Abstract
During the development of transformability (competence), Bacillus subtilis synthesizes a set of proteins that mediate both the uptake of DNA at the cell poles and the recombination of this DNA with the resident chromosome. Most, if not all, of these Com proteins localize to the poles of the cell, where they associate with one another, and are then seen to delocalize as transformability declines. In this study, we use fluorescence microscopy to analyze the localization and delocalization processes. We show that localization most likely occurs by a diffusion-capture mechanism, not requiring metabolic energy, whereas delocalization is prevented in the presence of sodium azide. The kinetics of localization suggest that this process requires the synthesis of a critical protein or set of proteins, which are needed to anchor the Com protein complex to the poles. We further show that the protein kinase proteins McsA and McsB are needed for delocalization, as are ClpP and either of the AAA+ (ATPases associated with a variety of cellular activities) proteins ClpC or ClpE. Of these proteins, at least McsB, ClpC and ClpP localize to the cell poles of competent cells. Our evidence strongly suggests that delocalization depends on the degradation of the postulated anchor protein(s) by the McsA-McsB- (ClpC or ClpE)-ClpP protease in an ATP-dependent process that involves the auto-phosphorylation of McsB. The extent of cell-pole association at any given time reflects the relative rates of localization and delocalization. The kinetics of this dynamic process differs for individual Com proteins, with the DNA binding proteins SsbB and DprA exhibiting less net localization.
Keywords: competence, cell poles, McsB, transformation, protein degradation, Clp proteins
Introduction
Bacterial development is often accompanied by a programmed accumulation of proteins and protein complexes at the poles of asymmetric cells and by the delocalization of these proteins from the poles. These movements, sometimes together with selective protein degradation, have been studied notably in the developmental cycle of Caulobacter crescentus and during the formation of spores (Shapiro et al., 2002), but also during the development of competence in Bacillus subtilis (Hahn et al., 2005, Kidane & Graumann, 2005, Kramer et al., 2007, Tadesse & Graumann, 2007). In B. subtilis, genetic competence is marked by the synthesis of a number of cytosolic and membrane-localized proteins that assemble into a polar-localized molecular machine dedicated to the internalization, processing and chromosomal integration of transforming DNA (Hahn et al., 2005, Kramer et al., 2007, Kidane & Graumann, 2005). DNA uptake during transformation consequently takes place mostly at the poles of the rod-shaped bacilli. As the cells lose the capacity to take up DNA, the competence (Com) proteins leave the poles (Hahn et al., 2005). Remarkably, at least some of the Com proteins co-localize with one-another not only at the poles, but also in helical tracks that extend along the long axis of the cell. Many questions concerning the localization and delocalization of Com proteins remain. If the Com proteins move to the poles from their sites of synthesis, do they do so individually and then assemble to form the DNA uptake machines or do complexes of Com proteins move to the poles? Is pole-ward movement directed or driven by diffusion and capture? Are unknown proteins required for this localization? How is delocalization accomplished and does it require protein degradation? The present study addresses these questions. We report that complexes assemble away from the poles, and that these ensembles move, probably by diffusion, to take up residence at the cell poles. In contrast to this diffusive process, delocalization seems to require metabolic energy. We show that the McsA/McsB protein kinase (Kirstein et al., 2008, Kirstein et al., 2007, Kirstein et al., 2005) is required for delocalization, and that delocalization most likely depends on the phosphorylation of McsB. We propose that an unknown anchor protein is degraded by a proteolytic complex consisting of McsA/McsB and ClpP, in complex with either one of two AAA+ (ATPases associated with various cellular activities (Ogura & Wilkinson, 2001)) proteins ClpC or ClpE.
Results
Localization of ComGA
Competence genes are expressed in B. subtilis beginning at T0 (the time at which a culture departs from exponential growth). In most of the experiments reported here, we have used the protein encoded by comGA, a traffic ATPase, as representative of competence (Com) proteins. ComGA is required for transformation and also for the assembly of the competence pseudopilus, a structure formed outside the cell membrane (Chen et al., 2006). ComGA is peripherally associated with the inner face of the membrane (Chung et al., 1998) and accumulates at the cell poles, as the cells become maximally transformable (Hahn et al., 2005). To follow the polar localization of ComGA, time-lapse experiments were conducted in which cells expressing ComGA-GFP under competence control were incubated at 37 °C on an agarose pad containing conditioned competence medium (selected frames are displayed in Fig. 1 and a representative time-lapse movie of additional cells may be seen in Fig. S1). In this experiment, the cells were grown in batch culture until 30 minutes after T0 (T0.5) and then placed on the agarose pads. In the earliest images, the fluorescent signals were predominantly non-polar (in about 75% of the competent cells) and then progressively localized to the poles in more than 70% of these cells. Inspection of these and other images shows that ComGA-GFP appears first as diffuse fluorescence, or in punctate foci, and that this fluorescence moves to the poles. In some cells, depopulation of the non-polar regions is discernible. The distribution of non-polar fluorescence is often non-uniform and in some cells is suggestive of helices (Hahn et al., 2005). Based on the examination of 18 individual cells, the average time from T0.5 to the first detection of a polar focus ranged from 0-20 minutes (6.1±11.5).
Fig. 1.

Localization of ComGA-GFP. BD2899, expressing comGA-gfp, was grown in competence medium until 30 minutes after T0 and an aliquot was placed on an agarose pad containing competence medium. Fluorescence images were collected every 30 seconds. Selected fluorescence images are depicted, together with DIC images taken at the beginning and end of the experiment. The numbers in the frames refer to minutes. The single competent cell in the field is marked with an arrow. For a similar time-lapse experiment in its entirety, see Fig. S1.
Interpretation of these images is complicated by several factors. First, continuing synthesis of ComGA-GFP occurs during this experiment, as determined by Western blotting on batch cultures (not shown). Second, for fluorescence to appear, maturation of the GFP domain must take place following synthesis, requiring several minutes. Third, it is possible that some turnover of ComGA-GFP may be occurring, although Western blotting after the addition of a protein synthesis inhibitor suggests that this protein is stable (see below). To determine whether the progressive accumulation of ComGA-GFP fluorescence at the poles shown in Figs. 1 and S1 represents movement of protein, rather than its synthesis at the poles, we used puromycin (Pm) to inhibit protein synthesis. Images from a typical time-lapse series are shown in Fig. 2 and the complete movie is presented in Fig. S2. These experiments permitted several conclusions. First, previously synthesized ComGA-GFP can move to the poles. We often noted non-polar fluorescent foci that moved erratically and eventually reached a pole, at which point their movement decreased. In addition, the impression of movement along a helical path was stronger in these images than in the absence of Pm, perhaps due to the absence of new ComGA-GFP synthesis, which would “fill-in” regions vacated by molecules that had moved to the helix or to a pole. Finally, in the presence of Pm, about five-fold more time was needed for ComGA to stably occupy a pole than in its absence; 32.1±8.7 minutes, based on measurements made on 12 cells, compared to 6.1±11.5 minutes in the absence of Pm (P<0.0001). The lower relative standard deviation of this measurement compared to that in the absence of Pm, is probably due to the greater ease of determining the end-point (localization) than when continuing synthesis of ComGA-GFP fills in non-polar areas of the cells.
Fig. 2.

Localization of ComGA-GFP in the presence of Pm. BD2899, expressing comGA-gfp, was grown in competence medium until 30 minutes after T0. Pm was added to an aliquot, which was then placed on an agarose pad containing competence medium with Pm. Images were collected at 2 minute intervals. Selected fluorescence images are shown, together with DIC images taken at the end of the experiment. The numbers in the frames refer to minutes. The single competent cell in the field is marked with an arrow. For a similar time-lapse experiment in its entirety, see Fig. S2.
We suggest that a critical anchor protein must be made for localization to take place. When protein synthesis is inhibited early in competence development, as in the experiment involving the addition of Pm, the concentration of the anchor protein is low and localization is correspondingly slow. This low rate of localization would be expected for a diffusion-capture mechanism, in which the probability of capture is dependent on the concentration of polar binding sites or of an anchor protein that forms an interacting surface on the Com protein complex. In rare cells, observed only in the presence of Pm, we have detected non-polar foci that move erratically and never settle at the pole. Perhaps the concentration of the anchor protein in these cells is insufficient to permit polar capture of the foci. The erratic movement of the non-polar foci, as well as their appearance and disappearance, is suggestive of a diffusion-capture mechanism. This mechanism is also strongly supported by the azide resistance of localization (discussed below). The slower localization observed in the presence of Pm, suggests that the probability of capture depends on the concentration of the hypothetical anchor protein. The relatively high standard deviations measured for the time-to-capture are also consistent with a stochastic (diffusive) process.
Delocalization is not due to Com protein degradation and requires metabolic energy, while localization does not
After T2, polar Com protein foci disappear, with retention of GFP fluorescence elsewhere in the cell, often as non-polar foci (Hahn et al., 2005). Previous experiments did not distinguish between the departure of protein from the poles and the loss of polar localization due to preferential degradation of polar Com proteins. Although the total fluorescent signal did not seem to decrease during delocalization, it was possible that preferential degradation of polar Com protein was masked by the synthesis of new protein. To study the delocalization of old protein, Pm and rifampicin (Rm) were added at T1, when about half of the cells already exhibited polar localization. (We have shown that Pm acts almost immediately to inhibit protein synthesis (see Supporting material)). Fig. 3A shows that pre-existing ComGA-GFP protein does de-localize. In the absence of Pm+Rm, the percent localization increased slightly during the 5-10 minutes following the initial point at T1 and then remained essentially constant, as expected from the observation that net delocalization does not occur until after T2 (Hahn et al., 2005). During the first two minutes after Pm+Rm addition, a slight increase in polar localization occurred, followed by a decrease in the percent localized cells from 62 to 39% of the population of competence-expressing cells. The delocalized fluorescence usually appeared as non-polar foci and similar results were obtained with the helicase-like competence protein ComFA-GFP in the presence of Pm+Rm (not shown). To determine if the delocalization of ComGA requires energy, a similar experiment was carried out with the addition of sodium azide (40 mM) as well as Pm+Rm (Fig. 3A). In the presence of NaN3, not only did delocalization fail to occur, but the percent cells exhibiting localization increased. These results can be explained as follows. When no inhibitors are present, ComGA synthesis and localization as well as delocalization occur, and the percent cells with localized ComGA is a result of these competing processes. In Fig. 3A, during the 20 minutes following T1, no net delocalization takes place. When Pm+Rm blocks synthesis, a slight increase in ComGA localization at the poles takes place, reflecting movement of protein formed prior to addition of the inhibitor. As this pool of un-localized protein is exhausted, a net decrease in the percent cells with polar foci occurs due to de-localization, un-obscured by further synthesis. When NaN3 is present, delocalization is blocked and all of the ComGA can be captured at the poles, resulting in an increase in the percent of localized cells. Western blotting revealed that during the course of incubation with Pm, no detectable degradation of ComGA-GFP took place (Fig. 3B). We conclude from these experiments that delocalization of “old” ComGA occurs without degradation and that delocalization requires metabolic energy. This conclusion is subject to the caveat that azide is an inhibitor of SecA (Oliver et al., 1990). However, the energy requirement for delocalization is strongly supported by the involvement of ATP requiring proteins, described below. It is interesting that in the presence of NaN3 the percent of localized cells actually increased, consistent with the idea that localization does not require metabolic energy, proceeding instead by diffusion and capture.
Fig. 3.
Kinetics of ComGA-GFP localization and delocalization. (A) BD2899, expressing comGA-gfp, grown in competence medium, was taken at T1 and incubated further in the presence of Pm+Rm (open circles), Pm+Rm+NaN3 (open squares) and with no inhibitor added open (triangles). The same experiment was performed with the isogenic mcsB strain BD3594 and the results are displayed with the equivalent closed symbols. The percent of competence-expressing cells showing polar localization of fluorescence was determined by counting at least 100 competence-expressing cells per sample. (B) Western blot of samples taken at the indicated times following addition of Pm+Rm, using anti-GFP antiserum to visualize native ComGA.
McsA and B are required for delocalization
We noticed that a clpC knockout strain expressing ComGA-GFP failed to exhibit delocalization. Because the clpC deletion in this strain also affected the immediate upstream open reading frame (Pan et al., 2001), encoding the protein kinase McsB (Fig. 4A), we tested a non-polar mcsB mutant for delocalization and observed a striking phenotype (Fig. 4B). First, at T2 localization was more complete than in the wild-type strain, with nearly all the detectable fluorescence confined to one or two foci in each cell, almost invariably at a cell pole. In contrast, as noted previously, wild-type cells exhibiting localization also possessed visible fluorescence located away from the poles, either distributed diffusely or localized to one or more non-polar foci. Second, in the mcsB mutant, ComGA-GFP failed to delocalize after T2, as seen in the T6 images in Fig. 4B, and in fact localization appeared to increase. In four experiments, in each of which at least 100 competent cells were counted, the wild-type and mcsB strains exhibited an average of 78±18% and 98±1.4% of competent cells with clear polar localization at T2. At T5, the percent of localized cells had decreased to an average of 29±8.7% in the wild-type strain and was 96±2.3% in the mcsB background. CtsR is a repressor, acting on the promoter upstream from the operon containing mcsB (Fig. 4A) and in its absence we might expect that all four genes in the operon, including mcsB, would be over-expressed. In accordance with this, we noted that in a ctsR knockout, localization of ComGA-GFP is decreased; few polar foci are observed and the fluorescence is distributed throughout the cell, although often with a punctate appearance (Fig. 4B). This is not surprising, because an increase in the cellular concentration of McsB due to the absence of the repressor might reasonably be expected to confer the reverse of the mcsB knockout phenotype, whatever the mechanism involved. Fig. 3A includes a control experiment with the mcsB strain BD3594 (filled symbols) identical to that performed with the wild-type strain (open symbols). As expected, no delocalization was detected with this strain during the 20 minutes following T1, showing that the delocalization observed with the wild-type strain in the presence of Pm was not an artifact caused by the inhibitor or a process unrelated to the role of McsB.
Fig. 4.
Localization and delocalization phenotypes of mcsB knockout mutants. (A) Map of the ctsR-mcsA-mcsB-clpC operon with a diagram summarizing its regulation. CtsR represses the transcription of the operon and is itself downregulated by the combined action of McsA, McsB, ClpP and either ClpC or ClpE. CtsR also represses the transcription of clpE and clpP. (B) Fluorescence images were collected at T2 and at T6 during growth in competence medium, from isogenic wild-type (BD2899), mcsB (BD3594) and ctsR (BD3656) strains, expressing comGA-gfp. (C) Fluorescence images were collected at T2 from wild-type (BD4429) and mcsB (BD4430) strains expressing divIVA-yfp and also from wild-type (BD3528) and mcsB (BD4136) strains expressing gfp from the comGA promoter. (D) Fluorescence images were collected at T2 from isogenic strains expressing comGA-yfp from a Pspac promoter in mcsB (BD4172) and mcsB comK (BD4173) backgrounds. The strains were grown in competence medium in the presence of 1 mM IPTG to induce comGA-yfp expression.
The mcsB phenotype is competence-specific
Several constructs tested the specificity of the mcsB effect on Com protein delocalization. When GFP is expressed from the comG promoter, it exhibits a diffuse distribution of GFP throughout the cell, which is unaffected by inactivation of mcsB (Fig. 4C). Also, images were collected from the non-competence related DivIVA-YFP fusion in wild-type and mcsB backgrounds. Like ComGA, DivIVA exhibits polar localization (Edwards & Errington, 1997), but this appears unaffected by inactivation of mcsB (Fig. 4C). We also expressed comGA-gfp from the isopropyl-thio-ß-D-galactoside (IPTG)-inducible Pspac promoter in mcsB and mcsB comK backgrounds. In the latter strain, no other Com proteins are present, because the transcription factor ComK is missing. In both strains, ComGA-GFP is synthesized in all the cells. In the Pspac-comGA-gfp strain the mcsB phenotype is evident in the cells expressing competence, but not in the mcsB comK strain, in which ComGA does not localize at all (Fig. 4D). This competence specificity supports the conclusion that McsB plays an important role in the delocalization of Com proteins.
Inactivation of mcsB affects neither the transcription nor translation of comGA
We next asked whether the mcsB mutant phenotype resulted in the altered expression of competence genes. For this, a strain carrying both comG-gusA and comK-lacZ in-frame fusions was used. This strain also expressed comK under Phyperspank control, and IPTG was used to induce expression. The two fusions were in-frame and were driven from their native ComK-dependent promoters. Introduction of the mcsB loss-of-function mutant had no effect on expression of either reporter (Fig. S3A). Also, Western blotting with anti-ComK antiserum showed no difference in the amounts of this protein (Fig. S3B). The mcsB delocalization defect was therefore not related to altered expression of the com genes on the levels of either transcription or translation. This experiment also provided evidence for the non-polarity of the mcsB mutation, since loss-of-function mutations in clpC, the gene immediately downstream of mcsB, would be expected to exhibit a dramatic increase in com gene expression (Dubnau & Roggiani, 1990). However, Western blotting with anti-ComGA anti-serum did show an increased concentration of this protein, presumably due to its greater stability in the mcsB background (Fig. S3C). The same has been observed for ComFA by Western blotting, and we will return to this point below, in the Discussion.
Inactivation of mcsB affects the delocalization of additional Com proteins
To test the generality of the mcsB delocalization phenotype, we studied several additional Com protein fusions to YFP (Fig 5). The mcsB delocalization defect was apparent for ComFA, YjbF, DprA, and SsbB (Fig. 5). The pattern observed for DprA in the mcsB+ background was different from that of the other Com proteins, because at T2, localization was observed in fewer of the competent cells than was the case for YjbF, ComFA, SsbB and ComGA. However, the effect of the mcsB mutation on the localization of YFP-DprA at T2 was quite pronounced and this dramatic difference persisted until T5. Although SsbB was more localized when mcsB was inactivated, it was not as dramatically affected as the other Com proteins and the apparently enhanced stability noted by Western blotting for ComGA and ComFA in the mcsB mutant was not observed for SsbB (Fig. S3D). ComGA, YjbF and DprA fail to localize when expressed in non-competent cells. Because SsbB can associate with the poles independently of other Com proteins (Kramer et al., 2007), we suspected that it might be needed for them to localize. However, an ssbB knockout strain appeared unaffected in the localization of ComGA-GFP (not shown).
Fig. 5.
Effect of mcsB knockout on localization of ComFA, SsbB, DprA and YjbF. Fluorescence images were collected at T2 and T5 from respective isogenic wild-type and mcsB strains expressing ssbB-yfp (BD2988 and BD4121), comFA-yfp (BD3799 and BD4119), yfp-yjbF (BD4183 and BD4200) and yfp-dprA (BD4184 and BD4197). The yfp-yjbF and yfp-dprA fusions were expressed from Pspank promoters and the strains were grown in competence medium with 1 mM IPTG.
The failure-to-delocalize phenotype of mcsA and mcsB mutants is not mediated by an effect on the repressor protein CtsR
mcsB and its immediate upstream neighbor mcsA (Fig. 4A) have been shown by the Turgay lab to encode proteins that bind to ClpC, targeting CtsR for degradation under heat-shock conditions (Kirstein et al., 2007, Kirstein et al., 2005). McsB is an auto-kinase and its kinase activity is stimulated by the presence of McsA. The complex of McsA and B phosphorylates CtsR, reversing its binding to DNA, and these proteins also act as adapter proteins, targeting the degradation of CtsR by complexes of ClpP with ClpC. It has been reported that not only ClpC, but also the related AAA+ protein ClpE is required for the full in vivo degradation of CtsR during heat shock (Miethke et al., 2006), suggesting that ClpE, like ClpC, may associate with McsA and B. In fact, it has been demonstrated in vitro that McsA and McsB can target CtsR for degradation by ClpE+ClpP (J. Kirstein and K. Turgay, personal communication). CtsR is the repressor of the ctsR-mcsA-mcsB-clpC operon (Fig. 4A), as well as of clpP and clpE (Derré et al., 1999, Derré et al., 2000, Krüger & Hecker, 1998). As noted above, we observed that in a ctsR mutant strain, the localization of ComGA-GFP is decreased (Fig. 4B). This observation raises the possibility that McsA and B are overproduced in the absence of CtsR, leading to decreased localization. However, it is also possible that CtsR itself regulates polar localization, while inactivation of mcsB acts indirectly through its effect on CtsR. By this logic, the absence of McsA or B would result in excess CtsR, which would then mediate increased localization, either directly, or by regulating some other gene. To exclude a role for CtsR accumulation in the failure-to-delocalize phenotype, we constructed a strain in which mcsB and ctsR were both inactivated. If the mcsB phenotype resulted from excess CtsR, the double mutant would reverse the failure-to-delocalize phenotype. Clearly, the opposite is true; inactivation of ctsR failed to reverse the delocalization phenotype of the mcsB mutant (Fig. 6) and we conclude that the delocalization phenotype is not mediated by an effect on CtsR.
Fig. 6.

Inactivation of ctsR does not reverse the mcsB phenotype. Fluorescence images were collected at T5 from isogenic strains expressing comGA-gfp in wild-type (BD2899), mcsB (BD3594) and mcsB ctsR (BD4541) backgrounds.
Phosphorylation of McsB is needed for delocalization of ComGA
The phosphatase YwlE has been shown to mediate the dephosphorylation of McsB-PO4 as well as other proteins (Mijakovic et al., 2003, Kirstein et al., 2005). If phosphorylation were involved in the McsB-dependent delocalization of Com proteins, we would expect that an increased cellular concentration of YwlE would mimic the mcsB phenotype by decreasing the level of McsB~PO4. To test this, we expressed ywlE from the IPTG-inducible Pspank promoter. In the presence of inducer, a clear increase in the localization of ComGA-GFP was observed, similar to the effect of mcsB inactivation (Fig. 7A). A ywlE loss-of-function mutant exhibited no obvious alteration in the pattern of ComGA-GFP localization (not shown), suggesting that under these conditions the cells do not limit the level of McsB-PO4 or that some other phosphatase accomplishes this task. A further prediction of the hypothesis that phosphorylation of McsB is involved in delocalization is that inactivation of mcsA would present a similar phenotype, because the protein encoded by this gene is needed to activate the auto-phosphorylation of McsB (Kirstein et al., 2005). This prediction was confirmed using a non-polar mcsA knockout, and the delocalization phenotypes of mcsA and mcsB knockouts are in fact indistinguishable (Fig. 7B). These experiments strongly suggest that phosphorylation of McsB is needed for delocalization.
Fig. 7.
Phenotypes of ywlE over-expressing and mcsA knockout mutants. (A) Fluorescence images are shown taken at T5, from a strain expressing comGA-yfp and carrying Pspank-ywlE (BD4770). The native ywlE locus was intact in this strain. Images are shown from this culture grown with and without I mM IPTG to induce expression of ywlE. (B) Fluorescence images are shown, taken at T5, from isogenic strains expressing comGA-gfp in wild-type (BD2899), mcsB (BD3594) and mcsA (BD3655) backgrounds.
ClpC, ClpE and ClpP contribute to delocalization
These results, together with the above mentioned role of McsA and B as adaptor proteins (Kirstein et al., 2007), suggest that they may facilitate Com protein delocalization by degrading one or more unknown proteins that are needed to anchor the Com proteins to the cell poles, consistent with the working model described above derived from the time-lapse experiments conducted in the presence and absence of Pm. If anchor protein degradation were the explanation for the mcs phenotype, we would expect loss-of-function mutants of clpP, clpC and clpE to exhibit delocalization defects similar to those of the mcsA and mcsB mutants. Although the individual clpE and clpC mutants had minor or no effects, a clpC clpE double mutant had a strong phenotype, consistent with the likely abilities of McsA and B to act with either of these AAA+ proteins (Fig. 8). To test the effect of clpP inactivation, we used an spx clpP double mutant, since clpP is needed for the full expression of competence while inactivation of spx bypasses this effect (Nakano et al., 2001, Nakano et al., 2002). Knockout of spx alone did not exhibit a delocalization defect (not shown), while the clpP spx strain exhibited a clear defect in delocalization (Fig. 8). As also shown in Fig. 8, inactivation of ctsR did not bypass the phenotype of a double clpC clpE mutant, just as it failed to reverse that of the mcsB mutant. These data strongly support the hypothesis that degradation of one or more anchor proteins is needed for delocalization and is also consistent with the energy requirement documented above, because AAA+ proteins require ATP hydrolysis to function and because McsB kinase activity is ATP-dependent.
Fig. 8.
Phenotypes of ComGA-GFP localization in clp mutants. Fluorescence images are shown, taken at T5, of isogenic strains expressing comGA-gfp in wild-type (BD2899 or BD3142), clpC (BD3579), clpE (BD4252), clpC clpE (BD4261), clpP spx (BD4530) and clpC clpE ctsR (BD4262) backgrounds. Note that the wild-type comparison with BD4530 is BD3142, expressing comGA-cfp. In many experiments we have seen no difference in the patterns of ComGA-GFP or ComGA-CFP localization.
McsB, ClpC and ClpP localize to the poles of competent cells
If these proteins act to degrade a presumptive anchor, they presumably do so at the cell poles. We would therefore expect them to exhibit polar localization and to co-localize with Com proteins. To investigate this, fusions of YFP to the C-termini of ClpC, McsB and ClpP, expressed from their native promoters, were used in a strain that also expressed comGA-CFP to identify the competence-expressing cells (Fig. 9). In such cells, the ClpC, ClpP and McsB fusions were localized at the poles and co-localized with ComGA-CFP. Although the McsB-YFP fusion does not interfere with heat-shock induction (Kirstein et al., 2008), it is not fully functional in the present assay, since in its presence, the ComGA-CFP fusion exhibited a mcsB phenotype. This is noticeable in Fig. 9A where localization is seen to persist at T5. Polar localization of McsB-YFP almost never occurred in the non-competent cells, which exhibited faint diffuse fluorescence, consistent with published results (Kirstein et al., 2008). Western blotting demonstrated that the amount of McsB protein is constant throughout growth in competence medium (not shown). We do not know whether the apparent increase in McsB-YFP fluorescence in the competent cells is due to the concentration of this protein at the poles, or to an increase in its synthesis or stability in the competent sub-fraction. Regardless of the mechanism, it appears that McsB is targeted to the poles in response to competence, possibly due to its affinity for one or more Com proteins. ClpC-YFP sometimes localized to the poles of non-competent cells, as previously reported (Kain et al., 2008, Kirstein et al., 2008, Simmons et al., 2008), but was almost always localized in competent cells where it co-localized with ComGA (Fig. 9B). In other experiments, we have determined that ClpC and McsB accumulate at the cell poles independently; ClpC-YFP is at the poles of a mcsB knockout strain and McsB-YFP is at the poles of clpC cells (not shown). In fact, the polar accumulation of McsB-YFP is more intense in a clpC knockout. Like MecA, another adapter protein that binds to ClpC, McsB is degraded by ClpCP and therefore accumulates when clpC is inactivated (Turgay et al., 1998, Kirstein et al., 2007). The ClpP-YFP protein was only partly functional, since its presence seemed to decrease the number of competent cells, as seen in a clpP knockout strain. Nevertheless, ClpP-YFP co-localized with ComGA in more than half of the rare competent cells, and localized less frequently in non-competent cells, where it usually had a punctate distribution and weaker fluorescence intensity than in the competent cells (Fig. 9C). It was difficult to obtain reliable numbers for the localization of ClpP-YFP because of the paucity of competent cells in the clpP strain.
Fig. 9.
Co-localization of McsB-YFP, ClpP-YFP and ClpC-YFP with ComGA-CFP. Fluorescence images were taken at T2 and T5 from isogenic strains expressing comGA-cfp with (A) mcsB-yfp (BD4507) and at T2 from strains expressing (B) clpC-yfp (BD3504) and (C) clpP-yfp (BD4768).
Inactivation of mcsB decreases transformability but not its programmed loss
To determine whether McsB is needed for transformability, we measured the transformation frequencies of otherwise isogenic wild-type and mcsB strains. An average derived from 13 experiments revealed that the frequency of transformation to leucine prototrophy was 7.2±2.4-fold lower in the mcsB strain. This effect was not due to the expression of com genes in fewer cells, or to lower expression per cell, since in mcsB mutants, like the wild-type, about 15% of the cells became competent (not shown), and because expression of comK and comG were not affected by inactivation of mcsB (Fig. S3).
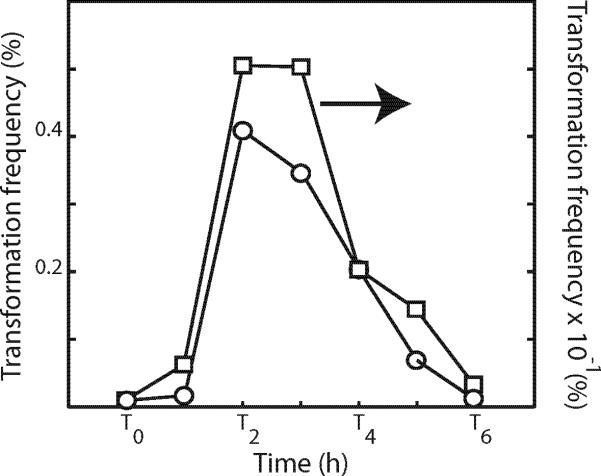
It has been shown that the appearance of Com proteins at the cell poles parallels the development of transformability and that the subsequent delocalization of Com proteins is approximately concomitant with a decrease in transformability (Hahn et al., 2005). The delocalization defect of the mcsB mutant provided an opportunity to ask whether delocalization per se is required for the decrease in transformability. Fig. 10 shows that transformability decreases, as it does in the wild type strain, even in the mcsB mutant, although the peak transformability reached in this experiment was about 8-fold lower in the mcsB culture. Clearly the loss of transformability does not depend on delocalization. Although delocalization is clearly not an absolute requirement for transformation, or for its programmed decrease, McsB is needed for both optimal transformability and for delocalization
Fig. 10.
Transformability of isogenic wild-type (BD2899, squares) and mcsB (BD3594, circles) strains during growth in competence medium. Note the ten-fold difference in the scales on the ordinates.
Discussion
We have shown that ComGA and probably other Com proteins localize to the poles by a mechanism that does not depend on metabolic energy and apparently proceeds by diffusion and capture. Because Com proteins co-localize even when non-polar (Hahn et al., 2005), and because the present experiments show that they move to the poles from non-polar locations, we conclude that at least some assembly of the Com machinery occurs away from the poles and that these complexes diffuse until they are captured at the poles. In some cells we have seen large foci form, presumably due to the coalescence of individual assemblies. These foci sometimes move to the poles and sometimes seen to disappear, perhaps due to disassembly. Although binding and uptake of transforming DNA occurs preferentially at the poles, we have detected less frequent transformation events along the lateral surfaces of the rod shaped cells (Hahn et al., 2005), consistent with non-polar assembly of the Com machinery. It is intriguing that the non-polar fluorescence of the Com fusion proteins is helically distributed and we are tempted to suggest that the pole-ward movement occurs along these helical paths. Indeed some of our movies can be interpreted in this way (see Fig. S2). However, it is also possible that the movement is not processive and that the helical tracks represent temporary binding sites. The Com proteins may attach and detach from these sites continually until they finally settle at the poles. Only high-speed video microscopy with sufficient spatial and temporal resolution can address this question.
It appears that localization is a dynamic process, in the sense that both localization and delocalization occur during competence development, with McsA, McsB, ClpP and ClpC or ClpE required for delocalization. Thus, in the mcsB knockout strain, in which delocalization is blocked, localization is more complete and seems to occur faster than in the wild-type strain. Also, in the presence of NaN3, which also blocks delocalization, more net localization takes place than in its absence (Fig. 3). The extent of localization is thus the result of competing processes and it is notable that following T2, the net localization declines. We do not know what regulates this equilibrium and what signals are involved. Perhaps the amounts of McsB and McsA or their phosphorylation states are regulated temporally. Another temporally regulated process is responsible for the decrease in transformability that occurs after T2, because this decrease occurs independently of McsB (Fig. 10). Although the regulation of the proteins involved in delocalization is complex (Fig. 4A), the repressor of all of the genes involved (CtsR) is not directly required for the delocalization process (Figs. 6 and 8), a ctsR loss-of-function mutant exhibits reduced polar localization (Fig. 4B). This can easily be explained by overproduction of mcsA, mcsB , clpP, clpC and clpE, consistent with the epistatic effects of each of these knockouts on the ctsR mutation.
An unidentified protein limits the rate of capture and is progressively synthesized or activated as the cells develop competence. Delocalization requires the action of McsA, McsB, ClpP and either ClpC or ClpE, presumably to degrade a protein or proteins required to secure the Com proteins at the cell poles. It is tempting to regard the rate-limiting capture protein and the McsB target as one and the same. However, binding of the Com proteins to the pole presumably requires at least two interacting surfaces, one at the pole and another on the Com complex, and either of these surfaces may be formed by the rate limiting protein or the proteolysis target. However, one line of reasoning suggests that the McsB proteolysis target may be part of the Com protein complex itself, rather than a protein forming a binding site at the poles. Although inactivation of mcsB does not affect the transcription or translation of comK or comG, the amounts of ComGA and ComFA are increased (Fig. S3), suggesting that these proteins are stabilized in the mutant. Similarly, these proteins are stabilized when expressed in conjunction with other Com proteins. Our observations therefore suggest that McsB targets a protein that facilitates the assembly of the Com machinery and is required to maintain its integrity, perhaps acting as a scaffold. We also suspect that this rate-limiting scaffold protein is under ComK control, because its amount appears to increase during the development of competence. It is worth pointing out that about a hundred genes are upregulated in the presence of ComK (Berka et al., 2002, Hamoen et al., 2002, Ogura et al., 2002), and that the roles of many of their encoded proteins remain undefined. These are all candidates for a scaffold protein. Clearly a fuller understanding of these processes will require the experimental identification of proteins needed for the polar localization of the Com proteins.
The requirement for McsA, McsB, ClpP and either ClpC or ClpE for delocalization implies strongly that the target protein is degraded using a mechanism similar to that used to degrade CtsR, the only documented substrate for the McsAB adaptor proteins. Both ClpC and ClpE bind to McsB (Kirstein et al., 2007, Kirstein et al., 2005) and ClpC and ClpE both are required for the regulation of CtsR (Miethke et al., 2006). These results are consistent with the data in this report and suggest strongly that McsB acts as an adaptor protein for both ATPases. Thus we propose that McsB, activated by its partner McsA, transfers one or more phosphoryl groups to the rate limiting protein, targeting it for degradation by the ClpC-ClpP or ClpE-ClpP proteases. Over-production of the YwlE phosphatase which is known to act on McsB~P (Kirstein et al., 2005) mimics the phenotype conferred by inactivation of mcsA or mcsB, lending strong support to this model. Also highly suggestive are the localizations of the degradation proteins. At least McsB, ClpP and ClpC are present near the poles of competent cells and all three seem to co-localize with ComGA. Particularly intriguing is the observation that McsB is only targeted to the poles in competent cells. The localization we have observed for ClpC is consistent with three previous studies (Kain et al., 2008, Kirstein et al., 2008, Simmons et al., 2008), showing ClpC at or near the poles in some, but not all cells. One of these studies examined cells in a medium that would permit the development of competence (Simmons et al., 2008). In this medium, ClpC and ClpE were associated with the nucleoids in most cells, but were at the poles in 11-17% of the cells. The present study would suggest that these cells might be competent.
Clearly McsB is not required for transformation, although in its absence the yield of recoverable transformants is depressed about 7-fold. The lack of an absolute requirement for McsB argues that the competence machinery can assemble and function correctly in its absence. What might be the reason for the decrease in transformability? Although the Com proteins establish multiple contacts with one another in competent cells, the competence machinery is most likely not a static assembly. For instance, DprA is not as completely associated with the poles as are other Com proteins in mcsB+ cells (Fig. 5), although it does establish contacts with other Com proteins (Kramer et al., 2007). DprA is a DNA binding protein and clearly interacts with the incoming single-stranded DNA following uptake. Indeed it has been shown to protect this DNA from degradation (Berge et al., 2003, Mortier-Barriere et al., 2007) It is likely that DprA associates with the uptake apparatus during transport of DNA across the membrane, so as to be positioned to contact the incoming strands, but then departs to accompany these DNA strands to the chromosome where it facilitates recombination, possibly by helping the DNA interact with RecA (Mortier-Barriere et al., 2007). It makes sense therefore, that the association of DprA with the uptake apparatus is relatively loose, as observed. In the mcsB knockout, DprA associates dramatically with the other Com proteins at the cell poles (Fig. 5), presumably because accumulation of the postulated scaffold protein favors this association. If as we suggest, loose association of DprA with the uptake proteins were important for recombination, the lowered rate of transformation of the mcsB mutant would be easily explained. More generally, we suggest that the complex must be dynamic to function optimally.
From the current state of knowledge, a picture emerges of complex regulated movement of proteins to particular cellular locations, targeted and regulated proteolysis and dynamic assembly-disassembly processes that lead to the proper development and loss of transformability.
Experimental procedures
Strains and microbiological methods
All the B. subtilis strains used in this study were derived from the 168 derivative BD630 (his leu met) (Table S1). Strains were constructed by combining mutant constructs using transformation by standard methods, always with selection for the antibiotic resistance markers listed in Table S1. The individual mutant constructs were made as described in the references given in the table, except for the Phyperspank-ywlE, clpC-yfp and comGA-yfp constructs, which are described in Supporting Material. Strains were grown to the indicated growth stages in competence medium (Albano et al., 1987). When needed, Pm and Rm were added at 200 μg/ml.
Microscopy
For microscopy, cells taken at the indicated times were deposited either on polylysine-coated slides or on 1.2% agarose pads, containing competence medium and inhibitors as indicated. Images were collected in a Nikon 90i microscope using a TIRF Plan Neo-Fluor 100x oil immersion objective (NA 1.45). Filter sets appropriate for each fluorophore were from Semrock Optical. For the time-lapse experiments, a home-built temperature-controlled chamber that encloses the entire microscope was used (constructed by K.B.), and maintained at 37 °C. Chambers for time-lapse were constructed from Chambered Cover glasses (Lab-Tek) as described in Supporting information.
Western blotting
Western blots were carried out as described previously (Kramer et al., 2007), using polyclonal anti-sera raised in rabbits. Rabbit anti-SsbB and anti-McsB antisera were kind gifts from Patrice Pollard and Kursad Turgay, respectively.
Supplementary Material
Acknowledgements
This work was supported by NIH grant GM057720. We thank U. Gerth, Qi Pan, R. Losick, L. Hamoen, P. Polard, M. Dias, D. Rudner, M. Nakano and P. Zuber for donating strains and anti-sera and N. Mirouze for the Pm luciferase experiment. We are also grateful to all the members of the Dubnau and Neidtich labs for frequent and valuable discussions. We particularly thank K. Turgay and J. Kirstein for donating strains and anti-sera prior to publication and for sharing ideas and information.
Footnotes
Supporting information. Supporting information contains the details of plasmid construction, time-lapse Quick-time movies (Fig. S1A and S2), an accompanying DIC images (Fig. S1B), Fig. S3 and the list of strains (Table S1).
References
- Albano M, Hahn J, Dubnau D. Expression of competence genes in Bacillus subtilis. J. Bacteriol. 1987;169:3110–3117. doi: 10.1128/jb.169.7.3110-3117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge M, Mortier-Barriere I, Martin B, Claverys JP. Transformation of Streptococcus pneumoniae relies on DprA- and RecA-dependent protection of incoming DNA single strands. Mol Microbiol. 2003;50:527–536. doi: 10.1046/j.1365-2958.2003.03702.x. [DOI] [PubMed] [Google Scholar]
- Berka RM, Hahn J, Albano M, Draskovic I, Persuh M, Cui X, Sloma A, Widner W, Dubnau D. Microarray analysis of the Bacillus subtilis K-state: genome-wide expression changes dependent on ComK. Mol Microbiol. 2002;43:1331–1345. doi: 10.1046/j.1365-2958.2002.02833.x. [DOI] [PubMed] [Google Scholar]
- Chen I, Provvedi R, Dubnau D. A Macromolecular Complex Formed by a Pilin-like Protein in Competent Bacillus subtilis. J Biol Chem. 2006;281:21720–21727. doi: 10.1074/jbc.M604071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YS, Breidt F, Dubnau D. Cell surface localization and processing of the ComG proteins, required for DNA binding during transformation of Bacillus subtilis. Mol. Microbiol. 1998;29:905–913. doi: 10.1046/j.1365-2958.1998.00989.x. [DOI] [PubMed] [Google Scholar]
- Derré I, Rapoport G, Msadek T. CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in gram-positive bacteria. Mol Microbiol. 1999;31:117–131. doi: 10.1046/j.1365-2958.1999.01152.x. [DOI] [PubMed] [Google Scholar]
- Derré I, Rapoport G, Msadek T. The CtsR regulator of stress response is active as a dimer and specifically degraded in vivo at 37 degrees C. Mol Microbiol. 2000;38:335–347. doi: 10.1046/j.1365-2958.2000.02124.x. [DOI] [PubMed] [Google Scholar]
- Dubnau D, Roggiani M. Growth medium-independent genetic competence mutants of Bacillus subtilis. J. Bacteriol. 1990;172:4048–4055. doi: 10.1128/jb.172.7.4048-4055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DH, Errington J. The Bacillus subtilis DivIVA protein targets to the division septum and controls the site specificity of cell division. Mol Microbiol. 1997;24:905–915. doi: 10.1046/j.1365-2958.1997.3811764.x. [DOI] [PubMed] [Google Scholar]
- Hahn J, Maier B, Haijema BJ, Sheetz M, Dubnau D. Transformation proteins and DNA uptake localize to the cell poles in Bacillus subtilis. Cell. 2005;122:59–71. doi: 10.1016/j.cell.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamoen LW, Smits WK, de Jong A, Holsappel S, Kuipers OP. Improving the predictive value of the competence transcription factor (ComK) binding site in Bacillus subtilis using a genomic approach. Nucleic Acids Res. 2002;30:5517–5528. doi: 10.1093/nar/gkf698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain J, He GG, Losick R. Polar localization and compartmentalization of ClpP proteases during growth and sporulation in Bacillus subtilis. J Bacteriol. 2008;190:6749–6757. doi: 10.1128/JB.00589-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidane D, Graumann PL. Intracellular protein and DNA dynamics in competent Bacillus subtilis cells. Cell. 2005;122:73–84. doi: 10.1016/j.cell.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Kirstein J, Dougan DA, Gerth U, Hecker M, Turgay K. The tyrosine kinase McsB is a regulated adaptor protein for ClpCP. EMBO J. 2007;26:2061–2070. doi: 10.1038/sj.emboj.7601655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein J, Strahl H, Moliere N, Hamoen LW, Turgay K. Localization of general and regulatory proteolysis in Bacillus subtilis cells. Mol Microbiol. 2008;70:682–694. doi: 10.1111/j.1365-2958.2008.06438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein J, Zuhlke D, Gerth U, Turgay K, Hecker M. A tyrosine kinase and its activator control the activity of the CtsR heat shock repressor in B. subtilis. EMBO J. 2005;24:3435–3445. doi: 10.1038/sj.emboj.7600780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer N, Hahn J, Dubnau D. Multiple interactions among the competence proteins of Bacillus subtilis. Mol Microbiol. 2007;65:454–464. doi: 10.1111/j.1365-2958.2007.05799.x. [DOI] [PubMed] [Google Scholar]
- Krüger E, Hecker M. The first gene of the Bacillus subtilis clpC operon, ctsR, encodes a negative regulator of its own operon and other class III heat shock genes. J Bacteriol. 1998;180:6681–6688. doi: 10.1128/jb.180.24.6681-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miethke M, Hecker M, Gerth U. Involvement of Bacillus subtilis ClpE in CtsR degradation and protein quality control. J Bacteriol. 2006;188:4610–4619. doi: 10.1128/JB.00287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijakovic I, Poncet S, Boel G, Maze A, Gillet S, Jamet E, Decottignies P, Grangeasse C, Doublet P, Le Marechal P, Deutscher J. Transmembrane modulator-dependent bacterial tyrosine kinase activates UDP-glucose dehydrogenases. EMBO J. 2003;22:4709–4718. doi: 10.1093/emboj/cdg458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier-Barriere I, Velten M, Dupaigne P, Mirouze N, Pietrement O, McGovern S, Fichant G, Martin B, Noirot P, Le Cam E, Polard P, Claverys JP. A key presynaptic role in transformation for a widespread bacterial protein: DprA conveys incoming ssDNA to RecA. Cell. 2007;130:824–836. doi: 10.1016/j.cell.2007.07.038. [DOI] [PubMed] [Google Scholar]
- Nakano MM, Hajarizadeh F, Zhu Y, Zuber P. Loss-of-function mutations in yjbD result in ClpX- and ClpP-independent competence development of Bacillus subtilis. Mol Microbiol. 2001;42:383–394. doi: 10.1046/j.1365-2958.2001.02639.x. [DOI] [PubMed] [Google Scholar]
- Nakano MM, Nakano S, Zuber P. Spx (YjbD), a negative effector of competence in Bacillus subtilis, enhances ClpC-MecA-ComK interaction. Mol Microbiol. 2002;44:1341–1349. doi: 10.1046/j.1365-2958.2002.02963.x. [DOI] [PubMed] [Google Scholar]
- Ogura M, Yamaguchi H, Kobayashi K, Ogasawara N, Fujita Y, Tanaka T. Whole-genome analysis of genes regulated by the Bacillus subtilis competence transcription factor ComK. J Bacteriol. 2002;184:2344–2351. doi: 10.1128/JB.184.9.2344-2351.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T, Wilkinson AJ. AAA+ superfamily ATPases: common structure--diverse function. Genes Cells. 2001;6:575–597. doi: 10.1046/j.1365-2443.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- Oliver DB, Cabelli RJ, Dolan KM, Jarosik GP. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc Natl Acad Sci U S A. 1990;87:8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Garsin DA, Losick R. Self-reinforcing activation of a cell-specific transcription factor by proteolysis of an anti-sigma factor in B. subtilis. Mol Cell. 2001;8:873–883. doi: 10.1016/s1097-2765(01)00362-8. [DOI] [PubMed] [Google Scholar]
- Shapiro L, McAdams HH, Losick R. Generating and exploiting polarity in bacteria. Science. 2002;298:1942–1946. doi: 10.1126/science.1072163. [DOI] [PubMed] [Google Scholar]
- Simmons LA, Grossman AD, Walker GC. Clp and Lon proteases occupy distinct subcellular positions in Bacillus subtilis. J Bacteriol. 2008;190:6758–6768. doi: 10.1128/JB.00590-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse S, Graumann PL. DprA/Smf protein localizes at the DNA uptake machinery in competent Bacillus subtilis cells. BMC Microbiol. 2007;7:105. doi: 10.1186/1471-2180-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgay K, Hahn J, Burghoorn J, Dubnau D. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 1998;17:6730–6738. doi: 10.1093/emboj/17.22.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.