Abstract
Non-melanoma skin cancers (NMSCs) such as basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are the most common form of human cancer worldwide, and their incidence is increasing. Photodynamic therapy (PDT), mediated by topically applied aminolevulinic acid (ALA) and subsequent exposure to light (either a laser or a noncoherent source), is being increasingly used for the treatment of dermatological disorders, including BCC and SCC. However, therapeutic responses of NMSCs to ALA-PDT are currently not superior to standard therapies, although the latter have undesirable side effects including scarring. In this study, we report that preconditioning of skin tumors with calcitriol (active form of Vitamin D; Vit D) prior to ALA-PDT, significantly improves the treatment outcome. In BCC and UVB-induced SCC mouse models, we identified an increase in tumor-specific accumulation of ALA induced photosensitizer (protoporphyrin IX, PpIX) due to Vit D preconditioning, of up to 6-fold in vivo. In addition, increased expression of differentiation (145 fold, p < 0.02) and proliferation (42 fold, p < 0.005) markers were identified in BCC tumors, all leading to increased tumor destruction (18.3 fold, p < 0.03) with the combination approach, as compared to ALA-PDT alone. Histomorphological changes identified using hematoxylin and eosin staining, and results of TUNEL staining, together documented a beneficial effect of Vit D pretreatment upon tumor cell death. We conclude that this new combination approach with Vit D and ALA-PDT has great potential to achieve complete remission of NMSC tumors, with excellent cosmetic results and an overall beneficial impact upon patient care.
Keywords: Calcitriol, Vitamin D, Photodynamic Therapy, Non-melanoma Skin Cancer, Squamous Cell Carcinoma, Basal Cell Carcinoma, Aminolevulinic acid, Protoporphyrin IX, Non-invasive imaging
1. INTRODUCTION
Cancers originating from the skin, either Basal Cell Carcinoma (BCC or Squamous Cell Carcinoma (SCC), are the most common form of all types of cancer. In America, > 2 million cases of BCC and SCC are diagnosed yearly, caused mainly by cumulative exposure to ultraviolet (UV) light 1,2. Surgical excision, radiation therapy, and chemotherapy are some of the common methods employed in the treatment of BCC and SCC. However, scarring, painful inflammation, drug resistance and secondary cancers are some of the side effects with these therapies. In the last few decades, Photodynamic Therapy (PDT), a non-invasive and non-scarring treatment, has become an important alternative for treating squamous precancers in the U.S.A., and also BCC and SCC in Europe 3.
PDT is a well-documented modality that uses a photosensitizer (PS), tissue oxygen, and light of particular wavelengths to kill malignant cells. The energy transferred from a light-activated photosensitizer to oxygen molecules results in the formation of reactive oxygen species (ROS) that are responsible for cell death 4–6. PDT with ALA using blue light (400–450 nm) 7,8 and red light (~630 nm) 9–12 sources has been reported as an effective treatment modality in curing superficial and nodular BCCs. However, due to the incomplete treatment response and recurrence of tumors, conventional ALA-PDT in the treatment of BCC is not considered as superior to current standard medical therapies 3. Although a variety of mechanisms may lead to relative inefficiency of ALA-PDT treatment, in all instances treatment failure involves a subpopulation of cells that manage to escape cell death.
To improve the efficacy of ALA-PDT induced cell killing, one promising approach developed by our group is to treat the malignant cells with differentiation enhancing agents prior to ALA-PDT. The preconditioning approach is based on the fact that differentiation treatment redirects the aberrant pathways and forces the cancer cells to undergo normal growth arrest, differentiation, and apoptosis 13–16. In our previous studies, we showed that nontoxic doses of Vit D (1,25(OH)2D3) can increase the expression of porphyrin-synthetic enzymes in the heme biosynthesis pathway, resulting in the increased accumulation of protoporphyrin IX (PpIX), the PS synthesized preferentially in ALA-treated tumor cells 17–19. In addition, our mechanistic studies have also revealed that Vit D pretreatment enhances the activation of extrinsic apoptotic pathways, thereby improving tumor selective cell killing by ALA-PDT 18.
The current study was designed to investigate the ability of Vit D to increase the cytotoxic effects of ALA-PDT in BCC and SCC mouse models in which the tumors are grown in their native connective tissue matrix. Additionally, we studied the in vivo imaging capacity of Maestro EX IVIS® to quantify ALA induced PpIX non-invasively.
2. MATERIALS & METHODS
2.1 Reagents
Corn oil and Tamoxifen were obtained from Sigma-Aldrich (St. Louis, MO). Levulan® Kerastick® For Topical Solution, 20% and Vectical™ (calcitriol) Ointment 3 mcg/g were purchased from DUSA Pharmaceuticals, Inc. (Wilmington, MA) and Galderma Laboratories, L.P. (Fort Worth, TX) respectively. Ketaset® (Ketamine HCl Injection, USP 100 mg/ml, Boehringer Ingelheim Vetmedica, Inc. (St. Joseph, MO), AnaSed® Injection (Xylazine, 100 mg/ml, LLOYD Laboratories, Shenandoah, Iowa), and Isoflurane (1-chloro-2,2,2-trifluoroethyldifuoromethyl ether, Piramal Critical Care, Inc. Bethlehem, PA) were obtained from the hospital pharmacy of the Cleveland Clinic.
2.2 Generation of mouse models
To create a BCC model, we bred mice with the K14-Cre-ER transgene 20 and mice with a floxed p53 allele 21 with Ptch1+/− mice to generate Ptch1+/− K14-Cre-ER p53 flox/flox mice. These mice were treated with 100 mg/day of tamoxifen intraperitoneally (i.p.) for three consecutive days at age 6 weeks. (For these treatments, 10 μl of 10 μg/μl tamoxifen-ethanol stock solution preserved at −20 ºC was mixed with 300 μl of corn oil right before injection). Tamoxifen activates the Cre recombinase enzyme and thereby deletes p53 by DNA cleavage at the two flox sites that flank p53, and in the absence of the p53 tumor suppressor gene, BCC tumor formation is accelerated. At 8 weeks of age, the mice were exposed to 4 Gy of ionizing radiation (IR), to mutate the only remaining allele of the Ptch gene. This was done under anesthesia (Ketamine 100 mg/kg i.p., and Xylazine 10 mg/kg i.p. body weight). Mice then developed multiple visible BCC tumors at age 5–6 months 22. Animal care activities and protocols were approved and monitored by our Institutional Animal Care and Use Committee (IACUC).
To create an SCC model, SKH-1 hairless male and female mice at 8 weeks of age were UV-irradiated three times weekly starting from week 1, using a UVB apparatus. The spectral irradiance of the ultraviolet lamps was 280–400 nm, providing 80% UVB and 20% UVA. UV exposure started with 90 mJ/cm2 at week 1 and the dose was increased by 10% per week until it reached a maximum of 175 mJ/cm2, after which the dose was maintained at 175 mJ/cm2 until week 20. Tumors began to appear rapidly on the dorsal skin of the mice starting at week 1523.
2.3 Vit D preconditioning and ALA-PDT
After identifying palpable BCC tumors on Ptch1+/− K14-Cre-ER p53 flox/flox mice at 5–6 months of age, hair overlying the tumors was trimmed using electric clippers. Using Q-tips, a thin layer of Aquaphor or Vectical was applied to the skin overlying the tumors for three consecutive days. On the fourth day, ALA (Levulan) was applied to induce PpIX synthesis in the tumors.
At 4 h after Levulan application, mice were irradiated at a fluency rate of 0.43 W/cm2, using a 633 nm noncoherent light source (LumaCare USA, Newport Beach, CA) calibrated with a FieldMate laser power meter (Coherent Inc., Santa Clara, CA). Each tumor received 250 J/cm2 of 633 nm light over ~10 minutes.
2.4 Fluorescence imaging of PpIX in vivo and ex vivo
A Maestro EX multispectral in vivo fluorescence imaging system (PerkinElmer, Inc. Waltham, MA) was used to detect the presence of ALA-induced PpIX in BCC tumor tissues. PpIX fluorescence images were acquired before and 4 h after applying Levulan®. Mice were placed in the light tight camera box with continuous exposure to 1–3 % isoflurane. Fluorescence images were obtained in 10 nm steps from 500 to 720 nm using a blue filter set (excitation range, 435 to 480 nm; and emission filter, 490 nm long pass). The images obtained pre- and post-Levulan® application were used to unmix the autofluorescence spectra present in the images and thereby obtain the PpIX specific fluorescence. Spectral processing (i.e., unmixing) and quantification of PpIX fluorescence was done as per the manufacturer’s established protocols using Mestro EX 3.0 Image Processing software.
After 4 h of Levulan application, the mice were sacrificed, tumors harvested, and the tissue embedded in O.C.T. medium (Tissue-Tek; Sakura-Finetek) for frozen sectioning. PpIX-specific fluorescence (λex 633 nm; λem 650–780 nm) from 10 μm thick cryosections was observed by confocal microscopy (Leica Microsystems, Buffalo Grove, IL) and quantified using IPLab image processing software (Signal Analytics, Vienna, VA) as previously described 24. Regions of interest, representative of the whole image, were cropped from each image and the levels of PpIX were analyzed by setting the background signal threshold level such that only fluorescence from PpIX was apparent. These PpIX-specific levels were expressed as arbitrary fluorescence units (total pixels) per region of interest.
2.5 Immunohistochemistry
Mice were euthanized at different time points (4 h post ALA application or 24 h post PDT), and the tumors and surrounding skin were excised, fixed in formalin, and embedded in paraffin. Histological sections (5 μm) were analyzed by Hematoxylin and Eosin (H&E) staining, terminal dUTP nick end labeling (TUNEL) (Roche Applied Science, Indianapolis, IN), or immunstaining for E-cadherin (Santa Cruz Biotechnology, Santa Cruz, CA) and Ki-67 (Neomarkers Inc., Fremont, CA) per the manufacturer’s directions. Digital images from these H&E or immunofluorescently stained specimens were obtained using a Leica SCN400 system, and analyzed using IP Lab image processing software and Image J, as described earlier 24.
2.6 Statistical Analysis
Two-sample t-test was used to compare differences in PpIX accumulation, in vivo fluorescence and other IHC staining data between different treatment groups. P-values ≤ 0.05 were considered statistically significant.
3. RESULTS
3.1 Mouse models for BCC and SCC closely resemble the human skin cancers
Ptch1+/− K14-Cre-ER p53 flox/flox mice developed 6–8 mm diameter solid tumors (Fig. 1A) after subjecting to tamoxifen and IR. During their development process, tumors in these mice showed an admixture of superficial, infiltrative, micronodular, and nodular BCC architectural growth patterns. H&E image shown in fig. 1A represent the initial tumor formation stage in which the tumors are poorly circumscribed and tumor nests are variable in shape and size with jagged contours, also characterized by retraction of proliferating cells from the stroma. Tumor cells then colonize forming nodular BCC tumors with discrete rounded tumor cell nests embedded in a collagenized fibrous stroma similar to human nodular BCC.
Fig. 1.
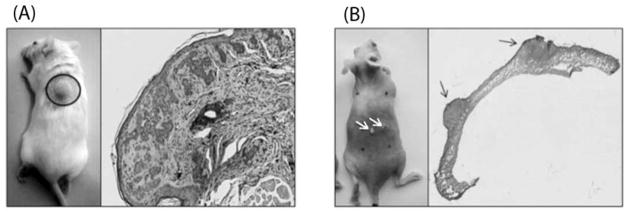
BCC and SCC mouse models, and the histomorphology of the tumors. (A), Left image shows a nodular BCC tumor developing on a PTCH1+/− K14-Cre-ER p53 flox/flox mouse at 5–6 months of age following Tamoxifen and IR treatment. Right image shows features of a typical BCC characterized by proliferating tumor nests and cleft-like retractions of the tumor stroma. (B), Left image, white arrows point to papillomatous-like SCC tumors developing on an SKH-1 hairless mouse after exposure to UVB for 20 weeks. Right image shows the histology of two SCC lesions.
After being irradiated with UVB for 20 weeks at a controlled dose, SKH-1 hairless mice has developed multiple nodular and hyperplasia lesions (1–5 mm; fig 1B) on the dorsal surface of animals between 15–20 weeks. Histological examination (fig. 1B H&E image) showed that these large and thick superficial ulcers simulate papillomatosis-like SCC pathology, which could continue to grow aggressively.
3.2 Vit D pretreatment increases ALA induced PpIX synthesis
PpIX concentration in tumors was estimated using in vivo (Maestro EX IVIS®) and ex vivo imaging (confocal microscopy). First, to establish the “gold standard” measurement by directly examining PpIX in tumor biopsy samples, Figure 2A and 2B shows that pretreatment of tumors with Vit D prior to ALA application increases PpIX production in BCC and SCC, respectively. Thus, confocal microscopy and digital quantification of the ALA induced PpIX signal showed an increase of 64 fold (p < 0.05) and 21 fold (p < 0.05) in Vit D pretreated BCC tumors and SCC tumors, respectively, relative to Aquaphor (vehicle) treated controls.
Fig. 2.

Ex vivo analysis of Vit D effect on PpIX synthesis, using Confocal Microscopy. (A) Left half of figure shows BCC tumors, (B) Right side of figure shows SCC tumors. For each half, the phase contrast (upper panels), fluorescence images (middle panels), and fluorescence quantification (lower panels) illustrates PpIX levels in tumor sections after 3 days of Aquaphor or Vit D treatment, followed by 4 h application of ALA. Number of tumors per treatment group is shown in parenthesis. Mean ± SEM of three representative images from each tumor; P-value from unpaired two-sided t-test.
In vivo analysis of BCC tumors shows that these changes in PpIX levels can be reliably be measured non-invasively in the living animal. In Figure 3, the normalized PpIX fluorescence images from the Maestro EX IVIS® device reflect a large and significant increase in the levels of photons emitting in Vit D treated tumors, compared to Aquaphor treated tumors (controls). Quantification of the total fluorescence signal per unit area in Vit D treated tumors was 5.8 fold (p < 0.00001) higher than in Aquaphor treated tumors. Therefore, both the in vivo and ex vivo imaging approaches show highly significance levels of PpIX due to Vit D treatment. Although the relative increase in PpIX fluorescence with Vit D appears to be much higher with the ex vivo confocal approach (64 fold, Fig. 2A) than with Maestro EX® in vivo imaging (5.8 folds, Fig. 3C), this discrepancy may be partly due to differences in how the threshold for the signal background signal is selected in the two different techniques.
Fig. 3.

In vivo imaging of PpIX in BCC tumors using Maestro EX IVIS®. (A), White light images, and (B), fluorescence images showing BCC tumors and their corresponding ALA induced PpIX levels after three days of pretreatment with Aquaphor and Vit D. (C), Quantification of PpIX specific fluorescence (total signal/area) calculated using Maestro EX 3.0 Image Processing Software. Mean ± SEM of three representative images from each of three tumors per treatment group is shown as fold change. Number of tumors per treatment group is shown in parenthesis. P-value is from unpaired two-sided t-test.
3.3 Vit D induces differentiation, proliferation, and cell death to enhance PDT
The effects of Vit D on differentiation and proliferation status of BCC tumors were analyzed by examining the expression of E-cadherin and Ki-67, respectively. Immunohistochemical staining of paraffin embedded tumor sections reveal that most cells in the Vit D preconditioned tumors expressed E-Cadherin (Fig. 4A) and Ki-67 (Fig. 4B), whereas in Aquaphor-treated tumors, expression of these markers was either completely absent or minimal. Quantification of fluorescence images showed a 145-fold (p < 0.02) and 42-fold (p < 0.005) enhancement in E-cadherin and Ki-67, respectively, in tumors that received Vit D application as compared to Aquaphor.
Fig. 4.
Effect of Vit D on differentiation, proliferation, and cell death due to ALA-PDT in BCC tumors. Fluorescence images and quantification of signal in tumor sections immunostained with secondary antibodies to (A), differentiation marker, E-cadherin, (B), proliferation marker, Ki-67, and (C), apoptotic marker, TUNEL 24 h post PDT. Mean ± SEM of three representative images from each of three tumors per treatment group is shown as fold change. Number of tumors per treatment group is shown in parenthesis. P-value, from unpaired two-sided t-test.
Tumors harvested at 24 h post ALA-PDT were also analyzed for cell death using TUNEL (which labels dying nuclei) on paraffin sections. The majority of the cells in tumors that received both Vit D and ALA-PDT were TUNEL positive (Fig. 4C, right), as compared to Aquaphor-ALA-PDT treated tumors (Fig. 4C, left). Quantitatively, tumors that received Vit D treatment prior to ALA-PDT caused 18.3 fold (p < 0.03) higher TUNEL labeling than traditional ALA-PDT treatment. As expected, the cell death in absolute control tumors (no PDT) or in normal tissues surrounding PDT-treated areas showed negligible amounts of TUNEL fluorescence (figure not included).
3.4 H&E confirms the increased efficacy of the combination approach
The cytotoxic effects of Vit D and PDT were also apparent from histological examination of the tumors. The morphology of untreated control BCC tumors showed normal staining, with nests of closely packed, large, rounded cells surrounded by thick collagenous fibrous stroma (Fig. 5, left panels). Tumors that received only ALA-PDT showed a decrease in overall staining and cell size, as well as thinning of the stroma (Fig. 5, middle panels). With the combined treatment (Vit D and ALA-PDT), these changes became more prominent, and consisted of pyknotic/shrunken nuclei, a complete loss of collagen in and around tumor cell nests (hypochromicity), and a loss of cells as reflected by an increase in dead/empty spaces (Fig. 6, right panels).
Fig. 5.

Histomorphological analysis of BCC tumors at 24 h post PDT, elucidating the tumor cell death-promoting effects of Vit D. Panels show H&E stained images of paraffin embedded tumor sections obtained from absolute controls (left panels), 24 h post-PDT with Aquaphor pretreatment (middle panels), and 24 h post PDT with Vit D treated tumors (right panels). Top and bottom panels show typical microscopic fields at low and high magnification, respectively.
4. CONCLUSIONS
Our in vivo and ex vivo imaging results demonstrate the potential of Vit D as a combination treatment to enhance ALA induced PpIX synthesis, resulting in enhanced tumor cell death following PDT. The increase in PpIX levels, and its detection using the in vivo imaging technique demonstrated here, suggests a promising approach for following tumor responses non-invasively and for optimizing treatment protocols. Furthermore, due to the enhanced efficacy of Vit D mediated ALA-PDT, this particular combination approach could be a good candidate to pursue for FDA approval for skin cancer treatment, and could also be explored as a new option for other epithelial cancers that do not respond well to traditional procedures such as surgery, radiation, and chemotherapy.
Acknowledgments
We thank Dr. Vinod Labhasetwar (Lerner Research Institute, Cleveland Clinic) for offering Maestro IVIS® to use in our experiments, Dr. Judy Drazba (Lerner Research Institute, Cleveland Clinic) for help with digital imaging, and Dr. Tayyaba Hasan for her continuing mentorship and support. This project was carried out with support from National Cancer Institute/NIH (Grant P01-CA84203, Dr. Tayyaba Hasan and Dr. Edward V. Maytin) and a Dissertation Research Award from Cleveland State University (Kishore Rollakanti).
References
- 1.de Vijlder HC, Sterenborg HJ, Neumann HA, Robinson DJ, de Haas ER. Light fractionation significantly improves the response of superficial basal cell carcinoma to aminolaevulinic acid photodynamic therapy: five-year follow-up of a randomized, prospective trial. Acta Derm Venereol. 2012;92(6):641–647. doi: 10.2340/00015555-1448. [DOI] [PubMed] [Google Scholar]
- 2.Xia YH, Li M, Fu DD, Xu SL, Li ZG, Liu D, Tian ZW. Effects of PTTG down-regulation on proliferation and metastasis of the SCL-1 cutaneous squamous cell carcinoma cell line. Asian Pac J Cancer Prev. 2013;14(11):6245–6248. doi: 10.7314/apjcp.2013.14.11.6245. [DOI] [PubMed] [Google Scholar]
- 3.Morton CA. Photodynamic therapy for nonmelanoma skin cancer--and more? Arch Dermatol. 2004;140(1):116–120. doi: 10.1001/archderm.140.1.116. [DOI] [PubMed] [Google Scholar]
- 4.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 5.Wilson BC. Photodynamic therapy for cancer: principles. Can J Gastroenterol. 2002;16(6):393–396. doi: 10.1155/2002/743109. [DOI] [PubMed] [Google Scholar]
- 6.Vrouenraets MB, Visser GW, Snow GB, van Dongen GA. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003;23(1B):505–522. [PubMed] [Google Scholar]
- 7.Itkin A, Gilchrest BA. delta-Aminolevulinic acid and blue light photodynamic therapy for treatment of multiple basal cell carcinomas in two patients with nevoid basal cell carcinoma syndrome. Dermatol Surg. 2004;30(7):1054–1061. doi: 10.1111/j.1524-4725.2004.30317.x. [DOI] [PubMed] [Google Scholar]
- 8.Dijkstra AT, Majoie IM, van Dongen JW, van Weelden H, van Vloten WA. Photodynamic therapy with violet light and topical 6-aminolaevulinic acid in the treatment of actinic keratosis, Bowen’s disease and basal cell carcinoma. J Eur Acad Dermatol Venereol. 2001;15(6):550–554. doi: 10.1046/j.1468-3083.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang I, Bendsoe N, Klinteberg CA, Enejder AM, Andersson-Engels S, Svanberg S, Svanberg K. Photodynamic therapy vs. cryosurgery of basal cell carcinomas: results of a phase III clinical trial. Br J Dermatol. 2001;144(4):832–840. doi: 10.1046/j.1365-2133.2001.04141.x. [DOI] [PubMed] [Google Scholar]
- 10.Thissen MR, Schroeter CA, Neumann HA. Photodynamic therapy with delta-aminolaevulinic acid for nodular basal cell carcinomas using a prior debulking technique. Br J Dermatol. 2000;142(2):338–339. doi: 10.1046/j.1365-2133.2000.03404.x. [DOI] [PubMed] [Google Scholar]
- 11.Varma S, Wilson H, Kurwa HA, Gambles B, Charman C, Pearse AD, Taylor D, Anstey AV. Bowen’s disease, solar keratoses and superficial basal cell carcinomas treated by photodynamic therapy using a large-field incoherent light source. Br J Dermatol. 2001;144(3):567–574. doi: 10.1046/j.1365-2133.2001.04085.x. [DOI] [PubMed] [Google Scholar]
- 12.Hurlimann AF, Hanggi G, Panizzon RG. Photodynamic therapy of superficial basal cell carcinomas using topical 5-aminolevulinic acid in a nanocolloid lotion. Dermatology. 1998;197(3):248–254. doi: 10.1159/000018006. [DOI] [PubMed] [Google Scholar]
- 13.Momma T, Hamblin MR, Hasan T. Hormonal modulation of the accumulation of 5-aminolevulinic acid-induced protoporphyrin and phototoxicity in prostate cancer cells. Int J Cancer. 1997;72(6):1062–1069. doi: 10.1002/(sici)1097-0215(19970917)72:6<1062::aid-ijc22>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Ortel B, Sharlin D, O’Donnell D, Sinha AK, Maytin EV, Hasan T. Differentiation enhances aminolevulinic acid-dependent photodynamic treatment of LNCaP prostate cancer cells. Br J Cancer. 2002;87(11):1321–1327. doi: 10.1038/sj.bjc.6600575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bikle DD. Vitamin D. 2. Elsevier Academic Press; Waltham, MA: 2005. Vitamin D: Role in skin and hair. [Google Scholar]
- 16.van Leeuwen JPTM, Pols HAP. Vitamin D. 2. Elsevier Academic Press; Waltham, MA: 2005. Vitamin D: Cancer and differentiation. [Google Scholar]
- 17.Anand S, Rollakanti KR, Horst RL, Hasan T, Maytin EV. Combination of oral vitamin D3 with photodynamic therapy enhances tumor cell death in a murine model of cutaneous squamous cell carcinoma. Photochem Photobiol. 2014;90(5):1126–1135. doi: 10.1111/php.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand S, Wilson C, Hasan T, Maytin EV. Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer Res. 2011;71(18):6040–6050. doi: 10.1158/0008-5472.CAN-11-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rollakanti KR, Anand S, Maytin EV. In-vivo luminescence model for the study of tumor regression and regrowth following combination regimens with differentiation-promoting agents and photodynamic therapy. Proc SPIE 8568. 2013:8568OY. [Google Scholar]
- 20.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29(4):418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 21.Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP, Epstein EH., Jr Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;(11):1285–1291. doi: 10.1038/15242. [DOI] [PubMed] [Google Scholar]
- 22.Tang JY, Xiao TZ, Oda Y, Chang KS, Shpall E, Wu A, So PL, Hebert J, Bikle D, Epstein EH., Jr Vitamin D3 inhibits hedgehog signaling and proliferation in murine Basal cell carcinomas. Cancer Prev Res (Phila) 2011;4(5):744–751. doi: 10.1158/1940-6207.CAPR-10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He G, Muga S, Thuillier P, Lubet RA, Fischer SM. The effect of PPARgamma ligands on UV- or chemically-induced carcinogenesis in mouse skin. Mol Carcinog. 2005;43(4):198–206. doi: 10.1002/mc.20111. [DOI] [PubMed] [Google Scholar]
- 24.Anand S, Honari G, Hasan T, Elson P, Maytin EV. Low-dose methotrexate enhances aminolevulinate-based photodynamic therapy in skin carcinoma cells in vitro and in vivo. Clin Cancer Res. 2009;15(10):3333–3343. doi: 10.1158/1078-0432.CCR-08-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]



