Abstract
In previous studies, we mapped glomerular layer 2-deoxyglucose uptake evoked by hundreds of both systematically related and chemically distinct odorants in rat olfactory bulbs. To determine which principles of chemotopic organization revealed in these studies may be more fundamental and which may be more species-typical, we now have characterized patterns of responses to 30 of these odorants in mice. We found that only a few odorants evoked their multiple foci of peak activity in exactly the same locations in the two species. In mice, as in rats, odorants that shared molecular features evoked overlapping patterns, but the locations of the feature-responsive domains often differed in rats and mice. In rats, increasing carbon number within a homologous series of aliphatic odorants is generally associated with rostral and ventral progressions of activity within domains responding to odorant functional group and/or hydrocarbon backbone. Such chemotopic progressions were not obvious in mice, which instead showed more abrupt differences in activated glomeruli within the domains for odorants differing by a single methylene group. Despite the differences, quantitative relationships between overall uptake patterns exhibited a similar organization with respect to odorant chemistry for the two species, probably due to partial overlaps of peak domains and more extensive overlaps in large, low-activity areas for rats and mice. We conclude that clustering responses to shared odorant features may be a general strategy for odor coding, but that the specific locations of high-activity domains may be unique to a species.
Indexing terms: 2-deoxyglucose, odors, imaging techniques, mapping
When odorants are detected by sensory neurons in the nose, they evoke spatial patterns of neural activity in the olfactory bulb due to the orderly projection of axons into the bulbar glomerular layer (Buck, 1996), and these glomerular activity patterns are systematically related to odorant chemical structure (Johnson and Leon, 2007). In rats, overlapping clusters of glomeruli respond to odorants that share molecular features such as functional groups and/or aspects of hydrocarbon structure, so that the glomerular surface can be rendered in terms of molecular-feature response domains (Johnson and Leon, 2000a; 2007; Johnson et al., 2002; Leon and Johnson, 2003; Mori et al., 2006). Within certain domains of the rat bulb, there is a further chemotopic organization in which glomeruli that respond to longer aliphatic odorants are systematically located more ventrally and rostrally (Johnson et al., 1998; 1999; 2004; Rubin and Katz, 1999; Johnson and Leon, 2000b; Meister and Bonhoeffer, 2001; Belluscio and Katz, 2001; Ho et al., 2006a). Both the clustering of feature-specific glomeruli into domains and the systematic positioning of glomeruli within domains according to odorant molecular properties may function to juxtapose related responses so that they might be better sharpened by way of lateral inhibitory networks involving periglomerular and granule cells (Yokoi et al., 1995; Johnson and Leon, 2007).
Much of what we know about chemotopic organization in the mammalian olfactory bulb has come from studies of 2-deoxyglucose (2DG) uptake in the rat, because this quantitative method allows equal access to all parts of the glomerular layer, thereby identifying all peak responses and their intensities relative to other glomerular responses. For the most part, other methods of measurement of glomerular activity are in agreement with 2DG results, including immediate early gene expression (Guthrie et al., 1993; Johnson et al., 1995) and optical imaging of intrinsic signals on the lateral aspect of the bulb (Mori et al., 2005). There are similarities in responses to some odorants that have previously been studied using 2DG in both rats and mice, such as isovaleric acid and amyl acetate (mice: Royet et al., 1987; Sicard et al., 1989; rats: Johnson and Leon, 2000b; Johnson et al., 2004), although there also have been indications of possible differences between the species (Salcedo et al., 2005).
The possibility of differences in responses between species as closely related as rats and mice is interesting because such differences might elucidate which aspects of the chemotopic organization are more fundamental for odor information processing and which aspects are more species-typical. Furthermore, because recombinant mice provide unique opportunities for testing the involvement of particular odorant receptors and other proteins in odor perception (Youngentob et al., 2001; 2003; 2004; Kobayakawa et al., 2007), it would be useful to determine whether the mouse olfactory bulb is organized chemotopically in the same way as the rat’s.
To compare chemotopic organization in the two species, we now have mapped 2DG uptake in mouse olfactory bulbs using a set of odorant chemicals for which we already have determined response patterns in rats. We selected odorants that represent a breadth of odorant chemical structure and that together would stimulate most of the glomerular layer in the rat. We also have investigated complementary sets of odorants that represent systematic, incremental variations in chemical structure to compare finer aspects of the chemotopic organization. Finally, we have included one odorant concentration series involving a type of odorant chemical that is perceived differently at different concentrations and that evokes different patterns of activity in the rat bulb at different concentrations. As we expected, our results identified both conserved and species-typical aspects of organization.
MATERIALS AND METHODS
Animals
The University of California, Irvine Institutional Animal Care and Use Committee approved all procedures using animals. Adult mice (strain C57BL/6J, purchased from The Jackson Laboratory, Bar Harbor, ME) were obtained at 6 weeks of age and were housed in groups of five per cage. Their age at time of use ranged from 7–12 weeks (7.6 ± 1.2 weeks, 21.2 ± 2.1 g, mean ± sd). On each experimental day, one mouse per cage was exposed to air or mineral-oil solvent, and each other mouse in the cage was exposed to a different odorant condition. The order of presentation of different odorant conditions was varied on each day of a study. The overall project consisted of ten smaller studies as shown in Table 1. The study involving different concentrations of 2-hexanone used 5 mice for each condition. All other studies involved exposures of 4 mice to each odorant. Exposures for each smaller study generally were completed over a 2–3 week span.
Table 1.
Odorants used in the study.
| Study | Odorant | CAS #1 | mL | Flow rate (mL/min) | Dilution |
|---|---|---|---|---|---|
| 1 | Decanal | 112-31-2 | 100 | 250 | 8 |
| 1 | Valeric acid #1 | 109-52-4 | 100 | 125 | 16 |
| 1 | Methyl benzoate | 93-58-3 | 100 | 250 | 8 |
| 2 | Guaiacol | 90-05-1 | 100 | 250 | 8 |
| 2 | L-Carvone | 6485-40-1 | 100 | 250 | 8 |
| 2 | L-Bornyl acetate | 5655-61-8 | 100 | 250 | 8 |
| 3 | Isoamyl acetate | 123-92-2 | 100 | 250 | 8 |
| 3 | 1-Pentanol | 71-41-0 | 100 | 250 | 8 |
| 3 | Acetone | 67-64-1 | 100 | 250 | 8 |
| 3 | β-Pinene | 18172-67-3 | 100 | 250 | 8 |
| 4 | Ethylbenzene | 100-41-4 | 100 | 250 | 8 |
| 4 | 2-Propanol | 67-63-0 | 100 | 250 | 8 |
| 4 | Pentadecane | 629-62-9 | 50 | 250 | 8 |
| 4 | Santalol | 11031-45-1 | 100 | 250 | 8 |
| 5 | Hexanal | 66-25-1 | 100 | 250 | 8 |
| 5 | Heptanal | 111-71-7 | 100 | 250 | 8 |
| 5 | Octanal | 124-13-0 | 100 | 250 | 8 |
| 5 | Nonanal | 124-19-6 | 100 | 250 | 8 |
| 6 | 2-Heptanone | 110-43-0 | 100 | 5 | 391 |
| 6 | 2-Heptanone | 110-43-0 | 100 | 16 | 127 |
| 6 | 2-Heptanone | 110-43-0 | 100 | 51 | 39 |
| 6 | 2-Heptanone | 110-43-0 | 100 | 158 | 13 |
| 7 | Methyl valerate | 624-24-8 | 100 | 250 | 8 |
| 7 | Propyl acetate | 109-60-4 | 100 | 250 | 8 |
| 7 | Isoamyl propionate | 105-68-0 | 100 | 250 | 8 |
| 7 | Ethyl propionate | 105-37-3 | 100 | 250 | 8 |
| 8 | Iodoform2 | 75-47-8 | 250 | 100 | 10 |
| 8 | 2,5-Dimethylpyrazine2 | 123-32-0 | 250 | 100 | 10 |
| 9 | Propionic acid | 79-09-4 | 100 | 50 | 40 |
| 9 | Butyric acid | 107-92-6 | 100 | 50 | 40 |
| 9 | Valeric acid #2 | 109-52-4 | 100 | 50 | 40 |
| 9 | Caproic acid | 142-62-1 | 100 | 50 | 40 |
| 10 | Ethyl acetate | 141-78-6 | 100 | 50 | 40 |
| 10 | 1-Decanol | 112-30-1 | 100 | 250 | 8 |
CAS #, chemical abstract services registry number.
Diluted 1/10 in light mineral oil (Sigma).
Odorant Exposures
We used procedures very similar to those in our studies on rat pups (Johnson et al., 1998; 1999). On the night before each experiment, bedding was changed to decrease odors from the home cage being carried over into the apparatus. Unlike for rat pups, isolation of mice in a clean cage only an hour before exposure was not effective in decreasing background odors. The mice were removed individually from the cage, weighed, injected intraperitoneally with [14C]2-DG (Sigma, 3 μL/g mouse of 50 nCi/μl, 54 mCi/mmol in 0.85% saline), placed immediately into a 2-L Mason jar, and exposed for 45 minutes to volatilized odorant.
Odorants were volatilized by bubbling high-purity, research-grade nitrogen through liquid in gas-washing bottles. Iodoform and 2,5-dimethylpyrazine were first diluted in mineral oil prior to use in 500-mL gas-washing bottles. All other odorants were used neat in 125-mL gas-washing bottles. Table 1 shows volumes of odorants, as well as the flow rate of nitrogen and the dilution of the vapors using ultra-zero grade air prior to delivery to the mice. Flow rates of nitrogen were measured using Gilford flowmeters. Separate sections of Teflon tubing were dedicated to each odorant to prevent cross-contamination. Exposures were terminated by switching the odorant line to carbon dioxide until the animals stopped breathing, at which time they were decapitated and their brains were removed and frozen in isopentane at −45°C.
Histology
Olfactory bulbs were cut using a cryostat at −20°C into 20-μm sections taken perpendicular to the long axis of the bulb. Because mouse bulbs were smaller than rat bulbs, we collected on glass coverslips every fourth section for autoradiographic measurement of 2DG uptake (instead of every sixth section used for rat pups) in order to map the whole structure using a similar number of measurements. We estimate from a sample of 22 mouse bulbs that 44 ± 2 sections (mean ± sd) contributed to the average mouse data analysis. This number can be compared with 38 ± 3 sections for rat pups estimated by sampling 24 bulbs distributed across 10 years of experiments on this species. Adjacent 20-μm sections were collected on glass slides for Nissl staining using cresyl violet.
Mapping
We mapped uptake of 2DG in images of autoradiographs using the same procedure that we have used for rat bulbs (Johnson et al., 1999; 2002). This method involves sampling uptake at intersections between grid lines and the glomerular layer, which is located in an image of the adjacent Nissl-stained section. Different grids are applied at different intervals between rostral-caudal landmarks to accommodate changes in the perimeter of the glomerular layer. An illustration of 2DG uptake in sections from a mouse bulb is shown in Figure 1, which also indicates the contribution of data from individual sections to the overall activity pattern obtained for that bulb. After mapping, data matrices are standardized to contain equal numbers of sections between landmarks.
Fig. 1.
Uptake of 2DG in a mouse exposed to valeric acid odorant. A: Images of cresyl violet-stained sections at three levels through the olfactory bulb (l, lateral; d, dorsal; m, medial; v, ventral). B: Pseudocolor-enhanced images of autoradiograms representing radiolabeled 2DG uptake in sections adjacent to those in A (warmer colors indicate higher levels of uptake). Outlines indicate boundaries of the glomerular layer determined from the cresyl violet-stained sections. Arrowheads denote lateral foci of evoked 2DG uptake, while arrows indicate medial foci. Open symbols indicate foci that respond to carboxylic acids, while closed symbols indicate foci that respond to a variety of aliphatic odorants with similar hydrocarbon structure in rats. C: A color-coded contour chart expressing levels of 2DG uptake across the glomerular layer of the single bulb that contributed the sections in A and B. Lines extending upward from the chart indicate which columns contain the data from the individual sections in B. Anatomical orientation is shown in the left panel. The locations of the same foci shown in B are indicated using the same type of arrow or arrowhead. A chart showing the blank-subtracted data averaged across both bulbs of four mice exposed to valeric acid is shown in the first panel of Figure 2A; the similarity of these patterns is a reflection of the consistency of the maps across different animals. D: Images of a three-dimensional reconstruction wherein an image of the contour chart in C has been applied as a texture over a model of the surface of the rat glomerular layer (see http://leonserver.bio.uci.edu; r, rostral; c, caudal; d, dorsal; v, ventral). Arrows and arrowheads are as in B and C. This figure can be compared to Figure 1 of Johnson et al. (1999) to appreciate more directly certain similarities in autoradiograms between mice and rats.
We used exactly the same grids and landmarks in mice as we did in rats. In mice, the first AOB, which is used as an internal landmark, was located at 0.53 ± 0.02 times (mean ± sd) the distance between the first external plexiform layer (the first landmark) and the end of the medial mitral cell layer (the final landmark), which can be compared with a value of 0.58 ± 0.04 in rat pups (range of 0.49 to 0.57 in our sample for mice compared to a range of 0.50 to 0.63 in the rat sample). Images of the smaller mouse autoradiographic sections were taken at a higher magnification (132 pixels/mm) than we used for rats (108 pixels/mm) so that the number of pixels between gridline intersections with the glomerular layer were approximately equal in the two species.
We initially were concerned about the possibility that there would be systematic differences between rats and mice in overall glomerular layer anatomy that would limit our ability to make meaningful, direct quantitative comparisons of data matrices generated using the same grid system. However, we found that high-uptake foci evoked by particular odorants were in some cases almost entirely superimposable between mice and rats (e.g., valeric acid-evoked foci in anterior-dorsal and posterior mid-lateral and mid-medial locations, decanal-evoked foci in ventral locations on both lateral and medial bulbar aspects, and methyl benzoate in dorsomedial locations). The similarity in responses to valeric acid can be appreciated further by comparing Figure 1 to the similar figure in our first description of this mapping method as applied to rats (Fig. 1 of Johnson et al., 1999). These overlaps, as well as other local and overall similarities in activity patterns that are described in this paper, suggested that the differences in activity patterns detected for other odorants do not reflect technical deficiencies in applying the rat mapping system to mice but rather indicate true, species-typical differences in the chemotopic organization of the olfactory systems.
Data Analysis
Data analysis was conducted as described previously (Johnson et al., 1999; 2002). Briefly, measurements taken in units of grayscale were transformed to nCi of [14C]2DG/g wet tissue by comparison with radioactivity standards (ARC 146A-CG from American Radiolabeled Chemicals, Inc., St. Louis, MO) exposed to each film. Data from individual sections were concatenated into matrices, which were standardized to give equal numbers of sections (columns) for each bulb. Data matrices from the left and right bulb of each mouse were averaged and further transformed into a ratio of glomerular layer (GL) uptake to uptake in the subependymal zone (SEZ) at predetermined locations in the bulb. For each study, the average GL/SEZ matrix from vehicle air-exposed mice was subtracted from each individual matrix from an odorant-exposed mouse. Examples of 2DG uptake in animals exposed to air and to vapors from mineral oil are shown in Supplementary Figure 1 along with patterns of 2DG uptake evoked during exposures to odorants in the same studies. Patterns in air-exposed mice involved less uptake than was evoked by odorants, and they tended to differ across different mice taken from different cages. The light mineral oil that was used as solvent for 2,5-dimethylpyrazine and iodoform evoked a more reproducible pattern that was distinct from the patterns obtained for mice exposed to odorants dissolved in mineral oil.
Blank-subtracted matrices were standardized further by calculating z-scores comparing uptake at each location to the mean and standard deviation of uptake across the entire glomerular layer of the same animal. Contour charts representing odorant-evoked activity patterns were generated by averaging the z-scored data matrices for all mice exposed to the same odorant condition within a smaller study. We have chosen a ventral-centered format for these charts, as we have for most of our studies on rats, to reduce the visual impact of missing values on the dorsal surface that are occasionally caused by loss of tissue on the cryostat blade during sectioning. Alternative figures containing dorsal-centered versions of all charts are provided as supplementary material at the journal’s website (Wiley: please insert URL here).
Together with each mouse pattern we show in this paper, we include a corresponding rat pattern from our archive of previous results (e.g., http://leonserver.bio.uci.edu). When more than one rat pattern evoked by a given odorant was available, we chose the best match to the mouse pattern for these qualitative illustrations. For quantitative comparisons leading to principal components analysis, however, we calculated correlation coefficients between each mouse pattern and every rat pattern evoked by the same odorant, and then we used the average of the resulting correlation coefficients as input for the factor analysis.
Photomicrograph Production
Images of cresyl violet-stained sections in Figure 1 were captured and digitized using trans-illumination, a Sony XC-77 CCD camera fitted with a macro lens stack, an LG-3 Scientific PCI frame grabber card (Scion Corporation), a Macintosh G3 computer, and NIH IMAGE 1.62 software. The contrast and brightness of the images were adjusted and the images were sharpened twice using Adobe Photoshop CS2, v. 9.02. The autoradiographic images in Figure 1 were colorized using the 32-color palette in NIH IMAGE 1.62, adjusting the slope to employ the full range of colors. The RGB colors then were converted to CMYK color space in Photoshop. Images were labeled and combined with charts and drawings in Adobe Illustrator CS2, v. 12.01.
RESULTS
General Observations
Each odorant tested in mice evoked a characteristic spatial pattern of activity across the glomerular layer that was distinct from the patterns generated by other odorants. Nevertheless, odorants that were similar in chemistry appeared to generate related patterns of activity, thus recapitulating a principal theme of spatial representations revealed by mapping 2DG uptake in rats (Johnson and Leon, 2007). The details of these relationships will be discussed below.
Whereas the locations of peak activity evoked by some odorants (e.g., valeric acid and decanal in Fig. 2A) were nearly superimposable in mice and rats, others only partially overlapped, apparently sharing some foci of peak activity but not others (e.g., methyl benzoate and guaiacol in Fig. 2D). Still other patterns differed dramatically in the locations of all peaks of activity (e.g., β-pinene in Fig. 2C and acetone in Fig. 3A).
Fig. 2.
Anatomically standardized contour charts illustrate the distribution of 2DG uptake across the entire glomerular layer in mice (top rows in each panel) and rats (bottom rows in each panel) exposed to the same odorants. A: Two odorants yielding nearly superimposable patterns in the two species. B: A set of aliphatic esters differing in carbon number, bond position, and branching. Dotted outlines denote the location of paired domains that were identified previously in rats as responding to aliphatic esters of various hydrocarbon structures. The corresponding lateral responses in mice appear to be displaced caudally and ventrally (arrows). C: A set of bicyclic odorants. Ventral areas were activated by these odorants in rats (dotted outlines). Corresponding areas in mice were not activated. Instead, intense 2DG uptake occurred in the posterior third of the midlateral and midmedial aspects of the mouse bulb (arrows). D: A set of aromatic odorants. In rats, methyl benzoate and 2,5-dimethylpyrazine activated a pair of dorsal domains (solid outlines). The dorsomedial domain in mice also was activated by these odorants, but the corresponding area of activation in the lateral bulb appeared to be displaced rostrally and ventrally (closed arrows). The phenolic odorant guaiacol activated a pair of dorsal domains in similar locations in mice and rats (dotted outlines), but posterior glomeruli also were activated in mice (closed arrowheads). The dorsomedial domain activated by the aromatic hydrocarbon ethylbenzene in rats (solid outline) was not activated in mice. Instead, more posterior mouse glomeruli showed uptake (open arrowheads). Each ventral-centered contour chart displays an average of z score matrices from several animals after subtraction of blanks from air-exposed cage mates. Dorsal-centered versions are available online as Supplementary Figure 2.
Fig. 3.
Anatomically standardized contour charts illustrate the distribution of glomerular layer 2DG uptake in mice (top rows in each panel) and rats (bottom rows in each panel) exposed to additional odorants. A: A set of small, water-soluble odorants that stimulated a pair of caudally located domains in rats (solid outline). In mice, less uptake occurred in these locations, but activity was detected more rostrally in areas that overlapped for acetone and 2-propanol (arrows) and that were distinct for iodoform (arrowheads). B: Two odorants with long hydrocarbon chains activated ventral glomeruli in both species (open arrowheads). In mice, decanol also stimulated glomeruli that were not as ventral in location (solid arrows). C: Responses to 1-pentanol and L-carvone showed further differences between rats and mice. Pentanol evoked some uptake in parts of the mouse bulb corresponding to paired domains previously shown in rats to respond to various alcohols (dotted outlines), but it also activated posterior, ventral glomeruli (solid arrows). The ketone- and aromatic-responsive domains activated by carvone in rats (solid outlines) were not activated in mice; nor was the region that was enantiomer-selective in rats (open arrow). Instead, mice showed uptake in other locations (solid arrowheads). The scale and orientation are the same as for Figure 2. A dorsal-centered version of this figure is available online as Supplementary Figure 3.
Molecular Feature Representation in Glomerular Modules
Aliphatic esters
In our mapping studies with rats, aliphatic odorants with ester bonds consistently evoked 2DG uptake in a pair of domains located in the lateral and medial aspects of the glomerular layer (indicated by dotted outlines in Fig. 2B). Each ester also activated glomeruli in other parts of the layer that were more specific to the individual odorant’s hydrocarbon structure and ester bond position (Johnson et al., 1998; 2002; 2004; 2005a). In contrast, mice did not exhibit consistent focal uptake in the same location as was activated in rats. Most of these esters, however, did generate foci of uptake located more posterior and ventral than the rat domains (arrows in Fig. 2B). Thus, the overall impression was that mice also possess a glomerular domain generally responsive to the ester molecular feature, but that this domain was shifted slightly in location.
Bicyclic odorants
In rats, various odorants with bicyclic structures and woody odors stimulate uptake in glomerular clusters located along the ventral extremity of the bulb (Johnson et al., 2006). Often, a very anterior cluster is activated together with either a single posterior cluster or a pair of lateral and medial, posterior clusters (indicated by dotted outlines in Fig. 2C). In mice, the ventral domains were entirely absent from the activity patterns evoked by the three bicyclic odorants we tested. On the other hand, all three bicyclic odorants evoked strong foci of uptake in a pair of overlapping areas in the lateral and medial bulbar aspects in mice (arrows in Fig. 2C), which again suggests that the bicyclic feature may be recognized by a glomerular domain in mice as in rats, but that the domain is present in a very different location.
Aromatic odorants
In rats, aromatic odorants such as methyl benzoate and 2,5-dimethylpyrazine activate dorsomedial and dorsolateral glomerular clusters (solid outlines in Fig. 2D; Johnson et al., 2005b; Farahbod et al., 2006). In mice, methyl benzoate and 2,5-dimethylpyrazine activated a dorsomedial region closely overlapping the domain that is stimulated in rats, but the paired lateral response in mice appeared to be shifted somewhat ventrally and rostrally relative to the location in the rat (solid arrows in Fig. 2D).
In rats, phenolic odorants such as guaiacol stimulate glomeruli that are located more ventrally than those responding to aromatic odorants with other oxygenic functional groups (dotted outlines in Fig. 2D; Johnson et al., 2002). In mice, guaiacol stimulated glomeruli in the same areas activated in rats on both the lateral and the medial aspects, but guaiacol also activated glomeruli in paired posterior domains that were not stimulated by guaiacol in rats (solid arrowheads in Fig. 2D).
In rats, high concentrations of the aromatic hydrocarbon ethylbenzene stimulate activity in the dorsomedial module that also responds to aromatic odorants with oxygenic functional groups (solid outline in Fig. 2D; Johnson et al., 2005b; Farahbod et al., 2006). In mice, ethylbenzene did not activate glomeruli in this module, but it did activate glomeruli located more caudally (open arrows in Fig. 2D), which is the area stimulated by many other aromatic hydrocarbons in rats (Farahbod et al., 2006).
Water-soluble odorants
In rats, odorants that possess very high water solubility, by virtue of either a small size or the presence of multiple oxygenic functional groups, stimulate glomeruli at the posterior extremities of the midlateral and midmedial bulbar aspects (Johnson et al., 2007). Such responses are exemplified in the patterns evoked by acetone and 2-propanol (Fig. 3A, outlines) as well as the response to ethyl acetate (Fig. 2B). In mice, although ethyl acetate stimulated a pattern that included posterior glomeruli and although 2-propanol evoked a small amount of uptake in the region, the water-soluble odorant acetone did not activate the posterior module strongly (Fig. 3A). Instead, acetone activated a region located more rostrally than the rat module, an area also stimulated by 2-propanol in mice (arrows in Fig. 3A). Iodoform, which is small and simple in structure without being very water soluble, also activated the posterior modules in rats (Fig. 3A; Johnson et al., 2007). Although iodoform evoked a small amount of uptake in a similar posterior region in mice, it also stimulated additional, more rostral glomeruli in mice (Fig. 3A, arrowheads).
Long-chain aliphatic odorants
In rats, alkanes and straight-chained aliphatic odorants possessing a variety of functional groups and hydrocarbon chains between 9 and 15 carbons stimulate glomeruli in the ventral part of the bulb (Ho et al., 2006a). This is exemplified by rat responses to decanal (Fig. 2A), 1-decanol (Fig. 3B), pentadecane (Fig. 3B), and nonanal (Fig. 4B). In mice, these long aliphatic odorants also stimulated ventral glomeruli. For decanal (Fig. 2A) and nonanal (Fig. 4B), the locations of the responses were very similar in the two species, whereas for 1-decanol and pentadecane, the overlap in location was more approximate (Fig. 3B, open arrowheads). In mice, 1-decanol also clearly stimulated additional glomeruli that were not particularly ventral (Fig. 3B, solid arrows).
Fig. 4.
Anatomically standardized contour charts illustrate the responses of mice (top row) and rats (bottom row) to homologous series of (A) aliphatic acids and (B) aldehydes. Lines are superimposed on charts in A to show the gradual ventral progression of uptake with increasing carbon number in the dorsomedial acid-responsive domain in rats. In mice, centroids of uptake in this domain did not differ significantly with carbon number, the average response to caproic acid being somewhat more ventral than the almost completely overlapping responses to the three smaller acids. Activity in a more posterior domain (black outline) also shifted in an overlapping progression related to carbon number in rats, but changed in location erratically with carbon number in mice. The black outline in B shows the lateral domain that is principally activated by aldehydes in rats. Uptake in this region progressed rostrally and ventrally with increasing carbon number in rats. In mice, aldehydes activated glomeruli scattered more widely throughout the layer (red outline), and the activated area changed erratically with odorant carbon number. The scale and orientation are the same as for Figure 2. A dorsal-centered version of this figure is available online as Supplementary Figure 4.
1-Pentanol
In rats, the primary alcohol 1-pentanol activates the same domain that responded to phenols such as guaiacol (Fig. 3C), which resemble alcohols by possessing a hydroxyl group (Johnson and Leon, 2000a). 1-Pentanol also stimulated glomeruli in this region in mice (dotted outline in Fig. 3C). However, the glomeruli that showed the most uptake in response to 1-pentanol in mice were located in regions that were not activated in rats (Fig. 3C, arrows).
Ketones
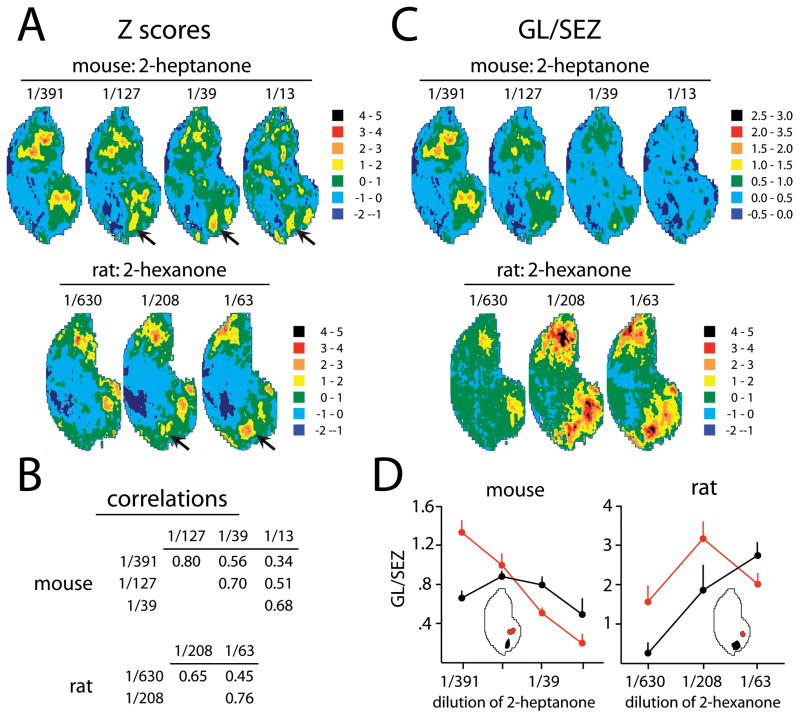
In rats, high concentrations of ketones stimulate a pair of dorsolateral and dorsomedial domains that overlap with those stimulated by aromatic odorants with oxygenic groups (Johnson and Leon, 2000a; Johnson et al., 2002; 2004; 2005a,b). Activity in these domains is exemplified in the response patterns to L-carvone (Fig. 3C, solid outlines) and 170 ppm 2-hexanone (Fig. 5A, arrows). In mice, L-carvone did not stimulate these dorsal domains (Fig. 3C). Nor did it stimulate the unpaired posterior ventral domain that responded to L-carvone but not D-carvone in rats (Linster et al., 2001) (Fig. 3C, white arrow). Instead, the mouse response to L-carvone involved glomeruli in a location not activated in rats (Fig. 3C, arrowheads). In contrast, the aliphatic ketone 2-heptanone did stimulate mouse glomeruli in the dorsomedial region activated by ketones in rats (Fig. 5A, arrows). The dorsolateral member of the paired response to high concentrations of ketones in rats is not as obvious in mice. It may be present more ventrally, as was observed for the aromatic odorants that activated the dorsomedial module.
Fig. 5.
A, C Anatomically standardized contour charts illustrate the responses of mice (top rows) and rats (bottom rows) to different concentrations of aliphatic ketones. Charts are shown using two different types of standardization. A: Contour charts of Z-score transformed matrices illustrate the relative pattern of 2DG uptake independently of the amount of uptake by expressing the number of standard deviations above or below the mean uptake measured throughout the glomerular layer. In both species, high concentrations of aliphatic ketones activated dorsal glomeruli (arrows) that were not activated at lower concentrations. B: Cell-by-cell Pearson correlations across the average matrices underlying the contour charts in A were used to assess the quantitative similarities between activity patterns evoked by the same odorant at different concentrations. The patterns in the two species were found to diverge to a similar extent with similar steps in odorant concentration. C: Contour charts showing the ratio of uptake in the glomerular layer (GL) to uptake in the subependymal zone (SEZ) illustrate the amount of uptake by glomeruli compared to a reference area thought to be unaffected by odorant exposure. In mice, increasing concentrations of 2-heptanone starting at a 1/391 dilution caused a decrease in the amount of 2DG taken up by glomeruli, while rats showed increased uptake followed by small decreases only in some regions. Orientation is the same as for Figure 2. D: Uptake calculated as in C was averaged across dorsomedial or midmedial modules in each animal (insets), and the mean values were expressed as a function of odorant dilution to illustrate further the different dependence of uptake on odorant concentration in rats and mice. Error bars denote the standard error of the mean across animals. A dorsal-centered version of the contour charts in this figure is available online as Supplementary Figure 5.
Representations of Homologous Series
Aliphatic acids
Aliphatic carboxylic acids evoked activity in two pairs of domains in the mouse bulb (Fig. 2A, Fig. 4A), and these domains were located very similarly to the two pairs of domains activated by the same acids in rats (Johnson et al., 1999; Johnson and Leon, 2000b). In rats, the average uptake in these domains shifts ventrally in a steady progression with increasing carbon number, which can be related to the molecular length of the hydrocarbon chain (Johnson et al., 1999; Johnson and Leon, 2000b). The shift is most obvious in the anterior, dorsomedial module (Fig. 4A). In mice, however, uptake in this module occurred in about the same location for acids of three, four, and five carbons, finally shifting abruptly ventral for the six-carbon acid. In contrast to the case for the rat responses (Johnson et al., 1999), there was no significant difference in the centroid of this response for odorants of different carbon number when analyzed using ANOVA.
Aliphatic aldehydes
In rats, increasing carbon number in other straight-chained aliphatic odorants (alkanes, aldehydes, primary and secondary alcohols, ethyl esters, acetates, and ketones) also has been shown to be associated with progressively more rostral and ventral activity in multiple domains related to the odorant functional group and hydrocarbon chain (Johnson et al., 2004; Ho et al., 2006a). This shift can be seen for the lateral response to aliphatic aldehydes between six and nine carbons in Figure 4B. In mice, this carbon number-related chemotopic progression was not observed for aliphatic aldehydes ranging from 6 to 9 carbons (Fig. 4B). Instead, there was little overlap in the activated regions for odorants differing by a single methylene group, despite the fact that the responses all occurred in an area similar in location to the chemotopically organized domain in rats. A similar observation applied to the posterior, lateral domain activated by carboxylic acids: overlapping responses in rats can be contrasted with the responses in mice, where we found abrupt changes in activated glomeruli whose locations were not systematically related to carbon number (Fig. 4A, solid outlines).
Concentration-Dependent Activity Patterns
In rats, some odorant molecules that humans perceive as having distinct odors at different concentrations also show changes in their evoked activity patterns with concentration (Johnson and Leon, 2000a). An example is 2-hexanone, which activates primarily ventral glomeruli at lower concentrations, but which activates primarily dorsal glomeruli at higher concentrations (Fig. 5A), a phenomenon that also has been observed for the longer aliphatic ketone 2-octanone (Johnson et al., 2004). To test if this phenomenon also would be observed in mice, we tested various concentrations of the aliphatic ketone 2-heptanone. Indeed, higher concentrations of 2-heptanone activated dorsal mouse glomeruli while a lower concentration activated only ventral glomeruli (Fig. 5A). The amount of change in pattern across comparable changes in concentration was similar for rats and mice as judged from cell-by-cell Pearson correlation analysis on the average z-score matrices (Fig. 5B).
Activity in Figure 5A is shown in units of z scores, which relate the 2DG uptake at each location in the glomerular layer to the average uptake across the entire layer. When activity is expressed instead as a ratio of glomerular uptake to uptake in the subependymal zone to give a measure that corrects for variations in the volume of 2DG injected but allows one to judge better the absolute amounts of activity, overall uptake in rats was seen to increase when the concentration of 2-hexanone was increased from 17 ppm to 50 ppm (Fig. 5C,D). A further increase in 2-hexanone concentration from 50 ppm to 170 ppm caused a slight further increase in uptake by dorsal glomeruli, but uptake by ventral glomeruli decreased with this concentration step, a change that might have been related to alterations in the active sniffing of the odorant by rats (Johnson and Leon, 2000a). In contrast, increased concentrations of 2-heptanone were associated with a steady decrease in 2-DG uptake in mice when uptake was expressed as the glomerular layer/subependymal zone ratio (Fig. 5C,D). In an early experiment in which mice were exposed to a 1/8 dilution of ethyl acetate odorant, we failed to detect evoked 2DG uptake (not shown), which contrasts with the robust activation that we observed at a five-fold lower concentration of ethyl acetate (Fig. 2B). Thus, the phenomenon of lower 2DG uptake at higher odorant concentrations is probably not specific for 2-heptanone, but may generalize to other odorants.
Reliability of Activity Patterns Across Individuals
In rats, spatial patterns of 2DG uptake evoked by odorants are very similar from animal to animal, so that even small differences in odorant chemical structure cause reliable changes in activity that are statistically significant when analyzed across different individuals. To determine if the spatial patterns of 2DG uptake in mice also are consistent across individual subjects and different across different odorants, we analyzed activity patterns generated in small studies involving four odorant conditions each. The data matrix representing an uptake pattern from an individual animal was compared separately to each other data matrix from the other animals in the same study. The pairwise comparisons involved Pearson correlation analysis where individual data points from corresponding positions in the two matrices were the X–Y pairs. The correlations matrix resulting from comparisons of all individual animals in the study then served as input for a principal components analysis that expressed the similarities and differences in the overall activity patterns in terms of a small number of extracted factors.
Results from three of the principal components analyses are shown in Figure 6, involving studies comparing four aliphatic esters (Fig. 6A), four aliphatic carboxylic acids (Fig. 6B), and four concentrations of 2-heptanone (Fig. 6C). Separated clusters of points involving individual mice exposed to the same odorant condition are apparent in each of the 2-dimensional plots. The loadings on the two factors that are plotted in each panel of Figure 6 were found to be significantly different with respect to type of odorant exposure when compared by ANOVA (Table 2). Thus, activity patterns are indeed consistent across different mice exposed to the same odorant, but significantly different across distinct odorants and different concentrations of 2-heptanone.
Fig. 6.
Scatter plots show results of principal components analyses on overall activity patterns evoked in mice in each of three studies involving (A) aliphatic esters, (B) aliphatic acids, and (C) different concentrations of 2-heptanone. The first two factors showing statistically significant differences among odorants in ANOVA (Table 2) are plotted in each panel. Individual points represent individual animals. Within each panel, different symbols represent different odorant exposure conditions. Clustered points involving the same odorant condition indicate the reliability of evoked patterns across different animals.
Table 2.
Significant differences among odorant exposure conditions judged by factor
| Study | Factor | % variance | ANOVA
|
||
|---|---|---|---|---|---|
| F | df | p | |||
| Esters (Fig. 5A) | 1 | 50.2 | 15.8 | 3, 11 | 0.0003 |
| 2 | 8.8 | 64.7 | 3, 11 | < 0.0001 | |
| 3 | 6.2 | 7.0 | 3, 11 | 0.0067 | |
| Acids (Fig. 5B) | 1 | 60.5 | 2.0 | 3, 11 | 0.1736 |
| 2 | 6.5 | 6.6 | 3, 11 | 0.0080 | |
| 3 | 5.2 | 4.1 | 3, 11 | 0.0357 | |
| 2-Heptanone concentration (Fig. 5C) | 1 | 48.1 | 63.1 | 3, 13 | < 0.0001 |
| 2 | 11.5 | 140.8 | 3, 13 | < 0.0001 | |
| 3 | 5.3 | 0.1 | 3, 13 | 0.9387 | |
loadings following principal components analysis.
Quantitative Relationships Among Overall Activity Patterns
Global chemotopy
In rats, odorants that are more similar in chemistry (e.g., by sharing functional groups and/or hydrocarbon structural features) evoke more similar overall activity patterns as judged by correlations analysis of matrices of 2DG uptake across the entire glomerular layer (Johnson et al., 2002; 2004; 2005a). We have referred to this quantitative relationship between stimulus and response as “global chemotopy.” To determine whether mice also display global chemotopy, we correlated patterns evoked by each odorant in mice with patterns evoked by each other odorant. For comparison, we also correlated the corresponding rat patterns with one another. Finally, we correlated each mouse pattern with each rat pattern.
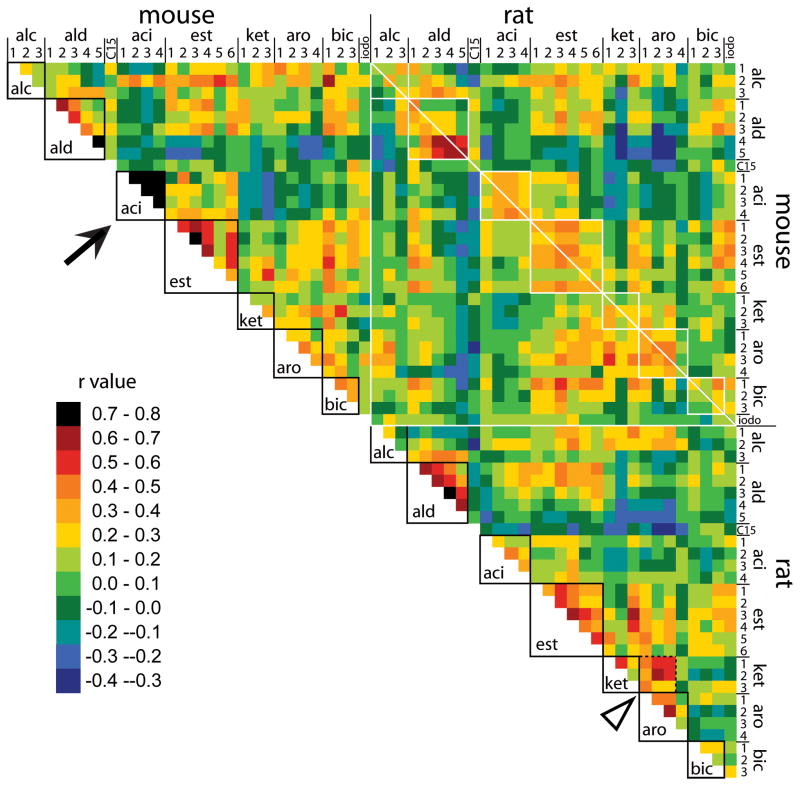
A correlations patchwork plot including all of these results is shown in Figure 7, where warmer colors are used to indicate higher positive correlations and where cooler colors indicate low positive correlations as well as negative correlations. A key for odorant identification can be found in Table 3. For each species, odorants have been ordered in the correlations patchwork plot so that chemicals sharing functional groups or hydrocarbon structures that were identified as features recognized by the rat olfactory system (Johnson and Leon, 2007) are located near one another. Within a set of odorants sharing such a feature, the chemicals are sorted further according to secondary characteristics such as carbon number that were found also to be characterized by global chemotopy in prior studies on rats. Therefore, pairs of odorants that are the most similar chemically tend to be located along the diagonal. In mice, as in rats, higher correlations are clustered on or near this diagonal, which is evidence of global chemotopy in both species (Fig. 7).
Fig. 7.
Color-coded patchwork plot showing the correlations matrix resulting from comparisons of activity patterns evoked in mice and rats exposed to the same set of odorants. Each correlation coefficient is rendered as a color according to the legend, higher coefficients being signified by warmer colors and lower or negative coefficients by cooler colors. Comparisons involving odorants sharing recognized molecular features such as functional groups or hydrocarbon structures that were previously identified in rats are enclosed in solid outlines. The white diagonal indicates comparisons of rats and mice exposed to the same odorants. The black arrow indicates the greater similarity between patterns evoked by aliphatic acids of different carbon number in mice than in rats. The open arrowhead and dotted outline indicate the higher correlations between ketones and aromatic odorants in rats than in mice.
Table 3.
Correlations between mouse and rat activity patterns.
| Class | Key | Odorant | Correlation vs rat (%ile) | Most similar rat pattern
|
|
|---|---|---|---|---|---|
| Odorant | r | ||||
| Alcohol | alc 1 | 2-Propanol | 34 | Heptane | 0.57 |
| Alcohol | alc 2 | 1-Pentanol | 70 | Nerolidol | 0.52 |
| Alcohol | alc 3 | 1-Decanol | 91 | Nonane | 0.40 |
| Aldehyde | ald 1 | Hexanal | 69 | 2,3-Dimethylhexane | 0.40 |
| Aldehyde | ald 2 | Heptanal | 83 | Heptane | 0.52 |
| Aldehyde | ald 3 | Octanal | 71 | Heptane | 0.57 |
| Aldehyde | ald 4 | Nonanal | 100 | Nonanal | 0.69 |
| Aldehyde | ald 5 | Decanal | 100 | Decanal | 0.70 |
| Alkane | C15 | Pentadecane | 93 | Tetradecane | 0.24 |
| Acid | aci 1 | Propionic acid | 91 | Valeric acid | 0.47 |
| Acid | aci 2 | Butyric acid | 99 | Valeric acid | 0.54 |
| Acid | aci 3 | Valeric acid #1 | 100 | Valeric acid | 0.52 |
| Acid | aci 3 | Valeric acid #2 | 100 | Valeric acid | 0.43 |
| Acid | aci 4 | Caproic acid | 93 | Valeric acid | 0.54 |
| Ester | est 1 | Ethyl acetate | 96 | Ethyl propionate | 0.56 |
| Ester | est 2 | Ethyl propionate | 100 | Ethyl propionate | 0.46 |
| Ester | est 3 | Propyl acetate | 97 | Nerolidol | 0.56 |
| Ester | est 4 | Methyl valerate | 79 | Nerolidol | 0.57 |
| Ester | est 5 | Isoamyl acetate | 94 | Isoamyl tiglate | 0.30 |
| Ester | est 6 | Isoamyl propionate | 86 | Nerolidol | 0.51 |
| Ketone | ket 1 | 2-Heptanone, 13 ppm | 78 | Heptane | 0.70 |
| Ketone | ket 1 | 2-Heptanone, 40 ppm | 89 | Trans-4-octene | 0.64 |
| Ketone | ket 1 | 2-Heptanone, 130 ppm | 80 | 2-Octyne | 0.58 |
| Ketone | ket 1 | 2-Heptanone, 400 ppm | 91 | 2-Octyne | 0.33 |
| Ketone | ket 2 | L-Carvone | 72 | 3-Hexanone | 0.41 |
| Ketone | ket 3 | Acetone | 67 | Trans-4-octene | 0.44 |
| Aromatic | aro 1 | Ethylbenzene | 96 | Cyclooctene | 0.48 |
| Aromatic | aro 2 | Methyl benzoate | 99 | 2-Hexanone | 0.60 |
| Aromatic | aro 3 | 2,5-Dimethylpyrazine | 91 | 2-Butanol | 0.60 |
| Aromatic | aro 4 | Guaiacol | 63 | Creosol | 0.44 |
| Bicyclic | bic 1 | β-Pinene | 80 | 2,3-Dimethylhexane | 0.69 |
| Bicyclic | bic 2 | L-Bornyl acetate | 46 | α-Phellandrene | 0.46 |
| Bicyclic | bic 3 | Santalol | 96 | 2,3-Butanedione | 0.39 |
| Halide | iodo | Iodoform | 83 | Pentanal | 0.27 |
Sets of correlations that involve odorant pairs sharing identified molecular features related to functional groups or hydrocarbon structures are indicated by square outlines in Figure 7. Figure 8 shows separate histograms of correlations between odorants sharing these features (same feature) or not (different features) in both mice (Fig. 8A) and rats (Fig. 8B). In both species, correlations between odorants possessing the same feature tend to be higher than correlations between odorants possessing different features, which also can be seen as a positive displacement of the curve in cumulative plots for same-feature odorant pairs compared to different-feature odorant pairs (insets in Fig. 8A and 8B). This finding constitutes further evidence for global chemotopy in both species.
Fig. 8.
Histograms showing distributions of correlation coefficients obtained for subsets of activity patterns. Insets show cumulative distributions of the same data. A: Comparisons of mouse patterns involving the same or different molecular features show that odorants with the same features tend to have more similar activity patterns. B: Comparisons of rat patterns yield correlation coefficients that are distributed similarly to the mouse data in A. C: Comparisons of patterns evoked in rats and mice by the same odorant yield higher correlation coefficients than do comparisons of patterns involving different odorants. The arrow shows the average correlation coefficient obtained for comparisons of patterns evoked in rats by the same odorants, but at different times in different studies (n = 65 comparisons).
Despite the presence of global chemotopy in both rats and mice, there were differences in terms of which odorants were best correlated in the two species. For example, correlation coefficients for pairs of aliphatic acid odorants of different carbon number were much higher in mice (solid arrow, Fig. 7) than in rats. Also, high correlations between ketones and aromatic odorants that have been observed for rats (open arrowhead and dotted outline, Fig. 7) were not apparent in mice.
Quantitative similarities between rats and mice
Correlations analysis showed that there was a strong quantitative similarity between the patterns evoked by the same odorants in mice and rats. The white diagonal line across the mouse versus rat portion of the patchwork plot in Figure 7 (upper right) indicates correlation coefficients relating the patterns evoked by the same odorants in the two species. These values are higher than most values that were obtained for comparisons of different odorants, as shown in the histograms of Figure 8C. Half of the comparisons involving different odorants yielded lower correlation coefficients that were less than the lowest correlation coefficient obtained for any pair of patterns involving the same odorant in the two species (Fig. 8C, cumulative plot in inset).
To simplify the information represented by the 900 correlation coefficients shown in Figure 7 and to understand simultaneously the relationships in odorant-evoked activity patterns both between and within the two rodent species, we performed a principal components analysis using the entire correlation matrix as input data. Figure 9 illustrates the loadings on the first four factors that were extracted by the analysis (factor 1 accounted for 18.7% of the variance; factor 2, 10.7%; factor 3, 9.0%; factor 4, 6.7%). Patterns evoked by odorants of the same chemical class clustered together in this analysis, independent of the species (Fig. 9, open symbols = mice, solid symbols = rats).
Fig. 9.
Scatter plots show results of principal components analyses involving the correlations matrix in Figure 7. The plots display the relative similarities and differences among overall activity patterns in only a few dimensions that simplify the multidimensional relationships existing in the original data. Each symbol represents a single combination of odorant and species. Data from mice are denoted by open symbols, while data from rats are indicated by closed symbols. Colors of symbols indicate the molecular features possessed by each odorant, as labeled in text of the same color. Individual odorants are identified by a combination of color and number (see key in Table 3). Both individual odorants and odorants sharing molecular features tend to cluster together in these plots independently of species, which indicates that there is a similar underlying chemotopic organization in the neural representation of odorants in the olfactory bulb in the two species. Some of these clusters are outlined in the colors that designate the molecular features.
Factor 1 separated the esters from most other odorants (orange outline and symbols), while factor 2 further separated most aldehydes (green), acids (red), and aromatic odorants (light blue) from each other. Factor 3 further segregated the acids from most other odorants, while factor 4 created a separate cluster of bicyclic odorants (black outline and symbols). The fact that patterns from mice and rats were similarly sorted by this unbiased, unsupervised analysis suggests not only that there is an underlying chemotopic organization in the two species, but also that this organization is very similar in the two species.
To further compare activity patterns in mice with those in rats, we investigated correlations between patterns in mice with our greater database of rat patterns. We have mapped responses to 365 different odorants in rats and have obtained 536 average patterns reflecting repetitions and different concentrations of these odorants. Of these, 323 of the odorants were judged to result in activity patterns that could be distinguished from those in blank odorant exposures (450 different patterns). We rank-ordered the correlations between each of the mouse patterns and each of the rat patterns.
For four of the odorants tested in mice, the best match to the mouse pattern was a rat pattern evoked by the same odorant (nonanal, decanal, valeric acid, and ethyl propionate). We expressed the best correlation with the corresponding rat pattern as a percentile score relative to all of the non-blank rat patterns (Table 3). In this table, if the most highly correlated rat pattern were evoked by the same odorant, the percentile score would be 100. In most cases, a rat pattern evoked by the same odorant was among the better matches to each mouse pattern. The mean percentile score was 85, with a standard deviation of 16 and a range of 34–100. The poorest match involved the odorant 2-propanol.
In a number of cases, the rat pattern providing the best match to the mouse pattern was evoked by an odorant that was chemically similar to the odorant used with the mice (Table 3). Mouse patterns evoked by pentadecane, butyric acid, caproic acid, and ethyl acetate were best correlated with rat patterns evoked by odorants with the same functional group, but differing by a single methylene group in their hydrocarbon chain. Mouse patterns evoked by heptanal and the lowest concentration of 2-heptanone best matched rat patterns evoked by heptane, which has the same hydrocarbon chain but which lacks a functional group. Finally, the mouse pattern evoked by guaiacol was best correlated with the rat pattern evoked by creosol. These phenolic odorants differ only in the presence of a methyl substituent on the benzene ring of creosol that is not present for guaiacol.
The chemotopic relationships revealed through these rank-order considerations were sometimes distinct from impressions about the absolute value of the correlation coefficient. For example, although the rat pattern for tetradecane was the pattern most highly correlated with the mouse pentadecane pattern, the correlation coefficient was only 0.24. Also, the top-ranked correlation between the mouse guaiacol pattern and the rat creosol pattern existed in spite of the fact that 37% of all rat patterns were better correlated with the mouse guaiacol pattern than was the rat pattern evoked by guaiacol itself. The difference seems to be that in rats, creosol, but not guaiacol, stimulated posterior glomeruli that overlap with those stimulated by guaiacol in mice (Fig. 2D).
Therefore, the quantitative analyses involving correlations of entire activity patterns revealed a remarkable similarity in chemotopic odorant representations in the rat and mouse olfactory bulbs. On the surface, this result seems to contradict the qualitative impression that we gleaned from the locations of peak activity in charts of 2DG uptake (Figs. 2–5), where it seemed that there were as many differences as there were similarities for the two species. It appears that overlaps and differences in the lower-activity parts of the patterns (blues and greens in the charts of Figures 2–5) may dominate the correlation analysis due to the greater area represented by lower uptake for most patterns.
DISCUSSION
Peak Activity and Overall Response Patterns
Although individual odorants evoked patterns of 2DG uptake that were well matched in most details in rats and mice, there were even more examples of partial overlaps or even entire mismatches in the locations of peak activity. Clearly, there would be some danger in uncritically using knowledge of activity patterns in rats to guide research in mice. However, the high-uptake foci did tend to be located in the same general areas in the two species, producing an extensive overlap in the larger, low-uptake portions of the patterns. In combination with partial overlaps in high-uptake foci evoked by many odorants, these similarities resulted in a “neural representation space” that was very similarly organized in rats and mice.
Given the established relationship between the neural space and perceptual space in rats (Linster et al., 2001; 2002; Cleland et al., 2002; Ho et al., 2006a,b; Youngentob et al., 2006), we would predict that mice and rats also might perceive similar relationships among odorants. Such an overall similarity in perceptual space across chemical classes would extend the commonalities of perception that have been revealed within series of related odorants in species such as honeybees, monkeys, and humans (Laska and Teubner, 1998; Laska et al., 1999).
Chemotopic Organization
As in rats, chemically related odorants activated overlapping high-uptake foci in mice, and quantitative analysis of overall activity patterns also showed that similar odorant chemicals evoked similar overall activity patterns. The spatial clustering of glomeruli that receive projections from sensory neurons expressing related odorant receptors may represent a fundamental organizational principle in larger olfactory systems, perhaps because the spatial proximity ensures that lateral inhibitory interactions can sharpen responses to related odorant chemicals (Johnson and Leon, 2007). The exact locations of the glomerular clusters responding to a given molecular feature often differed between rats and mice, which may indicate that spatial relationships at distances beyond the reach of lateral inhibitory influences are not fundamental for odor information processing.
We consistently have detected systematic molecular length-dependent shifts in the locations of 2DG uptake within domains responding to aliphatic odorants in rats (Johnson et al., 1998; 1999; 2004; Johnson and Leon, 2000b; Ho et al., 2006a). However, we did not observe such progressions in mice for homologous series of straight-chained, saturated carboxylic acids from three to six carbons or aldehydes from six to nine carbons. Others, however, have reported length-related rostral and ventral shifts in immediate early gene (Zif268) expression evoked in mice by acids and alcohols of three, six, and nine carbons (Inaki et al., 2002). Our set of acids did not extend to nine carbons, so it remains possible that the abrupt difference that we noticed in the position of the anterior, dorsomedial response between five and six carbons would continue to progress if we had included longer acids. If so, our results for this domain would be consistent with those of Inaki and coworkers (2002). However, with incremental increases in carbon number, posterior responses to acids and all responses to aldehydes showed erratic shifts back and forth within the general response domains, a phenomenon we never observed in rats. This observation suggests that smooth chemotopic progressions may not be fundamental aspects of odor information processing in all rodents. We previously hypothesized that the absence of chemotopic clustering of responses in honeybees (Sachse et al., 1999; Linster et al., 2005) might reflect the smaller size of the olfactory nervous system in that insect, where individual inhibitory interneurons span the entire antennal lobe and therefore can sharpen all responses without the need for nearest-neighbor arrangements of any kind (Johnson et al., 2007). A similar explanation may apply to the absence of length-related chemotopic progressions in mice. Smaller domains in the mouse bulb relative to the rat bulb may obviate the need for these detailed nearest-neighbor chemotopic relationships, although the mouse bulb may be large enough that chemotopic clusters remains useful for response sharpening.
Dorsal Responses
In rats, aromatic odorants and high concentrations of ketones evoke domains of peak 2DG uptake that include glomeruli located near the center of the dorsal surface of the olfactory bulb, which is easily accessible to optical imaging (Rubin and Katz, 1999). The medial partner to this response domain is located somewhat more ventrally and is not accessible from the top of the bulb. In mice, the medial domain is located in the same place as in rats, but the lateral partner appears to be displaced laterally and ventrally relative to the rat response, which raises the interesting possibility that there are unique dorsal glomeruli in mice that have displaced the glomeruli corresponding to the rat response domain(s). Indeed, dorsal glomeruli in mice appear to have a special involvement in innate responses to a wide variety of aversive odorants (Kobayakawa et al., 2007). Unfortunately, the dorsal glomeruli were often lost during our sectioning of fresh-frozen mouse tissue, a problem that may be related to the tougher dura mater surrounding the bulbs of adult mice compared to the weanling rats that have been the subject of our previous studies. The missing tissue in numerous animals resulted in noisy, statistically unreliable data in the dorsal regions of our uptake maps (see Supplementary Figures 2–5). Others have optically imaged responses to a very wide variety of odorants from the dorsal surface of adult mouse bulbs (Belluscio and Katz, 2001; Fried et al., 2002; Spors and Grinvald, 2002; Wachowiak and Cohen, 2003; Bozza et al., 2004; Lin et al., 2006; Spors et al., 2006; Kobayakawa et al., 2007), but these studies have not represented the average or variance of the responses across multiple animals, have not provided any anatomical references that can be used to orient them relative to our maps, and have not expressed the magnitude of the responses relative to responses occurring elsewhere in the bulb. For all of these reasons, it is not possible at this time to compare our data to the optically imaged responses, a comparison that probably would best be accomplished by using the two activity mapping methods in the same animals.
Additional Comparisons of Activity Patterns Obtained Using Different Methods
Maps of glomerular responses to amyl acetate measured using functional magnetic resonance imaging have been averaged across multiple mice (Schafer et al., 2006), and these average patterns have a reasonable resemblance to our average maps of 2DG uptake evoked by the closely related odorant isoamyl acetate; both patterns primarily contain responses in the midmedial and midlateral bulbar aspects (Fig. 2B). Unlike foci of 2DG uptake, fMRI responses were found to be highly variable in location across different individual mice (Schafer et al., 2006), which makes previous reports of fMRI responses to other odorants (Xu et al., 2000; 2003; 2005; Kida et al., 2002) more difficult to compare to our own data. Although the variability of fMRI responses across animals was taken as evidence for a different use of space for odor perception in different animals (Schafer et al., 2006), the consistency in the foci of 2DG uptake suggests the possibility that fMRI signals may reflect either inter-individual variations in blood supply or a greater impact of centrifugal input on the fMRI signal.
The posterior 2DG uptake we observed in response to ethyl acetate in mice closely resembled the pattern we obtained for rats, but it differed from the ventral pattern reported by others for glomerular c-fos mRNA responses to ethyl acetate in mice (Salcedo et al., 2005). Although this discrepancy could be evidence of strain differences or changes in pattern with odorant concentration, it also is possible that additional processing might impact the entirely postsynaptic, somatic response involved in the c-fos responses compared with 2DG uptake, which may be dominated by presynaptic activity in both axon terminals and dendrites (Johnson and Leon, 2007). We found previously that Fos-like responses to peppermint extract were disproportionately higher in ventral glomeruli when compared to 2DG uptake (Johnson et al., 1995), and this may be true of other odorants as well. Again, definitive comparisons of these methods probably will require using two mapping methods in the same animals.
Effects of Odorant Concentration
Like rats, mice showed differences in bulbar activity pattern at different concentrations of an aliphatic ketone. The conservation of this phenomenon across species is consistent with the observation that humans perceive different odor qualities at different concentrations of aliphatic ketones (Arctander, 1994; Johnson and Leon, 2000a). Although the change in relative pattern was quantitatively similar for rats and mice for a similar change in concentration, actual amounts of 2DG uptake showed a different concentration dependence in the two species, with uptake in mice decreasing in all regions of the bulb with increasing odorant concentration, while uptake in rats increased with increasing concentration in some glomeruli while first increasing and then decreasing in other glomeruli. The significance of this difference between the species is not entirely clear, but it may indicate a greater sensitivity of mice to odorants, resulting in suppressed respiration of what may become a noxious stimulus at higher concentrations, similar to what has been observed for other odorants in rats (Alarie, 1966; Johnson and Leon, 2000a). The fact that a loss of uptake was also observed at a high concentration of ethyl acetate suggests that the phenomenon may occur for a number of odorants. It may be interesting to compare rats and mice further with respect to their odor detection thresholds, the dependence of their glomerular activity on odorant concentration, and their respiratory reactions to odorants at different concentrations.
Similarities and Differences in Activity Patterns Among and Within Species
The existence of both similarities and differences in activity patterns between rats and mice raises the question of how genetically similar animals can be and yet still show systematic differences in odorant-evoked activity patterns as well as how genetically different animals can be and yet still show similarities in activity patterns. Different strains of mice differing in their sensitivity to isovaleric acid also differ significantly in the overall patterns of 2DG uptake evoked by that odorant (Sicard et al., 1989). Individual humans also can display specific anosmia or hyposmia for certain odorants, which is determined at least in part genetically, perhaps due to changes in particular odorant receptor genes (Whissell-Buechy and Amoore, 1973; Wysocki and Beauchamp, 1984). Changes in receptor expression are likely to impact activity patterns, each gene contributing to a very small degree, so that the impact on the overall pattern may be only a matter of the sensitivity of detection.
On the other hand, there is evidence that certain similarities between rats and mice extend to even more unrelated species. For example, activation of 2DG uptake in the posterior part of the olfactory bulb by acetoacetate has been observed for both guinea pigs (Astic and Saucier, 1983) and rats (Jourdan et al., 1980; Astic and Cattarelli, 1982; Johnson et al., 2007), raising the possibility of conservation throughout the rodent order. Also, responses to aliphatic acids recorded electrophysiologically from the rabbit olfactory bulb (Mori et al., 1992) are in a dorsomedial location similar to where one can detect responses to these same odorants in rats and mice, suggesting conservation throughout the superorder to which rodents and lagomorphs belong. A broader phylogenetic survey of response patterns to a systematic set of odorants would be required to determine if the similarity in response patterns to acids is either more broadly shared (e.g., because of basic anatomical constraints such as zonal relationships to odorant receptor classes) or more narrowly confined to fewer species due to a selection of these responses in a common ancestor.
Conclusions
Chemotopic clustering of glomerular responses was found in both rats and mice, although mice did not show clear nearest-neighbor progressions within these molecular feature-related response domains. Exact locations of primary response domains differed in rats and mice, and yet the overall relationships in response patterns were largely the same in the two species. Thus, the overall spatial organization of odorant chemical information may be fundamental for odor information processing, while details concerning the locations of primary responses to odorants may be unique to a given species.
Supplementary Material
Acknowledgments
Supported by United States Public Health Service Grants DC03545, DC006391, and DC006516.
We thank Dr. Cynthia Woo for a critical reading of the manuscript.
LITERATURE CITED
- Alarie Y. Irritating properties of airborne materials to the upper respiratory tract. Arch Environ Health. 1966;13:433–449. doi: 10.1080/00039896.1966.10664593. [DOI] [PubMed] [Google Scholar]
- Arctander S. Perfume and Flavor Chemicals (Aroma Chemicals) Carol Stream, IL: Allured Publishing Company; 1994. [Google Scholar]
- Astic L, Cattarelli M. Metabolic mapping of functional activity in the rat olfactory system after a bilateral transection of the lateral olfactory tract. Brain Res. 1982;245:17–25. doi: 10.1016/0006-8993(82)90335-3. [DOI] [PubMed] [Google Scholar]
- Astic L, Saucier D. Ontogenesis of the functional activity of guinea-pig olfactory bulb: autoradiographic study with the 2-deoxyglucose method. Brain Res. 1983;312:257–263. doi: 10.1016/0165-3806(83)90142-6. [DOI] [PubMed] [Google Scholar]
- Belluscio L, Katz LC. Symmetry, stereotypy, and topography of odorant representations in mouse olfactory bulbs. J Neurosci. 2001;21:2113–2122. doi: 10.1523/JNEUROSCI.21-06-02113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42:9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- Buck LB. Information coding in the vertebrate olfactory system. Annu Rev Neurosci. 1996;19:517–44. doi: 10.1146/annurev.ne.19.030196.002505. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116:222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Farahbod H, Johnson BA, Minami SS, Leon M. Chemotopic representations of aromatic odorants in the rat olfactory bulb. J Comp Neurol. 2006;496:350–366. doi: 10.1002/cne.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried HU, Fuss SH, Korsching SI. Selective imaging of presynaptic activity in the mouse olfactory bulb shows concentration and structure dependence of odor responses in identified glomeruli. Proc Natl Acad Sci U S A. 2002;99:3222–3227. doi: 10.1073/pnas.052658399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie KM, Anderson AJ, Leon M, Gall C. Odor-induced increases in c-fos mRNA expression reveal an anatomical “unit” for odor processing in olfactory bulb. Proc Natl Acad Sci U S A. 1993;90:3329–3333. doi: 10.1073/pnas.90.8.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SL, Johnson BA, Leon M. Long hydrocarbon chains serve as unique molecular features recognized by ventral glomeruli of the rat olfactory bulb. J Comp Neurol. 2006a;498:16–30. doi: 10.1002/cne.20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SL, Johnson BA, Chen AL, Leon M. Differential responses to branched and unsaturated aliphatic hydrocarbons in the rat olfactory system. J Comp Neurol. 2006b;499:519–532. doi: 10.1002/cne.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaki K, Takahashi YK, Nagayama S, Mori K. Molecular-feature domains with posterodorsal-anteroventral polarity in the symmetrical sensory maps of the mouse olfactory bulb: mapping of odourant-induced Zif268 expression. Eur J Neurosci. 2002;15:1563–1574. doi: 10.1046/j.1460-9568.2002.01991.x. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Modular glomerular representations of odorants in the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 2000a;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Odorant molecular length: One aspect of the olfactory code. J Comp Neurol. 2000b;426:330–338. doi: 10.1002/1096-9861(20001016)426:2<330::aid-cne12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Chemotopic odorant coding in a mammalian olfactory system. J Comp Neurol. 2007;503:1–34. doi: 10.1002/cne.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Duong H, Nguyen V, Leon M. A learned odor evokes an enhanced Fos-like glomerular response in the olfactory bulb of young rats. Brain Res. 1995;699:192–200. doi: 10.1016/0006-8993(95)00896-x. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Leon M. Spatial coding of odorant features in the glomerular layer of the rat olfactory bulb. J Comp Neurol. 1998;393:457–471. doi: 10.1002/(sici)1096-9861(19980420)393:4<457::aid-cne5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Hingco EE, Pham KL, Leon M. Multidimensional chemotopic responses to n-aliphatic acid odorants in the rat olfactory bulb. J Comp Neurol. 1999;409:529–548. [PubMed] [Google Scholar]
- Johnson BA, Ho SL, Xu Z, Yihan JS, Yip S, Hingco EE, Leon M. Functional mapping of the rat olfactory bulb using diverse odorants reveals modular responses to functional groups and hydrocarbon structural features. J Comp Neurol. 2002;449:180–194. doi: 10.1002/cne.10284. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Xu Z, Saber S, Leon M. Local and global chemotopic organization: general features of the glomerular representations of aliphatic odorants differing in carbon number. J Comp Neurol. 2004;480:234–249. doi: 10.1002/cne.20335. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Saber S, Leon M. Effects of functional group position on spatial representations of aliphatic odorants in the rat olfactory bulb. J Comp Neurol. 2005a;483:192–204. doi: 10.1002/cne.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Leon M. Interactions between odorant functional group and hydrocarbon structure influence activity in glomerular response modules in the rat olfactory bulb. J Comp Neurol. 2005b;483:205–216. doi: 10.1002/cne.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Xu Z, Kwok J, Pancoast P, Ong J, Leon M. Differential specificity in the glomerular response profiles for alicyclic, bicyclic and heterocyclic odorants. J Comp Neurol. 2006;499:1–16. doi: 10.1002/cne.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Arguello S, Leon M. Odorants with multiple oxygen-containing functional groups and other odorants with high water solubility preferentially activate posterior olfactory bulb glomeruli. J Comp Neurol. 2007;502:468–482. doi: 10.1002/cne.21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan F, Duveau A, Astic L, Holley A. Spatial distribution of [14C]2-deoxyglucose uptake in the olfactory bulbs of rats stimulated with two different odours. Brain Res. 1980;188:139–154. doi: 10.1016/0006-8993(80)90563-6. [DOI] [PubMed] [Google Scholar]
- Kida I, Xu F, Shulman RG, Hyder F. Mapping at glomerular resolution: fMRI of rat olfactory bulb. Magn Reson Med. 2002;48:570–576. doi: 10.1002/mrm.10248. [DOI] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, Mori K, Sakano H. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- Laska M, Teubner P. Odor structure-activity relationships of carboxylic acids correspond between squirrel monkeys and humans. Am J Physiol. 1998;274:R1639– R 1645. doi: 10.1152/ajpregu.1998.274.6.R1639. [DOI] [PubMed] [Google Scholar]
- Laska M, Galizia CG, Giurfa M, Menzel R. Olfactory discrimination ability and odor structure-activity relationships in honeybees. Chem Senses. 1999;24:429–438. doi: 10.1093/chemse/24.4.429. [DOI] [PubMed] [Google Scholar]
- Leon M, Johnson BA. Olfactory coding in the mammalian olfactory bulb. Brain Res Rev. 2003;42:23–32. doi: 10.1016/s0165-0173(03)00142-5. [DOI] [PubMed] [Google Scholar]
- Lin DY, Shea SD, Katz LC. Representation of natural stimuli in the rodent main olfactory bulb. Neuron. 2006;50:937–949. doi: 10.1016/j.neuron.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Morse A, Yue E, Xu Z, Hingco EE, Choi Y, Choi M, Messiha A, Leon M. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neurosci. 2001;21:9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Morse A, Yue E, Leon M. Spontaneous versus reinforced olfactory discriminations. J Neurosci. 2002;22:6842–6845. doi: 10.1523/JNEUROSCI.22-16-06842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Sachse S, Galizia CG. Computational modeling suggests that response properties rather than spatial position determine connectivity between olfactory glomeruli. J Neurophysiol. 2005;93:3410–3417. doi: 10.1152/jn.01285.2004. [DOI] [PubMed] [Google Scholar]
- Meister M, Bonhoeffer T. Tuning and topography in an odor map on the rat olfactory bulb. J Neurosci. 2001;21:1351–1360. doi: 10.1523/JNEUROSCI.21-04-01351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Mataga N, Imamura K. Differential specificities of single mitral cells in rabbit olfactory bulb for a homologous series of fatty acid odor molecules. J Neurophysiol. 1992;67:786–789. doi: 10.1152/jn.1992.67.3.786. [DOI] [PubMed] [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the Mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- Royet JP, Sicard G, Souchier C, Jourdan F. Specificity of spatial patterns of glomerular activation in the mouse olfactory bulb: computer-assisted image analysis of 2-deoxyglucose autoradiograms. Brain Res. 1987;417:1–11. doi: 10.1016/0006-8993(87)90173-9. [DOI] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Sachse S, Rappert A, Galizia CG. The spatial representation of chemical structures in the antennal lobe of honeybees: steps towards the olfactory code. Eur J Neurosci. 1999;11:3970–3982. doi: 10.1046/j.1460-9568.1999.00826.x. [DOI] [PubMed] [Google Scholar]
- Salcedo E, Zhang C, Kronberg E, Restrepo D. Analysis of training-induced changes in ethyl acetate odor maps using a new computational tool to map the glomerular layer of the olfactory bulb. Chem Senses. 2005;30:615–626. doi: 10.1093/chemse/bji055. [DOI] [PubMed] [Google Scholar]
- Schafer JR, Kida I, Xu F, Rothman DL, Hyder F. Reproducibility of odor maps by fMRI in rodents. Neuroimage. 2006;31:1238–1246. doi: 10.1016/j.neuroimage.2005.12.060. [DOI] [PubMed] [Google Scholar]
- Sicard G, Royet JP, Jourdan F. A comparative study of 2-deoxyglucose patterns of glomerular activation in the olfactory bulbs of C57 BL/6J and AKR/J mice. Brain Res. 1989;481:325–334. doi: 10.1016/0006-8993(89)90810-x. [DOI] [PubMed] [Google Scholar]
- Spors H, Grinvald A. Spatio-temporal dynamics of odor representations in the mammalian olfactory bulb. Neuron. 2002;34:301–315. doi: 10.1016/s0896-6273(02)00644-x. [DOI] [PubMed] [Google Scholar]
- Spors H, Wachowiak M, Cohen LB, Friedrich RW. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J Neurosci. 2006;26:1247–1259. doi: 10.1523/JNEUROSCI.3100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Correspondence between odorant-evoked patterns of receptor neuron input and intrinsic optical signals in the mouse olfactory bulb. J Neurophysiol. 2003;89:1623–1639. doi: 10.1152/jn.00747.2002. [DOI] [PubMed] [Google Scholar]
- Whissell-Buechy D, Amoore JE. Odour-blindness to musk: simple recessive inheritance. Nature. 1973;242:271–273. doi: 10.1038/242271a0. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Beauchamp GK. Ability to smell androstenone is genetically determined. Proc Natl Acad Sci U S A. 1984;81:4899–4902. doi: 10.1073/pnas.81.15.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Kida I, Hyder F, Shulman RG. Assessment and discrimination of odor stimuli in rat olfactory bulb by dynamic functional MRI. Proc Natl Acad Sci U S A. 2000;97:10601–10606. doi: 10.1073/pnas.180321397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Liu N, Kida I, Rothman DL, Hyder F, Shepherd GM. Odor maps of aldehydes and esters revealed by functional MRI in the glomerular layer of the mouse olfactory bulb. Proc Natl Acad Sci U S A. 2003;100:11029–11034. doi: 10.1073/pnas.1832864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Schaefer M, Kida I, Schafer J, Liu N, Rothman DL, Hyder F, Restrepo D, Shepherd GM. Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J Comp Neurol. 2005;489:491–500. doi: 10.1002/cne.20652. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Margolis FL, Youngentob LM. OMP gene deletion results in an alteration in odorant quality perception. Behav Neurosci. 2001;115:626–631. doi: 10.1037//0735-7044.115.3.626. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF, Margolis FL. OMP gene deletion results in an alteration in odorant-induced mucosal activity patterns. J Neurophysiol. 2003;90:3864–3873. doi: 10.1152/jn.00806.2002. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Pyrski MM, Margolis FL. Adenoviral vector-mediated rescue of the OMP-null behavioral phenotype: enhancement of odorant threshold sensitivity. Behav Neurosci. 2004;118:636–642. doi: 10.1037/0735-7044.118.3.636. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Johnson BA, Leon M, Sheehe PR, Kent PF. Predicting odorant quality perceptions from multidimensional scaling of olfactory bulb glomerular activity patterns. Behav Neurosci. 2006;120:1337–1345. doi: 10.1037/0735-7044.120.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.