Abstract
Atherosclerosis is a chronic inflammatory disease of the artery wall. Adaptive immunity plays a key role in the pathogenesis of atherosclerosis. Recently, modulation of the immune response against atherosclerotic plaque antigen(s) has attracted attention as a potentially preventive and therapeutic approach. Here we review a series of studies on immunization with various antigens targeting treatment and prevention of atherosclerosis. Atherosclerosis-related antigens include oxidized LDL, apolipoprotein B-100, and heat shock protein 60/65. Accumulating evidence supports the idea that immunization with these antigenic proteins or peptides may reduce atherosclerosis. In this review, we discuss the current status of immunization studies and possible associated mechanisms of atheroprotection.
Keywords: Atherosclerosis, immunization, apolipoprotein B-100, low density lipoprotein, heat shock protein 65
Introduction
Atherosclerosis is a chronic inflammatory disease characterized by intimal deposition of lipoproteins in large and medium-sized arteries. It is accompanied by innate and adaptive immune responses (1–3). Atherosclerosis is thought to be caused by a chronic response to arterial injury. In the last two decades, the role of local arterial tissue inflammation has been hypothesized to be a major underlying mechanism in the development of atherosclerosis (4–6). Subsequent investigations into the pathogenesis of atherosclerosis have revealed that both innate (7–9) and adaptive (3, 10) immunity play a significant role in the development and progression of atherosclerosis (4, 11, 12). While several auto-antigens have been suggested to trigger adaptive immune responses, the top three candidates for activating T cell mediated immune responses are oxidized LDL (oxLDL) (13), apolipoprotein B-100 (ApoB-100) (14, 15) and heat shock protein (HSP) 60/65 (16).
The current management of atherosclerotic vascular disease includes statins, antiplatelet drugs and antihypertensive compounds (17). However, several randomized clinical trials of statins and other preventive treatments suggest that it is difficult to achieve relative risk reductions exceeding 40% with current strategies (15). Accordingly, modulation of immune responses against atherosclerotic plaque antigens has attracted attention as a novel therapeutic strategy. In this review, we discuss recent progress in our understanding of the role of adaptive immunity in atherosclerosis, which provides a rationale and blueprint for the development of vaccines for modulation of adaptive immunity and prevention of cardiovascular disease.
Adaptive and humoral immunity in atherosclerosis
Adaptive immunity’s role in atherosclerosis was first hypothesized based on correlations between immunological biomarkers from blood samples of heart disease patients and examinations of atherosclerotic lesions (1). Serum titers of antibodies against HSP60 or HSP65 (18) and against oxLDL (19, 20) were found to be elevated in cardiovascular disease. Additional evidence suggests that T cells mediate proinflammatory and regulatory immune responses in atherosclerosis. In Rag1−/− Ldlr−/− mice, development of atherosclerotic lesions was significantly reduced compared to immunocompetent Ldlr−/− mice (21), suggesting that lymphocytes play a role in atherosclerosis. Zhou et al showed that Apoe−/− mice with severe combined immunodeficiency (SCID) developed less severe atherosclerosis compared to immunocompetent Apoe−/− mice (22). Importantly, transfer of CD4+ T cells from Apoe−/− to immunodeficient Apoe−/− mice aggravated atherosclerosis (22), strongly suggesting involvement of CD4+ T cells.
Patients with autoimmune diseases including systemic lupus erythematosus and rheumatoid arthritis have a significantly enhanced risk of atherosclerotic cardiovascular disease (23), which also supports the notion that adaptive immune responses are involved in its pathogenesis.
Humoral immunity
As mentioned above, patients with clinical evidence of atherosclerosis develop high titers of IgG and IgM antibodies to (modified) LDL (19, 20). In some studies, antibody titers were found to be inversely related to atherosclerosis (24), but other studies found a positive correlation (25, 26); thus, the relation between antibody titers and atherosclerotic disease burden and progression remains unclear. While so-called “natural” IgM antibodies can be produced by B1 cells without T cell help (27), most IgG production is dependent on isotype switching and requires T cell help (28). Specifically, follicular helper CD4+ T cells (TFH) are required for isotype switching (28).
B-1 cells secrete natural antibodies that are predominantly IgM and IgA (2). Several studies suggest that natural IgM antibodies have atheroprotective effects. Direct experimental evidence has shown that Ldlr−/− mice deficient in serum IgM developed larger atherosclerotic lesions when compared with wild-type Ldlr−/− mice (29). In humans, levels of plasma IgM reactive to oxLDL have an inverse relation to carotid atherosclerotic plaque size (24) and to coronary artery disease (20).
IgG antibodies are produced by B-2 cells in a TFH–dependent manner (2). T-helper (Th)1 and Th2 cells specifically activate mature B cells to produce IgG2a and IgG1 subclasses, respectively (30). In human studies, controversial results have been obtained with regard to association between anti-oxLDL IgG antibody and coronary artery disease. Some studies showed a positive correlation (26, 31), whereas other study showed a negative relationship (32).
T cells
How T cells affect the development and progression of atherosclerosis has been an area of intense study. T cells express either γδ or αβ T cell receptors. In general, αβ T cells express either CD4, a co-receptor for MHC-II, or CD8, a co-receptor for MHC-I. CD4+ T cells are further broadly categorized into functional subsets, such as into Th1, Th2, Th17, regulatory T cells (Treg) and TFH cells. TFH typically do not leave the lymph node, but rather stay in or near the germinal centers.
Th1 subsets are predominant in atherosclerotic plaque in humans and mice (33, 34), and cytokines associated with this subsets (such as IFN-γ) have been shown to play an important role in the pathogenesis of atherosclerosis based on several studies. Inhibition of Th1 polarization in Apoe−/− mice results in significant reduction of atherosclerotic lesion (35). While administration of IFN-γ increases atherosclerotic plaque (36), reduction of atherosclerotic lesion have been observed in IFN-γ deficient mice (37, 38) and IFN-γ receptor knockout mice (39). Consistently, deficiency of T-bet, which is the key transcription factor determining Th1 lineage, results in significant decrease of atherosclerosis in Ldlr−/− mice as well as shift of immune response toward Th2 (40). These observations support the evidence for the pathogenic role of Th1 cells in atherosclerosis.
Tregs are negative regulators of immune effector T cells. Natural Treg cells (nTreg cells) are produced in the thymus, and recognize auto-antigens. Naive T cells in the periphery can acquire Foxp3 expression and consequently Treg function, which are called induced Treg (iTreg). Although both nTregs and iTregs express CD4, CD25 (IL-2R), Foxp3, and CTLA-4 and have overlapping function, each subset differentiates distinctly and has different epigenetic status (41). The differentiation of nTreg cells in the thymus is involved with increased interactions with self-peptide-MHC complexes, while the differentiation of iTreg cells likely occurs in response to non-self antigens including allergens, food, and the microbiota (42). The balance between effector and regulatory T cells is believed to vary based on the antigen, the type of antigen-presenting cell (APC) and the co-stimulatory molecules. Besides Foxp3+ Tregs, it is thought that other types of Treg cells are induced from naive T cells in the periphery (43). CD4+ T cells secreting IL-10 and TGF-β, called Tr1 cells, are produced by antigenic stimulation of naive T cells in the presence of IL-10 (44, 45). Antigen-specific TGF-β-secreting T cells, called Th3 type Tregs, are TGF-β dependent and were discovered in the context of oral tolerance (46). Several studies have reported that Tregs may be protective in atherosclerosis. Adoptive transfer of Tr1 cells induced a significant decrease in Th1 responses revealed by reduced IFN-γ production, increased IL-10 production and significant reduction in atherosclerotic lesion size when compared with control mice (47). Adoptive transfer of CD4+CD25+ nTregs into Apoe−/−Rag2−/− mice ameliorated atherosclerosis in the aortic root (48).
The possible involvement of Th2 cells to progression of atherosclerosis remains to be elucidated. Both IL-4−/−Apoe−/− mice and IL-4−/−Ldlr−/− mice have shown no significant difference in atherosclerotic lesion compared to IL-4 wild-type control mice (49). In contrast, bone marrow transplantation from IL-5−/− mice into Ldlr−/− mice aggravated atherosclerosis, possibly due to decreased production of natural IgM antibody (50), suggesting IL-5 may be atheroprotective.
Th17 is a CD4+ T cell subset that produces IL-17A, IL-17F, and IL-22. The involvement of Th17 in atherosclerosis has been also investigated in recent years, but their role is still unclear (6). Treatment with mouse anti-mouse IL-17A antibody did not affect atherosclerosis development although the IL-17 signaling was abolished (51). Inconsistent results have been reported in several studies of genetic IL-17 deficiency in Apoe−/− mice, which include no difference (52), aggravation (53), and reduction (54, 55) of atherosclerotic lesion.
Although CD8+ T cells were found in human atherosclerotic plaque, the role of cytotoxic CD8+ T cells is not well investigated (56). A potential role for this cell type is suggested by the finding that cytotoxic and proinflammatory CD8+ T lymphocytes promote the development of vulnerable atherosclerotic plaques by perforin- and granzyme B-mediated apoptosis, resulting in necrotic core formation and inflammation (57).
Complex Immunogens: Immunization with oxLDL and MDA-LDL
Oxidation of lipoproteins (and oxidative processes in general) are thought to play an important role in the initiation and progression of atherosclerotic lesions (13). The oxidation of LDL results in various structural modifications and the formation of a large number of neoepitopes that may be recognized by antibodies (58). While the oxidation of LDL may be a trigger of the immune response, the mechanistic details are not known. Antibodies recognizing oxLDL-specific epitopes were detected in both human and rabbit atherosclerotic lesions (59). T cells reactive to oxLDL were also demonstrated from human plaque tissue two decades ago (60). Based on these findings, studies of protective immunity started with immunization with complex immunogens including native LDL, but also oxLDL, or malondialdehyde-modified LDL (MDA-LDL).
Palinski et al. reported that subcutaneous immunization with homologous MDA-LDL formulated in complete Freund’s adjuvant (CFA) and incomplete Freund’s adjuvant (IFA) reduces atherosclerosis in the thoracic aorta by 27% and in the abdominal aorta by 32% in Ldlr-deficient rabbits (61). These immunized rabbits had 10-fold higher MDA-LDL specific antibody titers (61). The ratio of antibody binding to MDA-LDL to antibody binding to native LDL was 10-fold, 2-fold, and equal in serum IgG, IgA, and IgM, respectively (61). Similarly, subcutaneous immunization with homologous LDL and copper-oxidized LDL reduced atherosclerotic lesions in high-cholesterol diet-fed rabbits in the proximal aorta by 74% and 48%, respectively (62).
Subsequently, several studies have supported the immunization concept in atherosclerotic mouse models. Freigang et al. reported that vaccination with both homologous native LDL and MDA-LDL reduced aortic atherosclerosis by 37% and 46%, respectively in Ldlr−/− mice (63). Induction of atheroprotection and high titers of anti-MDA-LDL IgG antibody by homologous MDA-LDL immunization was also observed in Apoe−/− mice (64, 65). It has been shown that administration of IgM with specificity for hypochlorite-oxLDL reduced atherosclerosis in Ldlr−/− mice (66) in a model of passive immunization. Interestingly, serum IgG binding to MDA-LDL and oxidized cholesterol was increased in MDA-LDL immunized mice, but not the native LDL group. In the MDA-LDL group, the titer of both IgG1 and IgG2a binding to MDA-LDL increased to the same extent. Since atheroprotection was observed in the LDL group in the absence of increased antibody titers, these data suggest that cellular immune responses may be involved with LDL-induced atheroprotection.
CD4+ T cells as a whole likely induce atherosclerosis. Apoe−/− mice deficient in CD4 developed less severe atherosclerotic lesion and low titers of IgG1, IgG2a, and IgM against MDA-LDL (67). When these mice were immunized with homologous MDA-LDL, a strong atheroprotective effect of CFA and IFA alone (adjuvant effect) with only weak additional atheroprotection by co-immunization with MDA-LDL was observed (67). These results suggest that CD4+ T cells as a whole play an important role in the progression of atherosclerosis and that atheroprotection induced by immunization may be mediated by CD4+ T cells.
However, several additional pieces of evidence suggest a potential dual role for CD4+ T cells. Adoptive transfer of oxLDL-pulsed dendritic cells (DCs) reduced atherosclerosis in Ldlr−/− mice (68). In contrast, some studies suggest that administration of oxLDL induces atherosclerosis. Adoptive transfer of bone marrow-derived DCs treated with MDA-LDL in vitro exacerbated atherosclerosis in Apoe−/− mice (69). In addition, transfer of CD4+ T cells isolated from oxLDL-immunized mice into Apoe−/− scid/scid mice induced increased fatty streak lesions in aortic root (70). These transferred T cells presumably include Th1 cells known to be atherogenic. This finding suggests that immunization with oxLDL potentially activates proinflammatory Th1 cells rather than Tregs. This may depend on the route and frequency of immunization and the adjuvants used.
Immunization with ApoB-100 Peptides recognized by autoantibodies
Researchers have focused on humoral immunity at an early stage because elevation of anti-ApoB antibody titers was frequently observed in patients and experimental animals. Three injections of antibody against MDA-modified ApoB-100 peptide (P45) improved atherosclerosis in Apoe−/− mice (71). The level of IgG against ApoB-100 peptides (P45 and P210) in serum from patients with cardiac disease was found to be lower than in healthy controls (32, 72). Taken together, these findings suggest that IgG reactive to ApoB-100 could be atheroprotective.
Because of the finding of naturally existing IgG and IgM to oxLDL, Fredrikson et al. tried to identify sequences from human ApoB-100 that are able to bind to IgG and IgM antibodies isolated from patients with acute coronary heart events (14). In their studies, several of these peptides have been used to immunize mice, including P45 (ApoB-100661–680)(73), P143 (ApoB-1002131–2150) (74), and P210 (ApoB-100 3136–3155) (74). Immunization with a mixture of P143 and P210 reduced atherosclerosis in the descending aorta of Apoe−/− mice by about 60% but induced slightly larger lesions in the subvalvular area (74). There was no difference in infiltrated macrophage count in plaque. The titer of IgG antibody against MDA-modified ApoB-100 was elevated, but the IgM titer was not changed (74). Similarly, immunization with another MDA-modified human ApoB-100 peptide (P45) reduced atherosclerotic plaque by 48% (73). Surprisingly, expression of IFN-γ, which is a proinflammatory Th1 cytokine and an exacerbating factor in atherogenesis, was highly elevated in aorta of P45 immunized mice (73). The authors argued that immunization with ApoB-100 peptide induce a shift in immune response from Th1 to Th2 because IgG1 against MDA-modified P45 was strongly induced. However, induction of inflammatory cytokines (i.e. IFN-γ) did not support this notion. Immunization with human ApoB-100 peptides (P45 and P210) was also examined in human ApoB-100 transgenic Ldlr−/− mice. Although injection of only native P45 significantly reduced aortic plaque by 66%, there was no detectable induction of peptide-specific antibodies and no difference in expression of Foxp3 and IL-10, both markers of Tregs (75). This is not too surprising, because P45 (and other peptides like P210) do not bind MHC-II and therefore cannot induce a CD4+ T cell response (6). In conclusion, the review of the data existing to date suggests that antibody responses are neither necessary nor sufficient to explain the disease modulation observed upon peptide immunizations.
Immunization with ApoB-100 Peptides and T cells responses
Hermansson et al. reported that T cells responsive to LDL express the T cell receptor cell receptorl(76). Depleting these T cells with an antibody against TRVB31 reduced atherosclerosis, suggesting that ApoB-100 reactive T cells may be involved with pathogenesis of atherosclerosis (76). In contrast, other studies have suggested that immunization with ApoB-100 causes an increase in Treg cells, resulting in reduction of atherosclerosis. Wigren et al have reported that immunization with human ApoB-100 peptide (P210) reduced plaque in the descending aorta by 37% and induced an increase in CD4+CD25+Foxp3+ cells in the spleen compared to control mice treated with PBS lacking adjuvant (77). Administration of anti-CD25 antibody (depleting Tregs) abrogated atheroprotection, which suggests that atheroprotection induced by this peptide involves Tregs. However, it should be taken into consideration that adjuvant alone could modulate immune response (78) and that anti-CD25 antibody treatment could abolish immune cells other than Tregs such as activated T cells and B cells. In a separate study, continuous subcutaneous injection of MDA-modified ApoB peptides (P210 and p240) reduced atherosclerosis in Apoe−/− mice and treatment with an anti-CD25 antibody again abrogated the atheroprotective effect (79). Elevated IL-10 production was also observed in plasma after immunization with human ApoB-100 peptides (80). In addition, adoptive transfer of DCs stimulated by ApoB-100 peptide P210 induced CD4+CD25+Foxp3+ cells in the spleen (80). Although these studies indicate Tregs might be involved with atheroprotection in immunized mice, further investigation is needed to clarify the role of Treg cell in immune response to peptide vaccination. Hermansson et al has examined atheroprotective effects of tolerogenic DCs treated with ApoB-100 protein and IL-10 (81). Injection of these tolerogenic DCs induced reduction of atherosclerosis, possibly due to suppressing activation of ApoB-100 reactive T cells (81). A vaccine containing human ApoB-100 peptide (P210) was in preclinical development for human safety and efficacy studies (82), but no active clinical trial is currently under way.
As mentioned above, the function of Th2 cells in atherosclerosis is unclear. It has been reported that elevation of IL-4 production in spleen correlated with reduction of atherosclerotic lesions in ApoB-100 immunized mice (73). However, another study has shown that Th2 cytokines and Th2-associated IgG1 against LDL induced by human ApoB-100 does not reduce atherosclerosis in Apoe−/− mice (83).
The role of CD8+ T cell on atherogenesis is also not well investigated. It was reported that adoptive transfer of CD8+ T cell from spleen of ApoB-100 peptide (P210) immunized mice attenuated atherosclerosis in Apoe−/− mice, although adoptive transfer of CD4+CD25+ cells did not affect atherosclerosis (84). In this study, it should be noted that the transferred CD8+ cell count was 3.3-fold higher than the transferred CD4+CD25+ cell count.
Immunization with MHC class II binding ApoB-100 Peptides
Although the peptides derived from human ApoB-100 were identified based on the binding capacity to human plasma antibody, it is interesting that these peptides also appear to induce Treg cells (77, 79, 80). The mechanism of this effect is unknown. To directly affect CD4+ helper T cell activation, the peptide would have to first bind MHC-II on an APC and be subsequently presented to a specific T cell whose T cell receptor recognizes the peptide–MHC complex (6). As we have reported (6), we found that peptide P210 has very low affinity for I-Ab (MHC class II in C57BL/6 mice) compared to well-known I-Ab-restricted T-cell epitopes, such as ovalbumin 323–339 peptide (85) and myelin oligodendrocyte glycoprotein 38–51 peptide (86). Likewise, for a CD4+ T cell to recognize molecules of (ox)LDL, APCs must first internalize oxLDL and present antigenic peptides in a manner that the T cell can recognize (Figure 1). For CD4+ T cells, this requires presentation by MHC-II. The protein component of LDL, ApoB-100, is processed into peptides by APCs, some of which bind MHC class II molecules, and are displayed on the APC cell surface (87). Indeed, ApoB-100 peptides are frequently displayed by HLA-DR (human MHC-II) molecules in cultured human lymphoblastoid cells (88).
Figure 1. Hypothetical adaptive immune response to atheroprotective immunization.
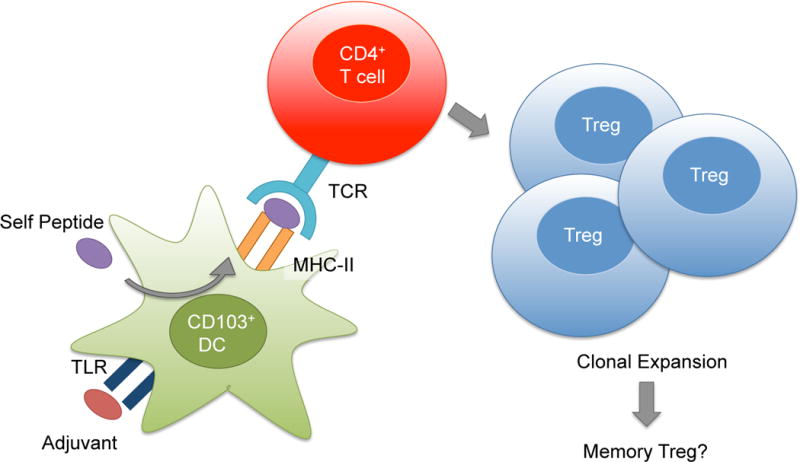
After self peptide (purple) is processed in CD103+ dendritic cells (DCs, green), antigen-MHC class II complex is presented to antigen-specific CD4+ T cells (red). Adjuvant stimulates toll-like receptors (TLR) and enables co-stimulation (not shown). Antigen presentation by CD103+ tolerogenic DCs likely induces the clonal expansion and the differentiation of antigen-specific CD4+ T cells to Treg cells, resulting in the suppression of inflammation and the reduction of atherosclerosis. Memory Tregs may form, persist and recirculate, resulting in durable atheroprotection.
To effectively induce a CD4+ T cell response, any antigenic peptide must bind MHC-II (I-Ab). Because of this requirement, we screened ApoB-100 peptides for binding to I-Ab using a competitive peptide binding assay to recombinant I-Ab purified from cell line (89). In our first study, two ApoB-100 peptides (ApoB3501-3516 [SQEYSGSVANEANVY] and ApoB978–993 [TGAYSNASSTESASY]) with high affinity for MHC class II were identified and shown to induce proliferation of peptide-specific T cells in vitro. These same peptides were effective in reducing atherosclerosis by about 40% in the aorta of Apoe−/− mice and one peptide also reduced lesions in the aortic roots (90). This study was aimed at prevention of atherosclerosis by immunizing around the time western diet was started. The mice were immunized with peptide in CFA followed by four booster injections with peptide in IFA. Among all cytokine mRNAs measured, only IL-10 mRNA was significantly elevated in the aortas of immunized mice. Although immunization induced highly peptide-specific IgG1 and IgG2c antibodies, these antibodies did not cross-react with native or modified LDL (90), suggesting that the protective mechanism is unlikely to be humoral. In an unpublished study, three more I-Ab binding peptides, but not a non-binding peptide, were found to be atheroprotective. These studies suggest that presentation by MHC-II is required for ApoB-100 peptides to induce atheroprotection and points to a likely Treg mechanism.
Immunization with HSP60/65
HSP60/65 is also mentioned as a candidate which can trigger an atheroprotective immune response (91). Human HSP60 shows mimicry with mycobacterial HSP65 and chlamydial HSP65 (92, 93). The expression of HSP60 is increased when cells are exposed to elevated temperatures or other stressors, and is the only HSP for which direct atherogenic potential has been proven in experimental and clinical studies (16). The titer of anti-HSP60 antibody is increased in blood sample in patients with hypertension and coronary artery disease (94, 95). When the therapeutic potential of HSP65 immunization was tested, controversial results were obtained. In fact, early studies suggested that HSP65 immunization increases atherosclerosis. In normocholesterolemic rabbits, atherosclerotic lesions developed after immunization with mycobacterial HSP65 in CFA and IFA adjuvant, although the levels of cholesterol in serum remained normal (96). In wild-type C57BL/6J mice, immunization with mycobacterial HSP65 led to an earlier onset of atherosclerotic lesions (97). To examine the involvement of T cell mediated immune response, Metzler et al immunized T cell depleted rabbits with mycobacterial HSP65 (98). Although arteriosclerotic lesions were observed in the aortic arch of immunocompetent rabbits as expected, rabbits depleted of T cells with anti-CD3 antibody developed significantly milder atherosclerosis (98). It has been reported that subcutaneous immunization with recombinant HSP65 and IFA as adjuvant promotes early atherosclerosis in Ldlr−/− mice (99). These immunized mice developed elevated titers of IgG antibody against HSP65, which suggests that this antibody alone is not atheroprotective. In addition, both adoptive transfer of HSP65-reactive lymph node cells and intraperitoneal administration of IgG derived from HSP65-immunized mice enhanced fatty-streak formation, suggesting the potential of HSP65 specific IgG to aggravate atherosclerosis (100). In addition, passive transfer of anti-HSP60 autoantibody from patients with coronary artery disease induced atherosclerosis in Apoe−/− mice (101).
In contrast, subcutaneous immunization with mycobacterial HSP65 in alum adjuvant and anti-CD45RB monoclonal antibody reduced atherosclerotic lesions in Apoe−/− mice (102). IgG1 antibody titer (a Th2 driven IgG) against HSP65 was also elevated (102). The discrepancy of these results with earlier studies is possibly due to the sort of adjuvant used or the stage of atherosclerosis. In fact, some studies indicate that adjuvant itself can modulate the immune response, usually leading to reduced atherosclerosis (78). Immunization with a combination ApoB peptide P45 (71) and HSP60 153-163 peptide (103) induced a significant reduction of atherosclerosis compared to P45 immunization alone (104), possibly due to reduced production of IFN-γ and increased production of IL-10.
Other immunization approaches
Several antigens other than ApoB-100 and HSP60/65 have been examined as potential immunogens. β2-glycoprotein I (β2GPI) has been thought to be the target of autoimmune anticardiolipin antibodies (105). In a study of subcutaneous immunization with human β2GPI, atherosclerosis was worsened in Ldlr−/− mice although the titer of anti-β2GPI antibody was elevated (106). Another group reported that intravenous injection of human β2GPI without adjuvant attenuated early atherosclerotic lesions in Ldlr−/− mice (107).
Binder et al found that antibodies against oxLDL cross-react with Streptococcus pneumonia (pneumococcus). Interestingly, subcutaneous immunization with pneumococcal immunogen decreased the extent of atherosclerosis and induced circulating oxLDL-reactive IgM (108). This finding suggests that vaccine-induced anti-pneumococcus antibody cross-reactive with oxLDL may have an atheroprotective potential. In two unconfirmed studies, immunization with complement C5a receptor peptide with alum adjuvant was atheroprotective compared to KLH with alum (109). Immunization with rat MDA-modified fibronectin reduced atherosclerosis in descending aorta and subvalvular lesion (110).
Interestingly, some studies suggest that administration of adjuvant alone can be atheroprotective. Freund’s adjuvant is commonly used, an emulsion of killed mycobacteria in mineral oil. CFA contains killed cells of Mycobacterium tuberculosis while IFA does not. Alum refers to adjuvants that comprise various aluminum salts. Alum is widely used for human vaccines. It has been reported that subcutaneous injection of CFA followed by intraperitoneal injection of IFA reduced atherosclerotic lesions in aortic roots of Apoe−/− mice (78). Intrapertitoneal injection of alum also induced atheroprotection (78). Similarly, subcutaneous injection of alum reduced atherosclerosis, possibly due to increased CD4+CD25+Foxp3+ T cells in the spleen (111).
Mucosal Immunization to Induce Tolerance
Mucosal immunization with antigens has been investigated in an effort to induce tolerance. Mucosal administration of antigen is known to generate tolerance and suppresses Th1 immune responses through production of IL-4, IL-10, and TGF-β, which induces unresponsiveness of immune system against a given antigen (112). Mucosal immunization has been explored using oxLDL and ApoB-100 peptides. Oral administration of human oxLDL and human MDA-LDL reduced atherosclerotic plaque in the aortic root and doubled the CD4+CD25+Foxp3+ cell count in spleen and mesenteric lymph node in Ldlr−/− mice (113). Nasal immunization with human ApoB-100 peptide (P210) fused to the B subunit of cholera toxin resulted in reduction of atherosclerosis in Apoe−/− mice (114). Although increased mRNA expression of Foxp3 was observed in the thoracic aorta of P210-immunized mice, there was no difference in the level of IL-10 expression between P210 and ovalbumin 323–339 peptide fused to cholera toxin.
While parenteral immunization with mycobacterial HSP65 is atherogenic, mucosal administration of HSP65 seems to induce atheroprotection. Nasal administration of mycobacterial HSP65 reduced atherosclerotic plaque as well as infiltration of CD4+ T cell and macrophages in Ldlr−/− mice (115). The titer of IgG1 antibody against HSP65 was increased in this study (115). Nasal administration of mycobacterial HSP65 reduced atherosclerosis also in high cholesterol diet-fed rabbits (116). In addition, oral administration of mycobacterial HSP65 reduced reactivity of lymphocytes to HSP65 and reduced atherosclerosis in Ldlr−/− mice (117). Similarly, oral administration of both mycobacterial HSP60 and HSP60 253-268 peptide reduced atherosclerosis (103). In the latter study, although the number of CD4+CD25+Foxp3+ T cells and expression of IL-10 and TGF-β were increased, causality was not tested. Oral administration of mycobacterial HSP65 delivered by Lactococcus lactis reduced atherosclerotic plaque in aortas of Ldlr−/− mice, accompanied by elevation of IL-10 production (118).
Recently, it has been reported that oral administration of a combination of human ApoB-100 peptide (P45) and HSP60 153-163 peptide induced enhanced reduction of atherosclerotic lesion compared to ApoB-100 peptide alone or HSP60 peptide alone in Ldlr−/− mice which express ApoB-100 but not ApoB-48 (119). The reason for this apparent synergistic effect is not known.
Conclusion
Over the past quarter century, immunization with the goal of preventing or reducing atherosclerosis has been explored in rabbits and mice. Immunization with complex antigens like oxLDL or MDA-LDL appears to be effective. Later, specific peptides derived from ApoB-100 were identified by reactivity with autoantibodies found in cardiovascular patients. These peptides, as well as several HSP60/65 peptides, were reported to be atheroprotective, although their ability to be presented by MHC-II was not tested. One of these peptides (P210) in fact does not bind mouse MHC-II, so it is surprising that these studies have suggested that the atheroprotective mechanism is primarily via increase in Foxp3+ Treg cells. Adjuvants commonly used in vaccination approaches can have strong atheroprotective effects independent of the antigen used. Oral tolerization has been reported to be successful in several studies, but the specificity of the tolerization was not assessed. Our report is the only one so far showing that MHC-II binding ApoB-100 peptides induce a CD4+ T cell response that is involved with the suppression of inflammation. Further work is needed to fully understand the mechanism of protective autoimmunity in atherosclerosis and to better understand the effects of immunization with self peptides in general.
Acknowledgments
Declaration of Interest: This work was supported by R01 HL121697 to Klaus Ley. Takayuki Kimura is funded by a research fellowship from Uehara Memorial Foundation.
References
- 1.Witztum JL, Lichtman AH. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol. 2014;9:73–102. doi: 10.1146/annurev-pathol-020712-163936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ait-Oufella H, Sage AP, Mallat Z, Tedgui A. Adaptive (T and B cells) immunity and control by dendritic cells in atherosclerosis. Circ Res. 2014;114:1640–1660. doi: 10.1161/CIRCRESAHA.114.302761. [DOI] [PubMed] [Google Scholar]
- 3.Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest. 2013;123:27–36. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 6.Tse K, Tse H, Sidney J, Sette A, Ley K. T cells in atherosclerosis. Int Immunol. 2013;25:615–622. doi: 10.1093/intimm/dxt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koltsova EK, Ley K. How dendritic cells shape atherosclerosis. Trends Immunol. 2011;32:540–547. doi: 10.1016/j.it.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koltsova EK, Hedrick CC, Ley K. Myeloid cells in atherosclerosis: a delicate balance of anti-inflammatory and proinflammatory mechanisms. Curr Opin Lipidol. 2013;24:371–380. doi: 10.1097/MOL.0b013e328363d298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2010;134:33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Ketelhuth DF, Hansson GK. Cellular immunity, low-density lipoprotein and atherosclerosis: break of tolerance in the artery wall. Thromb Haemost. 2011;106:779–786. doi: 10.1160/TH11-05-0321. [DOI] [PubMed] [Google Scholar]
- 12.Shimada K. Immune system and atherosclerotic disease: heterogeneity of leukocyte subsets participating in the pathogenesis of atherosclerosis. Circ J. 2009;73:994–1001. doi: 10.1253/circj.cj-09-0277. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 14.Fredrikson GN, Hedblad B, Berglund G, Alm R, Ares M, Cercek B, Chyu KY, Shah PK, Nilsson J. Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:872–878. doi: 10.1161/01.ATV.0000067935.02679.B0. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson J, Fredrikson GN, Bjorkbacka H, Chyu KY, Shah PK. Vaccines modulating lipoprotein autoimmunity as a possible future therapy for cardiovascular disease. J Intern Med. 2009;266:221–231. doi: 10.1111/j.1365-2796.2009.02150.x. [DOI] [PubMed] [Google Scholar]
- 16.Wick G, Jakic B, Buszko M, Wick MC, Grundtman C. The role of heat shock proteins in atherosclerosis. Nat Rev Cardiol. 2014;11:516–529. doi: 10.1038/nrcardio.2014.91. [DOI] [PubMed] [Google Scholar]
- 17.Fifth Joint Task Force of the European Society of, C., E. European Association of, I. European Association of Percutaneous Cardiovascular, A. European Heart Rhythm, A. Heart Failure, P. European Association for Cardiovascular, Rehabilitation, S. European Atherosclerosis, M. International Society of Behavioural, O. European Stroke, H. European Society of, D. European Association for the Study of, M. European Society of General Practice/Family, E. International Diabetes Federation, and N. European Heart. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): the Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur J Prev Cardiol. 2012;19:585–667. doi: 10.1177/2047487312450228. [DOI] [PubMed] [Google Scholar]
- 18.Grundtman C, Kreutmayer SB, Almanzar G, Wick MC, Wick G. Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:960–968. doi: 10.1161/ATVBAHA.110.217877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gounopoulos P, Merki E, Hansen LF, Choi SH, Tsimikas S. Antibodies to oxidized low density lipoprotein: epidemiological studies and potential clinical applications in cardiovascular disease. Minerva Cardioangiol. 2007;55:821–837. [PubMed] [Google Scholar]
- 20.Ravandi A, Boekholdt SM, Mallat Z, Talmud PJ, Kastelein JJ, Wareham NJ, Miller ER, Benessiano J, Tedgui A, Witztum JL, Khaw KT, Tsimikas S. Relationship of IgG and IgM autoantibodies and immune complexes to oxidized LDL with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk Study. J Lipid Res. 2011;52:1829–1836. doi: 10.1194/jlr.M015776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J Clin Invest. 2001;108:251–259. doi: 10.1172/JCI11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 23.Kahlenberg JM, Kaplan MJ. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu Rev Med. 2013;64:249–263. doi: 10.1146/annurev-med-060911-090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karvonen J, Paivansalo M, Kesaniemi YA, Horkko S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–2112. doi: 10.1161/01.CIR.0000092891.55157.A7. [DOI] [PubMed] [Google Scholar]
- 25.Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, Kornman KS, Berger PB. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48:425–433. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Hulthe J, Bokemark L, Fagerberg B. Antibodies to oxidized LDL in relation to intima-media thickness in carotid and femoral arteries in 58-year-old subjectively clinically healthy men. Arterioscler Thromb Vasc Biol. 2001;21:101–107. doi: 10.1161/01.atv.21.1.101. [DOI] [PubMed] [Google Scholar]
- 27.Tsiantoulas D, Diehl CJ, Witztum JL, Binder CJ. B cells and humoral immunity in atherosclerosis. Circ Res. 2014;114:1743–1756. doi: 10.1161/CIRCRESAHA.113.301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 29.Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 31.Dotevall A, Hulthe J, Rosengren A, Wiklund O, Wilhelmsen L. Autoantibodies against oxidized low-density lipoprotein and C-reactive protein are associated with diabetes and myocardial infarction in women. Clin Sci (Lond) 2001;101:523–531. [PubMed] [Google Scholar]
- 32.Sjogren P, Fredrikson GN, Samnegard A, Ericsson CG, Ohrvik J, Fisher RM, Nilsson J, Hamsten A. High plasma concentrations of autoantibodies against native peptide 210 of apoB-100 are related to less coronary atherosclerosis and lower risk of myocardial infarction. Eur Heart J. 2008;29:2218–2226. doi: 10.1093/eurheartj/ehn336. [DOI] [PubMed] [Google Scholar]
- 33.Uyemura K, Demer LL, Castle SC, Jullien D, Berliner JA, Gately MK, Warrier RR, Pham N, Fogelman AM, Modlin RL. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130–2138. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 35.Laurat E, Poirier B, Tupin E, Caligiuri G, Hansson GK, Bariety J, Nicoletti A. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2001;104:197–202. doi: 10.1161/01.cir.104.2.197. [DOI] [PubMed] [Google Scholar]
- 36.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol. 2000;157:1819–1824. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitman SC, Ravisankar P, Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J Interferon Cytokine Res. 2002;22:661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 38.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 39.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 45.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 46.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mallat Z, Gojova A, Brun V, Esposito B, Fournier N, Cottrez F, Tedgui A, Groux H. Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2003;108:1232–1237. doi: 10.1161/01.CIR.0000089083.61317.A1. [DOI] [PubMed] [Google Scholar]
- 48.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 49.King VL, Cassis LA, Daugherty A. Interleukin-4 does not influence development of hypercholesterolemia or angiotensin II-induced atherosclerotic lesions in mice. Am J Pathol. 2007;171:2040–2047. doi: 10.2353/ajpath.2007.060857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng X, Taleb S, Wang J, Tang TT, Chen J, Gao XL, Yao R, Xie JJ, Yu X, Xia N, Yan XX, Nie SF, Liao MY, Cheng Y, Mallat Z, Liao YH. Inhibition of IL-17A in atherosclerosis. Atherosclerosis. 2011;215:471–474. doi: 10.1016/j.atherosclerosis.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 52.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, Harrison DG. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1565–1572. doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Danzaki K, Matsui Y, Ikesue M, Ohta D, Ito K, Kanayama M, Kurotaki D, Morimoto J, Iwakura Y, Yagita H, Tsutsui H, Uede T. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:273–280. doi: 10.1161/ATVBAHA.111.229997. [DOI] [PubMed] [Google Scholar]
- 54.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012;110:675–687. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boyle JJ. Association of coronary plaque rupture and atherosclerotic inflammation. J Pathol. 1997;181:93–99. doi: 10.1002/(SICI)1096-9896(199701)181:1<93::AID-PATH696>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 57.Kyaw T, Winship A, Tay C, Kanellakis P, Hosseini H, Cao A, Li P, Tipping P, Bobik A, Toh BH. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation. 2013;127:1028–1039. doi: 10.1161/CIRCULATIONAHA.112.001347. [DOI] [PubMed] [Google Scholar]
- 58.Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew TE, Steinberg D, Witztum JL. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yla-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb Vasc Biol. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 60.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci U S A. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ameli S, Hultgardh-Nilsson A, Regnstrom J, Calara F, Yano J, Cercek B, Shah PK, Nilsson J. Effect of immunization with homologous LDL and oxidized LDL on early atherosclerosis in hypercholesterolemic rabbits. Arterioscler Thromb Vasc Biol. 1996;16:1074–1079. doi: 10.1161/01.atv.16.8.1074. [DOI] [PubMed] [Google Scholar]
- 63.Freigang S, Horkko S, Miller E, Witztum JL, Palinski W. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb Vasc Biol. 1998;18:1972–1982. doi: 10.1161/01.atv.18.12.1972. [DOI] [PubMed] [Google Scholar]
- 64.George J, Afek A, Gilburd B, Levkovitz H, Shaish A, Goldberg I, Kopolovic Y, Wick G, Shoenfeld Y, Harats D. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis. 1998;138:147–152. doi: 10.1016/s0021-9150(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 65.Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:108–114. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]
- 66.van Leeuwen M, Kemna MJ, de Winther MP, Boon L, Duijvestijn AM, Henatsch D, Bos NA, Gijbels MJ, Tervaert JW. Passive immunization with hypochlorite-oxLDL specific antibodies reduces plaque volume in LDL receptor-deficient mice. PLoS One. 2013;8:e68039. doi: 10.1371/journal.pone.0068039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou X, Robertson AK, Rudling M, Parini P, Hansson GK. Lesion development and response to immunization reveal a complex role for CD4 in atherosclerosis. Circ Res. 2005;96:427–434. doi: 10.1161/01.RES.0000156889.22364.f1. [DOI] [PubMed] [Google Scholar]
- 68.Habets KL, van Puijvelde GH, van Duivenvoorde LM, van Wanrooij EJ, de Vos P, Tervaert JW, van Berkel TJ, Toes RE, Kuiper J. Vaccination using oxidized low-density lipoprotein-pulsed dendritic cells reduces atherosclerosis in LDL receptor-deficient mice. Cardiovasc Res. 2010;85:622–630. doi: 10.1093/cvr/cvp338. [DOI] [PubMed] [Google Scholar]
- 69.Hjerpe C, Johansson D, Hermansson A, Hansson GK, Zhou X. Dendritic cells pulsed with malondialdehyde modified low density lipoprotein aggravate atherosclerosis in Apoe(−/−) mice. Atherosclerosis. 2010;209:436–441. doi: 10.1016/j.atherosclerosis.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Zhou X, Robertson AK, Hjerpe C, Hansson GK. Adoptive transfer of CD4+ T cells reactive to modified low-density lipoprotein aggravates atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:864–870. doi: 10.1161/01.ATV.0000206122.61591.ff. [DOI] [PubMed] [Google Scholar]
- 71.Schiopu A, Bengtsson J, Soderberg I, Janciauskiene S, Lindgren S, Ares MP, Shah PK, Carlsson R, Nilsson J, Fredrikson GN. Recombinant human antibodies against aldehyde-modified apolipoprotein B-100 peptide sequences inhibit atherosclerosis. Circulation. 2004;110:2047–2052. doi: 10.1161/01.CIR.0000143162.56057.B5. [DOI] [PubMed] [Google Scholar]
- 72.Fredrikson GN, Schiopu A, Berglund G, Alm R, Shah PK, Nilsson J. Autoantibody against the amino acid sequence 661–680 in apo B-100 is associated with decreased carotid stenosis and cardiovascular events. Atherosclerosis. 2007;194:e188–e192. doi: 10.1016/j.atherosclerosis.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 73.Fredrikson GN, Andersson L, Soderberg I, Dimayuga P, Chyu KY, Shah PK, Nilsson J. Atheroprotective immunization with MDA-modified apo B-100 peptide sequences is associated with activation of Th2 specific antibody expression. Autoimmunity. 2005;38:171–179. doi: 10.1080/08916930500050525. [DOI] [PubMed] [Google Scholar]
- 74.Fredrikson GN, Soderberg I, Lindholm M, Dimayuga P, Chyu KY, Shah PK, Nilsson J. Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler Thromb Vasc Biol. 2003;23:879–884. doi: 10.1161/01.ATV.0000067937.93716.DB. [DOI] [PubMed] [Google Scholar]
- 75.Fredrikson GN, Bjorkbacka H, Soderberg I, Ljungcrantz I, Nilsson J. Treatment with apo B peptide vaccines inhibits atherosclerosis in human apo B-100 transgenic mice without inducing an increase in peptide-specific antibodies. J Intern Med. 2008;264:563–570. doi: 10.1111/j.1365-2796.2008.01995.x. [DOI] [PubMed] [Google Scholar]
- 76.Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wigren M, Kolbus D, Duner P, Ljungcrantz I, Soderberg I, Bjorkbacka H, Fredrikson GN, Nilsson J. Evidence for a role of regulatory T cells in mediating the atheroprotective effect of apolipoprotein B peptide vaccine. J Intern Med. 2011;269:546–556. doi: 10.1111/j.1365-2796.2010.02311.x. [DOI] [PubMed] [Google Scholar]
- 78.Khallou-Laschet J, Tupin E, Caligiuri G, Poirier B, Thieblemont N, Gaston AT, Vandaele M, Bleton J, Tchapla A, Kaveri SV, Rudling M, Nicoletti A. Atheroprotective effect of adjuvants in apolipoprotein E knockout mice. Atherosclerosis. 2006;184:330–341. doi: 10.1016/j.atherosclerosis.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 79.Herbin O, Ait-Oufella H, Yu W, Fredrikson GN, Aubier B, Perez N, Barateau V, Nilsson J, Tedgui A, Mallat Z. Regulatory T-cell response to apolipoprotein B100-derived peptides reduces the development and progression of atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:605–612. doi: 10.1161/ATVBAHA.111.242800. [DOI] [PubMed] [Google Scholar]
- 80.Pierides C, Bermudez-Fajardo A, Fredrikson GN, Nilsson J, Oviedo-Orta E. Immune responses elicited by apoB-100-derived peptides in mice. Immunol Res. 2013;56:96–108. doi: 10.1007/s12026-013-8383-1. [DOI] [PubMed] [Google Scholar]
- 81.Hermansson A, Johansson DK, Ketelhuth DF, Andersson J, Zhou X, Hansson GK. Immunotherapy with tolerogenic apolipoprotein B-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation. 2011;123:1083–1091. doi: 10.1161/CIRCULATIONAHA.110.973222. [DOI] [PubMed] [Google Scholar]
- 82.Nilsson J, Wigren M, Shah PK. Vaccines against atherosclerosis. Expert Rev Vaccines. 2013;12:311–321. doi: 10.1586/erv.13.4. [DOI] [PubMed] [Google Scholar]
- 83.Engelbertsen D, Rattik S, Knutsson A, Bjorkbacka H, Bengtsson E, Nilsson J. Induction of T helper 2 responses against human apolipoprotein B100 does not affect atherosclerosis in ApoE−/− mice. Cardiovasc Res. 2014;103:304–312. doi: 10.1093/cvr/cvu131. [DOI] [PubMed] [Google Scholar]
- 84.Chyu KY, Zhao X, Dimayuga PC, Zhou J, Li X, Yano J, Lio WM, Chan LF, Kirzner J, Trinidad P, Cercek B, Shah PK. CD8+ T cells mediate the athero-protective effect of immunization with an ApoB-100 peptide. PLoS One. 2012;7:e30780. doi: 10.1371/journal.pone.0030780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, Snoke K, Serra HM, Kubo RT, Sette A, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–761. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 86.Petersen TR, Bettelli E, Sidney J, Sette A, Kuchroo V, Backstrom BT. Characterization of MHC- and TCR-binding residues of the myelin oligodendrocyte glycoprotein 38–51 peptide. Eur J Immunol. 2004;34:165–173. doi: 10.1002/eji.200324669. [DOI] [PubMed] [Google Scholar]
- 87.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 88.Chicz RM, Urban RG, Gorga JC, Vignali DA, Lane WS, Strominger JL. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sidney J, Southwood S, Moore C, Oseroff C, Pinilla C, Grey HM, Sette A. Measurement of MHC/peptide interactions by gel filtration or monoclonal antibody capture. Curr Protoc Immunol. 2013 doi: 10.1002/0471142735.im1803s100. Chapter 18: Unit 18 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tse K, Gonen A, Sidney J, Ouyang H, Witztum JL, Sette A, Tse H, Ley K. Atheroprotective Vaccination with MHC-II Restricted Peptides from ApoB-100. Front Immunol. 2013;4:493. doi: 10.3389/fimmu.2013.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wick G, Schett G, Amberger A, Kleindienst R, Xu Q. Is atherosclerosis an immunologically mediated disease? Immunol Today. 1995;16:27–33. doi: 10.1016/0167-5699(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 92.Wick G, Xu Q. Atherosclerosis–an autoimmune disease. Exp Gerontol. 1999;34:559–566. [PubMed] [Google Scholar]
- 93.Perschinka H, Mayr M, Millonig G, Mayerl C, van der Zee R, Morrison SG, Morrison RP, Xu Q, Wick G. Cross-reactive B-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1060–1065. doi: 10.1161/01.ATV.0000071701.62486.49. [DOI] [PubMed] [Google Scholar]
- 94.Zhu J, Quyyumi AA, Rott D, Csako G, Wu H, Halcox J, Epstein SE. Antibodies to human heat-shock protein 60 are associated with the presence and severity of coronary artery disease: evidence for an autoimmune component of atherogenesis. Circulation. 2001;103:1071–1075. doi: 10.1161/01.cir.103.8.1071. [DOI] [PubMed] [Google Scholar]
- 95.Kervinen H, Huittinen T, Vaarala O, Leinonen M, Saikku P, Manninen V, Mänttäri M. Antibodies to human heat shock protein 60, hypertension and dyslipidemia. A study of joint effects on coronary risk. Atherosclerosis. 2003;169:339–344. doi: 10.1016/s0021-9150(03)00229-6. [DOI] [PubMed] [Google Scholar]
- 96.Xu Q, Dietrich H, Steiner HJ, Gown AM, Schoel B, Mikuz G, Kaufmann SH, Wick G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb. 1992;12:789–799. doi: 10.1161/01.atv.12.7.789. [DOI] [PubMed] [Google Scholar]
- 97.George J, Shoenfeld Y, Afek A, Gilburd B, Keren P, Shaish A, Kopolovic J, Wick G, Harats D. Enhanced fatty streak formation in C57BL/6J mice by immunization with heat shock protein-65. Arterioscler Thromb Vasc Biol. 1999;19:505–510. doi: 10.1161/01.atv.19.3.505. [DOI] [PubMed] [Google Scholar]
- 98.Metzler B, Mayr M, Dietrich H, Singh M, Wiebe E, Xu Q, Wick G. Inhibition of arteriosclerosis by T-cell depletion in normocholesterolemic rabbits immunized with heat shock protein 65. Arterioscler Thromb Vasc Biol. 1999;19:1905–1911. doi: 10.1161/01.atv.19.8.1905. [DOI] [PubMed] [Google Scholar]
- 99.Afek A, George J, Gilburd B, Rauova L, Goldberg I, Kopolovic J, Harats D, Shoenfeld Y. Immunization of low-density lipoprotein receptor deficient (LDL-RD) mice with heat shock protein 65 (HSP-65) promotes early atherosclerosis. J Autoimmun. 2000;14:115–121. doi: 10.1006/jaut.1999.0351. [DOI] [PubMed] [Google Scholar]
- 100.George J, Afek A, Gilburd B, Shoenfeld Y, Harats D. Cellular and humoral immune responses to heat shock protein 65 are both involved in promoting fatty-streak formation in LDL-receptor deficient mice. J Am Coll Cardiol. 2001;38:900–905. doi: 10.1016/s0735-1097(01)01440-1. [DOI] [PubMed] [Google Scholar]
- 101.Foteinos G, Afzal AR, Mandal K, Jahangiri M, Xu Q. Anti-heat shock protein 60 autoantibodies induce atherosclerosis in apolipoprotein E-deficient mice via endothelial damage. Circulation. 2005;112:1206–1213. doi: 10.1161/CIRCULATIONAHA.105.547414. [DOI] [PubMed] [Google Scholar]
- 102.Klingenberg R, Ketelhuth DF, Strodthoff D, Gregori S, Hansson GK. Subcutaneous immunization with heat shock protein-65 reduces atherosclerosis in Apoe(−)/(−) mice. Immunobiology. 2012;217:540–547. doi: 10.1016/j.imbio.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 103.van Puijvelde GH, van Es T, van Wanrooij EJ, Habets KL, de Vos P, van der Zee R, van Eden W, van Berkel TJ, Kuiper J. Induction of oral tolerance to HSP60 or an HSP60-peptide activates T cell regulation and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2677–2683. doi: 10.1161/ATVBAHA.107.151274. [DOI] [PubMed] [Google Scholar]
- 104.Lu X, Chen D, Endresz V, Xia M, Faludi I, Burian K, Szabo A, Csanadi A, Miczak A, Gonczol E, Kakkar V. Immunization with a combination of ApoB and HSP60 epitopes significantly reduces early atherosclerotic lesion in Apobtm2SgyLdlrtm1Her/J mice. Atherosclerosis. 2010;212:472–480. doi: 10.1016/j.atherosclerosis.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 105.Tsutsumi A, Matsuura E, Ichikawa K, Fujisaku A, Mukai M, Kobayashi S, Koike T. Antibodies to beta 2-glycoprotein I and clinical manifestations in patients with systemic lupus erythematosus. Arthritis Rheum. 1996;39:1466–1474. doi: 10.1002/art.1780390905. [DOI] [PubMed] [Google Scholar]
- 106.George J, Afek A, Gilburd B, Blank M, Levy Y, Aron-Maor A, Levkovitz H, Shaish A, Goldberg I, Kopolovic J, Harats D, Shoenfeld Y. Induction of Early Atherosclerosis in LDL-Receptor Deficient Mice Immunized With 2-Glycoprotein I. Circulation. 1998;98:1108–1115. doi: 10.1161/01.cir.98.11.1108. [DOI] [PubMed] [Google Scholar]
- 107.De Haro J, Esparza L, Bleda S, Varela C, Sanchez C, Acin F. Attenuation of early atherosclerotic lesions by immunotolerance with beta2 glycoprotein I and the immunomodulatory effectors interleukin 2 and 10 in a murine model. J Vasc Surg. 2014 doi: 10.1016/j.jvs.2014.05.096. [DOI] [PubMed] [Google Scholar]
- 108.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 109.Lu X, Xia M, Endresz V, Faludi I, Mundkur L, Gonczol E, Chen D, Kakkar VV. Immunization with a combination of 2 peptides derived from the C5a receptor significantly reduces early atherosclerotic lesion in Ldlr(tm1Her) Apob(tm2Sgy) J mice. Arterioscler Thromb Vasc Biol. 2012;32:2358–2371. doi: 10.1161/ATVBAHA.112.253179. [DOI] [PubMed] [Google Scholar]
- 110.Duner P, To F, Beckmann K, Bjorkbacka H, Fredrikson GN, Nilsson J, Bengtsson E. Immunization of apoE−/− mice with aldehyde-modified fibronectin inhibits the development of atherosclerosis. Cardiovasc Res. 2011;91:528–536. doi: 10.1093/cvr/cvr101. [DOI] [PubMed] [Google Scholar]
- 111.Wigren M, Bengtsson D, Duner P, Olofsson K, Bjorkbacka H, Bengtsson E, Fredrikson GN, Nilsson J. Atheroprotective effects of Alum are associated with capture of oxidized LDL antigens and activation of regulatory T cells. Circ Res. 2009;104:e62–e70. doi: 10.1161/CIRCRESAHA.109.196667. [DOI] [PubMed] [Google Scholar]
- 112.Faria AM, Weiner HL. Oral tolerance: mechanisms and therapeutic applications. Adv Immunol. 1999;73:153–264. doi: 10.1016/s0065-2776(08)60787-7. [DOI] [PubMed] [Google Scholar]
- 113.van Puijvelde GH, Hauer AD, de Vos P, van den Heuvel R, van Herwijnen MJ, van der Zee R, van Eden W, van Berkel TJ, Kuiper J. Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation. 2006;114:1968–1976. doi: 10.1161/CIRCULATIONAHA.106.615609. [DOI] [PubMed] [Google Scholar]
- 114.Klingenberg R, Lebens M, Hermansson A, Fredrikson GN, Strodthoff D, Rudling M, Ketelhuth DF, Gerdes N, Holmgren J, Nilsson J, Hansson GK. Intranasal immunization with an apolipoprotein B-100 fusion protein induces antigen-specific regulatory T cells and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:946–952. doi: 10.1161/ATVBAHA.109.202671. [DOI] [PubMed] [Google Scholar]
- 115.Maron R, Sukhova G, Faria AM, Hoffmann E, Mach F, Libby P, Weiner HL. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation. 2002;106:1708–1715. doi: 10.1161/01.cir.0000029750.99462.30. [DOI] [PubMed] [Google Scholar]
- 116.Xiong Q, Li J, Jin L, Liu J, Li T. Nasal immunization with heat shock protein 65 attenuates atherosclerosis and reduces serum lipids in cholesterol-fed wild-type rabbits probably through different mechanisms. Immunol Lett. 2009;125:40–45. doi: 10.1016/j.imlet.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 117.Harats D, Yacov N, Gilburd B, Shoenfeld Y, George J. Oral tolerance with heat shock protein 65 attenuates Mycobacterium tuberculosis-induced and high-fat-diet-driven atherosclerotic lesions. J Am Coll Cardiol. 2002;40:1333–1338. doi: 10.1016/s0735-1097(02)02135-6. [DOI] [PubMed] [Google Scholar]
- 118.Jing H, Yong L, Haiyan L, Yanjun M, Yun X, Yu Z, Taiming L, Rongyue C, Liang J, Jie W, Li Z, Jingjing L. Oral administration of Lactococcus lactis delivered heat shock protein 65 attenuates atherosclerosis in low-density lipoprotein receptor-deficient mice. Vaccine. 2011;29:4102–4109. doi: 10.1016/j.vaccine.2011.03.105. [DOI] [PubMed] [Google Scholar]
- 119.Mundkur L, Mukhopadhyay R, Samson S, Varma M, Kale D, Chen D, Shivaprasad S, Sivanandan H, Soman V, Lu X, Kakkar VV. Mucosal tolerance to a combination of ApoB and HSP60 peptides controls plaque progression and stabilizes vulnerable plaque in Apob(tm2Sgy)Ldlr(tm1Her)/J mice. PLoS One. 2013;8:e58364. doi: 10.1371/journal.pone.0058364. [DOI] [PMC free article] [PubMed] [Google Scholar]


