Abstract
Schizophrenia can be conceptualized as a disorder of functional connectivity within the fronto-temporal (FT) and/or default-mode (DM) networks. Recent evidence suggests that dysfunctional integration between these large neural networks may also contribute to the illness, and that the ability to mentalize or have a ‘theory of mind’ (ToM) is discernibly impaired in patients with schizophrenia. Hence in this study, we examined whether impaired functional network connectivity (FNC) contributes to a compromise in the ability to mentalize in patients with schizophrenia. Functional magnetic resonance imaging data were acquired from 20 male schizophrenia patients and 19 matched healthy controls whilst performing a well-known ToM task. The study revealed that relative to non-ToM the engagement of ToM produced reduced neural activity in the lateral FT and insula networks in patients, as compared to healthy subjects. The findings also indicated that in comparison to healthy subjects the DM and medial FT networks are less suppressed in patients irrespective of the task (ToM/non-ToM). Further, FNC analyses showed that the degree of functional connectivity between task-positive (lateral FT and insula) and task-negative (medial FT, posterior DM) networks was significantly reduced in patients as compared to controls. Of note, a significant correlation between the functional connectivity strength of the lateral FT network with the medial FT and the degree to which this is modulated by the ToM task, suggest that mentalizing deficits in male schizophrenia patients may stem from impaired communication between neural networks that comprehend the mental states of self (medial FT) and others (lateral FT).
Keywords: functional network connectivity, FNC, independent component analysis, ICA, fMRI, schizophrenia, theory of mind
Introduction
Phenotypically, schizophrenia is a heterogeneous psychiatric disorder of neurodevelopmental origin (Murray and Lewis, 1987) that stems from disturbances in brain circuits and neurotransmitter systems (Weinberger, 1987). Likewise genetically, schizophrenia appears to be a complex trait that manifests via equally complicated endophenotypes (Flint and Munafo, 2007). However, clinically, one of its characteristic features is a diminished ability to empathise with others (Bleuler, 1950). This capacity to appraise the internal state of others is broadly referred to as ‘mentalizing’, and is often used interchangeably with the term “Theory of Mind” (ToM) (Premack and Woodruff, 1978) even though technically mentalizing has both emotional and cognitive components whereas ToM places greater focus on the cognition. Consequently, ToM is applied more specifically to the appraisal of others as opposed to one's self however, with the conceptualization of the mirror neuron system (MNS) ToM is now also thought to involve self-attribution. Therefore, mentalizing is actually the process by which ToM is acquired.
Applying these concepts somewhat broadly a growing number of neuropsychological studies indicate that ToM is perhaps impaired in schizophrenia (Bora et al., 2009; Brüne, 2005; Harrington et al., 2005), but few studies to date have used neuroimaging to identify the neurobiological substrate of any such impairment (Andreasen et al., 2008; Brüne et al., 2008; Brunet et al., 2003; Das et al., 2012; Russell et al., 2000; Walter et al., 2009). Those studies that have used functional MRI (fMRI) have relied primarily on the general linear model (GLM) to identify the brain regions that show task-related neural activity differences between healthy controls and schizophrenia patients. This approach is appropriate for localization but does not allow the identification of ‘interconnectedness’ per se, between functionally related brain regions, or networks and this is critical because current cognitive and affective models of impairment in schizophrenia suggest that the illness stems from abnormal functional connectivity (FC) between distant brain regions (Andreasen et al., 2008; Calhoun et al., 2009; Das et al., 2007; Friston and Frith, 1995; Garrity et al., 2007). Encompassing some of these regions is the fronto-temporal (FT) network, which includes both ventral and dorsal areas of the medial (MPFC) and lateral (LPFC) prefrontal cortices and superior temporal lobe (Friston and Frith, 1995; Wolf et al., 2007). This network is thought to have a pivotal role in schizophrenia but in recent years, the focus of research has shifted towards the ‘default-mode’ (DM) (Raichle et al., 2001) network (Bluhm et al., 2007; Calhoun et al., 2009; Garrity et al., 2007; Zhou et al., 2007), which by virtue of being more active during rest than during active cognitive processes is thought to provide the neural substrates of task-independent self-relevant information processing. Like the FT network the DM network also involves the MPFC that in turn includes the ventral anterior cingulate cortex (ACC). Other brain regions attributed to the DM network include the posterior cingulate cortex (PCC) extending as far as the precuneus (PC), and the lateral parietal cortex (Raichle et al., 2001). Abnormal activity and connectivity within the DM network in patients with schizophrenia has been identified (Bluhm et al., 2007; Calhoun et al., 2009; Garrity et al., 2007; Zhou et al., 2007). Interestingly, fMRI studies in healthy subjects have found that regions of the DM and FT networks such as MPFC, PC, PCC, the superior temporal sulcus (STS), temporo-parietal junction (TPJ) also contribute to mentalizing (Frith and Frith, 2003; Gallagher and Frith, 2003; Saxe and Wexler, 2005; Vogeley et al., 2001).
Consequently, the traditional view of schizophrenia as a disorder of functional connectivity within a network, is being revised and the disorder is being conceptualised as emerging from dysfunctional integration among neural networks that are spatially independent but functionally correlated (Demirci et al., 2009; Jafri et al., 2008). In support of this perspective, changes in the functional network connectivity (FNC) ‘between’ the major networks of the brain have been identified in patients with schizophrenia both at rest (Jafri et al., 2008), and when engaged in cognitive tasks (Demirci et al., 2009). One possible explanation for this is that in order to remain alert and attuned to changes in the external and internal environments the brain repeatedly toggles between “task-positive” and “task-negative” (or task-independent) oriented modes (Fransson, 2005; Raichle et al., 2001). Naturally any functional impairment within either mode, or between the two can impair essential information processing (Eichele et al., 2008; Fox et al., 2005).
In the current study, we set out to investigate whether ToM deficits in male patients with schizophrenia are due to impaired functional connectivity among task-positive and task-negative networks. In order to do this we used fMRI and a well-established ToM task. The details of this study are described in full in an earlier publication in this journal (Das et al., 2012) however, for convenience we have provided a detailed outline.
Subjects and methods
Participants & Clinical Assessment
Twenty-three right-handed male patients with schizophrenia (Mean age = 34.5 years, SD = ±8.4) and 22 healthy males (Mean age = 33.5 years, SD = ±8.4), matched on the basis of age and handedness participated in the study. Data from three subjects in each group could not be analysed (failure to complete task/movement artefact) and therefore the sample sizes for analysis were 20 and 19 respectively. Inclusion criteria for the study were that participants must be male, between 18-50 years of age, right handed, and able to give informed consent. Patients were also required to have a primary diagnosis of DSM-IV schizophrenia with no additional Axis-I/Axis-II psychiatric diagnosis. Exclusion criteria included: history of neurological disease, closed head injury, medical disorder necessitating treatment or a history of substance misuse. All participants provided written informed consent according to the Hospital and University ethics committee's protocols. A diagnosis of schizophrenia was assigned using the structured clinical interview for DSM-IV (SCID-P) (First, 1995) and clinical symptoms were rated using the positive and negative syndrome scale (PANSS) (Kay, 1986). Insufficient information in relation to duration of illness and clinical symptomatology was recorded on three patients. The remaining 17 patients had a mean illness duration of 9.4 years (SD = ±6.5) and scored 18.21 (SD = ±5.2) on the PANSS negative symptom subscale and 10.05 (SD = ±3.0) on the PANSS positive symptom subscale. Of those that underwent scanning all except one were taking antipsychotic medications and in addition, four patients were taking lithium and nine were taking sertraline. The latter two medications were prescribed for the treatment of mood symptoms in the context of schizophrenia.
fMRI task
The task used in this study has previously been used to investigate ToM deficits in schizophrenia (Das et al., 2012), bipolar disorder (Malhi et al., 2008), and autism (Castelli et al., 2002). This task is designed to capture the implicit components of mentalizing. It comprises a series of silent animations of two triangles, a large red triangle and a small blue triangle (Castelli et al., 2000) and involves the attribution of mental states to these moving shapes. Participants viewed two types of animations: those involving ToM and those not involving Tom, referred to as non-ToM. In ToM animations the two triangles mimicked human behaviour such as bluffing, persuading, surprising and mocking one another, whereas in non-ToM animations the triangles drifted and bounced off the walls randomly with no meaningful interaction between them. In total, participants viewed 16 blocks of animation, in which four distinct ToM and four random-motion (non-ToM) sequences were each presented twice. Each animated block lasted 36s and between juxtaposed animation blocks there was a six-second fade-in / fade-out segment. The ToM conditions alternated with non-ToM but were still counterbalanced. The ToM and random-motion animated sequences were matched as closely as possible for basic visual characteristics such as overall speed, shape and orientation (Castelli et al., 2000).
Prior to scanning each participant was instructed as follows: “You will see two triangles on the screen. One triangle will be larger than the other and both will move around with respect to each other. You will need to observe carefully how both triangles move around the screen and interact with each other and we will be asking you some questions about what you have been shown following the scan.” Immediately following the MRI scanning session, patients were again shown the animated stimuli and asked: “What was happening in the animation?” The verbal descriptions were noted and rated using specific criteria (Castelli et al., 2002) on two dimensions. The first, ‘intentionality’, captures the degree of appreciation of mental states and is rated from 0 (appreciation of a non-deliberate action) to 5 (appreciation of a deliberate action aimed at affecting another's mental state). The second dimension, ‘appropriateness’, assesses how well the underlying script in an animation is understood and is rated from 0 (in the event of no answer or a response of ‘don't know’) to 3 (an appropriate, clear answer). The complete procedures and further details for scoring have been published previously (Castelli et al., 2000).
Functional MRI Acquisition Parameters
Images were acquired using a 3T Siemens Trio scanner. Twenty-eight consecutive axial slices (5mm thickness with no gap) parallel to the anterior and posterior commissure covering the whole brain were imaged using a T2*-weighted gradient echo EPI sequence: TE = 35 ms; TR = 3000 ms; matrix = 64 × 64; flip angle = 90°; FOV=240mm, in-plane resolution = 3.75mm. For each functional run a total of 224 whole brain scans were collected. For anatomical reference, a high-resolution T1 weighted image was also acquired: TR = 1570ms; TE = 3.22 ms; flip angle = 15°; matrix 512 × 512 × 192.
fMRI Data Analysis
Analysis overview
First, 20 spatially independent but temporally correlated networks were determined from the pre-processed data of all participants using independent component analysis (ICA) (Calhoun et al., 2001b). This analysis captures the complex nature of fMRI data and produces consistent spatial components or networks (Turner and Twieg, 2005). Second, components for FNC analysis were selected by virtue of fulfilling one of two criteria: either component represented the DM or FT network or it displayed significantly differential modulation by the ToM task in the two groups. Third, FNC among these chosen networks was calculated for each subject and significant differences between groups in FNC were then identified. Finally, in order to understand whether any impairment in FNC contributes to ToM deficits, the relationship between FNC strength and the degree of modulation by the ToM task (compared to non-ToM task) was investigated.
a) Pre-processing
Pre-processing was performed using statistical parametric mapping (SPM5, version 958) (http://www.fil.ion.ucl.ac.uk/spm). Each subject's functional and structural images were first visually inspected for scanner artifacts and gross anatomical abnormalities, and then re-oriented so that the image origin lies within 3cm of the anterior commissure (AC). For each subject, images were first corrected for susceptibility-by-movement artefacts and then realigned to the first volume of the time series. The high-resolution structural MR image was then aligned to the mean of the T2* weighted functional images and then spatially normalized to the Montreal Neurological Institute (MNI) template. Parameter estimates determined from the spatial normalization of the structural image to the MNI template were then applied to spatially normalize functional images to the MNI template. The normalized functional data were then smoothed using a Gaussian smoothing kernel of 8mm full-width at half-maximum (FWHM) to improve the signal to noise ratio. Following spatial normalization, the data (originally acquired at 3.75 × 3.75 × 5 mm3) were slightly subsampled to 3 × 3 × 3 mm3, resulting in 53 × 63 × 46 voxels.
b) Identification of components that showed ToM related activity differences between groups
Group spatial ICA analysis was performed using the GIFT Toolbox, version 2.0d (http://icatb.sourceforge.net) on the data of all participants to identify 20 spatially independent networks. A complete description of the methods implemented in ICA has been published (Calhoun et al., 2001a; Calhoun et al., 2001b) but briefly, the time series data for each participant was first reduced by using principal components analysis (PCA), and then the data from all participants was temporally concatenated and further reduced by PCA. A group ICA was then performed on all the subjects at once using the infomax algorithm (Bell and Sejnowski, 1995). To ensure reliability of the components, the ICA algorithm was run 20 times using ICASSO software (http://www.cis.hut.fi/projects/ica/icasso). The resulting output is an independent component (IC) spatial map and a single associated ICA timecourse for every component and subject. Components were then spatially reconstructed and visually inspected for artifacts. To visualize the spatial maps of a component, all subjects' maps for that particular component were entered into a random-effect analysis model (1 sample t-test in SPM5). Brain regions were considered to be within each network if they met a height threshold of p< 0.00001 corrected for multiple comparisons using the family-wise error (FWE) and an extent threshold of 50 voxels.
To identify components that have shown experimental task relevance, a regression was performed on the ICA component time-course with the general linear model (GLM) design matrix taken from SPM5. This design matrix represents a combination of the experimental onsets convolved with a canonical hemodynamic response function. This resulted in a set of beta weights for every experimental regressor (ToM, non-ToM) associated with a particular subject and component. Beta weight associated with a particular regressor shows how much that component has been modulated by that regressor or task. To identify components that have been significantly differentially modulated by the contrast condition (ToM - non-ToM) in a group, first beta weights associated with the contrast were computed for each subject and then entered into a one sample t-test and thresholded at p<0.05. To identify among these components those that were significantly different between groups, differences in beta weights were entered into a series of two sample t-tests.
c) Components chosen for FNC analysis
Components that showed significant differences between groups in ToM activity along with FT and DM networks were chosen for the analysis.
d) Group differences in FNC
The procedure described by Jafri et al. (Jafri et al., 2008) was followed to determine FNC. For each subject, the time-series associated with the selected n components were extracted, temporally filtered via a band pass filter of 0.017-0.067 Hz, and paired to form n!((n-2)!*2) combinations. Correlations between pair-wise combinations were then calculated using a lagged-correlation approach where the lag was specified as ±6s. The maximal positive correlation value and corresponding lag were saved for each time course pair and later correlation values were transformed to a z-score. Correlation and lag values were averaged separately for control and patient groups, and a functional network connectivity map for each group was created. Significant correlations (corrected for multiple comparisons using a false discovery rate, p<0.05) were determined by using non-parametric tests.
To identify group differences in correlations, two sample t-tests were computed, and p-values were determined using a non-parametric permutation approach. For each component combination, a null distribution of group mean differences was created by randomly re-sampling 39 participants into two groups for 5000 times and calculating the group difference each time. An adjusted p-value was then created by calculating the percentage of times the null representation was higher than the observed mean difference, and a significant difference was determined by thresholding at p<0.05.
e) Relation between FNC strength and ToM activity
In order to understand whether impaired FNC contributes to ToM impairment in patients with schizophrenia FNC strengths shown to be significantly different between the groups (from step d) were correlated with the beta weights associated with the experimental contrast (ToM – non-ToM) for the components that have showed ToM related activity differences between groups (from step b).
Results
Identification of components that showed ToM related activity differences within and between groups
In both control and schizophrenia groups the within group analysis (ToM compared to non-ToM task) produced significant increased activity in the lateral visual (IC8), lateral FT (IC11), and occipito-temporal (IC12) networks and decreased activity in the medial visual (IC2) network (Table 1, Figure 1). Additional increased activity in the insula (IC5) network and decreased activity in the medial FT (IC20) (Table 1, Figure 1) was found only in controls. The between group (controls versus schizophrenia) analysis of differences, comparing ToM and non-ToM task activity, found less positive modulation of the lateral FT (IC11) (t = 2.5, p < 0.02) and insula (IC 5) (t = 2.3, p < 0.03) (Figure 2) networks in patients with schizophrenia compared to controls.
Table 1.
Showing brain regions within each component that showed significantly different modulation by the experimental contrast (ToM - non-ToM) in groups.
| Independent Component (Network) | BA | cluster size | tmax | Coordinate |
|---|---|---|---|---|
| IC 2 (Medial visual) | ||||
| Bi Lingual Gyrus | 17,18 | 4824 | 37.87 | -3, -82, -2 |
| L Precentral Gyrus | 33 | 7.75 | -45, -10, 55 | |
| IC5 (Insula) | ||||
| L Insula | 13 | 4681 | 27.87 | -39, 8, -11 |
| R Insula | 27.28 | 39, 14 -11 | ||
| IC8 (Lateral visual) | ||||
| R Middle Occipital Gyrus | 18 | 1455 | 31.72 | 33, -85, -5 |
| R Inferior/Middle Temporal Gyrus | 37 | 1397 | 29.5 | 45, -70, -2 |
| R Superior Parietal Lobule | 40 | 204 | 13.9 | 27, -58, 55 |
| L Superior Parietal Lobule | 40 | 136 | 13.12 | -27, -55, 55 |
| Postcentral Gyrus | 3 | 22 | 11.24 | 66, -19, 40 |
| Midbrain | 36 | 11.15 | -9, -25, -8 | |
| Medial Frontal Gyrus | 10 | 41 | 9.76 | -3, 50, -5 |
| Posterior Cingulate Gyrus | 23 | 13 | 9.67 | -3, -49, 22 |
| IC11(Lateral fronto-temporal) | ||||
| L Superior Temporal Gyrus | 22 | 2255 | 36.49 | -60, -55, 13 |
| L Inferior Frontal Gyrus | 44 | 15.34 | -60, 11, 7 | |
| R Superior Temporal Gyrus | 22 | 2629 | 27.47 | 60, -43, 10 |
| R Inferior Frontal Gyrus | 44 | 17.54 | 57, 26, 7 | |
| Bi Superior Frontal Gyrus | 9 | 212 | 15 | 3, 53, 28 |
| Bi Precuneus | 7 | 688 | 20.57 | 0, -52, 43 |
| L Cuneus | 18 | 42 | 13.37 | -9, -79, 16 |
| R Cuneus | 19 | 164 | 12.26 | 12, -82, 28 |
| Midbrain | 242 | 19.25 | 6, -25, -5 | |
| L Cerebellum | 195 | 19.17 | -21, -76, -35 | |
| R Cerebellum | 114 | 13.91 | 21, -73, -35 | |
| IC12 (Occipito-temporal) | ||||
| R Fusiform Gyrus | 37 | 1570 | 25.13 | 36, -40, -17 |
| L Occipitotemporal Gyrus | 37 | 601 | 19.68 | -48,-61,-14 |
| R Inferior Frontal Gyrus | 509 | 19.21 | 45, 8, 28 | |
| R Middle Temporal Gyrus | 390 | 15.14 | 45,-76,22 | |
| Caudate | 19 | 11.78 | -9,17,4 | |
| L Middle Frontal Gyrus | 28 | 10.64 | -45,26,22 | |
| R Postcentral Gyrus | 22 | 10.37 | 57,-25,49 | |
| R Precentral Gyrus | 28 | 10.33 | 27, -31,70 | |
| IC20 (Medial fronto-temporal) | ||||
| L Superior Temporal Gyrus | 41 | 2310 | 30.95 | -57, -22, 7 |
| R Superior Temporal Gyrus | 402 | 14.48 | 60, -1, 4 | |
| Bi Anterior Cingulate | 32 | 1539 | 20.69 | 3,44, -2 |
| Cerebellum | 16 | 10.01 | -9, -58, -17 | |
| Paracentral Lobule | 7 | 36 | 9.66 | 6, -40, 70 |
Figure 1.
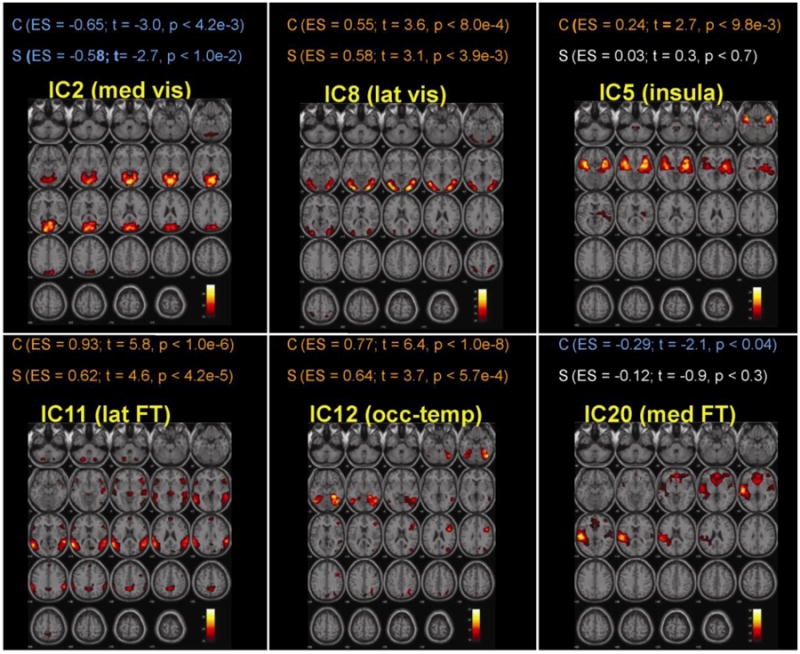
Within group analyses displayed significant positive modulation of the lateral visual (lat vis, IC8), lateral fronto-temporal (lat FT, IC11), and occipito-temporal (occ-temp, IC12) networks and negatively modulation of the medial visual (med vis, IC2) network by the experimental contrast (ToM – non-ToM) in both schizophrenia and control groups. In controls, the contrast also displayed positive modulation of the insula (IC5) and negative modulation of the medial fronto-temporal (med FT) networks. The nature of any modulation (positive, negative or lack of significant) is represented in different colours: Orange represents significant positive modulation, blue represents significant negative modulation and white represents non-significant modulation. Abbreviation used: C = Controls, S = Schizophrenia; ES = Effect Size.
Figure 2.
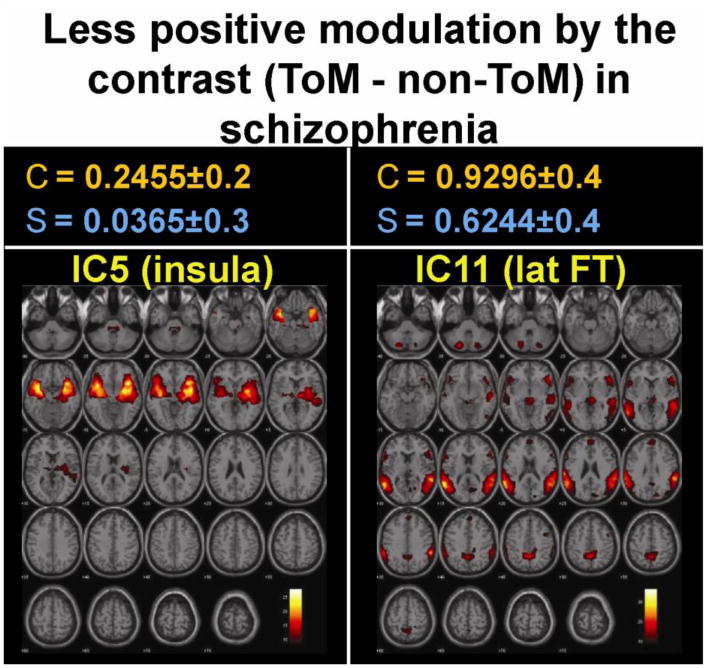
Between group analysis revealed that in comparison to controls, positive modulation of the lateral fronto temporal (lat FT) and insula networks by ToM (compared to non-ToM) task were significantly less in patients with schizophrenia. Top panel shows the mean effect size (± SD) of the contrast in controls (C) and schizophrenia (S).
Components that represented FT and DM networks
Components 9, 10 represented the anterior and posterior DM respectively (Table 2, Figure 3). Components 11 and 20 represented the lateral and medial FT networks respectively (Table 1, and Figure 1). Default mode networks did not show significantly different modulation by ToM compared to non-ToM tasks but fronto-temporal networks did. Both groups showed significantly greater positive modulation of the lateral FT (IC11) network by the ToM task relative to non-ToM task and this modulation was greater in controls (Figure 2). Significantly greater negative modulation (or suppression) of medial FT (IC20) network by ToM (relative to non-ToM) was seen only in controls (Figure 1). Irrespective of task (ToM or non-ToM) two DM networks, anterior (IC9) and posterior (IC10), and also medial FT (IC20) network were significantly less suppressed in patients compared to controls (Figure 3).
Table 2.
Brain regions of the anterior and posterior default mode networks that showed significant less suppression by both ToM and non-ToM tasks in schizophrenia patients (compared to healthy subjects).
| Independent Component (Network) | BA | Cluster size | ttmax | Coordinate |
|---|---|---|---|---|
| IC9 (Anterior default mode) | ||||
| Bi Anreior Cingulate Cortex | 24 | 6688 | 36.28 | -3,38,1 |
| Bi Posterior Cingulate Gyrus | 31 | 760 | 25.85 | -3,-55,28 |
| L Angular Gyrus | 39 | 201 | 19.54 | -48,-67,28 |
| R Angular Gyrus | 270 | 17.57 | 57, -58, 34 | |
| Cerebellum | 193 | 15.62 | 9, -52, -38 | |
| L Inferior Occipital Cortex | 18 | 288 | 14.99 | -24,-97,-11 |
| R Precentral Gyrus | 9 | 347 | 14.7 | 60,5,31 |
| IC10 (Posterior default mode) | ||||
| L Posterior Cingulate Cortex | 23 | 4802 | 45.63 | -3, -55, 22 |
| R Inferior Parietal Lobule | 39,40 | 463 | 23.68 | 39, -64, 40 |
| Bi Anterior Cingualte | ||||
| Cortex/Medial Frontal Gyrus | 10, 32 | 228 | 15.33 | 3, 53, -5 |
| R Middle Temporal Gyrus | 21 | 41 | 13.39 | 54, -7, -14 |
| L Middle Frontal Gyrus | 8 | 64 | 11.24 | -24, 26, 46 |
| R Middle Frontal Gyrus | 8 | 41 | 10.86 | 24, 29, 43 |
| R Supramarginal Gyrus | 40 | 95 | 10.34 | 51, -16, 16 |
| L Thalamus | 19 | 10.05 | -6, -19, -7 | |
| R Cerebellum | 17 | 11.13 | 24, -82, -20 | |
Figure 3.
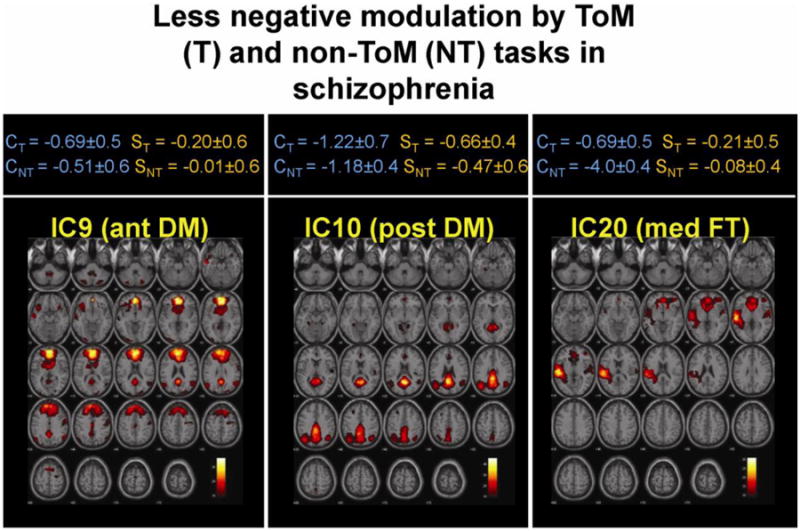
Between group analyses showed that in patients the anterior (ant DM) and posterior (post DM) and medial fronto temporal (Med FT) networks were less negatively modulated (or suppressed) by both ToM and non-Tom tasks. The top panel shows mean effect size (± SD) of modulation by ToM (T) and non-ToM (NT) tasks in controls (C) and schizophrenia (S).
Between group differences in FNC
In both groups a total of ten calculated component combinations (each combination represents coupling between two networks) displayed significant coupling. Among them three sets of coupling were significantly different between groups with controls displaying greater coupling than patients. Coupling 1: between the Insula (IC5) and posterior DM (IC10) networks; coupling 2: between the lateral FT (IC11) and medial FT (IC20) networks; and coupling 3: between the posterior DM (IC10) and medial FT (IC20) networks (Figure 4). These three sets of coupling were all significantly positively correlated among themselves. Correlation between coupling 1 and 2 were (r = .319, p=0.048), coupling 1 and 3 were (r = .566, p = 0.0005), and coupling 2 and 3 were (r = .503, p = 0.001).
Figure 4.
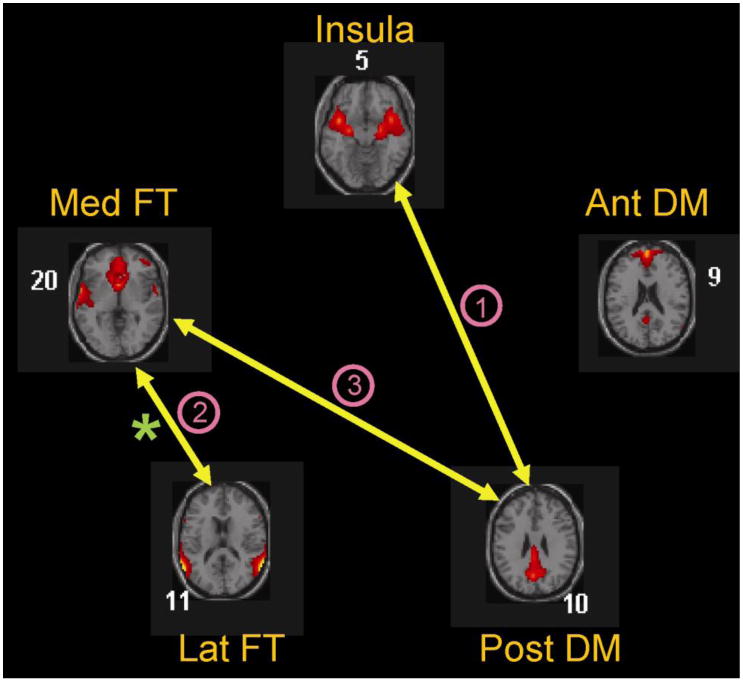
FNC analysis showed that compared to controls, schizophrenia patients display reduced coupling between: (1) the posterior DM (Post DM) and insula networks, (2) the two fronto-temporal networks, namely the medial (Med FT) and the lateral (Lat FT), and (3) the Post DM and Med FT networks. In addition a correlation analysis revealed that the degree of modulation of the Lat FT network by ToM correlated significantly with the degree to which this network was coupled with the Med FT network (denoted by asterisk).
Relation between FNC connectivity strength and the degree of modulation by the ToM task (compared to non-ToM task)
A significant correlation (r = 0.351, p=0.028) between the functional connectivity strength of the lateral FT network (IC11) with the medial FT network (IC20) (Coupling 2) and the degree to which this network (lateral FT displayed strongest modulation by ToM task in both groups) was modulated by the ToM task was observed.
Discussion
A key feature of schizophrenia is the compromise experienced by patients in their ability to mentalize and thereby understand social interactions. This study investigated whether this putative impairment is a result of miscommunication between spatially independent but temporally correlated large networks of the brain. Findings from this study suggest that in male schizophrenia patients this deficit is perhaps related to functional impairment between task-positive and task-negative networks.
The most significant difference between groups was seen in the lateral FT (IC11) network. Among all computed networks this network showed strongest modulation by the experimental contrast (ToM – non-ToM) in both patients and controls. Within this network robust activation was seen in the inferior frontal gyrus (IFG), superior temporal gyrus (STG) including temporo-parietal junction (TPJ), and precuneus (PC). This result is consistent with our earlier findings (Das et al., 2012) (using GLM in these same subjects) of reduced activity in the IFG and STG in patients with schizophrenia compared to healthy controls during ToM engagement. Among the activated regions of this network the STG showed the most significant activity. Interestingly, activity within the STG is implicated in reasoning concerning the contents of another person's mind (Saxe and Kanwisher, 2003). Maximum activity in this network in response to an implicit ToM task is consistent with the suggestion that the implicit automated component of ToM recruits the IFG while explicit mental state reasoning recruits the MPFC (Wolf et al., 2010). Of note, the IFG is also a component of MNS, an observation execution matching system that is thought to provide a neural mechanism for automatically understanding the actions and intentions of others (Rizzolatti and Craighero, 2004).
Diminished responsiveness in these key brain regions in patients as compared to controls when attempting to mentalize, coupled with reduced activity in the insula network (only found in the ICA analysis), is interesting because MNS activity in conjunction with limbic system processing is thought to subserve the comprehension of emotions in others (Leslie et al., 2004). In this context, the insula provides a conduit between these two systems that enables observed emotional behaviour to be internalized and assigned affective salience (Carr et al., 2003). Consequently, insula activation correlates with empathy (Singer et al., 2004), a form of social cognition governed by emotion. Reduced activity in these networks in patients with schizophrenia may therefore provide a neural explanation for their inability to mentalize and may also underpin their inability to empathize with others (Bleuler, 1950).
Networks that showed significantly less suppression by experimental tasks in schizophrenia patients were the two (anterior and posterior) DM networks (IC9 and IC10) and the medial FT network (IC20). These networks contain brain regions (midline MPFC, PCC, and PC) that are consistently associated with task-induced deactivations and all three networks overlap within the frontal anterior cingulate region. ACC dysfunction has been implicated in schizophrenia by numerous strands of scientific investigation. Our finding of less suppression of these networks in patients with schizophrenia is consistent with the findings of Whitfield-Gabrieli and colleagues (Whitfield-Gabrieli et al., 2009) who similarly found less suppression of midline regions of the DM network, such as the PCC/PC and the MPFC by a working memory task in schizophrenia patients as compared to healthy controls. This is important because suppression of the DM network has been found to be associated with better performance on an attention-demanding task in healthy subjects (Weissman et al., 2006). A lack of suppression that results in hyperactivity of the DM network in patients possibly reflects abnormal connectivity between the regions within the network (Bluhm et al., 2007; Whitfield-Gabrieli et al., 2009) or may be a consequence of abnormal connectivity of this network with other networks (Garrity et al., 2007; Zhou et al., 2007).
Compared to controls, patients with schizophrenia displayed reduced coupling between task positive and task negative networks and also between task-negative networks, but it is the reduced coupling between the task-positive and task-negative networks that showed significant correlation with the ToM activity in patients (Figure 4).
Impaired interaction between this task-positive and task-negative network in schizophrenia patients perhaps reflects an impairment of information processing (Eichele et al., 2008; Fox et al., 2005). The medial MPFC is thought to be involved in processing information about self and others in more abstract, evaluative terms that helps in understanding complex psychological aspects of others, whereas the more lateral FT network that includes the temporo-parietal junction (TPJ) may provide essential physical self-to-other mapping that is needed for comprehending the physical actions of intentional agents (Uddin et al., 2007). Interaction between these networks is therefore essential to the integration of information needed for maintaining self-other representations across multiple domains (Uddin et al., 2007). Therefore, one possibility is that the deficits in mentalizing in schizophrenia are a consequence of impairments in higher cognition, namely, the capacity to adopt a perspective different from the self, an ability that is fundamental to the comprehension of the mental states of one's self and others. This inference is in keeping with the problems observed in self-other differentiation clinically in patients with schizophrenia.
Study limitations
Before drawing firm conclusions from this study, it is important to acknowledge that there are many networks that are important to the pathophysiology of schizophrenia and subserve aspects of ToM, and that in this study we have not considered all of these. Further, though our findings cannot be generalized to female schizophrenia patients, the investigation of males in this study does have the advantage of minimising the potential confound of gender differences in ToM (Schulte-Ruther et al., 2008). Finally, as this was a real-world study there were significant differences in years of education, medication regimens, and clinical variables between patients and controls. These may also act as potential confounds in interpreting these preliminary findings, but it is of note that no significant correlations were found between these variables and functional connectivity strength.
Conclusion
In summary, our findings indicate that in patients with schizophrenia there is reduced coupling between task-positive and task-negative networks, especially between those networks that sub-serve the mapping of one's self to others. This reduction in coupling perhaps disrupts social construct information processing to the extent that it produces a clinically discernible deficit in mentalizing ability. As a consequence, patients with schizophrenia may experience discernible social compromise that makes it difficult for them to comprehend interpersonal interactions.
Acknowledgments
We are indebted to the participants that made this study possible and acknowledge the contributions of Dr. Lagopoulos, Dr. Henderson, and Dr. Coulston.
Role of the Funding Source: Funding for this study was provided by NHMRC Program Grant 510135.
Footnotes
Conflict of Interest: Dr Pritha Das and Dr Calhoun have no interest to declare. Professor Gin Malhi has served on a number of international and national pharmaceutical advisory boards, received funding for research and has been in receipt of honoraria for talks at sponsored meetings worldwide involving the following companies: AstraZeneca, Eli Lilly, Jansen-Cilag, Organon, Pfizer, and Wyeth.
Contributors: Authors Malhi and Das conceptualized the analysis;
Author Das performed the data analyses with assistance from author Calhoun and wrote the initial draft of the manuscript;
All authors contributed to and have approved the final manuscript.
References
- Andreasen NC, Calarge CA, O'Leary DS. Theory of mind and schizophrenia: a positron emission tomography study of medication-free patients. Schizophr Bull. 2008;34(4):708–719. doi: 10.1093/schbul/sbn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia Preacox or the Group of Schizophrenias. International Universities Press; Madison, CT: 1950. [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33(4):1004–1012. doi: 10.1093/schbul/sbm052. Epub 2007 Jun 1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109(1-3):1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Brüne M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005;31(1):21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- Brüne M, Lissek S, Fuchs N, Witthaus H, Peters S, Nicolas V, Juckel G, Tegenthoff M. An fMRI study of theory of mind in schizophrenic patients with “passivity” symptoms. Neuropsychologia. 2008;46(7):1992–2001. doi: 10.1016/j.neuropsychologia.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Brunet E, Sarfati Y, Hardy-Bayle MC, Decety J. Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia. 2003;41(12):1574–1582. doi: 10.1016/s0028-3932(03)00119-2. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, McGinty VB, Pekar JJ, Watson TD, Pearlson GD. fMRI activation in a visual-perception task: network of areas detected using the general linear model and independent components analysis. Neuroimage. 2001a;14(5):1080–1088. doi: 10.1006/nimg.2001.0921. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001b;14(3):140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Eichele T, Pearlson G. Functional brain networks in schizophrenia: a review. Front Hum Neurosci. 2009;3:17. doi: 10.3389/neuro.09.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(Pt 8):1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12(3):314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Das P, Kemp AH, Flynn G, Harris AW, Liddell BJ, Whitford TJ, Peduto A, Gordon E, Williams LM. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophr Res. 2007;90(1-3):284–294. doi: 10.1016/j.schres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Das P, Lagopoulos J, Coulston CM, Henderson AF, Malhi GS. Mentalizing impairment in schizophrenia: A functional MRI study. Schizophr Res. 2012;134(2-3):158–164. doi: 10.1016/j.schres.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Demirci O, Stevens MC, Andreasen NC, Michael A, Liu J, White T, Pearlson GD, Clark VP, Calhoun VD. Investigation of relationships between fMRI brain networks in the spectral domain using ICA and Granger causality reveals distinct differences between schizophrenia patients and healthy controls. Neuroimage. 2009;46(2):419–431. doi: 10.1016/j.neuroimage.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, von Cramon DY, Ullsperger M. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci U S A. 2008;105(16):6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P) (Version 2.0) NY: Biometrics Research, New York State Psychiatric Institute, New York; 1995. [Google Scholar]
- Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37(2):163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3(2):89–97. [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358(1431):459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164(3):450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Harrington L, Siegert RJ, McClure J. Theory of mind in schizophrenia: a critical review. Cogn Neuropsychiatry. 2005;10(4):249–286. doi: 10.1080/13546800444000056. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39(4):1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Multi-Health Systems; North Tonawanda, NY: 1986. [Google Scholar]
- Leslie KR, Johnson-Frey SH, Grafton ST. Functional imaging of face and hand imitation: towards a motor theory of empathy. Neuroimage. 2004;21(2):601–607. doi: 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Das P, Moss K, Berk M, Coulston CM. A functional MRI study of Theory of Mind in euthymic bipolar disorder patients. Bipolar Disord. 2008;10(8):943–956. doi: 10.1111/j.1399-5618.2008.00643.x. [DOI] [PubMed] [Google Scholar]
- Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed) 1987;295(6600):681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Chimpanzee problem-solving: a test for comprehension. Science. 1978;202(4367):532–535. doi: 10.1126/science.705342. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Russell TA, Rubia K, Bullmore ET, Soni W, Suckling J, Brammer MJ, Simmons A, Williams SC, Sharma T. Exploring the social brain in schizophrenia: left prefrontal underactivation during mental state attribution. Am J Psychiatry. 2000;157(12):2040–2042. doi: 10.1176/appi.ajp.157.12.2040. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19(4):1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43(10):1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Schulte-Ruther M, Markowitsch HJ, Shah NJ, Fink GR, Piefke M. Gender differences in brain networks supporting empathy. Neuroimage. 2008;42(1):393–403. doi: 10.1016/j.neuroimage.2008.04.180. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Turner GH, Twieg DB. Study of temporal stationarity and spatial consistency of fMRI noise using independent component analysis. IEEE Trans Med Imaging. 2005;24(6):712–718. doi: 10.1109/TMI.2005.846852. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci. 2007;11(4):153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happe F, Falkai P, Maier W, Shah NJ, Fink GR, Zilles K. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14(1 Pt 1):170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Walter H, Ciaramidaro A, Adenzato M, Vasic N, Ardito RB, Erk S, Bara BG. Dysfunction of the social brain in schizophrenia is modulated by intention type: an fMRI study. Soc Cogn Affect Neurosci. 2009;4(2):166–176. doi: 10.1093/scan/nsn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. Epub 2006 Jun 2011. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. Epub 2009 Jan 1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH, Gur RC, Valdez JN, Loughead J, Elliott MA, Gur RE, Ragland JD. Alterations of fronto-temporal connectivity during word encoding in schizophrenia. Psychiatry Res. 2007;154(3):221–232. doi: 10.1016/j.pscychresns.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf I, Dziobek I, Heekeren HR. Neural correlates of social cognition in naturalistic settings: a model-free analysis approach. Neuroimage. 2010;49(1):894–904. doi: 10.1016/j.neuroimage.2009.08.060. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97(1-3):194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]


