Abstract
There is a high interest in developing an 18F-labeled PET tracer that can aid in diagnosis and therapy monitoring of prostate cancer. In the current study, we have evaluated the potential of 2-18F-fluoropropionic acid (18F-FPA) as a PET tracer for imaging prostate cancer.
Methods
18F-FPA was synthesized starting from methyl-2-bromopropionate. Small-animal PET studies were performed on mice with CWR22rv1, PC-3, DU-145, and LNCaP prostate xenografts, and comparison of imaging characteristics of 18F-FPA with 18F-FDG uptake is reported. Biodistribution studies with 18F-FPA were performed on mice with CWR22rv1 xenografts and compared with 14C-acetate.
Results
18F-FPA was synthesized in 44% overall radiochemical yield (decay-corrected). Small-animal PET studies revealed that 18F-FPA can delineate both androgen-dependent and androgen-independent prostate xenografts with high tumor-to-background ratios. Comparative imaging studies demonstrate the superior performance of 18F-FPA over 18F-FDG for imaging prostate cancer, with excellent tumor-to-background contrast. Biodistribution studies show that tumor uptake of the tracer was 5.52 ± 0.35, 5.53 ± 0.42, 5.74 ± 0.54, and 5.34 ± 0.19 percentage injected dose (%ID) per gram at 1, 2, 3, and 4 h, respectively, after injection. The %ID/g values for 18F-FPA and 14C-acetate 1 h after tail vein injection were 7.08 ± 0.80 and 0.36 ± 0.08 in tumor, and the corresponding tumor-to-muscle ratios were 1.94 and 2.06, respectively.
Conclusion
The data presented here indicate that 18F-FPA accumulates in prostate cancers with high tumor-to-background ratios. 18F-FPA has potential for use in the clinical diagnosis of prostate cancer in humans.
Keywords: prostate cancer, PET, 11C-acetate, 2-18F-fluoropropionic acid, CWR22rv1
Prostate cancer is the most commonly diagnosed and second leading cause of cancer deaths in men in the United States (1). If detected early, prostate cancer treatment is highly successful, but at present there are no known treatments for advanced metastatic disease. For primary prostate tumors, determining the precise location and defining the target are critical for defining the clinical target volume for conformal radiotherapy (2). The field of noninvasive molecular imaging using PET isotopes has made rapid advances for the detection of primary and metastatic tumors, with 18F-FDG leading the way as a universal tumor-imaging agent. However, for prostate cancer, the results with 18F-FDG have been suboptimal (3). Hence, there is an urgent need to develop PET agents that can be used for routine detection and characterization of prostate tumors. Both 11C- and 18F-labeled radiotracers have been developed, including 11C-acetate, 11C-choline, 18F-fluoroacetate (4,5), and 18F-fluoroethylcholine (6–9), among others. 11C-acetate has shown promising results for the diagnosis of primary and metastatic prostate cancer, but the short half-life of 11C (20 min) means that 11C tracers can be used only at facilities that have an on-site cyclotron. 11C is impractical for use as a routine PET tracer in many imaging centers. 18F-labeled agents offer practical benefits due to a relatively long half-life (110 min). In this regard, 18F-fluoroacetate (4,5) and 18F-fluorine derivatives of choline (6–9) are currently being investigated as prostate tumor–imaging agents. 18F-fluoroacetate is a substrate for acetyl coenzyme A synthase and hence mimics 11C-acetate in the primary steps and has been proposed as a useful alternative for prostate cancer imaging. 18F-fluoroethylcholine mimics choline that is trapped inside the cells after phosphorylation by choline kinase, which is overexpressed in tumor cells. So far, no clear winner has emerged for prostate cancer.
In our continuing effort to identify novel PET tracers for oncologic applications (10–13), we have identified 2-18F-fluoropropionic acid (18F-FPA) as a possible radiotracer for imaging prostate cancer. The hypothesis is that 18F-FPA would mimic acetate in vivo in the same way that fluoroacetate mimics acetate (14,15) and hence would accumulate in prostate cancer and allow tumor delineation by PET. In support of this hypothesis, we present preliminary data including biodistribution and PET studies with 18F-FPA. The results suggest the potential use of 18F-FPA as a new PET radiotracer for imaging prostate cancer and provide a rationale for further laboratory and clinical characterization of this tracer.
MATERIALS AND METHODS
All reagents and solvents were purchased from either Aldrich Chemical Co. or Fisher Scientific and, unless stated otherwise, were used without further purification. All high-performance liquid chromatography (HPLC) solvents were filtered (0.45 µm, nylon, Alltech) before use. Water (ultrapure, ion-free, >18.2 MΩ · cm−1) was obtained from a Millipore α-Q Ultra-pure water system. HPLC was performed using a Shimadzu system equipped with a C-18 reversed-phase column (Phenomenex Luna analytic 4.6 × 250 mm or SemiPrep 21.2 × 100 mm, 5 µm, 1.0 or 6.0 mL/min, 0.2% acetic acid/CH3CN), 2 LC-10AT pumps, an SPD-M10AVP photodiode array detector, and a BioScan Flow Count PIN diode radiodetector. Radioactivity was assayed using a Capintec CRC-15R dose calibrator.
No-carrier-added 18F-fluoride ion was produced by the 18O(p,n)18F nuclear reaction by bombardment of an enriched 18O-H2O target with 11-MeV protons using an EBCO TR-19/9 cyclotron.
Synthesis of 18F-FPA
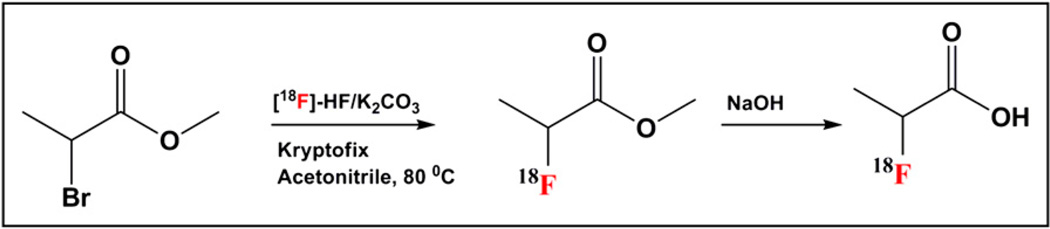
18F-FPA was synthesized using an established procedure (Scheme 1) (16). Briefly, 18F-fluoride ion (2.8 GBq = 75 mCi) in water was added to a vial containing 80 µL of 0.25 M potassium carbonate and 13 mg of Kryptofix dissolved in 1 mL of acetonitrile. Water was removed azeotropically with acetonitrile (3 × 1 mL) at 80°C. To the anhydrous 18F-KF/K2CO3 complexed with Kryptofix was added a solution of methyl-2-d,l-bromopropionate (3 mg) in 300 µL of anhydrous acetonitrile, and the vial was sealed and heated at 80°C for 6 min to give methyl-2-18F-fluoropropionate. The vial was allowed to cool to room temperature and diluted with 700 µL of water and purified using HPLC. The intermediate was purified on a semipreparative Phenomenex reversed-phase C-18 column (Gemini-NX, 100 × 21.2 mm, 5 µm, 110 A) using a mixture of 0.2% aqueous acetic acid:acetonitrile (60:40) as the eluant, with a flow rate of 6 mL/min. The product methyl-2-18F-fluoropropionate, with a retention time of 5.4 min, was collected. To the product, 50 µL of 10N sodium hydroxide was added and heated at 80°C for 10 min to give sodium 2-18F-fluoropropionate. The solvent was removed under reduced pressure, and the residue was neutralized with 85 µL of 6N HCl. The product was formulated in 0.9% saline and used for studies. For analysis of purity, 40 µL of product were acidified with 10 µL of 10N HCl and passed through a reversed-phase C-18 column (Luna, 250 × 4.6 mm, 5 µ, 100 A) using 0.2% aqueous acetic acid as the eluting solvent, with a flow rate of 1 mL/min. The final radiochemical yield was around 44% ± 3% (nonoptimized), with a specific activity of around 50 GBq/µM.
SCHEME 1.
Synthesis of 18F-FPA.
Octanol–Water Partition Coefficient Study (logP)
Octanol–water partition coefficients were determined for 18F-FPA at 4 different pH values—6.4, 7.0, 7.4, and 8.4—by measuring the distribution of radiolabeled compound in 1-octanol and phosphate-buffered saline. A 20 µL sample of 18F-FPA (~10–20 µCi) in saline was added to a vial containing 5 mL each of 1-octanol and phosphate-buffered saline. After being stirred in a vortex mixer for 1 min, the vial was centrifuged for 5 min to ensure complete separation of layers. Five hundred microliters of each layer were taken in a preweighed vial, and 18F counts (400- to 550-keVenergy range) were measured using a γ-counter (PerkinElmer 1480Wizard 3 Auto γ-counter). Counts per unit weight of sample were calculated, and logP values were calculated using the following formula:
log10P = log10 (counts in 1mL of octanol=counts in 1mL of water).
Generation of Tumor Xenografts
All animal experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of Memorial Sloan-Kettering Cancer Center and followed National Institutes of Health guidelines for animal welfare. Tumor cell lines were obtained from the American Type Culture Collection and cultured under conditions provided by the supplier. The cell lines included CWR22rv1, PC-3, LNCaP, and DU-145. Subcutaneous tumors were produced in nude mice (20–25 g; Taconic) by subcutaneous injection of 5 × 106 tumor cells in 200 µL consisting of 100 µL of cell culture medium and 100 µL of Matrigel (BD Biosciences) under 2% isoflurane anesthesia on the right forelimb of mice.
Small-Animal PET
Imaging was performed by use of a dedicated small-animal PET scanner (Focus 120 microPET; Siemens Medical Solutions USA, Inc.). The mice were maintained under 2% isoflurane anesthesia in oxygen at 2 L/min during the entire scanning period. Imaging was performed 1 h after administration of 11.1 MBq (300 µCi) of either 18F-FPA or 18F-FDG via injection in the tail vein. For a comparative study, mice were kept fasting for 4 h and imaged with 18F-FDG 1 h after intravenous injection. The activity was allowed to decay for 24 h, and then the mice were administered 18F-FPAvia the tail vein and imaged 1 h after injection. An energy window of 350–700 keV and a coincidence timing window of 6 ns were used. The image data were corrected for nonuniformity of the scanner response, dead-time count losses, and physical decay to the time of injection. No correction was applied for attenuation, scatter, or partial-volume averaging. The measured reconstructed spatial resolution of the Focus 120 scanner is approximately 1.6 mm in full width at half maximum at the center of the field of view. The counting rates in the reconstructed images were converted to activity concentrations (percentage injected dose [%ID] per gram of tissue) by use of a system calibration factor derived from the imaging of a mouse-sized water-equivalent phantom containing 18F.
In Vivo Biodistribution Studies
For single-isotope (18F) biodistribution studies, mice with CWR22rv1 tumors were injected in the tail vein with 3.7–5.5 MBq (100–150 µCi) of 18F-FPA in 200 µL of saline. Radioactivity in the syringe before and after administration was measured in an energy-calibrated dose calibrator (CRC-15R), and the exact quantity received by each animal was determined. The animals were euthanized at different time points, and then the organs were harvested. 18F radioactivity was measured in a calibrated γ-counter (1480 Wizard 3 Auto γ-counter) using a 400- to 600-keV energy window and decay correction. The counts were converted into activity, and %ID/g was calculated by dividing by decay-corrected injected activity and weight of the organ. For dual-isotope biodistribution studies, each animal was administered 200 µL of saline containing 3.7 MBq of 18F-FPA and about 0.0037 MBq (0.1 µCi) of 14C-acetate. Initially, 18F radioactivity was measured in a calibrated γ-counter (1480 Wizard 3 Auto γ-counter) using a 400- to 600-keV energy window and decay correction. For measuring 14C activity, all samples were solubilized (Soluene-350, Packard Instrument Co., Inc.) after the 18F radioactivity counting. The samples were then stored at 4°C for 1 d to allow for 18F decay. A scintillant agent (Insta-Fluor; Packard Instrument Co., Inc.) was added to solubilized samples, and 14C radioactivity was determined by liquid scintillation counting (Tri-Carb Liquid Scintillation Analyzer, model 1600TR; Packard Instrument Co., Inc.) using external standard quench corrections. The counts were converted into activity, and %ID/g was calculated by dividing by decay-corrected injected activity and the weight of the organ. For each time point, a minimum of 3 animals was used for the study, and the values are reported as mean ± SD of mean. The Student t test was performed, and differences were considered statistically significant at P < 0.05.
RESULTS
Synthesis
The overall synthesis time for 18F-FPA was 45 min, and the average radiochemical yields were about 44% ± 3% (decay-corrected). The radiochemical purity was at least 98%. The average specific activity was 50 GBq/µM.
Octanol–Water Partition Coefficient Study (logP)
Lipophilicity logP values of 18F-FPA at pH 6.4, 7.0, 7.4, and 8.4 are −2.89 ± 0.02, −3.08 ± 0.04, −2.97 ± 0.13, and −3.07 ± 0.03, respectively. As expected, for small carboxylic acids the logP is very low, indicating that the compound is very hydrophilic. The lipophilicity is not sensitive to changes in pH within the measured physiologically relevant pH ranges.
Small-Animal PET
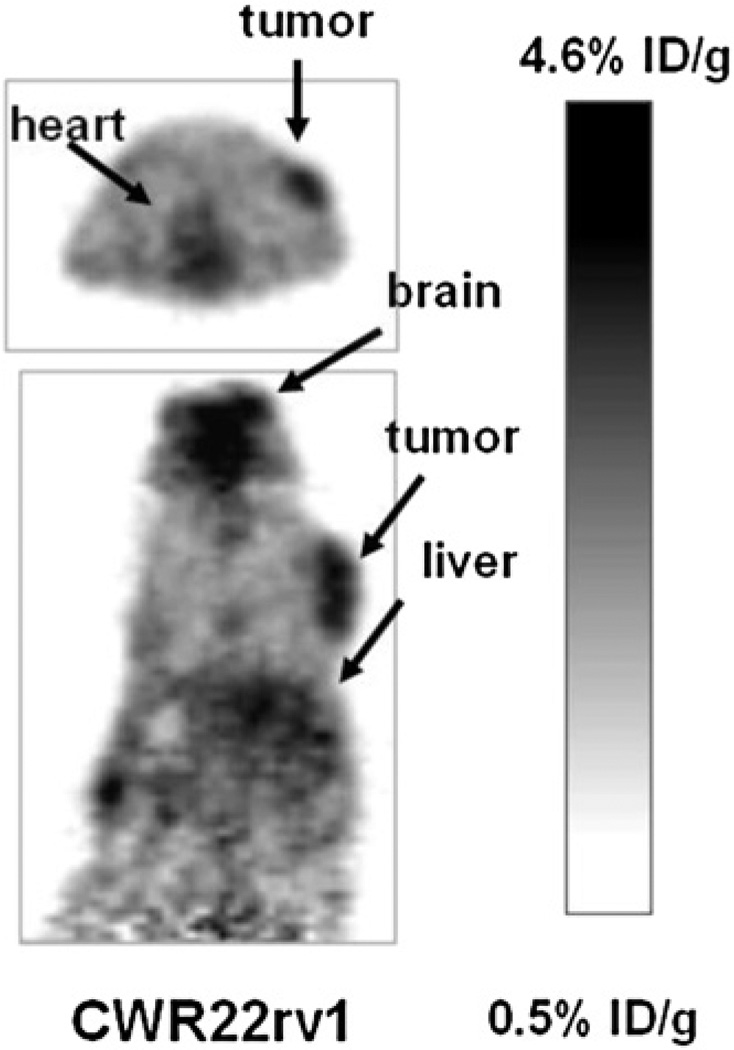
Small-animal PET was performed to evaluate the potential of 18F-FPA as a tumor-imaging agent in mice with prostate cancer CWR22rv1 xenografts. Figure 1 shows a small-animal PET image of 2 mice 1 h after administration of 18F-FPA via the tail vein. The acquisition time was 5 min. As shown in Figure 1, the CWR22rv1 tumor is clearly delineated with 18F-FPA. Uptake in the tumor at 1 h after administration was 4.4 %ID/g. In addition to the tumor, the heart and brain showed high uptake. The corresponding uptake values in the heart and brain were 4.0 and 4.6 %ID/g.
FIGURE 1.
Small-animal PET images of 18F-FPA in CWR22rv1 (androgen-independent) tumor–bearing mice 1 h after administration via tail vein. Images were acquired for 5 min. Tumor can easily be visualized in both transaxial and coronal slices.
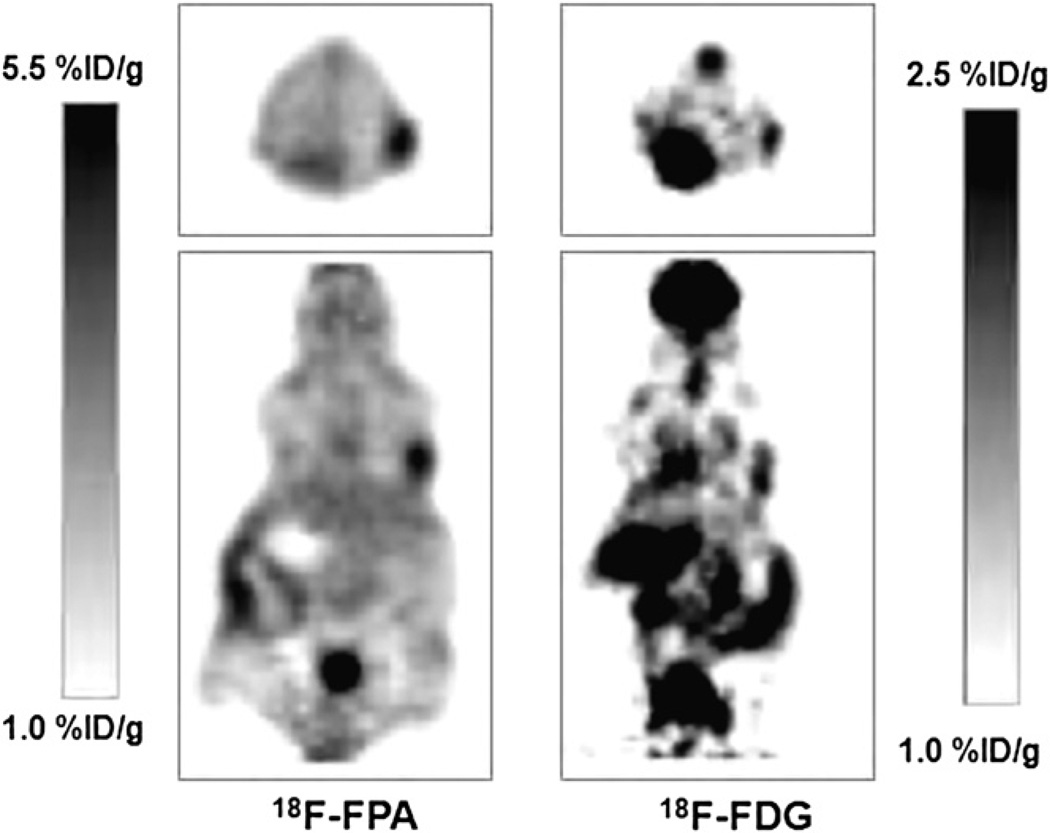
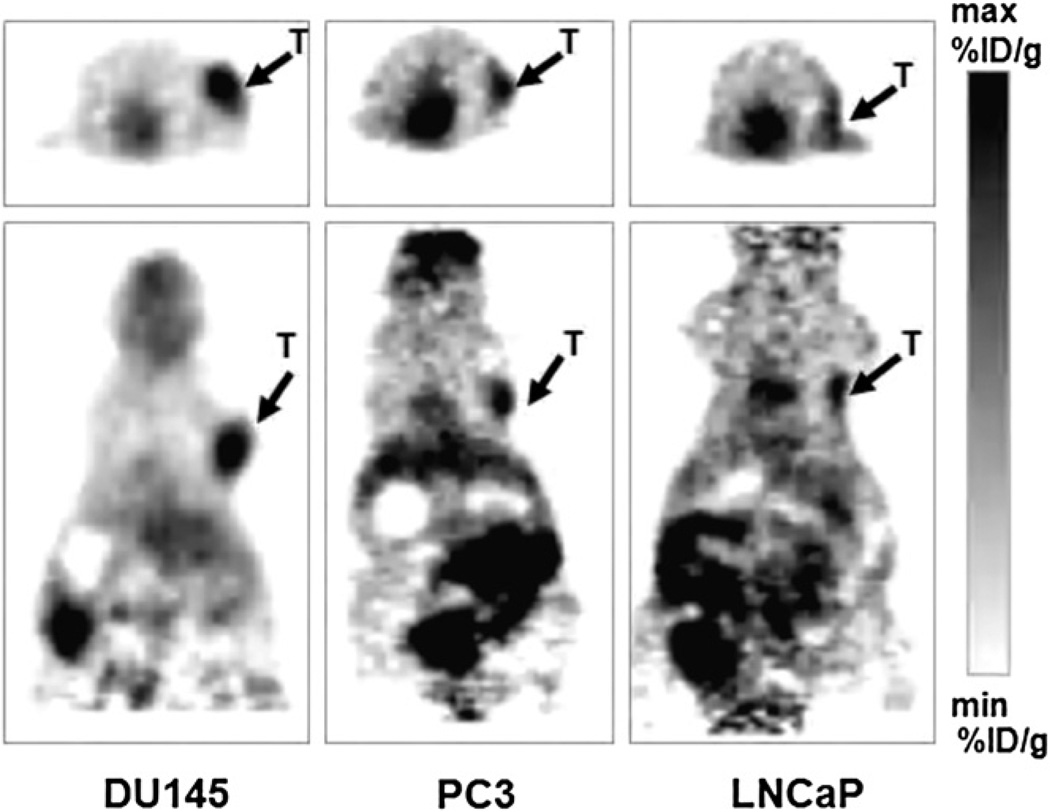
Figure 2 shows small-animal PET images of mice bearing CWR22rv1 tumor xenografts, imaged with 18F-FPA and 18F-FDG. First, the mice were kept fasting for 4 h, and then 18F-FDG was administered via the tail vein and imaged 1 h after administration. The activity was allowed to decay for 24 h, and the same mice were imaged with 18F-FPA 1 h after injection. In the case of 18F-FPA, the animals were not fasting. As shown in Figure 2, the tumors can be readily visualized with 18F-FPA, giving increased uptake and tumor contrast in comparison to 18F-FDG. 18F-FPA is taken up by the brain and kidneys but much less so than is 18FFDG. Figure 3 shows small-animal PET images of mice with different xenografts of prostate cancer origin. DU-145 is a cell line derived from brain metastases of prostate cancer and is androgen-independent. PC-3 is a highly metastatic androgen-independent cancer cell line derived from bone metastases. LNCaP is an androgen-sensitive cell line derived from lymph node metastases. It is apparent from PET studies that 18F-FPA shows a high accumulation in various tumor xenografts, and tumors can be clearly visualized because of a high tumor-to-background ratio.
FIGURE 2.
Small-animal PET images of nude mice with CWR22 xenografts imaged with either 18F-FPA or 18F-FDG 1 h after tail vein injection.
FIGURE 3.
Small-animal PET images of nude mice with DU-145, PC3, and LNCaP xenografts imaged with 18F-FPA 1 h after tail vein injection. Minimum and maximum values in small-animal PET images for DU-145, PC3, and LNCaP are 1.2 and 3.8, 1.5 and 3.8, and 2.0 and 4.0, respectively.
In Vivo Biodistribution
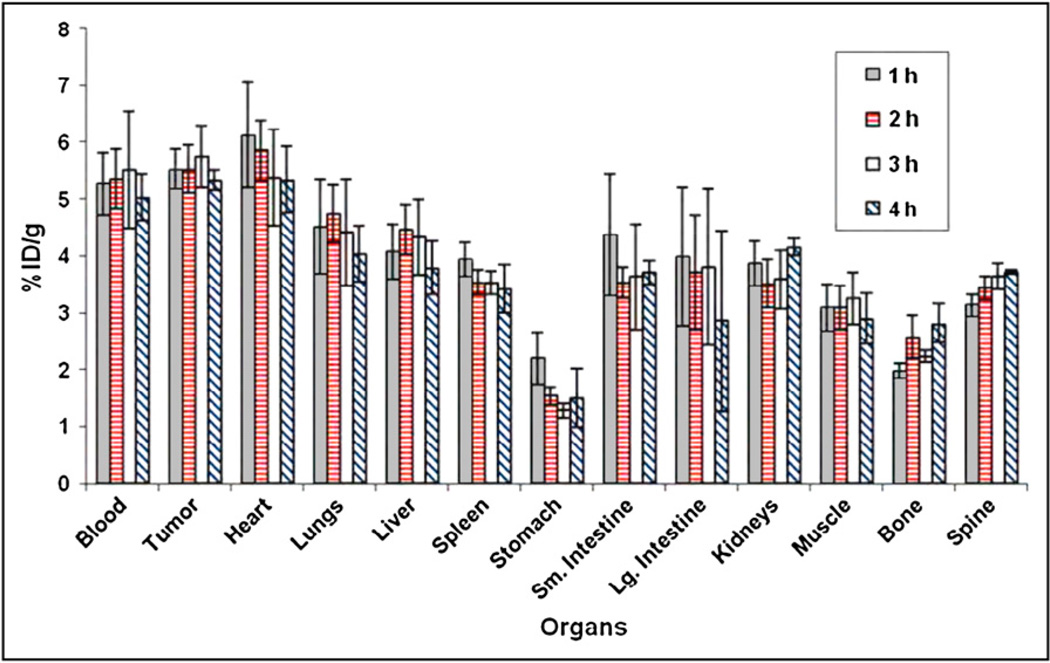
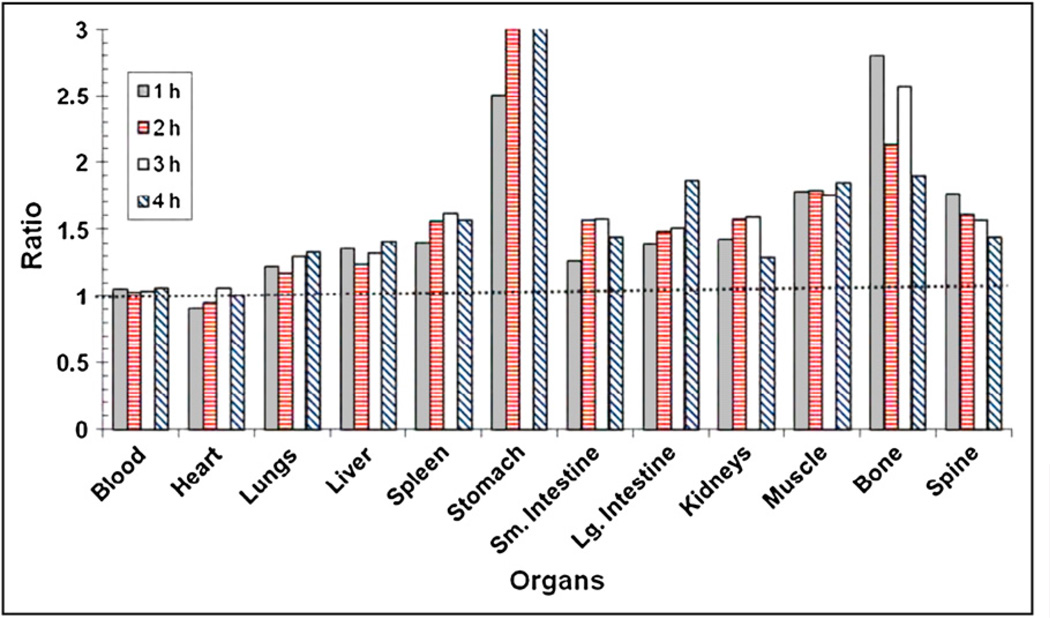
The in vivo biodistribution profile of 18F-FPA at 1, 2, 3, and 4 h after administration via the tail vein in nude mice with CWR22 xenografts is shown in Figure 4. It is apparent from these data that biodistribution remains constant throughout the study. There is considerable accumulation of tracer in tumor. Tumor uptake of the tracer was 5.52 ± 0.35, 5.53 ± 0.42, 5.74 ± 0.54, and 5.34 ± 0.19 %ID/g at 1, 2, 3, and 4 h, respectively, after injection. In addition to tumor, blood and heart showed high activity. Figure 5 shows the tumor-to-organ ratio of 18F-FPA at 1, 2, 3, and 4 h after injection. The tumor-to-organ ratio is greater than 1 for most of the organs with the exception of the heart, which is around 0.95.
FIGURE 4.
Biodistribution profile of 18F-FPA in nude mice with CWR22rv1 xenografts at 1, 2, 3, and 4 h after tail vein injection. Values are mean ± SD of mean (n = 3 for each time point). Lg. = large; Sm. = small.
FIGURE 5.
Tumor-to-organ ratio of 18F-FPA in nude mice with CWR22rv1 xenografts at 1, 2, 3, and 4 h after tail vein injection. Lg. = large; Sm. = small.
Comparison with 14C-Acetate
To compare the efficacy of 18F-FPA with acetate, we performed biodistribution studies 1 h after simultaneous administration of 18F-FPA and 14C-acetate via the tail vein (Table 1). The %ID/g values for 18F-FPA and 14C-acetate in tumor were 7.08 ± 0.80 and 0.36 ± 0.08, respectively, and the corresponding tumor-to-muscle ratios were 1.94 and 2.06, respectively. Two important points to note from the data are that the %ID/g values were much higher for 18F-FPA than for 14C-acetate and that the ratio of this type of tumor to some organs was more favorable for 18F-FPA than for 14C-acetate. The biodistribution values obtained with 14C acetate agreed well with 11C-acetate data in the literature (5).
TABLE 1.
Comparative Biodistribution Studies of 18F-FPA and 14C-Acetate in Mice
| %ID/g* | Tumor-to-organ ratio | |||
|---|---|---|---|---|
| Organ | 18F-FPA | 14C-acetate | 18F-FPA | 14C-acetate |
| Blood | 6.88 ± 0.64 | 0.02 ± 0.02 | 1.03 | 16.91 |
| Tumor† | 7.08 ± 0.80 | 0.36 ± 0.08 | 1.00 | 1.00 |
| Heart | 5.46 ± 0.63 | 0.25 ± 0.03 | 1.30 | 1.44 |
| Lungs | 4.00 ± 0.26 | 0.85 ± 0.11 | 1.77 | 0.42 |
| Liver | 5.61 ± 0.50 | 0.67 ± 0.17 | 1.26 | 0.53 |
| Spleen | 4.36 ± 0.85 | 0.68 ± 0.16 | 1.62 | 0.52 |
| Stomach | 2.53 ± 1.04 | 0.77 ± 0.42 | 2.80 | 0.47 |
| Small intestine | 4.37 ± 1.15 | 0.81 ± 0.13 | 1.62 | 0.44 |
| Large intestine | 3.71 ± 1.09 | 0.56 ± 0.20 | 1.91 | 0.64 |
| Kidneys | 4.17 ± 0.60 | 0.58 ± 0.14 | 1.70 | 0.62 |
| Muscle | 3.66 ± 0.50 | 0.17 ± 0.03 | 1.94 | 2.06 |
| Bone | 1.68 ± 0.54 | 0.29 ± 0.11 | 4.23 | 1.22 |
| Brain | 5.57 ± 0.33 | 0.30 ± 0.02 | 1.27 | 1.17 |
Mice euthanized 1 h after simultaneous administration of 18F-FPA and 14C-acetate via tail vein injection. Values are mean ± SD of mean (n = 4).
CWR22rv1 tumors.
DISCUSSION
The suboptimal performance of 18F-FDG for selective and sensitive detection of prostate tumors has spurred research in the development of alternative PET agents for detection of prostate cancers. 11C-acetate was initially developed as a PET tracer to measure myocardial metabolism (17,18) but was also found to be effective in detecting prostate tumors (19). Studies on the mechanism suggest a role for fatty acid synthase, which uses acetate as its substrate for the synthesis of long-chain fatty acids. Therefore, the increased uptake of 11C-acetate observed in prostate tumors has been attributed to fatty acid synthase activity (20). Despite the success of 11C-acetate for diagnosing prostate tumors, the short half-life of 11C is a problem for its use as a routine PET agent. In contrast, 18F-labeled tracers are advantageous because their relatively long half-life (2 h) does not require an onsite cyclotron.
Long-chain carboxylic acids labeled with 18F have been investigated as cardiac imaging agents for measuring myocardial uptake (21). To our knowledge, no such studies have been reported for short-chain carboxylic acids. 18F-FPA was initially developed as a radioderivatization agent for labeling small peptides with 18F (16). For example, the new radiotracer 18F-galacto-RGD, which is currently under clinical trials for imaging αvβ3 integrin expression, uses 18F-FPA as the prosthetic group for radiolabeling the peptide (22). The biodistribution and imaging properties of 18F-FPA itself have not been reported. Toxicologic studies in rats have shown that FPA caused no acute toxicity at 2.3 mmol/ kg (23). Sodium, potassium, and calcium salts of propionic acid are extensively used as food additives. Metabolic studies have indicated that propionate can be used as a precursor for synthesis of fatty acids, glycogen, amino acids, and others, depending on the species (14,15,24).
18F-FPA was synthesized using well-established procedures (14,15). Because the radiochemical yields obtained were high (44% ± 3% decay-corrected), further optimization of the yield was not attempted. Though the HPLC profile during sample purification indicated that the fluorination reaction was almost quantitative, the 44% overall radiochemical yield represents losses during fluoride transfers and the HPLC step. During the removal of solvent after hydrolysis of the methyl ester, it was observed that neutralization must be performed after the removal because FPA is fairly volatile and a significant amount of compound can be lost during the process even at 45°C. In addition, during analytic HPLC, the solution must be acidic (pH < 4) because the ionized and neutral forms of FPA have fairly different retention times under the conditions used. If the pH of the injectant is greater than 4, 2 separate peaks are observed. FPA has a low molar extinction coefficient in the ultraviolet region, making accurate calculations of specific activity difficult using a standard ultraviolet detector. The specific activity calculations were based on a minimal detectable concentration of FPA. Routinely during analysis, no ultraviolet absorption peaks (mass peaks) were observed during HPLC after the purification of 18F-FPA. Hence, in calculating the specific activity of 18F-FPA, it was assumed the mass amount of FPA was just below the detectable limit.
In vivo studies indicate that 18F-FPA can successfully detect prostate tumor xenografts in mice. Biochemical studies are under way to investigate the exact mechanism of uptake of 18F-FPA in prostate cancers. As shown in the PET images (Figs. 1–3) and biodistribution data (Figs. 4 and 5), the compound localizes in the tumor and is retained throughout 4 h of observation. In addition to tumor, the heart, brain, and bladder show a high amount of activity. 18F-FPA primarily clears through urine, as bladder shows a high amount of activity in PET images, similar to that seen with 18F-fluoroacetate or 18F-fluoroethylcholine. Similar to 18F-fluoroacetate, 18F-FPA has a fairly constant activity in tumor, and except for bone, the tumor-to-organ ratios do not differ much at the 30-min and 2-h time points (5). Unlike 18F-fluoroacetate, 18F-FPA appears to show no evidence of defluorination, and both PET and biodistribution studies show low bone uptake.
At later time points, activity was localized in the lower part of the gut. This region of the intestines has been shown to be one in which absorption of small carboxylic acids occurs. In our comparative imaging study with 18F-FDG, it was observed that for CWR22rv1 tumor xenografts, the smaller the tumor size was, the lower was the 18F-FDG uptake (unpublished data, December 2008). However, with 18F-FPA even the smaller tumors (<6 mm) were clearly delineated as shown in Figure 2.
CONCLUSION
In the present study, we have evaluated the potential of 18F-FPA as a PET tracer for imaging prostate cancer. Biodistribution and PET studies indicate that 18F-FPA accumulates in the tumors and produces high tumor-to-background ratios. In comparison with 18F-FDG, 18F-FPA shows better delineation of CWR22rv1 prostate cancer xenografts on PET studies. In addition, 18F-FPA has a much higher %ID/g than does acetate. These studies support our hypothesis that 18F-FPA is a promising PET tracer for prostate cancer imaging, and further studies are warranted to examine if 18F-FPA offers any advantages over existing tracers for prostate cancer.
ACKNOWLEDGMENTS
This work was supported in part by NIH grant P50 CA86438. Technical services provided by the MSKCC Small-Animal Imaging Core Facility, supported in part by NIH Small-Animal Imaging Research Program (SAIRP) grant R24 CA83084 and NIH grants 096945 P30 CA08748, are also gratefully acknowledged. Support from the radiochemistry and cyclotron core for production and supply of 18F is also acknowledged.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.John SS, Zietman AL, Shipley WU, Harisinghani MG. Newer imaging modalities to assist with target localization in the radiation treatment of prostate cancer and possible lymph node metastases. Int J Radiat Oncol Biol Phys. 2008;71(1, suppl):S43–S47. doi: 10.1016/j.ijrobp.2007.06.079. [DOI] [PubMed] [Google Scholar]
- 3.Bouchelouche K, Oehr P. Positron emission tomography and positron emission tomography/computerized tomography of urological malignancies: an update review. J Urol. 2008;179:34–45. doi: 10.1016/j.juro.2007.08.176. [DOI] [PubMed] [Google Scholar]
- 4.Matthies A, Ezziddin S, Ulrich EM, et al. Imaging of prostate cancer metastases with 18F-fluoroacetate using PET/CT. Eur J Nucl Med Mol Imaging. 2004;31:797. doi: 10.1007/s00259-003-1437-1. [DOI] [PubMed] [Google Scholar]
- 5.Ponde DE, Dence CS, Oyama N, et al. 18F-fluoroacetate: a potential acetate analog for prostate tumor imaging—in vivo evaluation of 18F-fluoroacetate versus 11C-acetate. J Nucl Med. 2007;48:420–428. [PubMed] [Google Scholar]
- 6.DeGrado TR, Coleman RE, Wang S, et al. Synthesis and evaluation of 18Flabeled choline as an oncologic tracer for positron emission tomography: initial findings in prostate cancer. Cancer Res. 2001;61:110–117. [PubMed] [Google Scholar]
- 7.Igerc I, Kohlfurst S, Gallowitsch HJ, et al. The value of 18F-choline PET/CT in patients with elevated PSA-level and negative prostate needle biopsy for localisation of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:976–983. doi: 10.1007/s00259-007-0686-9. [DOI] [PubMed] [Google Scholar]
- 8.Vees H, Buchegger F, Albrecht S, et al. 18F-choline and/or 11C-acetate positron emission tomography: detection of residual or progressive subclinical disease at very low prostate-specific antigen values (<1 ng/mL) after radical prostatectomy. BJU Int. 2007;99:1415–1420. doi: 10.1111/j.1464-410X.2007.06772.x. [DOI] [PubMed] [Google Scholar]
- 9.Kwee SA, DeGrado TR, Talbot JN, Gutman F, Coel MN. Cancer imaging with fluorine-18-labeled choline derivatives. Semin Nucl Med. 2007;37:420–428. doi: 10.1053/j.semnuclmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Veach DR, Namavari M, Pillarsetty N, et al. Synthesis and biological evaluation of a fluorine-18 derivative of dasatinib. J Med Chem. 2007;50:5853–5857. doi: 10.1021/jm070342g. [DOI] [PubMed] [Google Scholar]
- 11.Pillarsetty N, Cai S, Ageyeva L, Finn RD, Blasberg RG. Synthesis and evaluation of [18F] labeled pyrimidine nucleosides for positron emission tomography imaging of herpes simplex virus 1 thymidine kinase gene expression. J Med Chem. 2006;49:5377–5381. doi: 10.1021/jm0512847. [DOI] [PubMed] [Google Scholar]
- 12.Pal A, Glekas A, Doubrovin M, et al. Molecular imaging of EGFR kinase activity in tumors with 124I-labeled small molecular tracer and positron emission tomography. Mol Imaging Biol. 2006;8:262–277. doi: 10.1007/s11307-006-0049-0. [DOI] [PubMed] [Google Scholar]
- 13.Veach DR, Namavari M, Beresten T, et al. Synthesis and in vitro examination of [124I]-, [125I]- and [131I]-2-(4-iodophenylamino) pyrido[2,3-d]pyrimidin-7-one radiolabeled Abl kinase inhibitors. Nucl Med Biol. 2005;32:313–321. doi: 10.1016/j.nucmedbio.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Lorber SH, Komarov SA, Shay H. Effect of sham feeding on gastric motor activity of the dog. Am J Physiol. 1950;162:447–451. doi: 10.1152/ajplegacy.1950.162.2.447. [DOI] [PubMed] [Google Scholar]
- 15.Pritchard GI, Tove SB. Interrelationships between the metabolism of short-chain fatty acids by ruminant liver slices. Biochim Biophys Acta. 1960;41:130–137. doi: 10.1016/0006-3002(60)90378-4. [DOI] [PubMed] [Google Scholar]
- 16.Guhlke S, Coenen HH, Stocklin G. Fluoroacylation agents based on small Nca [F-18] fluorocarboxylic acids. Appl Radiat Isot. 1994;45:715–727. [Google Scholar]
- 17.Chan SY, Brunken RC, Phelps ME, Schelbert HR. Use of the metabolic tracer carbon-11-acetate for evaluation of regional myocardial perfusion. J Nucl Med. 1991;32:665–672. [PubMed] [Google Scholar]
- 18.Gropler RJ, Siegel BA, Geltman EM. Myocardial uptake of carbon-11-acetate as an indirect estimate of regional myocardial blood flow. J Nucl Med. 1991;32:245–251. [PubMed] [Google Scholar]
- 19.Dimitrakopoulou-Strauss A, Strauss LG. PET imaging of prostate cancer with 11C-acetate. J Nucl Med. 2003;44:556–558. [PubMed] [Google Scholar]
- 20.Vavere AL, Kridel SJ, Wheeler FB, Lewis JS. 1-11C-acetate as a PET radiopharmaceutical for imaging fatty acid synthase expression in prostate cancer. J Nucl Med. 2008;49:327–334. doi: 10.2967/jnumed.107.046672. [DOI] [PubMed] [Google Scholar]
- 21.Knust EJ, Kupfernagel C, Stocklin G. Long-chain F-18 fatty acids for the study of regional metabolism in heart and liver: odd-even effects of metabolism in mice. J Nucl Med. 1979;20:1170–1175. [PubMed] [Google Scholar]
- 22.Beer AJ, Haubner R, Wolf I, et al. PET-based human dosimetry of 18F-galacto-RGD, a new radiotracer for imaging alpha v beta3 expression. J Nucl Med. 2006;47:763–769. [PubMed] [Google Scholar]
- 23.Lock EA, Gyte A, Sturgess NC, Duffell S, Wyatt I. 2-Halopropionic acid-induced cerebellar granule cell necrosis in the rat: in vivo and in vitro studies. Neurotoxicology. 2001;22:363–374. doi: 10.1016/s0161-813x(01)00027-4. [DOI] [PubMed] [Google Scholar]
- 24.Buchanan JM, Hastings AB, Nesbett FB. The role of carboxyl-labeled acetic, propionic and butyric acids in liver glycogen formation. J Biol Chem. 1943;150:413–425. [Google Scholar]