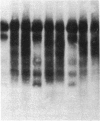
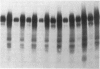
Abstract
Previous studies have shown that suramin is capable of disrupting autocrine growth involving coexpression of platelet-derived growth factor and its receptors in a fibroblast model for mesenchymal oncogenesis. Suramin is currently in use as an experimental drug for the treatment of patients with epithelial cell tumors. In the present study, we have investigated the efficacy of suramin in a carcinoma model system. Our findings demonstrate that suramin enhances cell surface signaling in A431 cells by activating an autocrine loop involving the receptor for epidermal growth factor (EGFR). The mechanism of suramin action was shown to be indirect, not affecting the ability of ligand to bind and activate the EGFR. Instead, suramin induced the release of membrane-bound transforming growth factor alpha, thereby increasing its potential to activate cell surface EGFRs. Since suramin potently blocks tyrosine phosphorylation induced by platelet-derived growth factor but can activate the growth pathway regulated by the EGFR, biological responses of tumor cells to suramin treatment may differ dramatically.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B. Efficient reversion of simian sarcoma virus-transformation and inhibition of growth factor-induced mitogenesis by suramin. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6440–6444. doi: 10.1073/pnas.83.17.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann R., Lindquist P. B., Nagashima M., Kohr W., Lipari T., Napier M., Derynck R. Transmembrane TGF-alpha precursors activate EGF/TGF-alpha receptors. Cell. 1989 Feb 24;56(4):691–700. doi: 10.1016/0092-8674(89)90591-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Derynck R., Goeddel D. V., Ullrich A., Gutterman J. U., Williams R. D., Bringman T. S., Berger W. H. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987 Feb 1;47(3):707–712. [PubMed] [Google Scholar]
- Derynck R., Roberts A. B., Winkler M. E., Chen E. Y., Goeddel D. V. Human transforming growth factor-alpha: precursor structure and expression in E. coli. Cell. 1984 Aug;38(1):287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- Di Marco E., Pierce J. H., Fleming T. P., Kraus M. H., Molloy C. J., Aaronson S. A., Di Fiore P. P. Autocrine interaction between TGF alpha and the EGF-receptor: quantitative requirements for induction of the malignant phenotype. Oncogene. 1989 Jul;4(7):831–838. [PubMed] [Google Scholar]
- EAGLE H. Propagation in a fluid medium of a human epidermoid carcinoma, strain KB. Proc Soc Exp Biol Med. 1955 Jul;89(3):362–364. doi: 10.3181/00379727-89-21811. [DOI] [PubMed] [Google Scholar]
- Fleming T. P., Matsui T., Molloy C. J., Robbins K. C., Aaronson S. A. Autocrine mechanism for v-sis transformation requires cell surface localization of internally activated growth factor receptors. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8063–8067. doi: 10.1073/pnas.86.20.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J. S., Coughlin S. R., Niman H. L., Tremble P. M., Giels G. M., Williams L. T. Blockade of autocrine stimulation in simian sarcoma virus-transformed cells reverses down-regulation of platelet-derived growth factor receptors. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7466–7470. doi: 10.1073/pnas.81.23.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. N., Lazar C. S. Increased phosphotyrosine content and inhibition of proliferation in EGF-treated A431 cells. Nature. 1981 Sep 24;293(5830):305–307. doi: 10.1038/293305a0. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Aaronson S. A. In vivo inoculation of RNA C-type viruses inducing regression of experimental solid tumors. J Natl Cancer Inst. 1973 Dec;51(6):1935–1938. doi: 10.1093/jnci/51.6.1935. [DOI] [PubMed] [Google Scholar]
- Hosang M. Suramin binds to platelet-derived growth factor and inhibits its biological activity. J Cell Biochem. 1985;29(3):265–273. doi: 10.1002/jcb.240290310. [DOI] [PubMed] [Google Scholar]
- LaRochelle W. J., May-Siroff M., Robbins K. C., Aaronson S. A. A novel mechanism regulating growth factor association with the cell surface: identification of a PDGF retention domain. Genes Dev. 1991 Jul;5(7):1191–1199. doi: 10.1101/gad.5.7.1191. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Todaro G. J. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science. 1984 Mar 9;223(4640):1079–1082. doi: 10.1126/science.6320373. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Twardzik D. R., De Larco J. E., Stephenson J. R., Todaro G. J. Transforming growth factors produced by retrovirus-transformed rodent fibroblasts and human melanoma cells: amino acid sequence homology with epidermal growth factor. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4684–4688. doi: 10.1073/pnas.80.15.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. Epidermal growth factor-like transforming growth factor. II. Interaction with epidermal growth factor receptors in human placenta membranes and A431 cells. J Biol Chem. 1983 Nov 25;258(22):13614–13620. [PubMed] [Google Scholar]
- Plowman G. D., Green J. M., McDonald V. L., Neubauer M. G., Disteche C. M., Todaro G. J., Shoyab M. The amphiregulin gene encodes a novel epidermal growth factor-related protein with tumor-inhibitory activity. Mol Cell Biol. 1990 May;10(5):1969–1981. doi: 10.1128/mcb.10.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins K. C., Leal F., Pierce J. H., Aaronson S. A. The v-sis/PDGF-2 transforming gene product localizes to cell membranes but is not a secretory protein. EMBO J. 1985 Jul;4(7):1783–1792. doi: 10.1002/j.1460-2075.1985.tb03851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor O., Sameshima J. H., Robbins K. C. Differential association of cellular proteins with family protein-tyrosine kinases. J Biol Chem. 1991 Apr 5;266(10):6462–6466. [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Spigelman Z., Dowers A., Kennedy S., DiSorbo D., O'Brien M., Barr R., McCaffrey R. Antiproliferative effects of suramin on lymphoid cells. Cancer Res. 1987 Sep 1;47(17):4694–4698. [PubMed] [Google Scholar]
- Stein C. A., LaRocca R. V., Thomas R., McAtee N., Myers C. E. Suramin: an anticancer drug with a unique mechanism of action. J Clin Oncol. 1989 Apr;7(4):499–508. doi: 10.1200/JCO.1989.7.4.499. [DOI] [PubMed] [Google Scholar]
- Wong S. T., Winchell L. F., McCune B. K., Earp H. S., Teixidó J., Massagué J., Herman B., Lee D. C. The TGF-alpha precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell. 1989 Feb 10;56(3):495–506. doi: 10.1016/0092-8674(89)90252-3. [DOI] [PubMed] [Google Scholar]