assessing HOMA-2 calculations because of missing insulin or glucose measurements: 19 participants had missing information for year 0, 9 for year 7, 5 for year 10, 2 for year 15, 1 for year 20 and 3 for year 25. We also excluded two participants at year 25 for HOMA-2 calculations for having an insulin value or a glucose value exceeding the accepted range for HOMA-2 calculations (insulin upper limit: 43.2 μIU/ml [300 pmol/L], glucose upper limit: 450 mg/dl [25 mmol/L]). In the present sample, BMI has a correlation of 0.87 with waist circumference. For the purpose of estimating the association between exposures to POPs and (an estimate of) fatness, we considered that presenting results for BMI would suffice given its equivalent correlation with fat mass (≈0.90) compared to that of waist circumference (Camhi et al., 2011).
POP concentrations in stored CARDIA year 2 serum samples (collected in 1987 and 1988) were measured in 2008 as part of the Young Adults Longitudinal Trends in Atheroclerosis (YALTA) ancillary study to CARDIA. Analyses were performed using solid-phase extraction and final determination using gas chromatography isotope dilution high-resolution mass spectrometry (Barr et al., 2003; Sjödin et al., 2004) at the Centers for Disease Control Environmental Health Laboratory. A total of 55 POPs were measured: 9 organochlorine pesticides, 35 PCB congeners, 10 polybrominated diphenyl ether (PBDE) congeners, and 1 polybrominated biphenyl (PBB) congener. Of the 55 measured POPs, we selected 32 which had at least 75% of values above the detection limit (8 OCPs, 23 PCBs, and PBB-153 congeners). The median concentrations of each of these POPs are presented in Supplementary Table 1. Non-detectable concentrations in this table were replaced with half of the detection limit. The levels of detection were dependent on the blood sample amount available for each participant and specific for each POP measured. The coefficients of variation of 4 POPs exceeded or were equal to 1 (mirex 1.79, γ-hexachlorocyclohexane 1.78, PBB153=1.97, PCB105=0.99). The remaining 28 had coefficients of variation ranging from 0.45 to 0.87. The concentrations of organochlorines and PCBs in CARDIA participants were approximately 2–4 times and 1.5–7 times greater than similar-age participants in 2003–2004 of the National Health and Nutrition Examination Survey (NHANES), respectively (Lee et al., 2010).
2.3. Statistical methods
Because in the general population background exposures to POPs occur as mixtures of many compounds (Porta et al., 2008), we were interested in assessing the associations of a composite of various POPs on glucose homeostasis. Since the concentrations of individual POPs varied substantially (e.g., the concentrations of the 8 most prevalent POPs were 25.6 times greater than the 8 least prevalent), we created a summary score for the 32 POPs detectable in ≥75% of participants (named 32 POP summary score). The summary score consisted of the sum over POPs of participant-specific log-transformed POP concentration/standard deviation of the corresponding log-transformed POP. We created similar summary variables for organochlorine pesticides (8 OCP summary score) and PCBs (23 PCB summary score). Overall, the correlations of the 32 POP summary score with the 23 PCB and 8 OCP summary scores were 0.98 and 0.80, respectively. Because of the high correlation between the 32 POP and 23 PCB summary scores, we only present results for the 23 PCB summary score. However, we present participant characteristics for the 32 POP summary score because we present results for three classes of POPs. The correlation between 23 PCB and 8 OCP summary scores was 0.70. We also log-transformed PBB-53 and analyzed its associations separately because it was the only congener of its class.
We applied repeated measures regression using a Toeplitz covariance matrix to explore the associations of each of the 32 POP, 23 PCB and 8 OCP summary scores with HbA1c, fasting glucose, insulin sensitivity, β-cell function and BMI over time. We included outcome information from the following examinations: for HbA1c, years 20 and 25; for BMI, years 0, 2, 7, 10, 15, 20 and 25; and for fasting glucose, insulin sensitivity and β-cell function, years 0, 7, 10, 15, 20 and 25. We include year 0 glucose, insulin sensitivity and β-cell function in longitudinal analyzes to estimate year 2 values (concurrent values with the measurement of POPs). Given that this study was originally designed as a nested case-control study, and diabetics and non-diabetics have significantly different dynamics of glucose metabolism, we analyzed the associations separately for diabetes cases and controls.
Statistical models defined a-priori adjusted for age, race, sex, exam center, exam period and concurrent BMI. A-posteriori, we considered concurrent glucose lowering medication use and menopause status (0=premenopause, 1=perimenopause, 2=menopause) as potential confounders; however, further adjustment for these variables (separately) yielded negligible changes (< 10% change) in the associations between POPs and all outcomes among all participants. For this reason, we did not include medication use or menopause status in the final model. Since HbA1c was only measured in years 20 and 25 of follow-up, associations between POPs and HbA1c were additionally adjusted for year 0 fasting glucose. Associations between POPs and β-cell function percent were adjusted for insulin sensitivity percent because basal insulin production varies according to the degree of insulin sensitivity (Wallace et al., 2004). We tested effect modification by age in the associations and conducted age-specific analyzes. We selected 4 age groups that included a roughly homogeneous number of participants in the youngest 3 groups and included at least 2/3 of available participants in the oldest age group. The selected age groups (in years) were 18–31, 32–39, 40–47 and 48–55. We calculated the adjusted predicted means using repeated measures regression of each outcome variable for each quartile of POP summary score and plotted the associations by each age stratum. We tested a linear term using a continuous POPs variable for each association. We also visually determined model fit and potential nonlinearity by comparing the plot of the statistical association with the plotted adjusted means of each outcome for the quartiles of the 23 PCB and 8 OCP summary scores. Quadratic fits were examined, but many clearly did not represent the functional shape well (data not shown).
Analyses of POPs have generally used POPs values standardized or corrected for lipids (POPs concentration divided by total lipid content) or statistical models that adjust for blood lipids given that POPs are predominantly carried in the lipid component of blood (Porta et al., 2009). However, POPs can disturb lipid metabolism (Lee et al., 2007c; Obana et al., 1987), which can in turn be a mechanism of disturbance of glucose homeostasis of POPs (Schisterman et al., 2005). Therefore, adjusting for lipids when analyzing the associations between POPs and glucose dysregulation may entail over-adjustment or adjustment for factors that are in the causal pathway. For these reasons, we present results unadjusted for blood lipids in Table 2, and as sensitivity analyses, we present results adjusted for cholesterol and triglycerides in Table 3.
Table 2.
Longitudinal associations between POP summary scores and measures of glucose homeostasis over 23 years across age groups and by participants with and without diabetesa.
| No diabetes
|
Diabetes
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nsubjects | All | Age group (years)
|
p-Age interaction |
All | Age group (years)
|
p-Age interaction |
|||||||
| 18–31 | 32–39 | 40–47 | 48–55 | 18–31 | 32–39 | 40–47 | 48–55 | ||||||
| 90 | 90 | 90 | 89 | 70 | 90 | 89 | 87 | 82 | 58 | ||||
| HbA1c (%) | Nobs | 163 | 67 | 91 | 139 | 60 | 72 | ||||||
| 23 PCB Summary | 0.005 (0.05) | 0.002 (0.49) | 0.008 (0.02) | 0.01 | 0.024 (0.04) | 0.035 (0.03) | 0.026 (0.07) | 0.38 | |||||
| 8 OCP Summary | 0.014 (0.20) | 0.008 (0.39) | 0.026 (0.13) | 0.09 | 0.113 (0.03) | 0.132 (0.08) | 0.111 (0.05) | 0.89 | |||||
| Fasting Glucose | Nobs | 622 | 216 | 175 | 134 | 97 | 560 | 212 | 151 | 119 | 78 | ||
| 23 PCB Summary | 0.06 (0.07) | −0.03 (0.41) | 0.01 (0.71) | 0.06 (0.14) | 0.24 (0.003) | <0.001 | 0.24 (0.15) | −0.05 (0.56) | 0.15 (0.48) | 0.16 (0.67) | 1.10 (0.03) | 0.01 | |
| 8 OCP Summary | 0.14 (0.27) | −0.04 (0.75) | −0.05 (0.66) | 0.29 (0.06) | 0.72 (0.04) | 0.001 | 1.01 (0.16) | −0.33 (0.36) | 0.66 (0.45) | 0.21 (0.89) | 3.72 (0.09) | 0.01 | |
| Insulin Sensitivity (%) | Nobs | 609 | 206 | 172 | 134 | 97 | 521 | 186 | 142 | 119 | 74 | ||
| 23 PCB Summary | −0.98 (< 0.001) | −0.29 (0.34) | −0.21 (0.16) | −0.48 (0.02) | −2.82 (< 0.001) | 0.01 | −0.18 (0.19) | 0.01 (0.92) | −0.17 (0.28) | −0.06 (0.67) | −0.31 (0.30) | 0.13 | |
| 8 OCP Summary | −1.47 (0.13) | −0.46 (0.71) | −0.83 (0.18) | −1.75 (0.04) | −3.73 (0.24) | 0.21 | −0.67 (0.24) | 0.18 (0.71) | −0.20 (0.76) | −0.26 (0.68) | −1.36 (0.26) | 0.15 | |
| β-cell Function (%) | Nobs | 609 | 206 | 172 | 134 | 97 | 521 | 186 | 142 | 119 | 74 | ||
| 23 PCB Summary | 0.10 (0.51) | 0.31 (0.13) | 0.08 (0.51) | −0.02 (0.86) | −0.43 (0.21) | 0.001 | 0.08 (0.78) | 0.36 (0.18) | −0.16 (0.61) | 0.07 (0.92) | −0.61 (0.46) | 0.22 | |
| 8 OCP Summary | 0.61 (0.31) | 1.30 (0.12) | 0.64 (0.22) | −0.43 (0.39) | −0.90 (0.51) | 0.05 | −0.29 (0.80) | 1.48 (0.18) | −0.83 (0.51) | 2.23 (0.46) | −6.26 (0.05) | 0.04 | |
| BMI (kg/m2) | Nobs | 622 | 216 | 175 | 134 | 97 | 567 | 213 | 154 | 121 | 79 | ||
| 23 PCB Summary | −0.10 (0.01) | −0.08 (0.01) | −0.09 (0.01) | −0.04 (0.38) | −0.08 (0.16) | 0.54 | −0.06 (0.06) | −0.04 (0.29) | −0.05 (0.14) | −0.08 (0.02) | −0.09 (0.08) | 0.04 | |
| 8 OCP Summary | −0.11 (0.48) | −0.15 (0.31) | −0.15 (0.35) | 0.03 (0.84) | −0.06 (0.81) | 0.23 | −0.14 (0.34) | −0.02 (0.92) | −0.11 (0.48) | −0.17 (0.26) | −0.33 (0.12) | 0.03 | |
Table entries are β (p-value for linear trend).
Nsubjects = Number of subjects; Nobs = Number of observations.
Adjusted for age, race, sex, exam center, exam period and concurrent BMI. Associations with HbA1c% are additionally adjusted for baseline glucose concentration.
Table 3.
Lipid-adjusteda longitudinal associations between POP summary scores and measures of glucose homeostasis over 23 years across age groups and by participants with and without diabetes.
| No diabetes
|
Diabetes
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Age group (years)
|
p-Age interaction |
All | Age group (years)
|
p-Age interaction |
||||||||
| 18–31 | 32–39 | 40–47 | 48–55 | 18–31 | 32–39 | 40–47 | 48–55 | ||||||
| Nsubjects | 89 | 89 | 89 | 88 | 69 | 87 | 86 | 84 | 79 | 55 | |||
| HbA1c% | Nobs | 161 | 66 | 90 | 134 | 58 | 69 | ||||||
| 23 PCB Summary | 0.003 (0.24) | 0.001 (0.72) | 0.006 (0.10) | 0.12 | 0.018 (0.21) | 0.030 (0.14) | 0.014 (0.41) | 0.27 | |||||
| 8 OCP Summary | 0.003 (0.84) | 0.005 (0.67) | 0.013 (0.51) | 0.60 | 0.105 (0.10) | 0.152 (0.17) | 0.086 (0.23) | 0.11 | |||||
| Fasting Glucose | Nobs | 615 | 213 | 173 | 133 | 97 | 540 | 204 | 146 | 115 | 75 | ||
| 23 PCB Summary | 0.04 (0.35) | −0.02 (0.55) | −0.03 (0.44) | 0.00 (0.92) | 0.14 (0.08) | 0.01 | 0.08 (0.69) | −0.14 (0.16) | 0.11 (0.67) | −0.11 (0.80) | 0.90 (0.16) | 0.18 | |
| 8 OCP Summary | −0.03 (0.87) | 0.01 (0.97) | −0.32 (0.03) | 0.06 (0.74) | −0.06 (0.87) | 0.10 | 0.64 (0.47) | −0.73 (0.10) | 0.65 (0.55) | −0.94 (0.64) | 3.38 (0.22) | 0.09 | |
| Insulin Sensitivity % | Nobs | 602 | 203 | 170 | 133 | 96 | 501 | 178 | 137 | 115 | 71 | ||
| 23 PCB Summary | −0.91 (0.001) | −0.19 (0.57) | −0.05 (0.77) | −0.43 (0.06) | −2.70 (< 0.001) | <0.001 | 0.00 (0.98) | 0.05 (0.72) | −0.09 (0.63) | 0.11 (0.54) | −0.19 (0.60) | 0.45 | |
| 8 OCP Summary | −0.27 (0.82) | 0.53 (0.73) | 0.13 (0.86) | −1.54 (0.14) | −0.58 (0.88) | 0.37 | 0.06 (0.93) | 0.36 (0.54) | 0.58 (0.47) | 0.56 (0.49) | −0.90 (0.55) | 0.56 | |
| β-cell Function % | Nobs | 602 | 203 | 170 | 133 | 96 | 501 | 178 | 137 | 115 | 71 | ||
| 23 PCB Summary | 0.14 (0.44) | 0.34 (0.14) | 0.21 (0.15) | 0.08 (0.54) | −0.20 (0.56) | 0.38 | 0.14 (0.68) | 0.39 (0.24) | −0.14 (0.70) | 0.77 (0.37) | −1.48 (0.13) | 0.94 | |
| 8 OCP Summary | 0.88 (0.24) | 1.68 (0.10) | 1.60 (0.01) | 0.04 (0.95) | 0.43 (0.79) | 0.98 | −0.43 (0.77) | 1.44 (0.32) | −1.13 (0.47) | 6.69 (0.08) | −10.32 (0.004) | 0.33 | |
| BMI | Nobs | 615 | 213 | 173 | 133 | 96 | 546 | 205 | 148 | 117 | 76 | ||
| 23 PCB Summary | −0.14 (0.001) | −0.10 (0.004) | −0.12 (0.01) | −0.07 (0.12) | −0.10 (0.12) | 0.02 | −0.06 (0.12) | −0.04 (0.35) | −0.05 (0.20) | −0.07 (0.10) | −0.07 (0.24) | 0.01 | |
| 8 OCP Summary | −0.28 (0.13) | −0.27 (0.11) | −0.25 (0.20) | −0.12 (0.57) | −0.13 (0.69) | 0.86 | -0.06 (0.74) | 0.10 (0.59) | −0.02 (0.92) | −0.04 (0.84) | −0.26 (0.34) | 0.06 | |
Table entries are β (p-value).
Nsubjects = Number of subjects; Nobs = Number of observations.
Adjusted for age, race, sex, exam center, exam period, concurrent BMI and year 2 cholesterol and triglyceride.
3. Results
3.1. Participant characteristics
Characteristics of participants with and without diabetes by quartiles of the 32 POP summary score are listed in Table 1. In both groups, participants with higher scores of the 32 POP summary were older, had higher fasting glucose and HbA1c values at year 25, and had higher year 2 cholesterol concentrations than participants with lower scores (unadjusted associations). The pre-valences of glucose lowering medication use among diabetics were 78% and 70% for years 20 and 25, respectively.
Table 1.
Characteristics among participants with and without diabetes and by quartiles of the year 2 32 POP summary score.
| Variable | No diabetes
|
Diabetes
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | All | 32 POP Summary quartiles
|
p-Trend | N | All | 32 POP Summary quartiles
|
p-Trend | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |||||||
| N | 26 | 17 | 25 | 22 | 19 | 28 | 20 | 23 | ||||||
| Cut-off (sum of standardized deviates of ln transforms) | 140.3–201.1 | 201.2–219.0 | 219.1–234.7 | 234.8–300.5 | 140.3–201.1 | 201.2–219.0 | 219.1–234.7 | 234.8–300.5 | ||||||
| Age Y0 (years) | 90 | 25.2 (3.3) | 23.3 (3.3) | 24.9 (3.0) | 26.1 (2.7) | 26.7 (3.2) | < 0.001 | 90 | 25.2 (3.6) | 24.1 (3.6) | 23.9 (3.8) | 26.1 (3.7) | 26.8 (2.6) | 0.01 |
| Race: White (%) | 90 | 51 | 54 | 59 | 44 | 50 | 0.69 | 90 | 31 | 37 | 32 | 20 | 35 | 0.92 |
| Sex: Female (%) | 90 | 69 | 77 | 71 | 60 | 68 | 0.62 | 90 | 54 | 53 | 64 | 60 | 39 | 0.10 |
| BMI Y0 (kg/m2) | 90 | 28.4 (6.1) | 30.3 (6.6) | 27.9 (6.4) | 27.7 (5.7) | 27.3 (5.5) | 0.07 | 90 | 29.6 (6.4) | 30.0 (7.9) | 31.8 (6.5) | 27.3 (6.3) | 28.7 (4.5) | 0.32 |
| BMI Y20 (kg/m2) | 90 | 33.0 (7.9) | 34.4 (7.1) | 34.9 (10.8) | 31.6 (7.1) | 31.5 (7.1) | 0.24 | 79 | 34.5 (7.7) | 35.5 (9.2) | 37.6 (7.2) | 31.1 (7.6) | 32.9 (5.9) | 0.07 |
| BMI Y25 (kg/m2) | 85 | 34.2 (8.6) | 35.1 (8.0) | 37.2 (11.0) | 33.0 (7.5) | 32.3 (8.1) | 0.29 | 72 | 34.0 (7.7) | 35.4 (11.1) | 36.4 (6.2) | 32.4 (7.6) | 31.7 (6.2) | 0.09 |
| Fasting Glucose Y0 (mg/dL) | 90 | 79.8 (6.0) | 80.5 (4.7) | 80.2 (6.6) | 79.8 (5.9) | 78.5 (7.3) | 0.06 | 90 | 86.1 (10.6) | 89.4 (11.2) | 87.8 (9.6) | 81.3 (6.0) | 85.5 (13.1) | 0.07 |
| Fasting Glucose Y20 (mg/dL) | 90 | 88.8 (7.6) | 87.7 (8.2) | 88.1 (9.9) | 89.7 (6.8) | 89.6 (5.4) | 0.11 | 78 | 149.0 (70.6) | 121.0 (36.0) | 166.8 (82.7) | 147.1 (66.2) | 148.5 (74) | 0.35 |
| Fasting Glucose Y25 (mg/dL) | 85 | 92.6 (14.4) | 89.2 (9.0) | 91.3 (9.7) | 91.4 (6.6) | 99.0 (25.1) | 0.03 | 71 | 164.5 (85.8) | 133.8 (71.2) | 161.9 (68.7) | 155.5 (80.7) | 196.1 (110.7) | 0.01 |
| Insulin Y0 (μIU/mL) | 86 | 9.1 (4.9) | 8.8 (3.5) | 7.1 (2.7) | 9.8 (4.1) | 10.1 (7.4) | 0.29 | 75 | 11.9 (6.5) | 11.0 (4.4) | 13.7 (8.2) | 10.9 (4.3) | 11.3 (6.8) | 0.97 |
| Insulin Y20 (μIU/mL) | 90 | 9.8 (5.5) | 9.3 (4.2) | 10.2 (5.5) | 8.7 (3.9) | 11.4 (7.8) | 0.15 | 78 | 17.7 (13.1) | 13.5 (6.6) | 19.7 (14) | 22.3 (19.2) | 14.5 (6.9) | 0.93 |
| Insulin Y25 (μIU/mL) | 85 | 11.6 (9.9) | 9.7 (7.1) | 15.5 (16.3) | 10.9 (7.8) | 11.6 (8.1) | 0.46 | 69 | 21.1 (32.4) | 16.9 (16.3) | 30.1 (53.0) | 19.8 (17.9) | 14.3 (9.4) | 0.44 |
| HbA1c Y20 (%) | 81 | 5.42 (0.40) | 5.40 (0.32) | 5.24 (0.44) | 5.42 (0.42) | 5.59 (0.41) | 0.12 | 69 | 7.48 (2.11) | 6.66 (0.93) | 7.83 (2.47) | 7.25 (2.16) | 7.75 (2.13) | 0.27 |
| HbA1c Y25 (%) | 82 | 5.54 (0.52) | 5.41 (0.28) | 5.47 (0.36) | 5.56 (0.35) | 5.72 (0.91) | 0.03 | 70 | 7.96 (2.24) | 7.31 (2.33) | 8.10 (1.89) | 7.71 (2.16) | 8.46 (2.68) | 0.10 |
| Insulin Sensitivity Y0 (%) | 86 | 96.0 (55.5) | 92.9 (41.2) | 125.3 (97.1) | 84.1 (35.0) | 89.4 (34.7) | 0.47 | 75 | 70.2 (27.7) | 69.9 (23.1) | 62.5 (25.2) | 74.4 (31.2) | 76.3 (30.5) | 0.37 |
| Insulin Sensitivity Y20 (%) | 90 | 99.6 (46.3) | 101.0 (48.8) | 94.5 (41.4) | 108.3 (48.5) | 92.1 (45.8) | 0.27 | 78 | 57.3 (37.2) | 74.8 (55.1) | 46.6 (23.3) | 56.4 (42.7) | 58.8 (26.6) | 0.71 |
| Insulin Sensitivity Y25 (%) | 85 | 111.0 (111.8) | 151 (171.1) | 83.6 (60.6) | 93.4 (60.6) | 106.9 (95.9) | 0.02 | 67 | 52.2 (41.6) | 68.1 (49.3) | 41.4 (27.0) | 53.9 (56.3) | 52.6 (34.3) | 0.48 |
| β-cell Function Y0 (%) | 86 | 145.1 (46.6) | 139.4 (40.1) | 123.1 (34.6) | 153.1 (37.9) | 160.4 (63.4) | 0.03 | 75 | 157.7 (60.2) | 144.0 (57.4) | 166.3 (75.9) | 155.4 (31.2) | 159.6 (61.0) | 0.35 |
| β-cell Function Y20 (%) | 89 | 108.6 (41.9) | 106.4 (24.6) | 117.7 (65.6) | 98.5 (29.1) | 115.4 (47.4) | 0.58 | 75 | 105.1 (137.6) | 98.4 (77.7) | 92.7 (75.6) | 140.8 (256.4) | 96.1 (89.2) | 0.90 |
| β-cell Function Y25 (%) | 90 | 120.5 (55.5) | 112.5 (51.9) | 144.1 (72.1) | 121.9 (52.3) | 109.5 (45.9) | 0.66 | 78 | 110.8 (152.6) | 148.0 (176.8) | 124.0 (201.3) | 117.3 (141.2) | 65.0 (39.1) | 0.22 |
| Cholesterol Y2 (mg/dL) | 89 | 180.3 (36.9) | 161.9 (35.9) | 166.9 (32.5) | 186.7 (24.0) | 206.2 (38.7) | <0.001 | 87 | 186.5 (34.2) | 167.2 (21.2) | 182.8 (31.8) | 181.1 (35.3) | 212.2 (32.2) | <0.001 |
| Triglycerides Y2 (mg/dL) | 89 | 77.9 (46.4) | 79.8 (32.3) | 57.9 (24.2) | 73.4 (53.3) | 97.1 (59.3) | 0.23 | 87 | 111.0 (71.9) | 72.3 (35.3) | 111.4 (54.1) | 78.3 (36.2) | 170.8 (96.3) | <0.001 |
| 32 POP Summary (Σ log[POPi]/log[SDig]) | 90 | 215.3 (26.4) | 181.6 (14.9) | 210.9 (5.2) | 226.1 (4.9) | 246.1 (8.8) | – | 90 | 220.5 (26.9) | 185.8 (13.1) | 211.0 (4.9) | 226.2 (5.1) | 255.9 (15.9) | – |
| 23 PCB Summary (Σ log[POPi]/log[SDig]) | 90 | 146.0 (20.8) | 119.8 (12.5) | 142.5 (5.1) | 154.6 (4.6) | 169.8 (7.4) | <0.001 | 90 | 149.4 (22.1) | 121.9 (11.6) | 141.3 (5.4) | 153.8 (5.9) | 178.0 (13.4) | <0.001 |
| 8 OCP Summary (Σ log[POPi]/log[SDig]) | 90 | 56.5 (5.7) | 50.8 (4.9) | 55.8 (3.4) | 58.0 (3.2) | 62.3 (3.2) | <0.001 | 90 | 58.0 (5.3) | 52.0 (4.3) | 57.2 (3.3) | 59.0 (3.2) | 63.2 (3.5) | <0.001 |
| Log(PBB-153) (log[pg/g]) | 90 | 2.90 (0.84) | 2.30 (0.37) | 3.00 (1.01) | 3.21 (0.73) | 3.19 (0.91) | <0.001 | 90 | 2.98 (0.66) | 2.75 (0.80) | 2.83 (0.74) | 3.08 (0.39) | 3.28 (0.52) | <0.001 |
Presented values are percentage or mean (standard deviation).
POPi: Concentration of each POP for each individual. SDig: Standard deviation of each POP for all participants.
3.2. POPs, HbA1c and fasting glucose
We observed significant effect modification by age in the associations of 23 PCB and 8 OCP summary scores with HbA1c% among participants without diabetes, and with fasting glucose in both participants with and without diabetes (Table 2 and Fig. 1). The associations of both summary scores with HbA1c% and fasting glucose were mostly significant among participants aged ≥ 40 years, and were strongest among participants ≥ 48 years of age, among both participants with and without diabetes. There were no associations between POPs and fasting glucose among participants < 40 years of age (associations with HbA1c were not conducted because only 12 participants had measurements before the age of 40). For instance, the associations between the 23 PCB summary score and fasting glucose among non-diabetics increased from β=−0.03 mg/dL per unit of score (p=0.41) among participants of ages 18–31, to β=0.06 (p=0.14) in participants of ages 40–47, and to β= 0.24 (p=0.003) in participants 48–55 years of age. Among diabetics, the associations ranged from β=−0.05 (p=0.56) to β=1.10 (p=0.03), in participants of ages 18–31 and 48–55, respectively. The associations between POPs and fasting glucose and HbA1c were larger in magnitude among participants with diabetes than among those without, but tended to flatten in ages 48–55 at high values of the PCB summary (Fig. 1), consistent with physician intervention at higher HbA1c and glucose levels. PBB-153 was not associated with HbA1c or fasting glucose (data not shown).
Fig. 1.
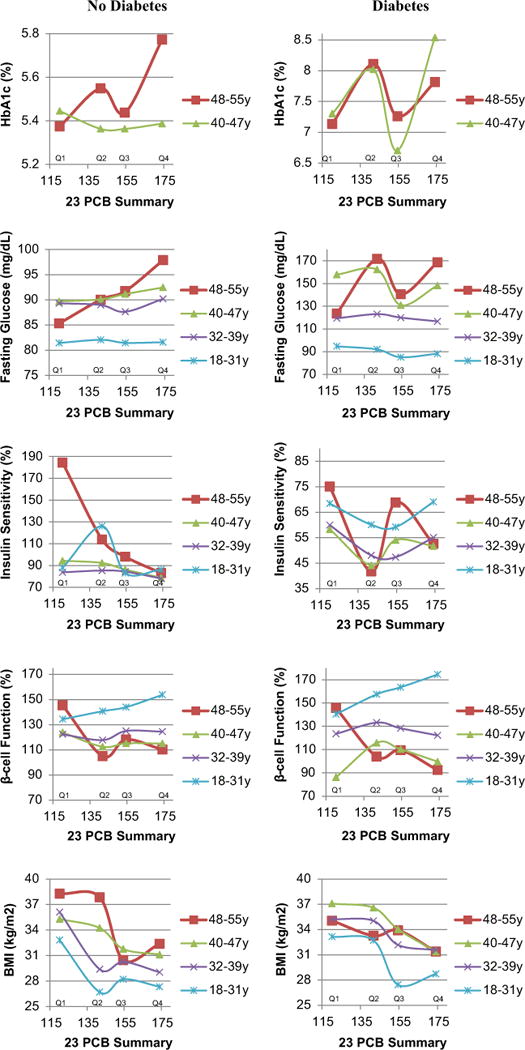
Adjusted a means depicting longitudinal associations between the 23 PCB summary score (quartiles) and glucose homeostasis over 23 years across age groups (years) and by participants with and without diabetes. Plotted points are connected as a visual aid. a Adjusted for age, race, sex, exam center, exam period and concurrent BMI. Associations with HbA1c% are additionally adjusted for baseline glucose concentration.
3.3. POPs, insulin sensitivity and β-cell function
There was indication of effect modification by age in the associations between 23 PCB and 8 OCP summary scores and insulin sensitivity among participants with and without diabetes: the associations strengthened negatively with increasing age (Table 2); however, the interaction term reached statistical significance only for the 23 PCB summary score (Pinteraction =0.01). Among persons without diabetes, the 23 PCB summary score had significant inverse associations with insulin sensitivity among participants older than 40 years of age, and the associations were strongest among participants of ages 48–55 (β=2.82, p < 0.001). Among persons with diabetes, the associations between both summary scores and insulin sensitivity were strongest, although non-significant, among participants of ages 48–55 compared to the rest.
There was also indication of effect modification by age in the associations between 23 PCB and 8 OCP summary scores and β-cell function (Table 2, Fig. 1). The associations strengthened negatively with increasing age but reached statistical significance only among participants aged 48–55 years for the 8 OCP summary score. All of these associations were negative among participants aged 48–55 years, and there was visual indication of inverse associations in this age group among participants with and without diabetes (Fig. 1). PBB-153 was not associated with insulin sensitivity or β-cell function (data not shown).
3.4. POPs and BMI
There was evidence of effect modification by age in the associations of the 23 PCB and 8 OCP summary scores with BMI among participants with diabetes (Table 2): the associations strengthened negatively with increasing age. The associations reached significance only with the 23 PCB summary among participants ≥ 40 years with diabetes. Among participants without diabetes, the 23 PCB summary score was also overall inversely associated with BMI (β=−0.10, p=0.01), although there was no effect modification by age. PBB-153 was strongly negatively associated with BMI (no effect modification by age) among participants with diabetes (β=−3.91, p=0.001) and without diabetes (β=−2.34, p=0.003, Supplementary Fig. 1). These associations were significant among participants of all age groups.
3.5. Sensitivity analyses: lipid adjustment
The associations between 23 PCB and 8 OCP summary scores and HbA1c, fasting glucose and insulin sensitivity mostly diminished with adjustment for year 2 cholesterol and triglyceride concentrations (Table 3). Remarkably, the directions of the associations remained coherent with the lipid unadjusted values. The associations involving the 23 PCB summary score remained significant or borderline significant among participants without diabetes. The associations of the 23 PCB and 8 OCP summary scores and β-cell function became stronger (8 OCP summary score) among diabetic adults of ages 48–55 years, whereas in participants without diabetes, the associations weakened and had no effect modification by age. The associations between the summary scores and BMI remained mostly unchanged with year-2 lipid adjustment.
4. Discussion
Our present longitudinal analyses show that serum concentrations of organochlorine pesticides and PCBs in early adulthood were positively associated with measures of decreased insulin sensitivity independently of BMI, after participants reached 40 years of age (and particularly at ≥ 48 years of age). These findings were observed in normoglycemic controls and among treated people with diabetes.
While many POPs have been banned in most countries, they are still detected in the food supply (Dougherty et al., 2000; Schafer and Kegley, 2002; Schecter et al., 2010b, 2010a) and in the general population (Lee et al., 2007a, 2010; Patterson et al., 2009), because of their long half-lives (2–15 years (Centers for Disease Control and Prevention, 2009; Smith, 1999)). POPs, such as the organochlorine pesticide 2,2-bis-(2-chlorophenyl)-1,1,1-trichloroethane (DDT), continues to be used for mosquito control to reduce the burden of malaria in some African countries; also, PBDEs have been used as flame retardants in nations including the US. None of the PBDEs measured were detectable in ≥ 75% of participants, likely because the serum samples were collected before their use.
Because CARDIA is a US population-based, multi-center study, the exposures to organochlorine pesticides and PCBs of study participants likely originate from background environmental exposures. While our analyses are limited to environmental exposures assayed in serum collected in 1987–1988, when study participants were between 20 and 32 years of age, exposure to organochlorine pesticides and PCBs likely occurred throughout their lives Based on high correlations of POPs with age (Lee et al., 2014), the long half-lives of these chemicals and renewing background environmental exposures, we suspect that POPs assayed in 1987–1988 would correlate highly with their subsequent, unmeasured values. Therefore, our findings are consistent with the hypothesis that lifetime exposure to background environmental and dietary concentrations of organochlorines and PCBs influence glucose metabolism after people reach about 40 years of age. Potential explanations for the latency or delay of the onset of glucose dysregulation include: multi-decade noxious effects of POPs to target organs, accumulation of POPs in tissues over time, interactions of POPs with emerging environmental exposures, and interactions of POPs with metabolic/physiologic processes associated with aging (present after 40 years of age) which initiate a process of decreased insulin sensitivity. Between the ages of 40–45 years, the prevalences of dyslipidemia, hypertension, cardiovascular disease and diabetes increase substantially (calendar period 1988–2010) (National Center for Health Statistics, 2013). We do not think that a birth-cohort effect explains the observed associations given that all participants are part of one generation (age difference between oldest and youngest participants is 13 years) and all participants were born before the bans on organochlorine pesticides and PCBs (1970s and 1980s). Exam period effects were accounted for in the statistical models.
With the exception of the inverse associations of POPs with BMI in our study, our findings support experimental findings in rats which showed positive associations between chronic high-fat diets with salmon oil contaminated with background levels of POPs and abdominal obesity, hepatosteatosis and decreased insulin sensitivity (measured through a euglycemic clamp), compared to rats fed with standard feed and high-fat diets with corn oil or purified salmon oil (Ruzzin et al., 2010). POPs, especially organochlorine pesticides, induced decreases of insulin action in cultured differentiated adipocytes and down-regulated insulin-induced gene-1 (Insig-1) and Lpin1, which are master regulators of lipid homeostasis. Parallel findings on obesity and decreased insulin sensitivity were seen in mice fed with high fat diets with commercially farmed POPs-contaminated salmon compared to mice fed with either standard feed, or high fat diets supplemented with corn oil or salmon filets with reduced POPs concentrations (Ibrahim et al., 2011).
To our knowledge, our study is the first to document associations of POP concentrations in young adulthood with measures of insulin sensitivity at various ages in humans. Our findings are in accord with those of population-based cross-sectional (Codru et al., 2007; Gasull et al., 2012; Lee et al., 2006, 2007a, 2007b; Uemura et al., 2008) and longitudinal studies worldwide (Turyk et al., 2009; Lee et al., 2011a; Bertazzi et al., 2001; Vasiliu et al., 2006; Wang et al., 2008), which have reported positive associations between background exposures to POPs (particularly PCBs and organochlorine pesticides) and insulin resistance, pre-diabetes and diabetes. In NHANES (n=2016), the association between POPs and diabetes or insulin resistance (HOMA-R > 90th percentile vs. lower) were strongest among overweight and obese individuals (Lee et al., 2006, 2007a). Similar but weaker findings were also reported in the Catalan Health Interview Survey (n=886) (Gasull et al., 2012) and in the Helsinki Birth Cohort (n=1988) (Airaksinen et al., 2011). In our study, PCBs and particularly PBB-153 were negatively associated with BMI. Although our limited sample size did not allow us to conduct stratified analyzes by BMI categories within each age group, we found that the associations between POPs and measures of decreased insulin sensitivity were independent of changes in BMI.
Our previous analyses of the CARDIA nested case-control study found non-monotonic (inverted U-shaped) associations of organochlorine pesticides and PCBs with diabetes (Lee et al., 2010). In extended analyses among controls, certain organochlorine pesticides and PCBs showed either inverted U-shaped or positive associations with HOMA-insulin resistance (which equates to 1/insulin sensitivity) 18 years after the measurement of POPs (calendar years of examination: 2005–2006) (Lee et al., 2011b). Our present analyses of this nested case-control study did not find non-monotonic associations, but rather positive associations between POPs and measures of insulin resistance. Since our current findings indicate that age is an important factor in these associations, we believe that the partial discrepant findings between our current and previous analyses occurred because the present study includes follow-up information of participants examined in 2010–2011, which is 5 years after the latest examination available during the previous analyses. The strongest associations between POPs and altered glucose metabolism occurred in participants aged 48–55. In 2005–2006, the oldest participants were 50 years of age and only 31% of participants were 48 years of age and older.
Our choice to conduct analyses of composite POP variables strengthened our findings in a few ways. It realistically estimates exposure given that background environmental concentrations are composed of a mix of POPs. In CARDIA, this was reflected by the substantial correlation (≈ 0.7) between the 23 PCB and 8 OCP summary scores. Furthermore, composite variables tend to be less variable than individual compounds. Composite variables also account, to some extent, for potential interactions between POPs, in that the sum of effects over all variables would comprise general information of interactive effects. However, composite variables do not allow us to assess interactions between specific POPs. Nonetheless, the high correlation of 23 PCB and 8 OCP summary scores still leaves room for unaccounted interactions between these two classes of POPs. Finally, we dramatically reduced the number of statistical analyses conducted by analyzing composite variables rather than individual POPs; thus, we reduced the likelihood of type-1 errors (finding associations when in fact there are none). A drawback of composite variables is that they include information of POPs that could be unrelated to the outcomes of interest, which therefore, could weaken the strength of the associations of POPs that are related.
A limitation of this investigation is the study population, which comprises individuals who became diabetic during the course of follow-up and controls who, by design, were restricted to having fasting glucose concentrations lower than 100 mg/dL at all examinations through year 20. This limits the generalizability of our findings to the general population. At year 25, 18% of controls had a fasting glucose concentration > 100 mg/dL and 2 became diabetic. People with and without diabetes have substantially different metabolism, and hypothetically different clearance rates of pollutants. We circumvented this by analyzing data of participants separately on the basis of diabetes status. It is remarkable that, despite the restricted range of fasting glucose among controls and (likely) treatment with glucose lowering medications among those with diabetes, we observed positive associations between POPs and fasting glucose and HbA1c. It is plausible that the associations between POPs and measures of glucose homeostasis were dampened among treated diabetic participants given that with increasing values of fasting glucose and HbA1c, physicians tend to strengthen glucose lowering regimes. Yet, such therapies do not seem to balance or fully counteract the adverse effects of POPs. These results support previous findings on POPs and risk of poor glycemic control among patients with diabetes (Lee et al., 2008).
While our study had a relatively small sample size, statistical power seemed sufficient for most outcomes. We were able to assess effect modification by age by comparing the same participants over 6 examinations (participants contributed data to most age groups); this strengthened our findings and power compared to other study designs with similar sample sizes. Nonetheless, it is plausible that insufficient power resulted in a lack of statistical significance for the associations between the 23 PCB summary and β-cell function, considering that: (a) there was significant effect modification by age; (b) there were negative parameter estimates for β-cell function among participants aged 48–55 years and lipid-unadjusted and lipid-adjusted analyses actually showed significant negative associations with the OCP summary score among diabetics of ages 48–55 years; and (c) Fig. 1 shows a negative relationship among both participants with and without diabetes. Due to our limited sample size, the number of participants within each quartile is relatively small; therefore, we gave more weight in our interpretations to the general pattern of the association than to any individual quartile. Both decreased insulin sensitivity and β-cell function are core pathophysiological defects of type 2 diabetes (Lin and Sun, 2010), and β-cell failure may trigger the progression from pre-diabetic to diabetic states (Muoio and Newgard, 2008). As more cases of pre-diabetes and type-2 diabetes will develop in later years of CARDIA, a longer follow-up may reveal stronger associations between POPs and β-cell function. An additional limitation of our study is the single measurement of POPs which predated outcome measurements by up to 23 years. Considering the long latency of glucose dysregulation from exposures to POPs and despite the likelihood of high correlations of body burdens of POPs over time, subsequent longitudinal studies should include quantifications of POPs at various ages.
4.1. Conclusions
Our findings suggest that impairments of glucose metabolism occur after decades of exposure to PCBs and organochlorine pesticides at background levels, independent of BMI and after participants reached the 5th decade of life. Larger longitudinal studies with long-term follow-up and repeated POPs assessments are needed.
Supplementary Material
Acknowledgments
The Coronary Artery Risk Development in Young Adults Study (CAR-DIA) is supported by Contracts HHSN268201300025C, HHSN268 201300026C, HHSN268201300027C, HHSN268201300028C, HHSN 268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005). The YALTA study is supported by R01HL53560. This investigation was further supported by the NHLBI T32HL007779 at the University of Minnesota.
Role of funding source
None.
Abbreviations
- AChE
acetylcholinesterase
- POPs
persistent organic pollutants
- PCB
polychlorinated biphenyl
- CARDIA
Coronary Artery Risk Development in Young Adults
- HbA1c
Hemoglobin-A1c
- HOMA
Homeostasis Model Assessment
- BMI
Body mass index
- YALTA
Young Adults Longitudinal Trends in Atheroclerosis
- OCP
organochlorine pesticides
- PCB
polychlorinated biphenyl congeners
- PBDE
polybrominated diphenyl ether
- PBB
polybrominated biphenyl
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.envres.2014.11.001.
Footnotes
Competing financial interests
None.
Institutional review board approval for research
The study was approved by the institutional review boards of the University of Minnesota, University of Alabama at Birmingham, Northwestern University, and the Division of Research at Kaiser Permanente Health Care Plan. Participants signed informed consent at every examination.
Contributor Information
Jose R. Suarez-Lopez, Email: jrsuarez@ucsd.edu.
Duk-Hee Lee, Email: lee_dh@knu.ac.kr.
Miquel Porta, Email: mporta@imim.es.
Michael W. Steffes, Email: steff001@umn.edu.
David R. Jacobs, Jr., Email: jacob004@umn.edu.
References
- Airaksinen R, Rantakokko P, Eriksson JG, Blomstedt P, Kajantie E, Kiviranta H. Association between type 2 diabetes and exposure to persistent organic pollutants. Diabetes Care. 2011;34:1972–1979. doi: 10.2337/dc10-2303. http://dx.doi.org/10.2337/dc10-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr JR, Maggio VL, Barr DB, Turner WE, Sjödin A, Sandau CD, et al. New high-resolution mass spectrometric approach for the measurement of polychlorinated biphenyls and organochlorine pesticides in human serum. J Chromatogr B: Analyt Technol Biomed Life Sci. 2003;794:137–148. doi: 10.1016/s1570-0232(03)00451-3. [DOI] [PubMed] [Google Scholar]
- Bertazzi PA, Consonni D, Bachetti S, Rubagotti M, Baccarelli A, Zocchetti C, et al. Health effects of dioxin exposure: a 20-year mortality study. Am J Epidemiol. 2001;153:1031–1044. doi: 10.1093/aje/153.11.1031. [DOI] [PubMed] [Google Scholar]
- Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring) 2011;19:402–408. doi: 10.1038/oby.2010.248. http://dx.doi.org/10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Environ Heal NCEH Pub. Atlanta, GA: 2009. Fourth National Report on Human Exposure to Environmental Chemicals; p. 529. [Google Scholar]
- Codru N, Schymura MJ, Negoita S, Rej R, Carpenter DO. Diabetes in relation to serum levels of polychlorinated biphenyls and chlorinated pesticides in adult Native Americans. Environ Health Perspect. 2007;115:1442–1447. doi: 10.1289/ehp.10315. http://dx.doi.org/10.1289/ehp.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty C, Holtz S, Reinert J. Dietary exposures to food contaminants across the United States. Environ Res. 2000;84:170–185. doi: 10.1006/enrs.2000.4027. [DOI] [PubMed] [Google Scholar]
- Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- Gasull M, Pumarega J, Téllez-Plaza M, Castell C, Tresserras R, Lee DH, et al. Blood concentrations of persistent organic pollutants and prediabetes and diabetes in the general population of Catalonia. Environ Sci Technol. 2012;46:7799–7810. doi: 10.1021/es300712g. http://dx.doi.org/10.1021/es300712g. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Bowsher RR, Mykkänen L, Hazuda HP, Mitchell BD, Valdez RA, et al. Proinsulin and specific insulin concentration in high- and low-risk populations for NIDDM. Diabetes. 1994;43:1490–1493. doi: 10.2337/diab.43.12.1490. [DOI] [PubMed] [Google Scholar]
- Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) Study. Control Clin Trials. 1987;8:68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Fjære E, Lock EJ, Naville D, Amlund H, Meugnier E, et al. Chronic consumption of farmed salmon containing persistent organic pollutants causes insulin resistance and obesity in mice. PLoS One. 2011;6:e25170. doi: 10.1371/journal.pone.0025170. http://dx.doi.org/10.1371/journal.pone.0025170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Jacobs DR, Steffes M. Association of organochlorine pesticides with peripheral neuropathy in patients with diabetes or impaired fasting glucose. Diabetes. 2008;57:3108–3111. doi: 10.2337/db08-0668. http://dx.doi.org/10.2337/db08-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR. Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2007a;30:622–628. doi: 10.2337/dc06-2190. http://dx.doi.org/10.2337/dc06-2190. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR. Extended analyses of the association between serum concentrations of persistent organic pollutants and diabetes. Diabetes Care. 2007b;30:1596–1598. doi: 10.2337/dc07-0072. http://dx.doi.org/10.2337/dc07-0072. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Porta M, Steffes M, Jacobs DR. Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among non-diabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetologia. 2007c;50:1841–1851. doi: 10.1007/s00125-007-0755-4. http://dx.doi.org/10.1007/s00125-007-0755-4. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker Ba, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006;29:1638–1644. doi: 10.2337/dc06-0543. http://dx.doi.org/10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lind PM, Jacobs DR, Salihovic S, van Bavel B, Lind L. Poly-chlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care. 2011a;34:1778–1784. doi: 10.2337/dc10-2116. http://dx.doi.org/10.2337/dc10-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Porta M, Jacobs DR, Vandenberg LN. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev. 2014;35:557–601. doi: 10.1210/er.2013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR. Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case-control study. Environ Health Perspect. 2010;118:1235–1242. doi: 10.1289/ehp.0901480. http://dx.doi.org/10.1289/ehp.0901480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One. 2011b;6:e15977. doi: 10.1371/journal.pone.0015977. http://dx.doi.org/10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Sun Z. Current views on type 2 diabetes. J Endocrinol. 2010;204:1–11. doi: 10.1677/JOE-09-0260. http://dx.doi.org/10.1677/JOE-09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. http://dx.doi.org/10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. http://dx.doi.org/10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Health United States 2012 CDC. 2013:1–489. [PubMed] [Google Scholar]
- Obana H, Hori S, Tanaka R. The effects of “yusho” type PCB on triglyceride lipase and fatty acid composition. Environ Res. 1987;42:500–508. doi: 10.1016/s0013-9351(87)80217-7. [DOI] [PubMed] [Google Scholar]
- Patterson DG, Wong LY, Turner WE, Caudill SP, Dipietro ES, McClure PC, et al. Levels in the U.S. population of those persistent organic pollutants (2003–2004) included in the Stockholm Convention or in other long range transboundary air pollution agreements. Environ Sci Technol. 2009;43:1211–1218. doi: 10.1021/es801966w. [DOI] [PubMed] [Google Scholar]
- Porta M, Jariod M, López T, Pumarega J, Puigdomènech E, Marco E, et al. Correcting serum concentrations of organochlorine compounds by lipids: alternatives to the organochlorine/total lipids ratio. Environ Int. 2009;35:1080–1085. doi: 10.1016/j.envint.2009.06.004. http://dx.doi.org/10.1016/j.envint.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Porta M, Puigdomènech E, Ballester F, Selva J, Ribas-Fitó N, Llop S, et al. Monitoring concentrations of persistent organic pollutants in the general population: the international experience. Environ Int. 2008;34:546–561. doi: 10.1016/j.envint.2007.10.004. http://dx.doi.org/10.1016/j.envint.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, et al. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect. 2010;118:465–471. doi: 10.1289/ehp.0901321. http://dx.doi.org/10.1289/ehp.0901321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer KS, Kegley SE. Persistent toxic chemicals in the US food supply. J Epidemiol Commun Health. 2002;56:813–817. doi: 10.1136/jech.56.11.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Colacino J, Haffner D, Patel K, Opel M, Päpke O, et al. Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ Health Perspect. 2010a;118:796–802. doi: 10.1289/ehp.0901347. http://dx.doi.org/10.1289/ehp.0901347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Haffner D, Colacino J, Patel K, Päpke O, Opel M, et al. Polybrominated diphenyl ethers (PBDEs) and hexabromocyclodecane (HBCD) in composite U.S. food samples. Environ Health Perspect. 2010b;118:357–362. doi: 10.1289/ehp.0901345. http://dx.doi.org/10.1289/ehp.0901345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Louis GMB, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect. 2005;113:853–857. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Lapeza CR, Focant JF, McGahee EE, Patterson DG. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal Chem. 2004;76:1921–1927. doi: 10.1021/ac030381+. http://dx.doi.org/10.1021/ac030381+ [DOI] [PubMed] [Google Scholar]
- Smith D. Worldwide trends in DDT levels in human breast milk. Int J Epidemiol. 1999;28:179–188. doi: 10.1093/ije/28.2.179. [DOI] [PubMed] [Google Scholar]
- Turyk M, Anderson H, Knobeloch L, Imm P, Persky V. Organochlorine exposure and incidence of diabetes in a cohort of Great Lakes sport fish consumers. Environ Health Perspect. 2009;117:1076–1082. doi: 10.1289/ehp.0800281. http://dx.doi.org/10.1289/ehp.0800281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura H, Arisawa K, Hiyoshi M, Satoh H, Sumiyoshi Y, Morinaga K, et al. Associations of environmental exposure to dioxins with prevalent diabetes among general inhabitants in Japan. Environ Res. 2008;108:63–68. doi: 10.1016/j.envres.2008.06.002. http://dx.doi.org/10.1016/j.envres.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Vasiliu O, Cameron L, Gardiner J, Deguire P, Karmaus W. Polybrominated biphenyls, polychlorinated biphenyls, body weight, and incidence of adult-onset diabetes mellitus. Epidemiology. 2006;17:352–359. doi: 10.1097/01.ede.0000220553.84350.c5. http://dx.doi.org/10.1097/01.ede.0000220553.84350.c5. [DOI] [PubMed] [Google Scholar]
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- Wang SL, Tsai PC, Yang CY, Guo YL. Increased risk of diabetes and polychlorinated biphenyls and dioxins: a 24-year follow-up study of the Yu-cheng cohort. Diabetes Care. 2008;31:1574–1579. doi: 10.2337/dc07-2449. http://dx.doi.org/10.2337/dc07-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


