Abstract
The past decade has seen several anti-cancer immunotherapeutic strategies transition from “promising preclinical models” to treatments with proven clinical activity or benefit. In 2013, the journal Science selected the field of Cancer Immunotherapy as the overall number-1 breakthrough for the year in all of scientific research. In the setting of cancer immunotherapy for adult malignancies, many of these immunotherapy strategies have relied on the cancer patient’s endogenous anti-tumor T cell response. While much promising research in pediatric oncology is similarly focused on T cell reactivity, several pediatric malignancies themselves, or the chemo-radiotherapy used to achieve initial responses, can be associated with profound immune suppression, particularly of the T cell system. A separate component of the immune system, also able to mediate anti-tumor effects and less suppressed by conventional cancer treatment, is the NK cell system. In recent years, several distinct immunotherapeutic approaches that rely on the activity of NK cells have moved from preclinical development into clinical testing, and some have shown clear antitumor benefit. This review provides an overview of NK cell-based immunotherapy efforts that are directed towards childhood malignancies, with an emphasis on protocols that are already in clinical testing.
Keywords: immunotherapy, natural killer cells, child, neoplasms
Background
Natural Killer (NK) cells comprise approximately 10% of lymphocytes in normal humans1,2 and their main function is to destroy virally infected, damaged, or transformed cells. NK cells also influence function of the adaptive immune system through the secretion of cytokines and chemokines that affect T cell function and dendritic cell (DC) maturation. Additionally, NK cell-mediated lysis of immature DCs selects for a more immunogenic subset of DCs during adaptive immune responses3,4.
In general, NK cells can be divided into two subsets, distinguished by density of CD56 surface expression (CD56 is an adhesion molecule known as Neural Cell Adhesion Molecule or NCAM). The CD56bright subset is localized primarily in lymph nodes and secondary lymphoid tissue and constitutes only 10% of circulating NK cells. This subset is proliferative and secretes abundant amounts of cytokines in response to cytokine stimulation5,6. The CD56dim subset is found predominantly in blood, bone marrow and spleen and comprises 90% of circulating NK cells. This subset: 1) expresses FcγRIIIa receptors (CD16) and mediates antibody-dependent cellular cytotoxicity (ADCC), 2) has limited proliferative capacity, and 3) secretes cytokines in response to target cell recognition5,6. Resting NK cells in the CD56dim subset are more cytotoxic against NK-sensitive targets compared to CD56bright NK cells. However, CD56bright NK cells have similar cytotoxic capability after stimulation with Interleukin-27.
NK Cell Functions
Cytokine production
NK cells secrete the cytokines Interferon- γ (IFN-γ), Tumor Necrosis Factor-α (TNF-α), Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF), Interleukin-10 (IL-10), Interleukin-13 (IL-13) and the chemokines Macrophage Inflammatory Protein-1α (MIP-1α), Macrophage Inflammatory Protein-1β (MIP-1β), and Regulated on Activation Normal T Cell Express and Secreted (RANTES) in response to stimulation5,8. NK cells in the CD56bright subset require co-stimulation for cytokine secretion (usually Interleukin-12 in addition to another cytokine or the ligation of an activation receptor [e.g., natural killer group 2, member D, or NKG2D]) while those in the CD56dim subset secrete cytokines in response to target cell recognition. Through the secretion of TNFα, GM-CSF, and IFN-γ, NK cells regulate DC maturation. The secretion of IFN-γ has a number of effects, including activation of macrophages, up-regulation of class I expression by antigen presenting cells (APC), Type 1 T-helper cell (Th1) polarization, and a direct antiproliferative effect on tumor cells. Thus, NK cells can directly inhibit tumor cell proliferation and augment the adaptive immune response to tumors via cytokine secretion.
Cytotoxicity
NK cells are able to kill virally infected, damaged and transformed cells by two mechanisms (Figure 1). The first involves the secretion of lytic granule contents and destroys the target within hours. The second involves the interaction of death receptor ligands expressed on NK cells with surface death receptors on transformed or damaged cells, inducing apoptosis of the latter within a day. In the first instance, NK cell secretory lytic granules containing perforin and granzymes are released into the intercellular space between the NK cell and its target at a zone of cell-to-cell contact, called the immune synapse. The immune synapse (IS) is a highly organized supra-molecular structure comprised of adhesion molecules, receptors, signaling molecules, co-stimulatory ligands, and cytoskeletal elements. The initial step in formation of a functional IS involves close association between NK cell and target cell that results in initial signaling events, and in the absence of overriding inhibitory signaling, firm adhesion occurs. Active cytoskeletal processes result in reorganization of cellular elements at the point of contact. A synaptic “cleft” is formed at the IS between the two cells into which cytolytic molecules are secreted. NK cell cytoskeletal reorganization results in the recruitment of lytic granules, that are distributed throughout the cell, toward the IS. Actin reorganization produces “conduits” within the NK cell cortex through which the lytic granules pass to accumulate at the synaptic cleft region of the IS. There, the lytic granules dock at the membrane with subsequent priming, fusion and secretion. Each intracellular event in the process, from initiation of IS formation to the directed release of lytic molecules, is highly regulated to enable NK cells to carefully direct their potent cytotoxic capability. The most well studied components of lytic granules are perforin and granzyme molecules. Perforin is a protein that forms pores in the target cell membrane allowing the entry of ions and small molecules. Cell death may ensue from the resultant disruption of osmotic equilibrium. Alternatively, target cell death can result from the action of granzymes that enter the cell through the perforin-induced pores or by endocytosis after granzyme-binding of mannose 6-phosphate receptors on the surface of the target cell. Interestingly, endocytosis in the target cell is increased due to cellular wound healing in the region of membrane disruption caused by perforin-induced pores. Granzymes entering the cell via endocytotic vesicles then gain entry to the cytoplasm via perforin-induced pores in the endocytotic vesicle membrane. Granzyme A induces cell death by indirectly generating single-stranded DNA nicks and Granzyme B produces rapid induction of caspase-dependent apoptosis9.
Figure 1.
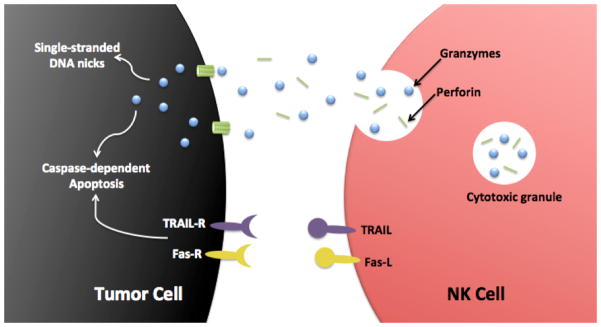
Two major mechanisms by which NK cells kill tumor cells: NK cells can use the perforin/granzyme containing granule exocytosis pathway or the TRAIL/Fas-L death-receptor pathway.
The second mechanism by which NK cells eliminate target cells involves the interaction of tumor cell surface death receptors with death receptor ligands expressed on NK cells (e.g., TNF-related apoptosis-inducing ligand or TRAIL, Fas-ligand) to induce apoptosis. When Fas-ligand (FasL) or TRAIL on NK cells bind their respective receptors on target cells, apoptosis of the target cell ensues through the activation of caspases 8 and 9 of the extrinsic pathway10,11. It has been suggested12,13 that NK cell-mediated lysis of tumor cells through ligation of death receptors may function without inhibitory KIR (killer immunoglobulin-like receptor) input. This may represent an important mechanism by which allogeneic NK cells may effectively kill tumor cells of a KIR/KIR ligand matched individual (see below).
Effect of inhibitory and activating receptors
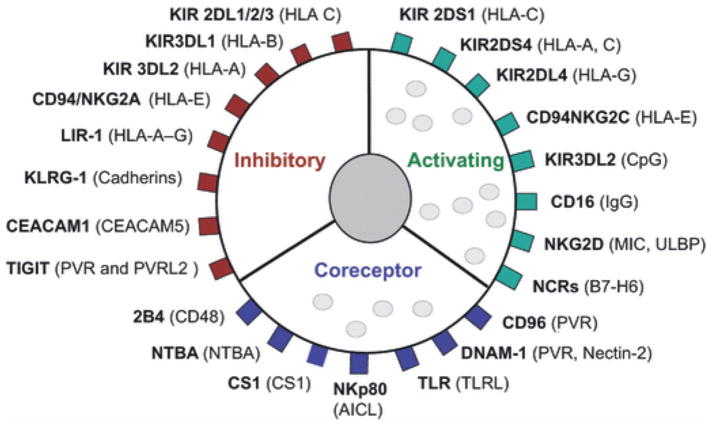
Whether an NK cell is activated to elicit effector functions (cytokine secretion or secretion of contents of lytic granules) depends on the balance of inhibitory and activating signals received through germ-line encoded, cell surface receptors (Figure 2). An important feature of the immune synapse is its role in integrating these signals through clustering of receptors and signal molecules at the point of cell-to-cell contact. Activating receptors include natural cytotoxicity receptors (e.g., NKp30, NKp44, NKp46), some C-type lectin-like receptors (e.g., NKG2D, NKG2C, NKG2E), CD2 family molecules (2B4, CRACC), receptors for nectin or nectin-like molecules (e.g., DNAM-1), several members of the KIR family, and Toll-like receptors 3 and 9 (figure 2)14,15. Another activating receptor on NK cells that is particularly important in the context of immunotherapy is FcγRIIIa (CD16). The CD56dim subset of NK cells possesses a high density of CD16, while the less mature CD56bright subset expresses limited CD16. The ligand for FcγRIIIa is the Fc domain of IgG antibody. Antibody bound to target cell surface antigens, via Fv domains, results in a lattice of bound antibody molecules with exposed Fc domains that essentially “tags” the tumor cell for destruction by NK cells by a process called antibody-dependent cellular cytotoxicity (ADCC).
Figure 2.
NK cell surface receptors and their ligands. Receptors are broadly classified based on their primary function (inhibitory receptors, activating receptors, and activating co-receptors). Known ligands are denoted in parentheses. Despite the multitude of receptors shown, other families of receptors are not illustrated, including cytokine receptors (e.g., IL-1, IL-2, IL-12, IL-15, IL-18, IL-21, IFNα), chemotactic receptors (CCR2, CCR5, CXCR1, CXCR3, CXCR4, CXCR6, CX3CR1 and Chem23R), adhesion receptors (CD2 and β1 and β2 integrins), and inhibitory co-receptors (CD300A, LAIR-1 and Siglec7). Adapted with permission from: Wing Leung, Use of NK cell activity in cure by transplant, British Journal of Haematology 155, 14–29, 2011, Blackwell Publishing Ltd.
Normal somatic cells do not generally express ligands for NK activating receptors but cells undergoing genotoxic or cellular stress, as occurs during transformation, do. For instance, many human cancer cells up-regulate the expression of a B7 gene family member designated B7-H6, a ligand for the NK cell activating receptor NKp30, while B7-H6 is absent from the surface of normal cells16. In addition, the ligands PVR (Poliovirus Receptor) and Nectin-2 that are present on some tumor cells, including freshly isolated neuroblastoma cells17 and neuroblastoma cell lines18, bind and activate DNAM-1 (DNAX Accessory Molecule 1) receptors on NK cells19. Moreover, many tumor cells express MICA and/or MICB (major histocompatibility complex class I-related chain glycoprotein A/B), ULBP-1 and/or ULBP-3 (UL-16 binding proteins 1/3), which are all ligands for the activating receptor, NKG2D20,21. In fact, tumors secrete NKG2D ligands as a form of NK cell immune evasion22. Interestingly, distinct signaling cascades are induced by different activating receptors in contrast to a common signaling pathway employed by most inhibitory receptors14.
The most well studied NK cell inhibitory receptors are members of the KIR family and CD94/NKG2A. The ligands for these receptors are major histocompatibility class (MHC) I molecules (classical and non-classical, respectively) which are expressed on all nucleated cells and therefore, serve as an excellent means by which NK cells can distinguish cells that are “self” from “non-self”. In fact, all NK cells that have been “licensed to kill” through the maturation process23, express at least one inhibitory receptor that recognizes an MHC class I molecule24,25. The binding affinity of ligands for inhibitory receptors is generally greater than that for ligands of activating receptors14. In this way, NK cell-mediated destruction of “self” is usually avoided unless self cells have reduced expression of MHC class I molecules or substantially increased expression of ligands for NK cell activating receptors, both of which frequently occur in the process of transformation. Many tumor cells have diminished MHC class I expression, likely through selective pressure to avoid recognition by T cells. Many neuroblastomas, for instance, have been shown to express very low levels of MHC class I molecules26–29.
When an NK cell is stimulated to kill a target cell, the target cell death that results is the sum of cytotoxicity induced by secretion of lytic granule contents and cytotoxicity induced through ligation of death receptors by NK cell surface TRAIL and FasL. In the absence of antibody, the NK cell-induced target cell lysis is called, “natural cytotoxicity” or antibody-independent cytotoxicity. For natural cytotoxicity, the cumulative integrated signal includes inputs from all NK cell receptors that are bound to target cell ligands at the IS except for FcγRIIIa receptors, since they are not bound to antibody. When antibody is bound to the target cell surface and NK cell FcγRIIIa receptors bind to the Fc domain, the resulting cytotoxicity is called ADCC. For ADCC, the cumulative integrated signal includes inputs from all NK cell receptors that are bound to target cell ligands at the IS including FcγRIIIa receptors. So in effect, the cytotoxicity in the presence of antibody is the sum of natural cytotoxicity (all receptors except FcγRIIIa receptors) plus the additional cytotoxicity resulting from stimulation of FcγRIIIa receptors. Interestingly, FcγRIIIa receptor ligation is the only activating input that does not require additional co-stimulation to result in an activation signal in NK cells30.
Enhancing NK Cell Function with Cytokines, Drugs and Toll-like receptor Ligands
Cytokines
Cytokines are secreted or membrane-bound molecular messengers that are produced by cells of the immune system to allow intercellular communication. Recombinant DNA manufacturing technology allows the production of sufficient quantities of these molecules for systemic administration for cancer immunotherapy. This section will focus on cytokines that influence NK cell proliferation, phenotype or function and thus, may produce anti-tumor responses, at least in part, through effects on NK cells.
Interleukin-2
Interleukin-2 (IL-2) is a well-studied gamma (c) cytokine that is FDA approved to treat renal cell carcinoma and melanoma. IL-2 stimulates the proliferation of NK cells, particularly the CD56bright subset, and promotes their functional maturation by inducing the expression of FcγRIIIa, NCRs (natural cytotoxicity receptors), NKG2D and production of perforin31–33. Thus, IL-2 renders this NK cell subset cytotoxic to NK-sensitive targets. Moreover, IL-2 activates CD56dim NK cells, augmenting the production of perforin15 and granzymes15,34, enhancing ADCC and antibody-independent (natural) cytotoxicity as well as IFN-γ production15,35–43. Activating receptors on this subset are also up-regulated by IL-2, including NCRs44–46, NKG2D15,33,44, and DNAM-144–46.
Although systemic IL-2 therapy has not demonstrated antitumor efficacy in pediatric trials as monotherapy47–49, it augments ADCC when administered with therapeutic antibodies50. In fact, IL-2 administered with alternating cycles of GM-CSF plus the mAb, ch14.18, along with standard therapy (isotretinoin) increased event-free survival by 20% after 2 years compared to standard therapy alone in children with high-risk neuroblastoma51. An ongoing multi-center clinical trial in Europe and Israel that is nearing completion (NCT01701479) is evaluating the long-term continuous infusion of an anti-GD2 mAb, ch14.18/CHO, along with subcutaneous IL-2 in children with high-risk neuroblastoma. In addition, a single center phase I trial is currently evaluating the combination of the anti-GD2 mAb, hu-3F8, along with subcutaneously administered IL-2 in children with GD2+ tumors (NCT01662804).
Monotherapy with inhaled IL-2 to treat pulmonary metastases of renal cell carcinoma was shown to be tolerable and controlled progressive disease for considerable periods of time52. There is an ongoing single institution, phase I/II trial that employs aerosolized IL-2 for the treatment of pulmonary metastases in individuals who are age 12 and older (NCT01590069).
IL-2 effectively activates NK cells ex-vivo for use in adoptive therapy regimens53 and several ongoing clinical trials employ ex-vivo activated allogeneic NK cells to treat cancer, mostly in adults. Chemotherapy is typically administered prior to allogeneic cell infusion in order to induce a state of lymphodepletion, which is thought to promote expansion of the adoptively transferred cells. In the setting of lymphodepletion, low-dose IL-2 therapy following administration of allogeneic NK cells facilitates the in vivo expansion of donor NK cells39,53,54. Successful, albeit transient, engraftment of donor NK cells using this approach has been demonstrated in adults53 and children whose acute myeloid leukemia responded to allogeneic NK cell therapy55. A number of current clinical trials involve the administration of low dose IL-2 in conjunction with NK cell adoptive therapy.
Interleukin-15
Interleukin-15 (IL-15) is also a member of the gamma (c) cytokine family and shares the use of two receptor subunits, IL2Rβ and γc (an intermediate affinity heterodimer), with IL-2 although they each bind to a unique alpha subunit (creating unique high affinity heterotrimers). Since the cytokines share an intermediate affinity receptor, IL-2 and IL-15 have similar functions, as might be expected. Like interleukin-2, IL-15 enhances ADCC56–64 and upregulates perforin65,66 and granzyme B67. In addition, IL-15 induces maturation67 and proliferation56 of the CD56bright NK cell subset and promotes NK cell survival through induction of Bcl-268.
IL-2 and IL-15 possess distinct roles as well, likely resulting from the differential distribution of their α-subunits, the distinct temporal and spatial patterns of expression of the two cytokines and the predominant mode of presentation of each cytokine. IL-15 is generally presented in trans by IL15Rα to neighboring cells bearing the IL2Rβ/γc receptor while IL-2 is usually presented by IL2Rα in cis to IL2Rβ/γc on the same cell via movements within microdomains of the extracellular membrane. IL-2 is involved in the elimination of self-reactive T cells by eliciting activation-induced cell death (AICD) and it promotes the activity and survival of regulatory T cells (Tregs), that function to regulate (or inhibit) immune responses to prevent autoimmunity. By contrast, IL-15 inhibits IL-2 induced AICD of CD8+ memory-phenotype T cells and does not activate Treg cells. Since IL-2 down-modulates the immune response by promoting AICD of T cells and by increasing Treg activity while IL-15 supports the maintenance of CD8+ memory T cells and does not have a significant effect on Treg cells, IL-15 may produce superior overall anti-tumor effects. IL-15 is currently being evaluated in phase I clinical trials in adults with solid tumors (NCT01727076) or with AML (NCT01385423) to determine safety and tolerability. An NCI sponsored phase I clinical trial (NCT01875601) is evaluating IL-15 in children and young adults with advanced solid tumors. Patients receive infusions of autologous NK cells that have been activated and expanded ex vivo followed by at least 12 doses of daily IL-15. A single institution clinical trial in Spain using IL-15 activated allogeneic NK cells in the transplant setting for pediatric refractory solid tumors was terminated to evaluate potential toxicity (NCT01337544).
Alpha-interferon (IFNα)
Alpha-interferon (IFNα) is a cytokine that likely exerts antitumor effects through a number of mechanisms involving cells of the innate (NK cells, DCs, macrophages) and adaptive (T cells) immune system. Although the antitumor mechanisms of action are not fully understood, IFNα has been shown to activate NK cells69,70. IFNα-2a is FDA approved for use in adults to treat melanoma, CML, hairy cell leukemia, and AIDS-related Kaposi’s sarcoma. IFNα-2a is also approved as combination therapy with bevacizumab for metastatic renal cell carcinoma. In children, a limited number of studies have evaluated the use of IFN-alphas to treat cancer. Tolerability and feasibility of high dose IFN-α2a administered for 4 weeks followed by a lower maintenance dose for 48 weeks was demonstrated in children with stage III melanoma71. A phase II study of pegylated IFN-α2a in 32 children with diffuse intrinsic pontine glioma was recently reported to delay the time to progression without significantly improving 2-year survival72.
A number of ongoing clinical trials are evaluating immunotherapy with IFN-α. A single institution phase I trial (NCT00855452) in Israel involves administration of low-dose IFN-α after allogeneic lymphocyte infusion in patients (12 years of age and older) with metastatic solid tumors. A phase II trial that incorporates pegylated IFN-α2b (NCT00539591) to treat children with high-risk melanoma is ongoing and pegylated IFN-α2b is being evaluated in the treatment of plexiform neurofibromas (NCT00678951). A combination of chemotherapy, pegylated IFN-α2b and surgery is being tested in a phase III COG trial to treat patients with osteosarcoma (NCT00134030) and a combination of IFN-α2b plus GM-CSF is being investigated to treat ALL, AML, blast phase CML and myelodysplastic syndrome in a phase I clinical trial (NCT00548847).
Drugs
Lenalidomide
Lenalidomide, a structural analog of thalidomide, was shown to increase the percentage and absolute number of NK cells in a dose-dependent manner in children with solid tumors or myelodysplastic syndrome73. After 3 weeks of lenalidomide therapy in a dose finding phase I trial, NK cells from these subjects demonstrated elevated granzyme content and enhanced antibody independent cytotoxicity. Interestingly, the authors reported a decrease in the percentage of Treg in these patients as well. Since Treg diminish antitumor immune responses elicited by tumor specific CD8+ cells74, tumor specific CD4+ cells75, and NK cells76, the authors suggest that lenalidomide-induced Treg inhibition may contribute to lenalidomide’s potential antitumor activity. These results were in agreement with preclinical findings of several studies that evaluated the effect of lenalidomide or thalidomide on NK cell number and function77–79.
Bortezomib
Bortezomib is an ubiquitin-proteasome pathway inhibitor that induces bcl-2 phosphorylation and is associated with G2-M phase cell cycle arrest and induction of apoptosis80. However, the mechanism(s) by which it elicits antitumor effects are not entirely clear. Bortezomib has been shown to upregulate surface expression of the death receptor TRAIL-R2 (DR5) on tumor cells sensitizing them to NK cell killing through the engagement of NK cell TRAIL81,82. Additionally, bortezomib decreases expression of MHC class I on tumor targets83 rendering them more sensitive to NK cell lysis.
b-AP15
A novel proteasome deubiquitinating inhibitor, currently called b-AP15, was also shown to sensitize numerous tumor cell lines to killing by NK cells through the upregulation of TRAIL-R2 on tumor cells in vitro84. Interestingly, T cell recognition of targets is reduced for tumor cells treated with bortezomib due to altered proteasomal processing and presentation of antigens81. b-AP15 inhibits the deubiquitinating activity of a different proteasomal regulatory particle than does bortezomib leaving immunoproteasome processing and presentation of antigenic peptides to T cells intact. Thus, b-AP15 may enhance NK cell killing without attenuating antigen processing and T cell-mediated killing84. It is not yet being tested in clinical trials.
Toll-Like Receptors
Toll-Like Receptors (TLRs) bind to molecules that possess highly conserved structural and molecular patterns associated with pathogens (e.g., double stranded DNA or lipopolysaccharide) or self-peptides released during cellular damage or death (alarmins). Identification of these self-peptides, known as damage associated molecular patterns or DAMPs, is an active area of investigation. TLR ligands can cause NK cell activation indirectly via binding of TLRs on other immune cells (e.g., DCs, macrophages) that become activated and secrete NK cell activating cytokines (e.g., IL-12, IL-18). Alternately, TLR ligands may produce direct NK cell activation through binding of intracellular and cell surface TLRs in and on NK cells. Sivori and colleagues demonstrated the presence of TLR3 and TLR9 in NK cells85. In the presence of cytokines, CpG and double-stranded RNA induce NK cell secretion of IFN-γ and TNF-α and enhance NK cell cytotoxicity of tumor cells. Several groups have reported the expression of TLR2 and TLR4 on NK cells86,87 and TLR4 receptor binding on NK cells was associated with release of IFN-γ and TNF-α, upregulation of perforin and enhanced cytotoxicity87. The cell-wall skeleton of Bacillus Calmette-Guerin (BCG) is a TLR2 and TLR4 agonist that is FDA approved for the treatment of bladder cancer in adults. Preclinical studies showed that BCG enhanced NK cell antibody independent cytotoxicity and ADCC88 and increased NK cell lysis of NK-resistant tumor targets89. A phase I clinical trial in children with GD2+ tumors that combined BCG treatment with the anti-idiotypic mAb A1G4 (NCT00003023) is now complete although results have not yet been reported.
Enhancing NK Cell Function with Monoclonal Antibodies
Normal cells are resistant to NK cell lysis due to the presence of MHC class I molecule expression and the lack of activating ligands (Figure 2). In this situation, inhibitory signals predominate, NK cell natural cytotoxicity does not occur and the cell is considered “NK cell-resistant”. As mentioned above, tumor cells frequently express activating ligands and reduced levels of MHC class I, which results in the tumor cell being sensitive to NK cell natural cytotoxicity. Unfortunately, in some instances, tumor cells are resistant to NK cell natural cytotoxicity due to inhibitory signals predominating over activating signals and NK cell natural cytotoxicity does not occur. However, if antibodies are bound to the target cell, as occurs when tumor specific antibodies are employed as cancer immunotherapy, then activating signals through NK FcγRIIIa receptor ligation may predominate and result in ADCC.
Selectively targeting tumor cells for destruction via ADCC elicited by immune effector cells (e.g., NK cells, monocytes, neutrophils) became possible through hybridoma technology and the development of monoclonal antibodies (mAb) for which Milstein and Kohler received the Nobel Prize in 198490. Transformed cells frequently express abnormal molecules, or abnormal amounts of normal molecules on their cell surface, and monoclonal antibodies (mAb) can be generated to specifically bind antigenic determinants on these molecules. The ideal tumor antigen against which a therapeutic antibody is generated is one that is not expressed, or expressed in very limited amounts, by normal cells. An example of such an antibody is RAbDMvIII91 that binds to the most common variant of the epidermal growth factor receptor known as EGFRvIII that is exclusively expressed on certain malignant cells including some pediatric gliomas92. Alternatively, tumor antigens can serve as excellent targets if tumor cells are exposed to circulating mAb while normal cells (bearing the same antigen) are sequestered from circulating mAb. For example, anti-GD2 mAbs (e.g., 3F8, ch14.18, hu14.18) are specific for the disialoganglioside, GD2, which is expressed on cells of neuroectodermal origin. Expression of GD2 on normal tissues is generally restricted to cells that are protected from circulating mAb by the blood-brain-barrier or blood-nerve-barrier.
Many studies have shown that mAbs are capable of inducing ADCC of tumor cells by immune effector cells in vitro93. The importance of the antibody-FcR interaction for mAb antitumor efficacy in vivo has been demonstrated in pre-clinical models. Using murine xenograft models of HER2+ human breast carcinoma and CD20+ human B-cell lymphoma, Clynes et al.94 showed that the antitumor effect of Herceptin and Rituximab®, respectively, were attenuated in FcγR knockout mice compared to wild-type. Similar results were obtained using an immunocompetent, syngeneic melanoma murine model95. Although mouse models lend strong support for the role of ADCC in the antitumor effect of mAbs, the most convincing evidence that activating FcRs play a role in the clinical response to therapeutic antibodies (likely through ADCC, but potentially also via augmented antigen presentation) comes from studies evaluating the relationship between certain activating FcγR polymorphisms and clinical benefit of mAb. A polymorphism of the activating FcγR, FcγRIIIa, exists at position 158 where the V/V (homozygous for valine at position 158) genotype results in an FcγRIIIa that binds human IgG1 antibody with high affinity. The F/F (homozygous for phenylalanine at position 158) and V/F genotypes result in a low and intermediate affinity FcγRIIIa, respectively. In non-Hodgkin’s Lymphoma, Rituximab® was most effective in those patients with the 158 V/V genotype and thus, those patients with the high affinity FcRγIIIa96. Likewise, HER2+ metastatic breast cancer patients treated with Trastazumab97 and metastatic colorectal cancer patients treated with Cetuximab98 showed greater response to therapy if they had the 158 V/V genotype. If ADCC plays no role in the therapeutic efficacy of these antibodies, then polymorphisms of FcγRIIIa would not be expected to be associated with clinical outcome. Moreover, antitumor efficacy of antibodies induced by immunization to idiotypic antigens on B cell tumors99 and antigens on colon cancer100 are associated with possession of the genotypes for high affinity FcγRIIa and FcγRIIIa. However, some have argued that higher affinity FcRs might provide clinical benefit via enhanced antigen processing which may augment an adaptive immune response, rather than facilitating ADCC and this issue has not been resolved.
Therapeutic mAbs can be engineered in a number of ways to increase their utility for clinically relevant NK-mediated ADCC. First, they can be engineered to be less foreign to the human immune system, thus attenuating the development of anti-antibodies that may neutralize the therapeutic mAb. The constant region of a human immunoglobulin gene can be grafted to the variable region of mouse immunoglobulin genes to create a chimeric mAb (e.g., ch14.18; ~80% human). Alternately, a humanized antibody can be created by grafting the nucleotide sequence for the complementarity-determining region (CDR) of a murine antibody to the appropriate CDR location of a human immunoglobulin gene (e.g., hu14.18; ~98% human). Additional modifications of therapeutic mAbs include modified glycosylation of Fc-linked oligosaccharides to enhance ADCC (e.g., hu14.18K322A) and amino acid substitution in the heavy chain constant region to alter the binding affinity of mAb to complement (e.g., hu14.18K322A).
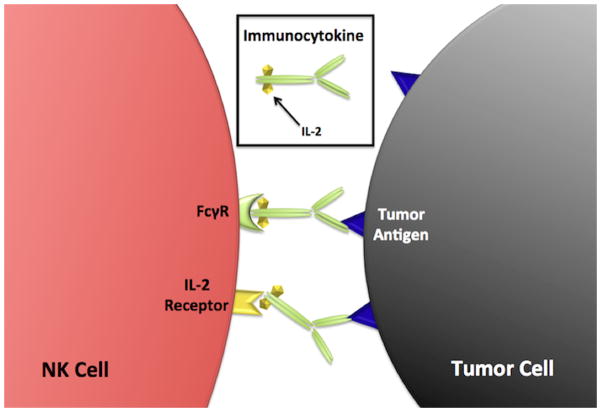
Therapeutic mAbs can also be conjugated to cytokines (e.g., ch14.18-IL2, hu14.18-IL2) by fusing the human gene for the cytokine to the mAb gene. By linking cytokine directly to the tumor specific mAb, the immunomodulatory effect of the cytokine should be more localized to the tumor microenvironment. For example, ch14.18-IL2 has two functional molecules of interleukin-2 attached to the Fc terminal end of the ch14.18 mAb. Preclinical studies showed that this fusion protein (or immunocytokine) had far superior antitumor activity when compared to comparable doses of ch14.18 mAb and IL-2 given simultaneously as individual agents101. A study evaluating immunocytokine-facilitated conjugate formation between NK cells and tumor cells demonstrated that tumor cells coated with immunocytokine were bound to NK cells not only through the Fc region of the immunocytokine but also through the IL-2 moiety102,103. NK cells bound to tumor cells formed activated immune synapses, defined by the localization of LFA-1 and CD2, along with clustering of NK cell CD25 into the synapse. In fact, in the presence of immunocytokine, NK cells with minimal or no FcγRIIIa were able to form conjugates with tumor cells demonstrating an important role for IL2Rα. Thus, immunocytokine therapy may: 1) target NK cells to tumor via tumor antigen-mAb moiety-FcγIIIa interactions, 2) deliver immune-modulatory cytokine directly to the tumor microenvironment, and 3) participate in immune synapse formation via the IL2 component of the immunocytokine bound by the IL2 receptors on the NK cells (Fig. 3).
Figure 3.
Schematic representation of immunocytokine-facilitated tumor cell-NK cell conjugate formation. Tumor cells coated with immunocytokine bind NK cells via interaction between 1) Fc region of the immunocytokine and the NK cell FcγR, and 2) IL-2 moiety of immunocytokine and the NK cell IL-2 receptor. Bound FcγRs and IL-2Rs transmit activation signals leading to enhanced NK cell function.
A recent approach to augment ADCC employing the use of monoclonal antibodies involves a separate means to activate NK cells in vivo. When NK cells become activated following engagement of their FcRs by tumor bound mAb, they up-regulate expression of cell surface CD137 molecules, which are activating receptors. Provision of an agonistic anti-CD137 mAb at this critical time, in vivo, can augment ADCC capabilities and function of the NK cells with improved in vivo anti-tumor effects in tumor bearing mice104. This approach is now being pursued clinically in studies of Rituximab® for lymphoma104,105.
Table 1 shows open or recently completed clinical trials employing mAb therapy for pediatric cancers that may involve NK-mediated mechanisms. Excellent recent reviews that include all mAb therapy for pediatric cancers are available106–108
Table 1.
Open and Recently Completed Clinical Trials for Pediatric Cancers Employing Monoclonal Antibodies and Immunocytokines that may involve NK-mediated mechanisms
| Clinical Trial Number | Title | Sponsor |
|---|---|---|
| NCT01055314 | Temozolomide, Cixutumumab, and Combination Chemotherapy in Treating Patients With Metastatic Rhabdomyosarcoma | National Cancer Institute |
| NCT01598454 | Use of Racotumomab in Patients With Pediatric Tumors Expressing N- glycolylated Gangliosides | Laboratorio Elea S.A.C.I.F. y A. |
| NCT01595048 | Combination Chemotherapy With or Without Rituximab in Treating Younger Patients With Stage III-IV Non-Hodgkin Lymphoma or B-Cell Acute Leukemia | Children’s Oncology Group |
| NCT01279707 | Monoclonal Antibodies in Recurrent or Refractory B Cell Acute Lymphoblastic Leukaemia (ALL) (MARALL) | Queen Mary University of London |
| NCT01900496 | Study of Rituximab and Brentuximab Vedotin for Relapsed Classical Hodgkin Lymphoma | Sidney Kimmel Comprehensive Cancer Center |
| NCT01552434 | Bevacizumab, Temsirolimus, Valproic Acid, Cetuximab | M.D. Anderson Cancer Center |
| NCT01767194 | Irinotecan Hydrochloride and Temozolomide With Temsirolimus or Monoclonal Antibody Ch14.18 in Treating Younger Patients With Refractory or Relapsed Neuroblastoma | National Cancer Institute |
| NCT00026312 | Isotretinoin With or Without Dinutuximab, Aldesleukin, and Sargramostim Following Stem Cell Transplantation in Treating Patients With Neuroblastoma | National Cancer Institute |
| NCT01711554 | Lenalidomide and Dinutuximab With or Without Isotretinoin in Treating Younger Patients With Refractory or Recurrent Neuroblastoma | National Cancer Institute |
| NCT01526603 | High Dose Chemotherapy and Autologous Transplant for Neuroblastoma | Masonic Cancer Center, University of Minnesota |
| NCT01419834 | Humanized 3F8 Monoclonal Antibody (Hu3F8) in Patients With High-Risk Neuroblastoma and GD2-Positive Tumors | Memorial Sloan-Kettering Cancer Center |
| NCT01662804 | Humanized 3F8 Monoclonal Antibody (Hu3F8) When Combined With Interleukin-2 in Patients With High-Risk Neuroblastoma and GD2-positive Solid Tumors | Memorial Sloan-Kettering Cancer Center |
| NCT00877110 | Anti-GD2 3F8 Antibody and Allogeneic Natural Killer Cells for High-Risk Neuroblastoma | Memorial Sloan-Kettering Cancer Center |
| NCT01183429 | 3F8/GM-CSF Immunotherapy Plus 13-Cis-Retinoic Acid for Consolidation of First Remission After Non-Myeloablative Therapy in Patients With High-Risk Neuroblastoma | Memorial Sloan-Kettering Cancer Center |
| NCT01757626 | Combination Therapy of Antibody Hu3F8 With Granulocyte- Macrophage Colony Stimulating Factor (GM-CSF) in Patients With Relapsed/Refractory High-Risk Neuroblastoma | Memorial Sloan-Kettering Cancer Center |
| NCT01183897 | 3F8/GM-CSF Immunotherapy Plus 13-Cis-Retinoic Acid for Primary Refractory Neuroblastoma in Bone Marrow | Memorial Sloan-Kettering Cancer Center |
| NCT01183884 | 3F8/GM-CSF Immunotherapy Plus 13-Cis-Retinoic Acid for Consolidation of Second or Greater Remission of High-Risk Neuroblastoma | Memorial Sloan-Kettering Cancer Center |
| NCT00445965 | Iodine I 131 Monoclonal Antibody 3F8 in Treating Patients With Central Nervous System Cancer or Leptomeningeal Cancer | Memorial Sloan-Kettering Cancer Center |
| NCT01704716 | High Risk Neuroblastoma Study 1 (1.5) of SIOP-Europe (SIOPEN) | St. Anna Kinderkrebsforschung, Austria |
| NCT01701479 | Long Term Continuous Infusion ch14.18/CHO Plus s.c. Aldesleukin (IL-2) (LTI) | St. Anna Kinderkrebsforschung, Austria |
| NCT01576692 | A Safety/Feasibility Trial of the Addition of the Humanized Anti-GD2 Antibody (hu14.18K322A) With and Without Natural Killer Cells to Chemotherapy in Children and Adolescents With Recurrent/Refractory Neuroblastoma (GD2NK) | St. Jude Children's Research Hospital |
| NCT00743496 | A Phase I Trial Of The Humanized Anti-GD2 Antibody In Children And Adolescents With Neuroblastoma, Osteosarcoma, Ewing Sarcoma and Melanoma | St. Jude Children's Research Hospital |
| NCT01857934 | Therapy for Children With Advanced Stage Neuroblastoma | St. Jude Children's Research Hospital |
| NCT01592045 | Ch14.18 Pharmacokinetic Study in High-risk Neuroblastoma | United Therapeutics |
| NCT01748721 | MORAb-004 in Treating Young Patients With Recurrent or Refractory Solid Tumors or Lymphoma | Morphotek |
| NCT01334515 | Biological Therapy, Sargramostim, and Isotretinoin in Treating Patients With Relapsed or Refractory Neuroblastoma | COG |
| NCT00003750 | Biological Therapy in Treating Children With Refractory or Recurrent Neuroblastoma or Other Tumors | aaaaaaaaaCOG |
Antitumor effects of NK cells in Allogeneic HSCT
The rationale for using allogeneic hematopoietic stem cell transplant (HSCT) in an attempt to improve survival for individuals with recurrent or refractory hematologic malignancies is two-fold. First, stem cell rescue provides the opportunity to use high doses of chemotherapy and radiation that may potentially overcome drug resistance but are likely to produce severe myelosuppression. Second, donor immune cells can react to and destroy recipient tumor cells producing a “graft versus leukemia” (GVL) effect. Unfortunately, donor immune cells may also attack normal recipient tissues resulting in graft versus host disease (GVHD). Initial pediatric allogeneic HSCTs were generally performed in patients who had a human leukocyte antigen (HLA)-matched sibling donor. The frequent lack of a suitable HLA-matched donor led to the use of parents as a donor. Most parents share only one HLA haplotype with their child (haploidentical) and this degree of HLA mismatch would be expected to cause severe GVHD. However, since T lymphocytes are predominantly responsible for eliciting GVHD, the depletion of T cells from haploidentical grafts reduces the incidence and severity of this untoward effect. Although T lymphocytes were known to play a significant role in mediating the GVL effect of allogeneic HSCT, T-depleted grafts were still associated with an anti-leukemic effect109. This antitumor activity was mediated, in large part, by NK cell effector mechanisms. Interestingly, although NK cells are able to elicit a GVL (or graft versus tumor) effect, they do not appear to cause GVHD.
Because the genes for HLA and KIR are on different chromosomes and they sort independently, matching for HLA genes between donor and recipient does not result in matched KIR genes. In fact, ~50–75% of transplants from HLA identical sibling donors will be KIR/KIR ligand mismatched. The probability of KIR/KIR ligand mismatch in the haploidentical transplant setting is similarly 50–75%. A pivotal study by Ruggeri and colleagues110 clearly demonstrated the effectiveness of KIR-mismatched donor NK cells, predicted to be alloreactive, to mediate a graft-versus-leukemia effect in adults with acute myelogenous leukemia (AML). Using haploidentical HSCT, they showed that susceptibility of tumor cells to NK cell cytotoxicity correlated with the predicted existence of a mismatch between KIR receptors expressed by donor NK cells and MHC class I alleles expressed by the recipient. Another study in the setting of haploidentical stem cell transplant showed that NK cell alloreactivity appeared to be a major factor influencing the risk of relapse for pediatric patients with both acute lymphoblastic leukemia (ALL) and AML suggesting that a GVL effect, mediated by allogeneic NK cells, was not limited to patients with AML111. A recent study in children with high-risk leukemias who received haploidentical HSCT showed that donor-derived alloreactive NK cells are generated and persist for many years in the transplanted recipients112.
Interestingly, when NK cell reconstitution is monitored following haploidentical HSCT using CD34+-selected cells, it appears that immature, poorly functioning KIR-NKG2A+ NK cells predominate in the early transplant period112,113. Thus, allogeneic HSCT using CD34+ selected grafts may have limited GVL effect in the immediate post-transplant period due to the lack of mature donor NK cells in the graft. This finding provides a rationale for graft processing methods that selectively remove unwanted GVHD-inducing cells (α/β T cells) and thus, leave desirable GVL effector cells in the graft (e.g., NK cells, γ/δ T cells)114. Alternatively, mature donor NK cells can be adoptively transferred during the post-transplant period. A number of ongoing clinical trials are using haploidentical HSCT, with and without donor NK cell adoptive therapy, for the treatment of both hematologic and solid tumor malignancies (Table 2).
Table 2.
Open and Recently Completed Clinical Trials for Pediatric Cancers Employing Adoptive NK cell therapy
| Clinical Trial Number | Title | Sponsor |
|---|---|---|
| NCT00582816 | Haploidentical Transplant With NK Cell Infusion for Pediatric Acute Leukemia and Solid Tumors | University of Wisconsin, Madison |
| NCT00640796 | Pilot Study of Expanded, Donor Natural Killer Cell Infusions for Refractory Non-B Lineage Hematologic Malignancies and Solid Tumors | St. Jude Children's Research Hospital |
| NCT01287104 | A Phase I Study of NK Cell Infusion Following Allogeneic Peripheral Blood Stem Cell Transplantation From Related or Matched Unrelated Donors in Pediatric Patients With Solid Tumors and Leukemias | National Cancer Institute |
| NCT01386619 | NK DLI in Patients After HLA-haploidentical HSCT | University Hospital, Basel, Switzerland |
| NCT01700946 | Therapy for Pediatric Relapsed or Refractory Precursor B-Cell Acute Lymphoblastic Leukemia and Lymphoma | St. Jude Children's Research Hospital |
| NCT01875601 | NK White Blood Cells and Interleukin in Children and Young Adults with Advanced Solid Tumors | National Cancer Institute |
| NCT01795378 | Safety and Efficacy Study of Donor Natural Killer Cells Given After Haploidentical Hematopoietic Cell Transplantation (DNKI-II) | Asan Medical Center |
| NCT00896701 | Relationship Between Natural Killer Cells' Ability to Kill Leukemia Cells and the Outcome of Patients With Acute Myeloid Leukemia Previously Treated With Interleukin-2 | Cancer and Leukemia Group B |
| NCT00789776 | Fludarabine Phosphate, Cyclophosphamide, Total-Body Irradiation, and Donor Bone Marrow Transplant Followed by Donor Natural Killer Cell Therapy, Mycophenolate Mofetil, and Tacrolimus in Treating Patients With Hematologic Cancer | Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium |
| NCT01823198 | Natural Killer (NK) Cells With HLA Compatible Hematopoietic Transplantation for High Risk Myeloid Malignancies | M.D. Anderson Cancer Center |
| NCT00877110 | Anti-GD2 3F8 Antibody and Allogeneic Natural Killer Cells for High-Risk Neuroblastoma | Memorial Sloan-Kettering Cancer Center |
| NCT00526292 | Chemotherapy and a Donor Natural Killer Cell Infusion in Treating Patients With Relapsed or Persistent Leukemia or Myelodysplastic Syndrome After a Donor Stem Cell Transplant | Memorial Sloan-Kettering Cancer Center |
| NCT01621477 | T-Cell Replete Haploidentical Donor Hematopoietic Stem Cell Plus Natural Killer (NK) Cell Transplantation in Patients With Hematologic Malignancies Relapsed or Refractory Despite Previous Allogeneic Transplant | St. Jude Children's Research Hospital |
| NCT01576692 | A Safety/Feasibility Trial of the Addition of the Humanized Anti-GD2 Antibody (hu14.18K322A) With and Without Natural Killer Cells to Chemotherapy in Children and Adolescents With Recurrent/Refractory Neuroblastoma (GD2NK) | St. Jude Children's Research Hospital |
| NCT00145626 | HLA-Nonidentical Stem Cell and Natural Killer Cell Transplantation for Children Less the Two Years of Age With Hematologic Malignancies | St. Jude Children's Research Hospital |
| NCT01857934 | Therapy for Children With Advanced Stage Neuroblastoma | St. Jude Children's Research Hospital |
| NCT00995137 | Genetically Modified Haploidentical Natural Killer Cell Infusions for B- Lineage Acute Lymphoblastic Leukemia | St. Jude Children's Research Hospital |
| NCT00703820 | Clofarabine Plus Cytarabine Versus Conventional Induction Therapy And A Study Of NK Cell Transplantation In Newly Diagnosed Acute Myeloid Leukemia | St. Jude Children's Research Hospital |
| NCT01807611 | KIR Mismatched Haploidentical Donor Hematopoietic Progenitor Cell and NK Cell Transplantation for Hematologic Malignancy | St. Jude Children's Research Hospital |
The influence of NK cell alloreactivity on post-transplant survival is not limited to the setting of haploidentical HSCT, but has also been demonstrated in patients receiving HLA matched sibling or unrelated donor grafts. Hsu et al. documented a significant improvement in disease-free survival among AML and MDS patients who were KIR-KIR ligand mismatched with their HLA matched sibling donors115. This survival benefit was not observed for patients with ALL or CML, suggesting that the leukemias that benefit from donor NK cell alloreactivity depends on multiple variables. First, it should be noted that HSCT therapy in various studies is dissimilar with respect to: 1) conditioning regimen 2) graft composition and dose 3) donor type, 4) cell source (peripheral blood, bone marrow, umbilical cord blood), and 5) use of post-transplant immunosuppression. These disparate HSCT processes will affect NK cell recovery, proliferation, maturation, and activation in the post-transplant period. Second, other families of inhibitory receptors on NK cells besides inhibitory KIRs may play a role in the anti-leukemia effect of NK cells. Miller and colleagues116 have shown that families of inhibitory receptors (e.g., NKG2A, LIR-1) present on both KIR+ and KIR− NK cells are involved in leukemia cell killing. They assert that blockade of multiple MHC class I-recognizing NK cell receptors may overcome ALL blast resistance to NK alloreactivity. Moreover, while most investigators have focused on the influence exerted by donor inhibitory KIRs, Cooley et al. found that donor activating KIRs influenced survival in AML patients following unrelated donor transplantation117. Patients transplanted from donors possessing the B KIR haplotype were found to have a 30% improvement in the relative risk of relapse-free survival compared to donors lacking this genotype. The B KIR haplotype is associated with a greater number of activating KIR genes.
Adoptive therapy with NK cells
In addition to utilizing the antitumor potential of adoptive NK cell therapy during the post-allogeneic hematopoietic transplant period, allogeneic NK cells have been successfully administered in the non-transplant setting. Patients are initially treated with lymphodepletive chemotherapy to avoid immediate rejection of allogeneic NK cells and to decrease competition for endogenous cytokines. The efficacy of this approach was first demonstrated by Miller and colleagues, who administered ex-vivo IL-2 stimulated, allogeneic NK cells to lymphodepleted patients along with IL-2 therapy53. In this study, 75% of patients who were KIR/KIR ligand mismatched (in the graft-versus-host direction) achieved complete remission while only 13% of patients achieved complete remission in the group without KIR/KIR ligand mismatch status. Another trial to treat AML in adults involved the infusion of unstimulated, KIR/KIR ligand mismatched haploidentical donor NK cells along with IL-2 administration. The complete response rate in this elderly adult cohort was encouraging118. Patients with solid tumors, for example ovarian or breast cancer, have also been treated with allogeneic NK cells in the non-transplant setting119. A study in adults with advanced renal cell carcinoma or melanoma that involved the infusion of IL-2 stimulated, haploidentical donor NK cells along with IL-2 administration showed disease stabilization when measured six weeks following the infusion53. Iliopoulou et al., treated adult patients with advanced non-small cell lung cancer with repetitive infusions of donor NK cells in combination with chemotherapy and showed disease stabilization or a partial response in half of the patients. In this study, only one donor was reported to be KIR/KIR ligand mismatched to the recipient120. A recent report described this approach in children. Rubnitz and colleagues55 administered non-activated, haploidentical donor NK cells, followed by IL-2 administration, to children with AML (most with favorable risk disease) and reported that all patients remained in remission for at least 2 years following adoptive NK cell therapy. The donor NK cells were KIR/KIR ligand mismatched in 9/10 of these patients. There are several open pediatric clinical trials employing adoptive NK cell therapy in the non-transplant setting (Table 2).
Role of Autologous KIR/KIR ligand Mismatch
The genes for KIRs and MHC class I are located on different chromosomes and they segregate independently in human pedigrees121. Thus, approximately 60% of individuals have KIRs for which they have no corresponding MHC class I; these individuals are considered to be autologous, or “self-” KIR/KIR ligand mismatched. During the maturation process, an NK cell must express at least one inhibitory KIR that recognizes an MHC class I molecule to be “licensed to kill”23. NK cells that do not encounter an MHC class I ligand for one of its KIRs during development are unlicensed and functionally less potent than “licensed” NK cells, yet they are still somewhat reactive. Recent studies suggest that licensing may actually be a continual process by which NK cells respond to their environment122.
A study evaluating the relationship between KIR/KIR ligand mismatch and clinical outcome following autologous HSCT in children with solid tumors and lymphoma showed that relapse was significantly reduced in individuals with self-KIR/KIR ligand mismatch123. A similar study in high-risk neuroblastoma patients who had undergone autologous HSCT found that self-KIR/KIR ligand mismatch was associated with improved disease-free survival124. Another study in children with neuroblastoma who were treated with hu14.18-IL2 reported a better response to therapy in those that were self-KIR/KIR ligand mismatched125. These studies support the notion that unlicensed, hyporesponsive NK cells (self-KIR/KIR ligand mismatched) may gain potent effector function in the proper milieu that is provided in the setting of HSCT and subsequent immune reconstitution. The selection of appropriately KIR/KIR-ligand mismatched donors for allogeneic cell therapy, and selection of autologous KIR/KIR-ligand mismatched patients for NK based forms of immunotherapy (such as hu14.18-IL2 therapy) seems to be supported by the associations of improved outcome or responses for KIR/KIR-ligand mismatched individuals. One way to potentially circumvent the apparent disadvantage of a KIR/KIR-ligand matched setting may be the additional administration of a mAb that binds to and blocks inhibitory signals transmitted by inhibitory KIRs126. Preclinical models indicate that blocking inhibitory receptors can cause a potent NK cell mediated anti-tumor effect127.
Adoptive NK cell therapy using expanded, activated NK Cells
A limitation of NK cell adoptive therapy is the relatively small number of NK cells that can be obtained from apheresis of a healthy adult. In order to increase the number of NK cells available for adoptive therapy, several new protocols have been developed to expand NK cells ex-vivo. Co-culturing donor NK cells with irradiated “feeder” cells (that present activating antigens to NK cells) substantially increases NK cell numbers and their activity compared to simple IL-2 cytokine stimulation. Several of these feeder cell lines have been developed to expand donor NK cells and are currently in use. The first feeder cell line, EBV-TM-LCL (Epstein-Barr virus-transformed B-cell line), was reported to expand NK cells nearly 500-fold in 3 weeks128. Several different feeder cell lines are genetic modifications of the myelogenous leukemia cell line, K562, that have been induced to express different molecules on the cell surface. The K562-MICA-41BBL-mbIL15 feeder cell line was shown to expand NK cells 550-fold in about 3 weeks129 and the K562-mb15–41BBL feeder cell line expanded NK cells more than 275-fold in the same period of time130. An additional feeder cell line that has reported dramatic NK cell expansion in 3 weeks (median of > 21,000-fold) is K562-Clone9-mbIL21131.
NK cells that are activated ex vivo by these feeder cell methodologies show increased expression of activation receptors, including the activating receptors, DNAM-1, NKp46, NKp44, NKp30132–135 and NKG2D130,132,133,135. By up-regulating the density of activating surface receptors on the NK cell, the balance of signals at the NK cell interface with a ligand-expressing tumor cell may be “tipped” toward activation. Therefore, some authors have proposed that the rule of KIR mismatch do not apply to ex vivo expanded NK cells due to the high degree of activation135. Up-regulation of chemokine receptors, CXCR3, CXCR4 and CXCR6, has also been reported132,135, which may enhance NK localization to the tumor microenvironment. In addition, activated, expanded NK cells show increased expression of adhesion molecules CD54 and CD56132,135 which may enhance adherence to target cells, the first stage of immune synapse formation. Activated, expanded NK cells have also shown potent in vitro cytotoxicity against numerous tumor cell types130,132–136. Despite the high cytotoxicity against tumor cells demonstrated by these NK cells, they have been shown to spare healthy cells130,132,134.
In light of the finding that approximately 60% of individuals are self-KIR/KIR ligand mismatched, the notion of adoptive therapy using expanded, highly activated autologous NK cells is particularly attractive. In fact, Liu and colleagues131 have demonstrated that NK cells from children with neuroblastoma can be effectively expanded and activated using the irradiated K562-derived Clone9.mbIL21 feeder cell line. These cells were shown to possess potent anti-neuroblastoma activity, even following cryopreservation. The authors propose that clinical testing using autologous expanded and activated NK cells combined with ch14.18 in the minimal disease setting is warranted.
Four ongoing adult clinical trials use one of the aforementioned methodologies to expand and activate either autologous or allogeneic NK cells prior to NK cell infusion and two additional adult trials are registered but not yet open to accrual. A pediatric trial has recently been completed that involved the expansion and activation of haploidentical allogeneic NK cells ex vivo prior to adoptive therapy (NCT00640796).
NK cell CARs (Chimeric Antigen Receptors)
A first generation CAR is a genetically engineered “hybrid” receptor comprised of an extracellular single-chain antibody fragment, or scFv, (that recognizes the tumor associated antigen) linked by a transmembrane domain to an intracellular signaling moiety, usually CD3ζ. Second and third generation CARs also incorporate one or two costimulatory activating motifs (e.g., 2B4, CD28, 4-1BB, OX-40), respectively, into the intracellular segment of the CAR. The most common approach to introduce CARs into effector cells uses γ retroviruses that stably integrate the genetic sequence for the receptor into the target cell genome137,138. Alternate approaches use lentiviral transduction, direct RNA transfection and transposon-based systems.
The incorporation of tumor specific CARs into NK cells provides the opportunity for NK cells to directly bind a specific antigen on tumor cells. Receptor binding is agonistic in nature and an activation signaling cascade ensues. The nature of the immune synapse formed has not been explored but it appears that the activation signal resulting from CAR activation may be sufficient to override inhibitory receptor signals139–141. Several in vitro studies demonstrate markedly enhanced cytotoxicity of tumor cells by NK cells bearing CARs specific for tumor antigen140,142,143. A single institution, phase I trial (NCT00995137) in children with B-cell ALL is investigating the safety of NK cells bearing CD19-specific CARs. Donor NK cells are first expanded by co-culture with feeder cells (K562-41BBL-mbIL15) and then transduced to express the signaling CAR, anti-CD19-BB-zeta. Preclinical studies are exploring the antitumor activity of expanded cord blood NK cells bearing CD20-specific CARs143.
Summary
A number of immunotherapy strategies employ the effector functions of NK cells to kill malignant cells. NK cells can be activated by cytokines or specific TLR ligands, enhancing their proliferative capacity, survival and effector functions (cytotoxicity and secretion of cytokines and chemokines). In addition, select drugs can enhance the expression of proteins on the surface of tumor cells that serve as ligands for activating receptors on NK cells. Moreover, tumor cells can be targeted for destruction by NK cells using tumor specific mAbs. NK cells are also involved in the success of both autologous and allogeneic HSCT in treating malignancies and evidence supports a role for KIR/KIR ligand mismatch in the associated antitumor effect. Adoptive therapy with allogeneic NK cells, in the setting of transplant or lymphodepletion, has demonstrated some success, particularly in hematologic malignancies. An exciting new means by which to exploit the potent tumor killing potential of natural killer cells is through the introduction of tumor specific CARs into NK cells using genetic engineering. Finally, new methodologies that allow the production of large numbers of highly active NK cells may provide an avenue to utilize autologous, allogeneic, and tumor specific CAR possessing NK cells for cancer immunotherapy. Numerous clinical trials are ongoing to explore several possible therapeutic uses of NK cells to treat pediatric cancer. Some of these utilize technology that requires highly specialized clinical support/production laboratories (i.e., ex vivo expansion of NK cells for infusion, or ex vivo transfection with CARs), while others involve approaches that are “off the shelf” and readily administered in standard pediatric oncology clinics or inpatient units (i.e., infusions of NK-activating cytokines, or use of mAbs that facilitate NK induced ADCC). Many of these approaches are already showing clear evidence of antitumor activity or clinical benefit. This next decade will involve efforts of the pediatric oncology community to integrate these forms of NK based immunotherapy (along with other forms of immunotherapy) into the conventional treatment for childhood malignancies. The goal, as always, will be to improve disease free survival while minimizing acute and long-term toxicities from treatment.
Figure 4. Impact of KIR/KIR-Ligand relationship and FcγR genotype on tumor cell lysis following mAb treatment.

KIR molecules are depicted as matched (left boxes) or mismatched (right boxes) for the corresponding MHC class I molecule on the tumor cell. The NK cell FcγIIIa receptors (FcR) are depicted as lowest affinity (homozygous for phenylalanine at position 158, upper two boxes) or highest affinity (homozygous for valine at position 158, lower two boxes). This simplified example demonstrates the interplay of inhibitory and activating signals that are integrated within the NK cell to produce a lytic response. Adapted with permission from: Koehn TA et al., Increasing the clinical efficacy of NK and antibody-mediated cancer immunotherapy: potential predictors of successful clinical outcome based on observations in high-risk neuroblastoma, Frontiers in Pharmacology 3, Article 91, 2013, Nature Publishing Group.
Acknowledgments
Sources of Funding: This work was supported by National Institutes of Health Grants CA032685, CA87025, CA166105, CA14520, Department of Defense grant W81XWH-08-1-0559 and grants from the Midwest Athletes for Childhood Cancer Fund, Stand Up to Cancer, St. Baldrick’s Foundation, The Crawdaddy Foundation, The Evan Dunbar Foundation, Alex’s Lemonade Stand Foundation, Hyundai Hope on Wheels, and the University of Wisconsin-Madison Institute for Clinical and Translational Research (ICTR) TL1 Training Grant UL1TR000427.
Footnotes
Conflicts of Interest:
There are no conflicts of interests declared for any author.
References
- 1.Bisset LR, Lung TL, Kaelin M, et al. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol. 2004;72:203–212. doi: 10.1046/j.0902-4441.2003.00199.x. [DOI] [PubMed] [Google Scholar]
- 2.Blum KS, Pabst R. Lymphocyte numbers and subsets in the human blood. Do they mirror the situation in all organs? Immunol Lett. 2007;108:45–51. doi: 10.1016/j.imlet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Morandi B, Mortara L, Chiossone L, et al. Dendritic cell editing by activated natural killer cells results in a more protective cancer-specific immune response. PLoS One. 2012;7:e39170. doi: 10.1371/journal.pone.0039170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccioli D, Sbrana S, Melandri E, et al. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strowig T, Brilot F, Munz C. Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol. 2008;180:7785–7791. doi: 10.4049/jimmunol.180.12.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagler A, Lanier LL, Cwirla S, et al. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183–3191. [PubMed] [Google Scholar]
- 8.Fauriat C, Long EO, Ljunggren HG, et al. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieberman J. Mechanisms of granule-mediated cytotoxicity. Current Opinion inImmunology. 2003;15:513–515. [Google Scholar]
- 10.Chavez-Galan L, Arenas-Del Angel MC, Zenteno E, et al. Cell death mechanisms induced by cytotoxic lymphocytes. Cell Mol Immunol. 2009;6:15–25. doi: 10.1038/cmi.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terme M, Ullrich E, Delahaye NF, et al. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 13.Zamai L, Ponti C, Mirandola P, et al. NK cells and cancer. J Immunol. 2007;178:4011–4016. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- 14.Bryceson YT, March ME, Ljunggren HG, et al. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skak K, Frederiksen KS, Lundsgaard D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology. 2008;123:575–583. doi: 10.1111/j.1365-2567.2007.02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandt CS, Baratin M, Yi EC, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castriconi R, Dondero A, Corrias MV, et al. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004;64:9180–9184. doi: 10.1158/0008-5472.CAN-04-2682. [DOI] [PubMed] [Google Scholar]
- 18.Bottino C, Castriconi R, Pende D, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198:557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pende D, Bottino C, Castriconi R, et al. PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: involvement in tumor cell lysis. Mol Immunol. 2005;42:463–469. doi: 10.1016/j.molimm.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Kloess S, Huenecke S, Piechulek D, et al. IL-2-activated haploidentical NK cells restore NKG2D-mediated NK-cell cytotoxicity in neuroblastoma patients by scavenging of plasma MICA. Eur J Immunol. 2010;40:3255–3267. doi: 10.1002/eji.201040568. [DOI] [PubMed] [Google Scholar]
- 21.Raffaghello L, Prigione I, Airoldi I, et al. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia. 2004;6:558–568. doi: 10.1593/neo.04316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chitadze G, Bhat J, Lettau M, et al. Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications. Scand J Immunol. 78:120–129. doi: 10.1111/sji.12072. [DOI] [PubMed] [Google Scholar]
- 23.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. 2010;142:847–856. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 25.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–154. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 26.Lampson LA, Fisher CA, Whelan JP. Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol. 1983;130:2471–2478. [PubMed] [Google Scholar]
- 27.Lampson LA, Whelan JP, Fisher CA. HLA-A,B,C and beta 2-microglobulin are expressed weakly by human cells of neuronal origin, but can be induced in neuroblastoma cell lines by interferon. Prog Clin Biol Res. 1985;175:379–388. [PubMed] [Google Scholar]
- 28.Main EK, Lampson LA, Hart MK, et al. Human neuroblastoma cell lines are susceptible to lysis by natural killer cells but not by cytotoxic T lymphocytes. J Immunol. 1985;135:242–246. [PubMed] [Google Scholar]
- 29.Prigione I, Corrias MV, Airoldi I, et al. Immunogenicity of human neuroblastoma. Ann N Y Acad Sci. 2004;1028:69–80. doi: 10.1196/annals.1322.008. [DOI] [PubMed] [Google Scholar]
- 30.Bryceson YT, March ME, Ljunggren HG, et al. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–239. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 32.Ferlazzo G, Pack M, Thomas D, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konjevic G, Mirjacic Martinovic K, Vuletic A, et al. In-vitro IL-2 or IFN-alpha-induced NKG2D and CD161 NK cell receptor expression indicates novel aspects of NK cell activation in metastatic melanoma patients. Melanoma Res. 2010;20:459–467. doi: 10.1097/CMR.0b013e32833e3286. [DOI] [PubMed] [Google Scholar]
- 34.Trotta R, Ciarlariello D, Dal Col J, et al. The PP2A inhibitor SET regulates granzyme B expression in human natural killer cells. Blood. 2011;117:2378–2384. doi: 10.1182/blood-2010-05-285130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Handa K, Suzuki R, Matsui H, et al. Natural killer (NK) cells as a responder to interleukin 2 (IL 2). II. IL 2-induced interferon gamma production. J Immunol. 1983;130:988–992. [PubMed] [Google Scholar]
- 36.Hank JA, Kohler PC, Weil-Hillman G, et al. In vivo induction of the lymphokine-activated killer phenomenon: interleukin 2-dependent human non-major histocompatibility complex-restricted cytotoxicity generated in vivo during administration of human recombinant interleukin 2. Cancer Res. 1988;48:1965–1971. [PubMed] [Google Scholar]
- 37.He XS, Draghi M, Mahmood K, et al. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J Clin Invest. 2004;114:1812–1819. doi: 10.1172/JCI22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meropol NJ, Porter M, Blumenson LE, et al. Daily subcutaneous injection of low-dose interleukin 2 expands natural killer cells in vivo without significant toxicity. Clin Cancer Res. 1996;2:669–677. [PubMed] [Google Scholar]
- 39.Miller JS, Tessmer-Tuck J, Pierson BA, et al. Low dose subcutaneous interleukin-2 after autologous transplantation generates sustained in vivo natural killer cell activity. Biol Blood Marrow Transplant. 1997;3:34–44. [PubMed] [Google Scholar]
- 40.Siegel JP, Sharon M, Smith PL, et al. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987;238:75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- 41.Sondel PM, Kohler PC, Hank JA, et al. Clinical and immunological effects of recombinant interleukin 2 given by repetitive weekly cycles to patients with cancer. Cancer Res. 1988;48:2561–2567. [PubMed] [Google Scholar]
- 42.Trinchieri G, Matsumoto-Kobayashi M, Clark SC, et al. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984;160:1147–1169. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weil-Hillman G, Fisch P, Prieve AF, et al. Lymphokine-activated killer activity induced by in vivo interleukin 2 therapy: predominant role for lymphocytes with increased expression of CD2 and leu19 antigens but negative expression of CD16 antigens. Cancer Res. 1989;49:3680–3688. [PubMed] [Google Scholar]
- 44.Hromadnikova I, Pirkova P, Sedlackova L. Influence of in vitro IL-2 or IL-15 alone or in combination with Hsp-70-derived 14-mer peptide (TKD) on the expression of NK cell activatory and inhibitory receptors. Mediators Inflamm. 2013:405295. doi: 10.1155/2013/405295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitale M, Bottino C, Sivori S, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187:2065–2072. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yakes FM, Chinratanalab W, Ritter CA, et al. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- 47.Roper M, Smith MA, Sondel PM, et al. A phase I study of interleukin-2 in children with cancer. Am J Pediatr Hematol Oncol. 1992;14:305–311. [PubMed] [Google Scholar]
- 48.Bauer M, Reaman GH, Hank JA, et al. A phase II trial of human recombinant interleukin-2 administered as a 4-day continuous infusion for children with refractory neuroblastoma, non-Hodgkin's lymphoma, sarcoma, renal cell carcinoma, and malignant melanoma. A Childrens Cancer Group study. Cancer. 1995;75:2959–2965. doi: 10.1002/1097-0142(19950615)75:12<2959::aid-cncr2820751225>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 49.Lange BJ, Yang RK, Gan J, et al. Soluble interleukin-2 receptor alpha activation in a Children's Oncology Group randomized trial of interleukin-2 therapy for pediatric acute myeloid leukemia. Pediatr Blood Cancer. 2011;57:398–405. doi: 10.1002/pbc.22966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hank JA, Surfus J, Gan J, et al. Treatment of neuroblastoma patients with antiganglioside GD2 antibody plus interleukin-2 induces antibody-dependent cellular cytotoxicity against neuroblastoma detected in vitro. J Immunother Emphasis Tumor Immunol. 1994;15:29–37. doi: 10.1097/00002371-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merimsky O, Gez E, Weitzen R, et al. Targeting pulmonary metastases of renal cell carcinoma by inhalation of interleukin-2. Ann Oncol. 2004;15:610–612. doi: 10.1093/annonc/mdh137. [DOI] [PubMed] [Google Scholar]
- 53.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 54.Miller JS. Therapeutic applications: natural killer cells in the clinic. Hematology Am Soc Hematol Educ Program. 2013;2013:247–253. doi: 10.1182/asheducation-2013.1.247. [DOI] [PubMed] [Google Scholar]
- 55.Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi SS, Chhabra VS, Nguyen QH, et al. Interleukin-15 enhances cytotoxicity, receptor expression, and expansion of neonatal natural killer cells in long-term culture. Clin Diagn Lab Immunol. 2004;11:879–888. doi: 10.1128/CDLI.11.5.879-888.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lopez-Albaitero A, Lee SC, Morgan S, et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. 2009;58:1853–1864. doi: 10.1007/s00262-009-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luedke E, Jaime-Ramirez AC, Bhave N, et al. Cetuximab therapy in head and neck cancer: immune modulation with interleukin-12 and other natural killer cell-activating cytokines. Surgery. 2012;152:431–440. doi: 10.1016/j.surg.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moga E, Canto E, Vidal S, et al. Interleukin-15 enhances rituximab-dependent cytotoxicity against chronic lymphocytic leukemia cells and overcomes transforming growth factor beta-mediated immunosuppression. Exp Hematol. 2011;39:1064–1071. doi: 10.1016/j.exphem.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Moga E, Alvarez E, Canto E, et al. NK cells stimulated with IL-15 or CpG ODN enhance rituximab-dependent cellular cytotoxicity against B-cell lymphoma. Exp Hematol. 2008;36:69–77. doi: 10.1016/j.exphem.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen QH, Roberts RL, Ank BJ, et al. Enhancement of antibody-dependent cellular cytotoxicity of neonatal cells by interleukin-2 (IL-2) and IL-12. Clin Diagn Lab Immunol. 1998;5:98–104. doi: 10.1128/cdli.5.1.98-104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberti MP, Barrio MM, Bravo AI, et al. IL-15 and IL-2 increase Cetuximab-mediated cellular cytotoxicity against triple negative breast cancer cell lines expressing EGFR. Breast Cancer Res Treat. 2011;130:465–475. doi: 10.1007/s10549-011-1360-2. [DOI] [PubMed] [Google Scholar]
- 64.Wren L, Parsons MS, Isitman G, et al. Influence of cytokines on HIV-specific antibody-dependent cellular cytotoxicity activation profile of natural killer cells. PLoS One. 2012;7:e38580. doi: 10.1371/journal.pone.0038580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gamero AM, Ussery D, Reintgen DS, et al. Interleukin 15 induction of lymphokine-activated killer cell function against autologous tumor cells in melanoma patient lymphocytes by a CD18-dependent, perforin-related mechanism. Cancer Res. 1995;55:4988–4994. [PubMed] [Google Scholar]
- 66.Wang J, Sun ZM, Cao LL, et al. Biological characteristics of cord blood natural killer cells induced and amplified with IL-2 and IL-15. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20:731–735. [PubMed] [Google Scholar]
- 67.Zamai L, Del Zotto G, Buccella F, et al. Cytotoxic functions and susceptibility to apoptosis of human CD56(bright) NK cells differentiated in vitro from CD34(+) hematopoietic progenitors. Cytometry A. 2012;81:294–302. doi: 10.1002/cyto.a.22025. [DOI] [PubMed] [Google Scholar]
- 68.Cooper MA, Bush JE, Fehniger TA, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 69.Orange JS, Biron CA. Characterization of early IL-12, IFN-alphabeta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol. 1996;156:4746–4756. [PubMed] [Google Scholar]
- 70.Nguyen KB, Cousens LP, Doughty LA, et al. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat Immunol. 2000;1:70–76. doi: 10.1038/76940. [DOI] [PubMed] [Google Scholar]
- 71.Navid F, Furman WL, Fleming M, et al. The feasibility of adjuvant interferon alpha-2b in children with high-risk melanoma. Cancer. 2005;103:780–787. doi: 10.1002/cncr.20860. [DOI] [PubMed] [Google Scholar]
- 72.Warren K, Bent R, Wolters PL, et al. A phase 2 study of pegylated interferon alpha-2b (PEG-Intron((R))) in children with diffuse intrinsic pontine glioma. Cancer. 2012;118:3607–3613. doi: 10.1002/cncr.26659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berg SL, Cairo MS, Russell H, et al. Safety, pharmacokinetics, and immunomodulatory effects of lenalidomide in children and adolescents with relapsed/refractory solid tumors or myelodysplastic syndrome: a Children's Oncology Group Phase I Consortium report. J Clin Oncol. 2011;29:316–323. doi: 10.1200/JCO.2010.30.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishikawa H, Jager E, Ritter G, et al. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106:1008–1011. doi: 10.1182/blood-2005-02-0607. [DOI] [PubMed] [Google Scholar]
- 76.Ghiringhelli F, Menard C, Martin F, et al. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev. 2006;214:229–238. doi: 10.1111/j.1600-065X.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 77.Wu L, Adams M, Carter T, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–4657. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 78.Jungkunz-Stier I, Zekl M, Stuhmer T, et al. Modulation of natural killer cell effector functions through lenalidomide/dasatinib and their combined effects against multiple myeloma cells. Leuk Lymphoma. 2013;55:168–176. doi: 10.3109/10428194.2013.794270. [DOI] [PubMed] [Google Scholar]
- 79.Davies FE, Raje N, Hideshima T, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 80.Piperdi B, Ling YH, Liebes L, et al. Bortezomib: understanding the mechanism of action. Mol Cancer Ther. 2011;10:2029–2030. doi: 10.1158/1535-7163.MCT-11-0745. [DOI] [PubMed] [Google Scholar]
- 81.Lundqvist A, Berg M, Smith A, et al. Bortezomib Treatment to Potentiate the Anti-tumor Immunity of Ex-vivo Expanded Adoptively Infused Autologous Natural Killer Cells. J Cancer. 2011;2:383–385. doi: 10.7150/jca.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lundqvist A, Abrams SI, Schrump DS, et al. Bortezomib and depsipeptide sensitize tumors to tumor necrosis factor-related apoptosis-inducing ligand: a novel method to potentiate natural killer cell tumor cytotoxicity. Cancer Res. 2006;66:7317–7325. doi: 10.1158/0008-5472.CAN-06-0680. [DOI] [PubMed] [Google Scholar]
- 83.Shi J, Tricot GJ, Garg TK, et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood. 2008;111:1309–1317. doi: 10.1182/blood-2007-03-078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sarhan D, Wennerberg E, D'Arcy P, et al. A novel inhibitor of proteasome deubiquitinating activity renders tumor cells sensitive to TRAIL-mediated apoptosis by natural killer cells and T cells. Cancer Immunol Immunother. 2013;62:1359–1368. doi: 10.1007/s00262-013-1439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sivori S, Falco M, Della Chiesa M, et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci U S A. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saikh KU, Lee JS, Kissner TL, et al. Toll-like receptor and cytokine expression patterns of CD56+ T cells are similar to natural killer cells in response to infection with Venezuelan equine encephalitis virus replicons. J Infect Dis. 2003;188:1562–1570. doi: 10.1086/379196. [DOI] [PubMed] [Google Scholar]
- 87.Mian MF, Lauzon NM, Andrews DW, et al. FimH can directly activate human and murine natural killer cells via TLR4. Mol Ther. 2010;18:1379–1388. doi: 10.1038/mt.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saijo N, Irimajiri N, Ozaki A, et al. Effects of BCG and cyclophosphamide on the spontaneous and antibody-dependent cell-mediated cytotoxicity of peritoneal and spleen lymphocytes of ACI/N rats. Gann. 1982;73:270–277. [PubMed] [Google Scholar]
- 89.Brandau S, Riemensberger J, Jacobsen M, et al. NK cells are essential for effective BCG immunotherapy. Int J Cancer. 2001;92:697–702. doi: 10.1002/1097-0215(20010601)92:5<697::aid-ijc1245>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 90.Alkan SS. Monoclonal antibodies: the story of a discovery that revolutionized science and medicine. Nat Rev Immunol. 2004;4:153–156. doi: 10.1038/nri1265. [DOI] [PubMed] [Google Scholar]
- 91.Gupta P, Han SY, Holgado-Madruga M, et al. Development of an EGFRvIII specific recombinant antibody. BMC Biotechnol. 2010;10:72. doi: 10.1186/1472-6750-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bax DA, Gaspar N, Little SE, et al. EGFRvIII deletion mutations in pediatric high-grade glioma and response to targeted therapy in pediatric glioma cell lines. Clin Cancer Res. 2009;15:5753–5761. doi: 10.1158/1078-0432.CCR-08-3210. [DOI] [PubMed] [Google Scholar]
- 93.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 94.Clynes RA, Towers TL, Presta LG, et al. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 95.Clynes R, Takechi Y, Moroi Y, et al. Fc receptors are required in passive and active immunity to melanoma. Proc Natl Acad Sci U S A. 1998;95:652–656. doi: 10.1073/pnas.95.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 97.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 98.Bibeau F, Lopez-Crapez E, Di Fiore F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27:1122–1129. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 99.Weng WK, Czerwinski D, Levy R. Humoral immune response and immunoglobulin G Fc receptor genotype are associated with better clinical outcome following idiotype vaccination in follicular lymphoma patients regardless of their response to induction chemotherapy. Blood. 2007;109:951–953. doi: 10.1182/blood-2006-03-013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang B, Kokhaei P, Mellstedt H, et al. FcgammaR polymorphisms and clinical outcome in colorectal cancer patients receiving passive or active antibody treatment. Int J Oncol. 2010;37:1599–1606. doi: 10.3892/ijo_00000814. [DOI] [PubMed] [Google Scholar]
- 101.Lode HN, Xiang R, Dreier T, et al. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91:1706–1715. [PubMed] [Google Scholar]
- 102.Buhtoiarov IN, Neal ZC, Gan J, et al. Differential internalization of hu14. 18-IL2 immunocytokine by NK and tumor cell: impact on conjugation, cytotoxicity, and targeting. J Leukoc Biol. 2011;89:625–638. doi: 10.1189/jlb.0710422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gubbels JA, Gadbaw B, Buhtoiarov IN, et al. Ab-IL2 fusion proteins mediate NK cell immune synapse formation by polarizing CD25 to the target cell-effector cell interface. Cancer Immunol Immunother. 2011;60:1789–1800. doi: 10.1007/s00262-011-1072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kohrt HE, Houot R, Weiskopf K, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122:1066–1075. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Houot R, Kohrt HE, Marabelle A, et al. Targeting immune effector cells to promote antibody-induced cytotoxicity in cancer immunotherapy. Trends Immunol. 2011;32:510–516. doi: 10.1016/j.it.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 106.Capitini CM, Otto M, DeSantes KB, et al. Immunotherapy in pediatric malignancies: current status and future perspectives. Future Oncol. 2014;10:1659–1678. doi: 10.2217/fon.14.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shah NN, Dave H, Wayne AS. Immunotherapy for pediatric leukemia. Front Oncol. 2013;3:166. doi: 10.3389/fonc.2013.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Capitini CM, Mackall CL, Wayne AS. Immune-based therapeutics for pediatric cancer. Expert Opin Biol Ther. 2010;10:163–178. doi: 10.1517/14712590903431022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ruggeri L, Aversa F, Martelli MF, et al. Allogeneic hematopoietic transplantation and natural killer cell recognition of missing self. Immunol Rev. 2006;214:202–218. doi: 10.1111/j.1600-065X.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 110.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 111.Leung W, Iyengar R, Turner V, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172:644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 112.Pende D, Marcenaro S, Falco M, et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009;113:3119–3129. doi: 10.1182/blood-2008-06-164103. [DOI] [PubMed] [Google Scholar]
- 113.Rutella S, Rumi C, Laurenti L, et al. Immune reconstitution after transplantation of autologous peripheral CD34+ cells: analysis of predictive factors and comparison with unselected progenitor transplants. Br J Haematol. 2000;108:105–115. doi: 10.1046/j.1365-2141.2000.01824.x. [DOI] [PubMed] [Google Scholar]
- 114.Chaleff S, Otto M, Barfield RC, et al. A large-scale method for the selective depletion of alphabeta T lymphocytes from PBSC for allogeneic transplantation. Cytotherapy. 2007;9:746–754. doi: 10.1080/14653240701644000. [DOI] [PubMed] [Google Scholar]
- 115.Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Godal R, Bachanova V, Gleason M, et al. Natural killer cell killing of acute myelogenous leukemia and acute lymphoblastic leukemia blasts by killer cell immunoglobulin-like receptor-negative natural killer cells after NKG2A and LIR-1 blockade. Biol Blood Marrow Transplant. 2010;16:612–621. doi: 10.1016/j.bbmt.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cooley S, Trachtenberg E, Bergemann TL, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113:726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Curti A, Ruggeri L, D'Addio A, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118:3273–3279. doi: 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- 119.Geller MA, Cooley S, Judson PL, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iliopoulou EG, Kountourakis P, Karamouzis MV, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother. 2010;59:1781–1789. doi: 10.1007/s00262-010-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol. 2002;20:217–251. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 122.Joncker NT, Shifrin N, Delebecque F, et al. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 2010;207:2065–2072. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leung W, Handgretinger R, Iyengar R, et al. Inhibitory KIR-HLA receptor-ligand mismatch in autologous haematopoietic stem cell transplantation for solid tumour and lymphoma. Br J Cancer. 2007;97:539–542. doi: 10.1038/sj.bjc.6603913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Venstrom JM, Zheng J, Noor N, et al. KIR and HLA genotypes are associated with disease progression and survival following autologous hematopoietic stem cell transplantation for high-risk neuroblastoma. Clin Cancer Res. 2009;15:7330–7334. doi: 10.1158/1078-0432.CCR-09-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Delgado DC, Hank JA, Kolesar J, et al. Genotypes of NK cell KIR receptors, their ligands, and Fcgamma receptors in the response of neuroblastoma patients to Hu14. 18-IL2 immunotherapy. Cancer Res. 2010;70:9554–9561. doi: 10.1158/0008-5472.CAN-10-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kohrt H, Thielens A, Marabelle A, et al. Anti-KIR Antibody Enhancement of Anti-Lymmphoma Activity of Natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2013;122:abstr. 4417. doi: 10.1182/blood-2013-08-519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koh CY, Blazar BR, George T, et al. Augmentation of antitumor effects by NK cell inhibitory receptor blockade in vitro and in vivo. Blood. 2001;97:3132–3137. doi: 10.1182/blood.v97.10.3132. [DOI] [PubMed] [Google Scholar]
- 128.Berg M, Lundqvist A, McCoy P, Jr, et al. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy. 2009;11:341–355. doi: 10.1080/14653240902807034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gong W, Xiao W, Hu M, et al. Ex vivo expansion of natural killer cells with high cytotoxicity by K562 cells modified to co-express major histocompatibility complex class I chain-related protein A, 4–1BB ligand, and interleukin-15. Tissue Antigens. 2010;76:467–475. doi: 10.1111/j.1399-0039.2010.01535.x. [DOI] [PubMed] [Google Scholar]
- 130.Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu Y, Wu HW, Sheard MA, et al. Growth and activation of natural killer cells ex vivo from children with neuroblastoma for adoptive cell therapy. Clin Cancer Res. 2013;19:2132–2143. doi: 10.1158/1078-0432.CCR-12-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garg TK, Szmania SM, Khan JA, et al. Highly activated and expanded natural killer cells for multiple myeloma immunotherapy. Haematologica. 2012;97:1348–1356. doi: 10.3324/haematol.2011.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rujkijyanont P, Chan WK, Eldridge PW, et al. Ex Vivo Activation of CD56+ Immune Cells That Eradicate Neuroblastoma. Cancer Res. 2013;73:2608–2618. doi: 10.1158/0008-5472.CAN-12-3322. [DOI] [PubMed] [Google Scholar]
- 134.Voskens CJ, Watanabe R, Rollins S, et al. Ex-vivo expanded human NK cells express activating receptors that mediate cytotoxicity of allogeneic and autologous cancer cell lines by direct recognition and antibody directed cellular cytotoxicity. J Exp Clin Cancer Res. 2010;29:134. doi: 10.1186/1756-9966-29-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang H, Cui Y, Voong N, et al. Activating signals dominate inhibitory signals in CD137L/IL-15 activated natural killer cells. J Immunother. 2011;34:187–195. doi: 10.1097/CJI.0b013e31820d2a21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cho D, Shook DR, Shimasaki N, et al. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin Cancer Res. 2010;16:3901–3909. doi: 10.1158/1078-0432.CCR-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee DW, Barrett DM, Mackall C, et al. The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin Cancer Res. 2012;18:2780–2790. doi: 10.1158/1078-0432.CCR-11-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Klingemann HG. Natural killer cell-based immunotherapeutic strategies. Cytotherapy. 2005;7:16–22. doi: 10.1080/14653240510018000. [DOI] [PubMed] [Google Scholar]
- 140.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Altvater B, Landmeier S, Pscherer S, et al. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin Cancer Res. 2009;15:4857–4866. doi: 10.1158/1078-0432.CCR-08-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Esser R, Muller T, Stefes D, et al. NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med. 2012;16:569–581. doi: 10.1111/j.1582-4934.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chu Y, Ayello J, LLo L, et al. Expanded Natural Killer (NK) Cells Transfected with Anti-CD20 Chimeric Antigen Receptor (CAR) mRNA Have Significant cytotoxicity against poor risk B-Cell (CD20+) Leukemia/Lymphoma (B-L/L) Blood. 2012;120:abstr. 3007. [Google Scholar]