Abstract
Autoantibodies to ribosomal P-proteins are present in 12-16% of patients with systemic lupus erythematosus and are associated with neuropsychiatric disease. As the ribosomal P proteins are located in the cytoplasm, the pathogenic effects of their cognate autoantibodies are unclear. In this study affinity-purified anti-P autoantibodies were used to explore the cell surface of several types of human and animal cells. Immunofluorescence as well as EM immunogold analysis demonstrated, on the surface of human hepatoma cells, the presence of an epitope that is antigenically related to the immunodominant carboxy terminus of P-proteins. The presence of this epitope was also demonstrated on the surface of human neuroblastoma cells and, to a lesser extent, on human fibroblasts. Furthermore, the Western blot technique revealed in purified human and animal plasma membranes a 38-kD protein that is closely related or identical with ribosomal P0 protein. The availability of reactive P peptide on the surface of cells makes possible the direct effect of autoantibodies on the function and viability of cells that express this antigenic target. This delineates one of the possible impacts of anti-P antibodies in disease expression.
Full text
PDF

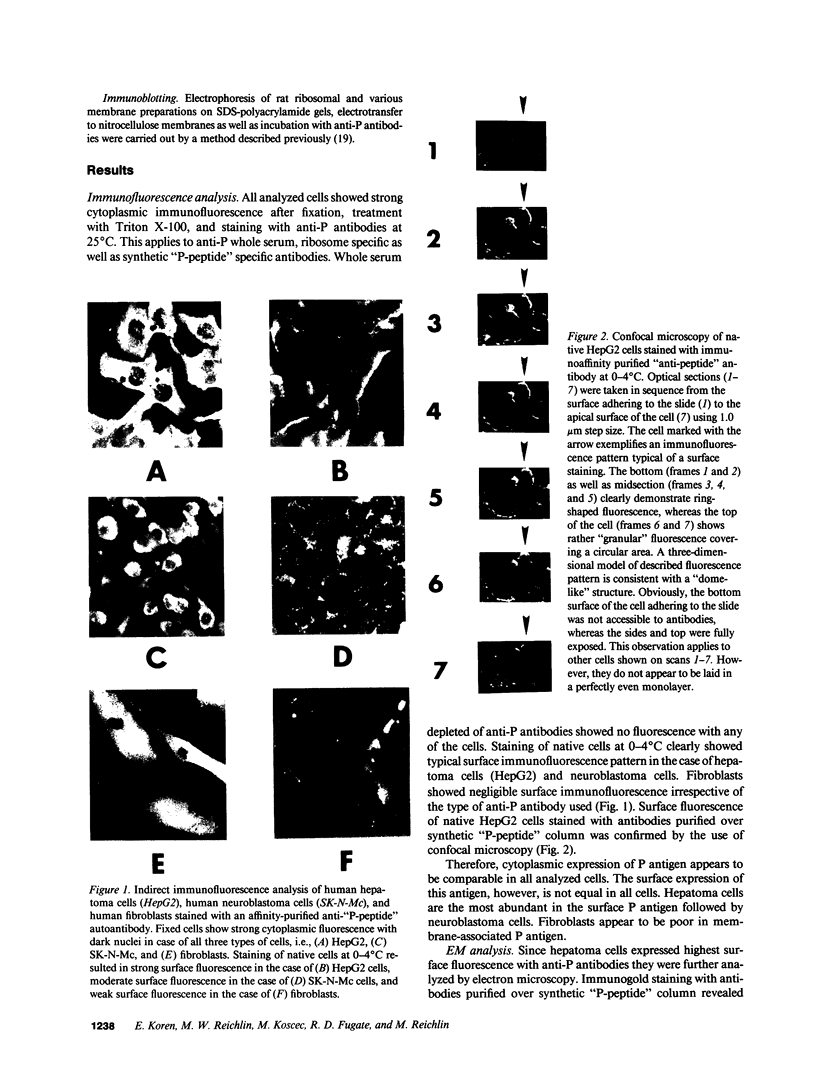



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham A. K., Khatim M. S. Kinetics of ribosomal protein S6 phosphorylation by HepG-2 cells in response to insulin. Biochem Biophys Res Commun. 1989 Jun 15;161(2):797–802. doi: 10.1016/0006-291x(89)92670-3. [DOI] [PubMed] [Google Scholar]
- Alarcon-Segovia D., Ruiz-Arguelles A., Fishbein E. Antibody to nuclear ribonucleoprotein penetrates live human mononuclear cells through Fc receptors. Nature. 1978 Jan 5;271(5640):67–69. doi: 10.1038/271067a0. [DOI] [PubMed] [Google Scholar]
- Alarcon-Segovia D., Ruiz-Arguelles A., Llorente L. Antibody penetration into living cells. II. Anti-ribonucleoprotein IgG penetrates into Tgamma lymphocytes causing their deletion and the abrogation of suppressor function. J Immunol. 1979 May;122(5):1855–1862. [PubMed] [Google Scholar]
- Bennett R. M., Kotzin B. L., Merritt M. J. DNA receptor dysfunction in systemic lupus erythematosus and kindred disorders. Induction by anti-DNA antibodies, antihistone antibodies, and antireceptor antibodies. J Exp Med. 1987 Oct 1;166(4):850–863. doi: 10.1084/jem.166.4.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfa E., Elkon K. B. Clinical and serologic associations of the antiribosomal P protein antibody. Arthritis Rheum. 1986 Aug;29(8):981–985. doi: 10.1002/art.1780290806. [DOI] [PubMed] [Google Scholar]
- Bonfa E., Golombek S. J., Kaufman L. D., Skelly S., Weissbach H., Brot N., Elkon K. B. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med. 1987 Jul 30;317(5):265–271. doi: 10.1056/NEJM198707303170503. [DOI] [PubMed] [Google Scholar]
- Casiano C., Matheson A. T., Traut R. R. Occurrence in the archaebacterium Sulfolobus solfataricus of a ribosomal protein complex corresponding to Escherichia coli (L7/L12)4.L10 and eukaryotic (P1)2/(P2)2.P0. J Biol Chem. 1990 Nov 5;265(31):18757–18761. [PubMed] [Google Scholar]
- Dashti N., Alaupovic P., Knight-Gibson C., Koren E. Identification and partial characterization of discrete apolipoprotein B containing lipoprotein particles produced by human hepatoma cell line HepG2. Biochemistry. 1987 Jul 28;26(15):4837–4846. doi: 10.1021/bi00389a035. [DOI] [PubMed] [Google Scholar]
- Elkon K. B., Parnassa A. P., Foster C. L. Lupus autoantibodies target ribosomal P proteins. J Exp Med. 1985 Aug 1;162(2):459–471. doi: 10.1084/jem.162.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K., Skelly S., Parnassa A., Moller W., Danho W., Weissbach H., Brot N. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7419–7423. doi: 10.1073/pnas.83.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holers V. M., Kotzin B. L. Human peripheral blood monocytes display surface antigens recognized by monoclonal antinuclear antibodies. J Clin Invest. 1985 Sep;76(3):991–998. doi: 10.1172/JCI112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Rader M. D., Reichlin M. Heterogeneity of the Ro/SSA antigen and autoanti-Ro/SSA response: evidence of the four antigenically distinct forms. Clin Exp Immunol. 1990 Jul;81(1):45–51. doi: 10.1111/j.1365-2249.1990.tb05289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Koren E., McConathy W. J., Lacko A. G., Knowles B. Detection of lecithin: cholesterol acyltransferase (LCAT) in a human hepatoma cell line. Cancer Biochem Biophys. 1985 Feb;7(4):301–307. [PubMed] [Google Scholar]
- Lee C. C., Cantor C. R., Wittmann-Liebold B. The number of copies of ribosome-bound proteins L7 and L12 required for protein synthesis activity. J Biol Chem. 1981 Jan 10;256(1):41–48. [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. Separation of large denatured peptides by reverse phase high performance liquid chromatography. Trifluoroacetic acid as a peptide solvent. J Biol Chem. 1980 Dec 10;255(23):11199–11203. [PubMed] [Google Scholar]
- Moldave K., Sadnik I. Preparation of derived and native ribosomal subunits from rat liver. Methods Enzymol. 1979;59:402–410. doi: 10.1016/0076-6879(79)59101-0. [DOI] [PubMed] [Google Scholar]
- Morris K. M., Goldberger G., Colten H. R., Aden D. P., Knowles B. B. Biosynthesis and processing of a human precursor complement protein, pro-C3, in a hepatoma-derived cell line. Science. 1982 Jan 22;215(4531):399–400. doi: 10.1126/science.7199205. [DOI] [PubMed] [Google Scholar]
- Möller W., Schrier P. I., Maassen J. A., Zantema A., Schop E., Reinalda H., Cremers A. F., Mellema J. E. Ribosomal proteins L7/L12 of Escherichia coli. Localization and possible molecular mechanism in translation. J Mol Biol. 1983 Feb 5;163(4):553–573. doi: 10.1016/0022-2836(83)90112-2. [DOI] [PubMed] [Google Scholar]
- Prabhakar B. S., Allaway G. P., Srinivasappa J., Notkins A. L. Cell surface expression of the 70-kD component of Ku, a DNA-binding nuclear autoantigen. J Clin Invest. 1990 Oct;86(4):1301–1305. doi: 10.1172/JCI114838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichlin M. Use of glutaraldehyde as a coupling agent for proteins and peptides. Methods Enzymol. 1980;70(A):159–165. doi: 10.1016/s0076-6879(80)70047-2. [DOI] [PubMed] [Google Scholar]
- Sato T., Uchiumi T., Kominami R., Arakawa M. Autoantibodies specific for the 20-KDal ribosomal large subunit protein L12. Biochem Biophys Res Commun. 1990 Oct 30;172(2):496–502. doi: 10.1016/0006-291x(90)90700-w. [DOI] [PubMed] [Google Scholar]
- Schneebaum A. B., Singleton J. D., West S. G., Blodgett J. K., Allen L. G., Cheronis J. C., Kotzin B. L. Association of psychiatric manifestations with antibodies to ribosomal P proteins in systemic lupus erythematosus. Am J Med. 1991 Jan;90(1):54–62. doi: 10.1016/0002-9343(91)90506-s. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Fridovich S. E., Knowles B. B., Lodish H. F. Characterization of the asialoglycoprotein receptor in a continuous hepatoma line. J Biol Chem. 1981 Sep 10;256(17):8878–8881. [PubMed] [Google Scholar]
- Towbin H., Ramjoué H. P., Kuster H., Liverani D., Gordon J. Monoclonal antibodies against eucaryotic ribosomes. Use to characterize a ribosomal protein not previously identified and antigenically related to the acidic phosphoproteins P1/P2. J Biol Chem. 1982 Nov 10;257(21):12709–12715. [PubMed] [Google Scholar]
- Tsurugi K., Collatz E., Todokoro K., Ulbrich N., Lightfoot H. N., Wool I. G. Isolation of eukaryotic ribosomal proteins. Purification and characterization of the 60 S ribosomal subunit proteins La, Lb, Lf, P1, P2, L13', L14, L18', L20, and L38. J Biol Chem. 1978 Feb 10;253(3):946–955. [PubMed] [Google Scholar]
- Uchiumi T., Traut R. R., Kominami R. Monoclonal antibodies against acidic phosphoproteins P0, P1, and P2 of eukaryotic ribosomes as functional probes. J Biol Chem. 1990 Jan 5;265(1):89–95. [PubMed] [Google Scholar]
- Uchiumi T., Wahba A. J., Traut R. R. Topography and stoichiometry of acidic proteins in large ribosomal subunits from Artemia salina as determined by crosslinking. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5580–5584. doi: 10.1073/pnas.84.16.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijg J., Mullaart E., Berends F., Lohman P. H., Knook D. L. UV-induced DNA excision repair in rat fibroblasts during immortalization and terminal differentiation in vitro. Exp Cell Res. 1986 Dec;167(2):517–530. doi: 10.1016/0014-4827(86)90191-6. [DOI] [PubMed] [Google Scholar]
- van Agthoven A. J., Maassen J. A., Möller W. Structure and phosphorylation of an acidic protein from 60S ribosomes and its involvement in elongation factor-2 dependent GTP hydrolysis. Biochem Biophys Res Commun. 1977 Aug 8;77(3):989–998. doi: 10.1016/s0006-291x(77)80075-2. [DOI] [PubMed] [Google Scholar]
- van Agthoven A., Kriek J., Amons R., Möller W. Isolation and characterization of the acidic phosphoproteins of 60-S ribosomes from Artemia salina and rat liver. Eur J Biochem. 1978 Nov 15;91(2):553–565. doi: 10.1111/j.1432-1033.1978.tb12709.x. [DOI] [PubMed] [Google Scholar]