Abstract
Over the past 20 years, the field of cognitive neuroscience has relied heavily on hemodynamic measures of blood oxygenation in local regions of the brain to make inferences about underlying cognitive processes. These same functional magnetic resonance imaging (fMRI) and functional near-infrared spectroscopy (fNIRS) techniques have recently been adapted for use with human infants. We review the advantages and disadvantages of these two neuroimaging methods for studies of infant cognition, with a particular emphasis on their technical limitations and the linking hypotheses that are used to draw conclusions from correlational data. In addition to summarizing key findings in several domains of infant cognition, we highlight the prospects of improving the quality of fNIRS data from infants to address in a more sophisticated way how cognitive development is mediated by changes in underlying neural mechanisms.
Keywords: brain, cognitive neuroscience, development, human infant, neuroimaging
Lulled in the countless chambers of the brain, our thoughts are linked by many a hidden chain; awake but one, and lo, what myriads rise!
—Samuel Rogers (1792)
1. Introduction
The intricacies of the human brain have been recognized for centuries as an unsurpassed achievement of evolution and culture. What enables this organ to exert such exquisite control over human behavior and over the mental events that are a correlate of its neural activity? Surely this control is the product of a cascade of genetic and experiential events that unfold during embryonic and postnatal development (Stiles 2008), resulting in both general-purpose and highly specialized neural circuitry. But what are the necessary and sufficient anatomical substrates and functional properties of the infant brain that support the rapid development of human cognition?
These questions have motivated developmental researchers to go beyond detailed studies of infant behavior in search of neural signals that play a causal role in generating those behaviors. Indeed, without direct evidence of these putative neural mechanisms, it remains unclear what the precise causal linkage is between brain and behavior during development. For example, if infants at two postnatal ages exhibit the same behavior, it is tempting to conclude that this behavior is mediated by the same neural mechanisms. Alternatively, if these same infants were to exhibit different behaviors, it is tempting to conclude that this development is the result of a qualitative change (e.g., a new functional area or neural computation) rather than a quantitative change (e.g., more neurons dedicated to an existing area or computation). To be clear, without a sophisticated understanding of the manner in which external stimuli elicit behavior, inferences about the causal role of neural activity would be impossible, and no neural correlate of behavior is sufficient for drawing causal inferences. Yet, the simultaneous collection of behavioral and neural data, and their resulting correlations, provides a starting point for conducting experimental manipulations from which causal inferences can be drawn. Because studies of human infants are not amenable to invasive manipulations, the field of developmental cognitive neuroscience is limited to neural-behavioral correlations. Nevertheless, these neural data serve as powerful constraints on how to interpret developmental changes in behavior.
Even though limited to noninvasive neural correlates of behavior, a variety of measures of neural activity, both direct and indirect, have been employed to study the brains of human infants. Here, we focus on two major indirect methods of measurement: functional magnetic resonance imaging (fMRI) and functional near-infrared spectroscopy (fNIRS). Both methods (see next section) rely on hemodynamic signatures (ferromagnetic and optical) from blood carried to local regions of neural tissue in response to the metabolic demand created by neural activity.
The principles of fMRI were first demonstrated in animals by Ogawa et al. (1990), and the principles of fNIRS were first demonstrated by Jöbsis (1977). The application of fMRI to humans was surprisingly rapid, with three studies published in 1992, whereas the application of fNIRS to humans did not appear until 1993 (see Boas et al. 2014, for a review). In mid-2014, Google Scholar lists 453,000 hits for “fMRI” and 75,060 for “NIRS” or “fNIRS.” fMRI is a much more pervasive and, as discussed below, precise measure of brain hemodynamics than fNIRS. Yet, for certain classes of participants, most notably human infants, fNIRS has certain advantages over fMRI (e.g., awake versus asleep to reduce motion artifacts), thereby providing a unique opportunity to record hemodynamic correlates of neural activity. Several recent reviews of fNIRS provide a comprehensive treatment of the underlying optical physics (e.g., Ferrari & Quaresima 2012, Scholkmann et al. 2014) and the utility of fNIRS for studies of human infants (e.g., Aslin 2012, Gervain et al. 2011, Lloyd-Fox et al. 2010, Minagawa-Kawai et al. 2008).
2. Techniques for Measuring Blood Oxygenation
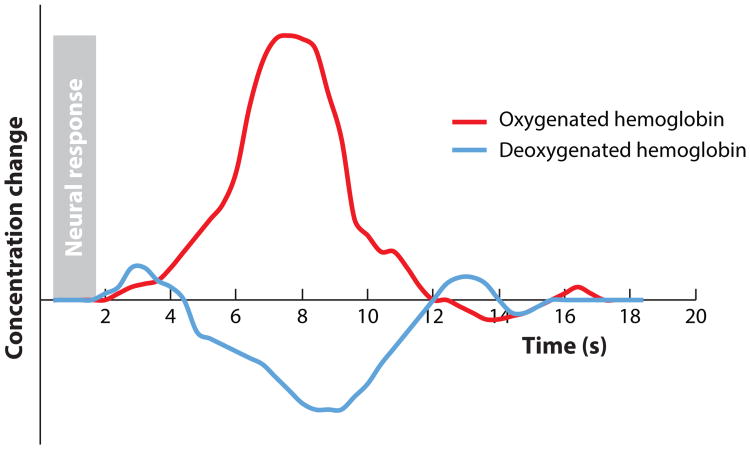
Neural firing is metabolically demanding and results first in a local increase in deoxygenated hemoglobin (deOxy-Hb), followed immediately by a compensatory sustained increase in oxygenated hemoglobin (Oxy-Hb) and subsequent decrease in deOxy-Hb (see Figure 1). The specific relationship of the circulatory system to local changes in neural activity is referred to as neurovascular coupling.
Figure 1.
Depiction of the canonical hemodynamic response, with decreases in deoxygenated hemoglobin and increases in oxygenated hemoglobin that are delayed by several seconds after a brief stimulus and its elicited neural activity (shaded region). Adapted from Gervain et al. (2011) with permission.
When a specific pool of neurons is active, the hemodynamic response is believed to be localized within 1–3 mm of this neural activity (e.g., Shmuel et al. 2007, using 7T in human occipital cortex, V1) and likely represents the local field potentials rather than spiking activity of clusters of neurons (Goense & Logothetis 2008). However, the hemodynamic response unfolds slowly compared with the rapid change in neural activity. Even after a brief burst of stimulus-evoked neural activity (<1 s), the adult hemodynamic response peaks approximately 6 s later and the concentrations of Oxy-Hb and deOxy-Hb do not return to baseline until more than 10 s after the stimulus was initially presented (Malonek & Grinvald 1996) (see Figure 1). These spatial-temporal aspects of the hemodynamic response greatly constrain the designs and interpretations that can be drawn from neuroimaging methods that rely on neurovascular coupling.
Although both fMRI and fNIRS record the same physiological substrate, they are based on quite different detection methods, have different spatial and temporal resolutions, and pose different constraints on experimental design and implementation, especially for use with infants and young children. Before summarizing the specifics of fMRI and fNIRS, we ask why one would choose to measure the hemodynamic response rather than obtaining a more direct measure of neural activity (see Lenartowicz & Poldrack 2010, for a review).
2.1. Comparison of fMRI and fNIRS to Other Methods
Noninvasive recordings of large populations of neurons using electroencephalography (EEG) have been widely used in studies of adults (e.g., Luck 2005) and infants (e.g., Nelson & McCleery 2008). Although EEG and its time-locked averaging to discrete stimuli—the event-related potential (ERP)—has excellent temporal resolution, reflecting the millisecond-by-millisecond unfolding of neural events, it has limited spatial resolution given uncertainties about how field potentials propagate through neural and non-neural tissues to reach electrodes on the surface of the scalp (Srinivasan & Nunez 2012). Although there has been recent progress in applying cortical source localization to studies of the infant brain (Reynolds & Richards 2009), these advances are beyond the scope of the present review.
Two other imaging modalities have been used with adults and infants, but each has substantial methodological challenges. Magnetoencephalography (MEG) capitalizes on the small changes in magnetic fields induced by electrical currents from neural activity (Cohen & Halgren 2009). Although MEG has somewhat better spatial resolution than EEG because of a more restricted sensitivity to the alignment of electrical currents, it also requires several assumptions to infer cortical source localization. MEG has been used with human infants (Cheour et al. 2004, Imada et al. 2006, Matuz etal. 2012), but, like fMRI, suffers from the high cost of the recording instrument (>$1 million) and the requirement of some head stabilization.
Positron emission tomography (PET) relies on the detection of radioactive decay of one of a variety of isotopes injected into the bloodstream as participants perform a brief task (Watson 1997). Early application to the study of brain development revealed substantial qualitative changes in glucose uptake between the newborn and the older child (Chugani et al. 1987). However, because of the invasive nature of injecting a radioactive tracer into the bloodstream, PET has been relegated to a tool for studies of clinical populations whose potential benefits from an assessment outweigh the risks of the imaging procedure (Kumar & Chugani 2013).
2.2. Recording the Hemodynamic Response with fMRI and fNIRS
This section summarizes the methodological differences between fNIRS and fMRI as they bear on recordings from typically developing infants. FMRI employs the presentation of radio wave pulses in a static magnetic field to record what is called the blood-oxygen-level-dependent (BOLD) response that varies with fluctuations in local concentrations of deOxy-Hb. By contrast, fNIRS simultaneously records changes in both Oxy-Hb and deOxy-Hb by employing two wavelengths of near-infrared light (e.g., 690 nm and 830 nm), which span the crossover point in their respective absorbance spectra.
While the spatial resolution of fMRI varies with the strength of the static magneticfield (B0; e.g., 1.5T, 3T, or 7T) and the specific scanning parameters, the typical fMRI voxel size of 3 mm3 is well matched to the resolution of the hemodynamic response itself. In contrast, current fNIRS systems have a much coarser spatial resolution. As shown in Figure 2, near-infrared light is delivered to the surface of the scalp via optical fibers (emitters). Surrounding each emitter are one or more optical fibers (detectors) that collect a small fraction of the photons that return to the surface of the scalp after passing through various layers of neural tissue between the emitter and the detector. Each pair of optical fibers that link an emitter and a detector is referred to as a fNIRS channel and samples the underlying tissue from which the hemodynamic response is being recorded. Photons leaving the emitter and reaching the detector are primarily absorbed in the surface layers above the cortex, including the scalp, skull, cerebral-spinal fluid, and surface vasculature (see Figure 2). The remaining photons travel along a banana-shaped trajectory that dips into the underlying cortex. The distance between the emitter and detector determines the lowest point of the banana-shaped trajectory; the greater the separation between the two, the deeper the trajectory through the cortex. However, greater separation also leads to greater attenuation of the signal. Current systems typically employ emitter-detector separations of 2–3 cm, which optimize cortical sampling and signal strength. However, photons must necessarily enter and exit the brain through the scalp and therefore are substantially modulated by absorption in the superficial layers of tissue between the surface of the cortex and the scalp. This introduces a substantial source of noise into what is a rather weak signal. Thus, signal averaging, as in the ERP, must be used to factor out noncortical noise (under the assumption that background systemic vascular effects are uncorrelated with the presentation of a stimulus). We return to the contaminating effects of non-neural surface vasculature in Section 3.1.
Figure 2.
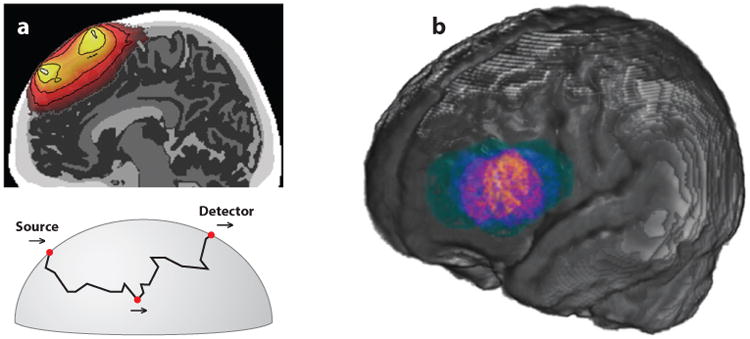
Illustration of the spatial locations sampled by a single NIRS channel (source-detector pair). (a) Top: Cross-section of the brain with a color-coded probability map of how photons move through a banana-shaped pathway from a source (emitter) to a detector location. Brighter colors indicate a higher relative probability of sampling that spatial location for this NIRS channel. Bottom: A possible path of a single photon going from the emitter to the detector as it dips to a specific point in the brain and returns to the surface. (b) Given the relatively large spatial extent of a single channel and variability in head size and probe placement across a population, this panel illustrates the spatial extent of recordings from a single NIRS channel in a sample of 6-month-old infants when coregistered to a standard brain template. Colors indicate the likelihood of sampling different brain regions. Brighter colors indicate greater representation of this region for the population. Panel a from Strangman et al. (2013), adapted with permission; panel b courtesy of Lauren Emberson, John Richards, and Richard Aslin.
The interrelationship between size of the fNIRS channel (the distance between the emitters and detectors) and the depth of penetration of the light provides a clear constraint on the spatial extent of cortical sampling by fNIRS. The region of cortex sampled by a fNIRS channel is less than the distance between the emitter and detector because the banana-shaped pathway passes through supracortical tissues to reach the cortex. Thus, the exact area of cortex recorded from a fNIRS channel is difficult to quantify. As noted above, there are intervening tissues (scalp, skull, cerebral spinal fluid) that vary in thickness across the head, rendering different path-lengths for fNIRS channels at different locations. Moreover, the detected absorption for each fNIRS channel is an average function of all absorbing molecules in the entire path of photons from the emitter to the detector, with a maximum penetration of approximately 15–18 mm from the surface of the scalp given current infrared safety standards. Thus, fNIRS does not provide depth information and represents a point-projection of the 3D path of the photons in the banana-shaped pathway. The current best estimate of spatial resolution is 2 cm (Cui et al. 2011), which is an order of magnitude coarser than fMRI. Finally, while fNIRS shares with fMRI the property of being spatially unambiguous (i.e., oxygenation is being sampled from a region between the emitter and detector, rather than some distant location), the spatial resolution of a fNIRS channel is much larger than the neurally triggered hemodynamic response itself. We return to prospects for enhancing the spatial resolution of fNIRS in Section 5.2.
One final consideration regarding spatial resolution of fNIRS is skull thickness. The thickness of the skull is directly related to the amount of cortex that is being recorded from in a given fNIRS channel. This is important not only because the skull is quite thin in neonates (6 mm on average) and becomes thicker with age (10 mm by age 7) but also because skull thickness varies across regions of the head within a given age (Beauchamp et al. 2011), creating a confound when examining absolute changes in cortical activation across ages or across brain regions (e.g., hemispheres). This underlying anatomy should be taken into consideration for studies that compare neural activity to a given stimulus across fNIRS channels without normalizing these activations to a second stimulus. In the future, anatomical data that compensate for this differential path length from fNIRS could help to prevent the false attribution of hemispheric and regional differences (unless only relative changes in activation comprise the dependent measure). In addition to variability in skull thickness, it is important to determine the distance from the tips of the optical fibers on the scalp to the depth of the cortical area that is being targeted in a given recording session. This anatomical variability can only be resolved by a structural MRI, either from each infant or based on age-appropriate templates (see Section 5.2).
Finally, the temporal resolution of fMRI is typically 0.5 Hz (i.e., a whole-brain sample every 2 s). This slow sample rate has proven to be sufficient for most applications because the underlying hemodynamic response is an order of magnitude slower. In contrast, fNIRS is typically recorded at 10 Hz and higher sampling rates are possible because detection of the optical response is not limited by the interaction between slice selection and gradient encoding in fMRI. Thus, fNIRS has much better temporal resolution than fMRI and in principle could provide a more accurate measure of the shape and timing of the hemodynamic response. In practice, however, that potential has not yet been realized, in part because of noise from noncortical, surface-vascular responses (which does not affect fMRI) and because infants cannot provide a sufficient number of stimulus blocks (or events) to average out the noise. Moreover, phased-array head coils have improved the sampling rates of fMRI (Keil et al. 2013) so that the intrinsic superiority of fNIRS in the temporal domain is not likely to be a significant advantage in the future.
2.3. Pros and Cons of fMRI and fNIRS for Studies of Infants
Although fMRI provides a rich picture (in pseudocolor) of the spatial distribution of estimated neural activity from the deOxy-Hb BOLD signal, it suffers from several disadvantages that are particularly severe for studies of human infants. First, MRI recordings require rigid head stabilization; currently available algorithms can tolerate head movements of only a few millimeters over a 5–10 min scan, resulting in elimination of scans that exceed this threshold. Moreover, the head coil and associated mirror for viewing visual stimuli constrain the angular size and proximity of visual displays, making it difficult to reliably present visual stimuli to infants. Second, the MRI scanner produces substantial acoustic noise from the gradients that, even with adequate hearing protection, can be distracting or induce startle responses that limit the ability to hear subtle acoustic differences, or attend to nonauditory stimuli. Third, safety issues inherent to any MR scan (e.g., heating of body tissues, especially if they come in contact with any ferrous metal, and damage to the auditory system from inadequate hearing protection), while posing limited dangers, are more difficult to mitigate in a nonverbal participant such as an infant. Despite taking special precautions to deal with these safety concerns, it has proven to be very difficult to eliminate movement artifacts in infants and very young children. Thus, the vast majority of fMRI studies with infants are conducted while they are sleeping. This enables the successful collection of structural MRI and fMRI recordings in this population without movement artifacts, but it severely limits the kinds of stimuli and behavioral paradigms that can be used with infants to study the neural correlates of cognitive development.
The foregoing disadvantages of fMRI are a counterpoint to the major advantages of fNIRS. First, the fNIRS cap that holds the fiber-optic bundles to deliver near-infrared light to the emitters and gather near-infrared light from the detectors can be lightweight and comfortable to wear. Second, because this cap attaches the emitters and detectors to the scalp, the fNIRS recordings do not require rigid head stabilization and therefore enable the infant to be positioned to maximize attention to experimental stimuli or to engage in motor responses that would not be feasible in the MRI scanner environment. Third, there is no acoustic noise from the fNIRS machine (in contrast to noisy MRI gradients), thereby reducing distractions and maximizing the fidelity of auditory stimuli. Fourth, there are no safety concerns because the intensity of the IR-light sources is well below the FDA guidelines for damage to neural tissue. Thus, fNIRS studies can readily be conducted with developmental and special populations who would not tolerate the constraints of the fMRI environment.
The disadvantages of fNIRS, while substantial in the past, have largely been overcome, and further improvements are on the horizon (see Section 5.2). Nevertheless, some disadvantages remain. First, the spatial resolution of fNIRS is currently 2–3 cm, and as noted above, the signal recorded from a given channel (an emitter-detector pair) samples a 3D volume that is not precisely known and projects that volume onto a midchannel location on the surface of the scalp. Second, fNIRS does not provide coregistration with anatomical images of the participant's brain. Rather, either a separate structural MRI must be obtained and the location of the emitters and detectors aligned with external skull landmarks, or average structural MRI templates for age- or headsize-appropriate infants can be used to provide approximate coregistration using average skull landmarks (Lloyd-Fox et al. 2013). Third, fNIRS can only record from the surface of the cortex (although sulci are clearly within the range of accessible depths). Thus, brain regions more than 2 cm from the surface of the scalp are outside the range of fNIRS recordings, including many cortical areas in the ventral temporal cortex (e.g., the fusiform gyrus) and all medial-brain structures (e.g., hippocampus). Fourth, fNIRS recordings obtained from participants across different ages must consider changes in skull thickness [affecting both the depth and the signal-to-noise (S/N) ratio of hemodynamic responses] and signal degradation introduced by the presence of hair (particularly if the hair is dense and highly pigmented). Finally, because fNIRS signals sample photons that passed twice through the scalp, skull, and surface vasculature as they travel from emitter to detector, changes in these signals are contaminated by these non-neural factors. Thus, without taking precautions to reduce the effects of systemic vascular responses (e.g., due to changes in blood pressure mediated by arousal), measurements of changes in cortical oxygenation may be masked by these much larger non-neural, supracortical signals.
Two general strategies have been employed to deal with the contaminating effects of these non-neural signals. First, one can use principal components analysis (PCA) to regress out the first or first + second principal components across channels, under the assumption that these components represent the shared variance of the systemic vascular (i.e., non-neural) signals (Zhang et al. 2005). While this analysis procedure certainly reduces the shared noise, it also reduces any shared variance in the signal. Moreover, the assumption that systemic vascular noise is uniformly distributed across the skull has been shown to be incorrect (Gagnon et al. 2014). Thus, a second method for dealing with non-neural noise is to directly sample the surface vascular signals using a channel with a small emitter-detector separation (e.g., 0.5 cm) at several locations on the skull. Then this surface noise can be regressed out of signals obtained from each 2–3 cm channel that samples from the cortex (Saager & Berger 2005, Saager & Berger 2008). Although this method has been verified in adults and via simulations on phantoms, it has yet to be applied to infants. However, there is every reason to expect an improvement in both S/N ratio and spatial selectivity in fNIRS recordings from infants using this near-channel regression technique (Saager et al. 2011).
3. Questions Asked Using fMRI and fNIRS
One of the most difficult questions confronted, but frequently overlooked, by infancy researchers who use neuroimaging methods is: What types of causal inferences can be drawn about cognition from a change in brain state? We briefly review the complexities of making correct causal inferences from fMRI and fNIRS data and then discuss why paradigms used with infants pose additional constraints on our confidence in these inferences. To be clear, these concerns can be overcome if careful attention is directed to using experimental designs with proper controls.
3.1. Linking Hypotheses Between Behavior and Hemodynamics
Researchers who study the neural correlates of cognition are deeply interested in the neural properties—both structural and functional—that mediate a particular underlying cognitive process. That process could involve any aspect of cognition that is studied with behavioral methods, including perception, learning, memory, reasoning, problem solving, language understanding, theory of mind, etc. In the case of fMRI and fNIRS, a single metric—a hemodynamic signature of neural activation—is available to make inferences about any (or all) of these cognitive processes. Thus, it is tempting to conclude that an increase in activation in a particular region of the brain, triggered by a task that is known to involve a cognitive process at the behavioral level, is indicative of neural activity that plays a causal role in that cognitive process. But of course there are two other logical possibilities: (a) The increase in activity is actually mediated by a different brain region that was either not measured or was masked by noise or insufficient spatial resolution of the hemodynamic signals, or (b) the brain region plays a causal role in the task, but the causal role is different than the one inferred by the researcher.
The foregoing interpretive challenges, which stem from uncertainty about the linking hypothesis between neural activation (as measured by hemodynamics) and cognitive process, raise a wide variety of possible causal inferences about fMRI or fNIRS data gathered from infants. Most linking hypotheses come from studies of adults that have revealed both spatial selectivity (i.e., activations to stimulus X in one brain region but not others) and stimulus specificity (i.e., activations to stimulus Z but not to stimulus Y). Given a wealth of information about these two aspects of neural activation, with parametric manipulations of stimulus and task variables as well as whole-brain analyses using fMRI, it is common to extend these linking hypotheses from adults to the infant brain. However, fNIRS studies of infants are currently limited to coverage of only a portion of the cortical surface, and the number of stimulus and task variables examined to date in this literature is extremely sparse. Thus, it is incumbent on infancy researchers to be cautious in assuming that the linking hypotheses accepted in studies of adults seamlessly extend to studies of infants.
An example of a common prediction made in infant fNIRS studies is that activation in a region of the infant brain to stimulus X that is similar in relative spatial location to a region of the adult brain also known to be activated by stimulus X (e.g., speech stimuli that activate the superior temporal sulcus of the temporal lobe) indicates the presence of a processing mechanism similar to that of adults. That is, the greater the activation of this developmentally homologous brain region by stimulus X, the greater the specificity or maturity of the cognitive process linked to this brain region. This modular linking hypothesis—that a brain region is tuned to stimulus X—is subject to a variety of alternative accounts. For example, in the targeted brain region, stimulus X may be more difficult to process than most other stimuli, leading to greater activation because of task difficulty rather than stimulus specialization. Or processing efficiency may improve with age or experience, thereby reducing activation in one brain region, which in turn frees up resources in a complementary brain region that now shows greater activation. Alternatively, activation may reflect a stimulus-general property (e.g., arousal or attention) that has a differential effect downstream from the brain region where stimulus X is processed (e.g., a memory consolidation process) or upstream because of a reduction of inhibition (e.g., a priming effect). Crucially, limited spatial coverage of the brain with fNIRS—both locally because spatial resolution is coarse (2 cm) and globally because deep brain regions are inaccessible—constrains the goal of achieving high spatial selectivity. While some of these uncertainties could be resolved, in principle, if a large number of repetitions of each stimulus or task variable were obtained, human infants are not ideal subjects for achieving this goal given their limited attention and cooperation in a single recording session (see next section). The foregoing discussion of linking hypotheses is not unique to studies of infants, but in fact has implications for the use of any neuroimaging method at any age.
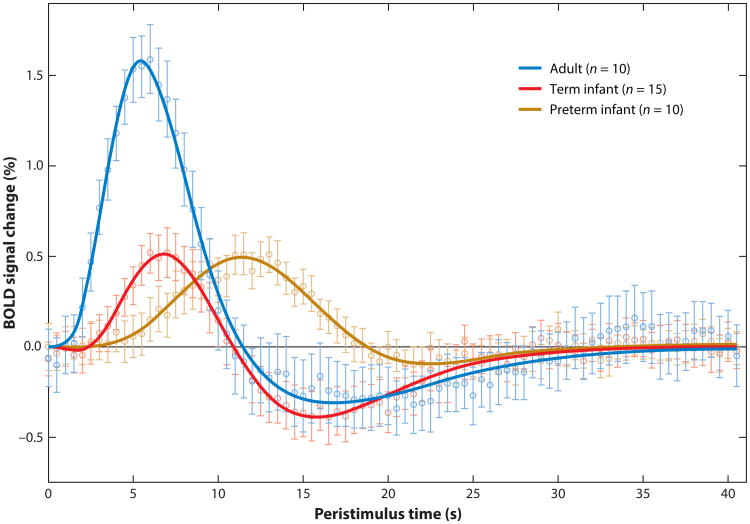
In addition, there are measurement problems common to both fMRI and fNIRS when attempting to study changes across age so that incorrect developmental conclusions are avoided. First, the physical size of fMRI voxels and fNIRS channels is fixed by the instrument rather than being scaled by brain size. Thus, each method includes a proportionately larger brain region in smaller brains, thereby increasing the risk of failing to detect a small region of activation in the infant brain. Second, the hemodynamic response function (HRF) in the infant is smaller in amplitude and delayed in time course compared to the adult (see Figure 3). Thus, with the same S/N ratio, the infant hemodynamic response will be less likely to be detected by the same stimulus condition in an event-related design than in adults. Third, there appears to be a substantial developmental change in neurovascular coupling during early infancy (Roche-Labarbe et al. 2012). One consequence of this change could be greater variability in the hemodynamic response in infants both across brain areas and across ages, including the occasional presence of a negative BOLD response. Given current analysis methods, especially in fMRI, this variability may result in false negatives or underestimating the neural activity correlated with the HRF. Moreover, careful controls and behavioral measures should be employed to ensure that age-related changes in hemodynamics are due to neural activity and not circulatory development.
Figure 3.
Variations in the time course and amplitude of the hemodynamic response function (HRF) between adults, infants, and preterm infants as derived from functional magnetic resonance imaging (fMRI) in the somatosensory cortex. Redrawn from Arichi et al. (2012), with permission. Abbreviation: BOLD, blood oxygen level–dependent.
Finally, although in principle fNIRS, with access to both Oxy-Hb and deOxy-Hb signals, could help disambiguate the reasons for a negative BOLD signal in fMRI, very few infant fNIRS studies have reported reliable deOxy-Hb signals (which in adults are closely correlated with the BOLD signal in fMRI). While it remains unclear why negative BOLD signals have been found in some fMRI studies with young infants, a few possibilities have been suggested: (a) The fact that most infant fMRI studies are conducted while they are sleeping, which is known to alter the direction of the HRF in adults (Born et al. 2002); (b) the canonical adult HRF, even when adjusted for a more sluggish time course in infants, can falsely lead to a negative BOLD response (Arichi et al. 2012); (c) infants may have a level of resting-state functional activity that is both higher and differentially distributed compared with adults, thereby leading to greater negative BOLD signals in certain cortical areas (Nilsson et al. 2013); and (d) the aforementioned change in neurovascular coupling in early infancy (Kehrer & Schöning 2009, Kozberg et al. 2013). Determining whether these developmental differences in the HRF create substantial difficulties for interpreting the linkage between hemodynamic signals and cognitive processes requires further studies.
3.2. Paradigms Used with Infants
As with any methodology, the design of an experimental paradigm is tailored to the types of signals that can be obtained. For example, reaction time and ERPs afford millisecond temporal resolution, and therefore paradigms using these measures can present hundreds of discrete events within a single testing session. In contrast, fMRI and fNIRS rely on the relatively sluggish hemodynamic response, and therefore have employed designs that either use blocks of multiple stimuli or event-related single stimuli separated by a jittered 3–8 s interstimulus interval. Clearly, the observed hemodynamic response in event-related designs is delayed by several seconds compared with the onset and duration of elicited neural activity, and so the number of stimulus events in fMRI or fNIRS studies is substantially fewer for a given duration of a recording session. One result of this design conflict, given the limited attention of infants, is that the effectiveness of signal averaging is reduced because there is less statistical power to eliminate the background noise that is uncorrelated with the stimulus events.
Another challenge for interpreting hemodynamic signals is the mismatch between the designs of fMRI or fNIRS paradigms and behavioral paradigms used to study infants. Neural signals have no obvious linkage to any underlying cognitive process unless they are referenced to clear behavioral outcomes. Unfortunately, canonical behavioral paradigms used with infants involve preference, discrimination, and violation of expectation paradigms (Aslin & Fiser 2005), typically involving visual attention as the dependent measure, that are not identical to blocked or event-related designs used with fMRI or fNIRS. For example, in a behavioral habituation paradigm infants look at a visual stimulus until they meet a criterion of looking-time decrement, and then view two types of test stimuli that are the same or different compared to the habituation stimulus. In contrast, fMRI or fNIRS paradigms assess discrimination with multiple interleaved blocks (or single events) that present one of two different stimuli. The dependent measure is a difference in activation between fMRI voxels or fNIRS channels that is correlated with the two different stimuli. Thus, discrimination in the behavioral paradigm is indexed by differential recovery of looking time after a period of encoding (the habituation phase), whereas discrimination in the hemodynamic paradigm is indexed by differential activation across a set of interleaved stimuli (without a habituation phase). When behavioral and hemodynamic paradigms yield different results, it is not clear if the discrepancy is due to the difference in paradigms or to a difference in the sensitivity of the two measures (see Turk-Browne et al. 2008). Thus, a goal for future neuroimaging studies is to more closely match the designs with behavioral paradigms (see Section 5.1).
4. Domain-Specific Findings about Infant Cognition
Although we often employ the unitary term brain, attempts to gain traction on the functional properties of this organ have naturally led to hypotheses about modules or areas of specialization, derived on anatomical and/or functional grounds. This research strategy enables us to examine different neural mechanisms that underlie separable aspects of cognitive processing in relative isolation from each other. For example, sensory and perceptual processing have been extensively studied within particular modalities (e.g., audition or vision) under the assumption that these unimodal sensory stimuli are processed independently of other modalities, while holding constant the rest of the brain.
In the following subsections, we describe representative studies that span the range from simple unimodal stimulus processing to complex object identification. We cover both fMRI and fNIRS studies, with an emphasis on the latter because of the sparse literature on the former due to the methodological constraints discussed earlier. In the final subsection, we discuss hypotheses about the changing cortical organization of the infant brain as revealed by studies of resting state functional connectivity using fMRI and fNIRS.
4.1. Unimodal Perceptual Processing
The literature on using the hemodynamic response to study infant cognition has a preponderance of studies in the auditory modality for a simple, pragmatic reason: Given the requirement of rigid head stabilization for fMRI and the early difficulty of eliminating motion artifacts for fNIRS, it was far easier to present auditory stimuli to sleeping infants than to grapple with presenting visual stimuli to awake infants. Moreover, the presentation of auditory stimuli can be continuous, even if the infant is not consistently attending to them, whereas to ensure attention to visual stimuli the consistency (and/or variance) of gaze must be established, typically with an eye-monitoring system (see Section 5.1). These constraints in both the visual and auditory modalities have prevented the field from successfully obtaining fMRI data from awake infants.
Most of the early fMRI and fNIRS studies of infants examined low-level perceptual processing, primarily because they were guided by clear, preexisting hypotheses about what kind of activations should be recorded from specific regions of the brain. For example, one of the first fNIRS studies of infants (Meek et al. 1998) used flashing visual stimuli, with the neural activation expected from occipital cortex. But the mere presence of a sluggish hemodynamic response from occipital channels cannot rule out influences via feedback pathways from other brain areas to early visual areas. A more definitive assay of feed-forward (i.e., sensory) activation in visual cortex consists of the signature contralateral hemi-field pattern of activation that is well established in retinotopic mapping studies using fMRI (see Wandell et al. 2007). Such patterns of activation have been obtained using fNIRS in adults (White & Culver 2010), but to date, have not been obtained with infants (but see Liao et al. 2012). The situation is even less clear with fMRI because of the necessity to eliminate head motion and deliver visual stimuli with open eyelids (see Seghier et al. 2006).
Similarly, in the auditory modality there are dozens of fNIRS studies using simple stimuli (Kotilahti et al. 2005, Sakatani et al. 1999, Zaramella et al. 2001), but the hypotheses about where in the brain one would expect to find focal activation are much less clear than in the visual system (e.g., pathways from cochlea to cortex are much less lateralized than in the visual system). Thus, activation to simple auditory stimuli elicits a rather diffuse pattern of bilateral frontal and temporal activation (see Taga et al. 2007). The situation is more clear-cut in the few studies of infants using fMRI (Anderson et al. 2001, Altman et al. 2001, Dehaene-Lambertz et al. 2002, Redcay et al. 2007), although these studies were conducted on sleeping infants.
The situation in the somatosensory system has the potential to be much more straightforward than in the visual or auditory systems because of the completely contralateral projection from surface receptors to cortex. Using stimulation such as limbflexion or passive manual movement, the literature is split between findings of contralateral neural activity, consistent with adults (fNIRS: Kusaka et al. 2011; fMRI: Arichi et al. 2010) and bilateral activation of somatosensory and motor cortices (fMRI: Erberich et al. 2006, Arichi et al. 2012).
While the foregoing results are partially confirmatory of expectations based on the organization of the adult brain, some studies suggest that activity elicited by a given sensory stimulus is widespread across many areas of the infant brain. The mere presence of widespread activation in the infant brain is not surprising as the raw (i.e., stimulus versus rest) patterns of activation in most adult fMRI studies are also quite diffuse. In adult paradigms where subtle stimulus contrasts are used with multiple fNIRS epochs or fMRI scanning runs, regression methods with stringent activation thresholds can yield focal patterns of activation. However, a number of findings suggest that this widespread activation is a developmentally relevant phenomenon. First, fNIRS paradigms, even with high-density arrays (e.g., 94 channels), have revealed widespread patterns of activation in occipital, occipitotemporal, and frontal areas in infants between birth and 3 months of age, for both simple visual and auditory stimuli (Taga et al. 2003, 2011; Watanabe et al. 2008, 2010). Second, in preterm infants, activation in the occipital cortex is less robust, and at times absent (Lee et al. 2012), and the extent of activation increases with age in visual (Born et al. 2000) and even somatosensory studies [unilateral activation in preterm infants versus bilateral activation in term infants (Arichi et al. 2012)]. Studies with comparatively older infants also suggest that activation to basic sensory stimuli may extend beyond the corresponding sensory cortex and may be widely distributed in the infant brain; infants between 3 and 7 months of age exhibit significant hemodynamic responses to acoustic stimuli not just in temporal cortices, but in occipital and frontal regions and the basal ganglia (Taga et al. 2011, Blasi et al. 2011). Finally, Taga et al. (2011) examined the temporal (phase) relations between activations in different areas: Channels both anterior and posterior to those over temporal cortex showed longer lags, suggesting gradients of hemodynamic activity away from primary auditory areas. Taken together, these findings raise the possibility that the infant brain, even in response to basic sensory stimuli, is not as specialized as in adults, and undergoes considerable developmental reorganization.
4.2. Multisensory Processing
When examining stimuli composed of elements from more than one modality, it would be ideal to compare hemodynamic activity to such multimodal (e.g., audio-visual) stimuli to their unimodal components (auditory alone, visual alone). This would allow us to understand if, and how, hemo-dynamic responses to multimodal stimuli are more than just the linear combination of evoked activity in each modality alone. An early study of multimodal processing in infants (Bortfeld et al. 2007) examined hemodynamic activity for speech stimuli, either by themselves, or accompanied by visual stimuli. Six- to 9-month-old infants viewed either visual-only (V, animated 3D objects) or audio-visual (A+V, animations accompanied by speech) stimuli in a blocked design. While fNIRS channels over occipital cortex were activated equivalently for both V and A+V conditions, temporal cortex activity varied by condition: There was significant activation in the temporal cortex in the A+V condition, but a significant deactivation in the V condition. A similar deactivation in channels over temporal cortex to A+V stimuli compared with V stimuli was observed in 3-month-olds by Watanabe et al. (2013), using a dense, 94-channel recording system. The stimuli were videos of a colorful mobile with bells and little objects, with or without the sounds of the bells ringing. Thus, over the first 6 postnatal months, infants exhibit deactivation of the temporal cortex in the presence of unimodal visual input, a pattern of cortical activation not found in adults.
Three possibilities could account for the pattern of deactivation observed in temporal cortex when auditory stimuli are presented in combination with visual stimuli. First, although infants show clear unimodal activations to V stimuli and to A stimuli in occipital and temporal cortex, respectively, they may preferentially direct their attention to the Vstimuliin the A+V combination because of an inability to attend to both A and V stimuli at the same time, and the resulting V bias may lead to a decrease in processing of the A stimulus. Second, the combined A+V stimulus may be processed by different cortical areas than the A and V components when they are presented alone (i.e., the compound is treated as a new stimulus), and differentially reduce activation in unimodal areas. Third, unimodal presentation might lead to deactivation in other cortical areas due to resource stealing that results from exceeding the metabolic capacity of the primary cortical area for each unimodal component. The results of the high-density study by Watanabe et al. (2013) suggest that neither the second nor the third possibilities are likely: Unique multimodal processing areas for A+V compounds were not evident, and there was greater activation in occipital areas to the A+V stimulus than to the V stimulus, suggesting that resource stealing was not triggered by inadequate metabolic capacity in the occipital cortex. This last finding provides further evidence for qualitative changes in how unimodal stimuli, classically processed only by corresponding unimodal pathways, may be distributed to a much wider array of cortical areas in infants than in adults. One possible reason for this more diffuse activation in early development is that the slow time course of the hemodynamic response may include large-scale reverberant (feedback) connections between cortical areas that become more finely tuned, both spatially and temporally, with postnatal experience.
4.3. Language Processing
Early studies examining the pattern of neural activity in infants to speech stimuli using both fNIRS and fMRI were directed to the question of cortical selectivity (i.e., lateralization) and to functional specialization [i.e., speech versus nonspeech (Peña et al. 2003, Dehaene-Lambertz et al. 2002)]. Subsequent studies have addressed three questions: (a) What are the acoustic properties of speech that engender language-like processing, (b) How does speech processing interact with other cognitive capacities such as memory and attention, and (c) What are the early learning mechanisms that might underlie the extraction of linguistic structure present in the speech input?
A number of studies have reported that there is a left hemisphere (LH) specialization for speech in newborns using fNIRS (Peña et al. 2003) and in 2- to 3-month-old infants using fMRI (Dehaene-Lambertz et al. 2002; but see Taga et al. 2007). Preferential LH-activation (temporal and frontal areas) early in infancy conforms to a similar pattern in adults and may suggest either an innate processing bias for speech signals in the LH or an innate LH-bias that captures fundamental properties of speech during prenatal exposure to maternal vocalizations. Similarly, Bortfeld et al. (2009) found a predominantly left-lateralized increase in Oxy-Hb to an audio-visual stimulus (speech+abstract animations), compared to an animation-only visual stimulus in 6-month-olds. Moreover, Redcay et al. (2008) found that sleeping 21-month-olds in a fMRI study recruited additional neural regions (e.g., frontal) when listening to forward versus backward speech. One possible interpretation of these results is that as the language system becomes more sophisticated and infants move from receptive-only to both understanding and producing speech, they may engage a more elaborated network of cortical areas as they process linguistic stimuli. Another possibility is that, similar to the broad and developmentally variable cortical activation seen in response to even simple perceptual stimuli, infants might exhibit experience-based development of the neural systems engaged in language comprehension.
Because prenatal infants have access to suprasegmental levels of speech information (e.g., prosody) and not segmental information (e.g., phonemes), one might expect that infants rely on slowly varying word- and phrase-level information rather than the rapidly changing acoustic cues that characterize consonants to distinguish forward from backward speech sentences (see Peña et al. 2003). However, other lines of research have suggested that it is the right hemisphere (RH) that is specialized for processing slowly modulated information (see Poeppel 2003, Hickok & Poeppel 2007 for a comprehensive review). Indeed, recent fNIRS studies with newborns (Arimitsu et al. 2011, Telkemeyer et al. 2009) and 3- and 6-month-old infants (Telkemeyer et al. 2011) find greater RH-activation for changes in prosody.
How does one reconcile these differences? Part of the answer might lie in the precise placement of probes on the scalp. For example, Arimitsu et al. (2011) found that, although processing of rapidly modulated units (phonemes) is bilateral in temporal areas, it is asymmetric in inferior parietal areas, with greater activation in the LH. A recent study of preterm infants of around 30-weeks gestational age suggests a weak, left-dominant processing of syllables in posterior temporal regions (Mahmoudzadeh et al. 2013).
Language acquisition is not just about rote memorization of speech input, but crucially depends on the ability to understand and to generate novel utterances. Studies with adults (e.g., Saffran et al. 1996, Endress & Bonatti 2007) and infants (e.g., Marcus et al. 1999, Gómez 2002, Marchetto & Bonatti 2013) have demonstrated that humans are capable of extracting relevant language like structures from brief exposure to speech. Several fNIRS studies have provided converging evidence that even the neonate brain responds to rudimentary structure in the input, such as the presence of adjacent repetitions of syllables. Gervain et al. (2008) presented newborns with blocks of trisyllabic sequences, separated by silence, that were either of the form ABB (e.g.,/mubaba/) or ABC (e.g.,/mubage/), and found greater fNIRS activation for the ABB blocks compared with the ABC blocks. Moreover, this effect was greater in the LH than the RH. A follow-up study by Gervain et al. (2012) showed that newborns also had differential fNIRS activations to late versus early repetitions (i.e., ABB versus AAB sequences). Wagner et al. (2011) examined developmental changes in the processing of such repetition grammars, and found that while 7-month-old infants showed a greater fNIRS response to ABB over ABC blocks, 9-month-olds showed a greater response to ABC over ABB blocks. One interesting speculation about why infants might show this developmental shift from greater activation to repetition to greater activation to nonrepetition is that repetition is both useful for learning and prevalent in early maternal speech directed to infants. In contrast, repetition of phonemes and syllables is much less common in maternal speech as infants acquire their native language, and a recent behavioral study by Gerken et al. (2014) provides evidence that 9-month-olds treat repetitions as unexpected.
4.4. Social Cognition
One of the reasons that studies of language have been a focus of research on infant cognition is that only humans naturally acquire such a complex system of reference and communication. Another domain that serves a crucial function in human development is the social interaction among parents and offspring. For immature infants who must rely on their parents for survival, recognition of classes of social agents (e.g., members of your species) as well as recognition of individual social agents (e.g., parents, siblings) is a species-specific ability that has its roots even in newborns [e.g., preferences for upright over inverted or scrambled faces (Goren et al. 1975, Farroni et al. 2005)]. The classic demonstrations of regions of the ventral temporal cortex specialized for the processing of individual faces [the fusiform face area (FFA) (Kanwisher et al. 1997)] and regions of the lateral occipital-temporal cortex specialized for the processing of configurations of face-like features [the occipital face area (OFA) (Gauthier et al. 2000)] raised the prospect that these two neural areas were specialized by evolutionary pressures and not by postnatal exposure to faces.
Unfortunately, understanding the origins of the FFA and OFA have been stymied by the inability to gather fMRI data from (awake) children younger than age 4 years (see Cantlon et al. 2011 for the earliest evidence of the FFA), and by the inability of fNIRS to capture data from the FFA because of its distance from the skull. Results from studies of the OFA using fNIRS are mixed. While there is some converging evidence for right posterior temporal areas being involved in face processing, several studies find significant bilateral activations.
Otsuka et al. (2007) found that upright faces elicited significant elevations of Oxy-Hb and total hemoglobin (Total-Hb) in the right lateral cortex in 5- to 8-month-old infants. Further, Total-Hb was significantly higher for upright faces compared with inverted faces in the right lateral channels. This RH-dominance for face processing was seen in subsequent experiments, although the effect was found to be more robust in older, 7- to 8-month-old infants, compared with 5- to 6-month-olds. Kobayashi et al. (2011) successfully implemented a fNIRS adaptation paradigm and found an increased hemodynamic response to blocks of different faces compared with blocks of the same face in bilateral temporal areas. The fNIRS adaptation paradigm was also used to show viewpoint-invariance and size-invariance in face perception (Kobayashi et al. 2012), and again adaptation effects were found bilaterally.
The neural correlates of social cognition have been further explored using fNIRS in three other domains: eye-gaze, emotion expression, and voice recognition. Grossmann et al. (2008) found that right posterior temporal and left fronto-polar cortices were preferentially activated for the socially more relevant, mutual-gaze face, compared to a face with an averted gaze in 4-month-old infants. These results were replicated in a subsequent study with 5-month-olds, where again a left prefrontal channel showed enhanced activation for an image of a face with direct gaze compared to averted gaze (Grossmann et al. 2010). These results are consistent with fMRI evidence from adults of a third face region in the superior temporal sulcus that is specialized for eye-gaze (Liu et al. 2009). Another aspect of eye-gaze—positive contrast polarity (black eyes on a white scleral background)—has been studied in 5- to 6-month-olds (Ichikawa et al. 2013). The authors found converging evidence for the importance of positive contrast polarity, with greater Oxy-Hb over RH temporal areas.
Face recognition has been studied by Nakato et al. (2011), who examined the processing of one's own mother's versus a stranger's face, and of happy versus angry faces in 7-month-old infants. For the own mother versus stranger comparison, the right temporal channels showed increased activation for both own mother (Oxy-Hb and Total-Hb) and for the stranger (Total-Hb only), thus again replicating the RH-advantage for faces. However, the own mother also significantly activated the left temporal channels. Similarly, left temporal channels also showed increased activation for happy but not angry faces (Nakato et al. 2011). It is possible that bilateral activations observed in fNIRS studies of faces might reflect the processing of multiple aspects of faces, including their affect and/or familiarity.
The paralinguistic vocal qualities correlated with specific individuals also carry important information about social agents. Recent fMRI studies have examined infant neural engagement for emotional speech (Graham et al. 2013) and nonspeech vocalizations (Blasi et al. 2011). Blasi et al. (2011) found that nonspeech vocal sounds selectively modulated activity in the anterior temporal cortex compared with nonvocal environmental sounds. Both Blasi et al. (2011) and Graham et al. (2013) found that regions of the infant brain are differentially engaged during the presence of emotional vocalizations. While these studies implicate different regions of the infant brain in this process, the studies both find that negative emotions (sad and angry) elicit increased activation.
In the Grossmann et al. (2010) fNIRS study mentioned earlier, 5-month-old infants showed enhanced activation for their own name over others' names in a left-frontal channel adjacent to the location where activation to direct-gaze was greater than activation to indirect-gaze. Similar areas also showed preferential activation for social aspects of visual stimuli. Posterior temporal activation in the LH was found in 5-month-olds for dynamic social stimuli (videos of faces) compared with dynamic, nonsocial stimuli [videos of moving, mechanical toys (Lloyd-Fox et al. 2009)]. In 12-month-olds, Minagawa-Kawai et al. (2009) found greater activation for a video of the infants' mother's face when it was smiling, compared with when it had a neutral expression, in a centrally placed channel over the orbitofrontal cortex.
Finally, it is widely acknowledged that autism spectrum disorders (ASD) include differences in the processing of social stimuli, including disinterest in face stimuli and insensitivity to gaze cues. Although currently ASD can be reliably clinically diagnosed only beyond the second year of life, researchers are increasingly turning to studying infant siblings of individuals clinically diagnosed with ASD, as this population is at high risk for autism, showing more than a 15-fold increase in the likelihood of a positive clinical outcome (e.g., Ozonoff et al. 2011). In addition to collecting behavioral measures that might correlate with later development of autism, a few studies have begun using fNIRS to investigate cortical differences between at-risk infants and low-risk or typically developing infants in the first year of life (Lloyd-Fox et al. 2013, Fox et al. 2013, Keehn et al. 2013; see also Redcay & Courchesne 2008, for a study of neural engagement during speech processing in 2–3-year-olds with autism). Keehn et al. (2013) raises the intriguing prospect that focal activation in key regions of the brain are less relevant to mediating the symptoms of ASD than imbalances in the maturation of functional connectivity among the network of regions subserving social cognition (see Section 4.7).
4.5. Object and Number Processing
Two other topics of substantial and longstanding interest in infant cognition involve studies of object and number perception. Since the classic work of Piaget on object permanence, and one of the very first fNIRS studies on that topic (Baird etal. 2002), the literature on the neural mechanisms of object recognition has focused on the inferotemporal cortex (IT) in nonhuman primates (Wang et al. 1996).
An infant's understanding that an object exists despite being temporarily out of sight requires that the characteristics of that object have been stored in short-term memory and matched with the test object that is currently in view. Behavioral evidence of object permanence has been reported for infants as young as 2–3 months of age (e.g., Aguiar & Baillargeon 1999), but the properties of the object that enable this ability vary with age and experience. For example, infants use shape to recognize a briefly occluded object before they use color (see Wilcox 1999).
In a series of experiments building on these behavioral findings, Wilcox and colleagues have used fNIRS to measure activation in visual and anterior temporal cortex in 6.5-month-old infants as they viewed dynamic events depicting an object that repeatedly passed back and forth behind an occluder. For example, if a red sphere with silver stars moves from left to right and briefly disappears behind an occluder, its reappearance on the right side of the occluder implies that a single object has moved from left to right. But if the red sphere with silver stars transforms into a green cube with black dots after passing behind the occluder, then more than a single object should be inferred (with the red sphere remaining behind the occluder). This process of perceiving one or two objects is called individuation. Wilcox et al. (2008) varied the color, shape, or texture of the object after it reappeared from behind the occluder. Using fNIRS channels over temporal cortex in 6.5-month-olds, Wilcox reported that a change of shape or texture led to increased activation in temporal cortex, but color did not, thereby providing converging evidence for previous behavioral findings (e.g., Wilcox & Chapa 2004).
Color is not used in object individuation until 9 months of age, unless it is predictive of the function of an object in a pretest phase (Wilcox & Chapa 2004, Wilcox et al. 2008). Wilcox et al. (2014) found increased activation of anterior temporal cortex when infants individuated objects based on this color-priming manipulation, again adding support to the hypothesized role of anterior temporal cortex in object individuation. This neural signature for object individuation has also been found in similar studies examining the role of spatiotemporal discontinuity in object individuation (Wilcox et al. 2009, 2010). Taken together, these results provide robust evidence that anterior temporal cortex is engaged in the process of object individuation.
Object individuation is closely related to the ability to compute exact numerosities, such as the difference between one and two objects regardless of their shape, color, or texture. Classic behavioral work by Wynn (1992) showed that 5-month-old infants could compute small numerosities in the range of one to three when one or more objects were briefly occluded. Based on extensive evidence from patients with deficits in counting, single-unit recordings, and fMRI studies of children as young as 4 years of age (Cantlon et al. 2011), the interparietal sulcus (IPS) of the RH has been strongly implicated in numerical judgments. Hyde et al. (2010) used fNIRS to target the IPS in 6-month-olds as they viewed visual displays containing sets of objects differing either in numerosity or shape. They found a significant increase in activity in the right IPS but not the left IPS for the change in numerosity but not the change in shape, suggesting that the right IPS has attained some of the functional specialization for numerosity present in older children and adults.
4.6. Learning and Memory
As discussed in the domain of language processing, an important aspect of extracting structure from linguistic stimuli involves a form of automatic (implicit) learning. Every behavioral paradigm requires some rudimentary form of learning, even if the task is simply to discriminate the current stimulus from another stimulus presented just a few seconds earlier. Similarly, the oddball paradigm used when recording ERPs relies on the ability of infrequently presented stimuli to elicit a discrepancy in the average waveform compared with more frequently presented stimuli. Nakano et al. (2008) adapted a variant of this habituation paradigm to study discrimination learning in infancy using high-density fNIRS. They compared activation patterns in 3-month-old sleeping infants who were exposed to auditory stimuli varying in pitch. One pitch predicted the upcoming presentation of a human voice, whereas the other pitch predicted silence. An enhanced Oxy-Hb response over bilateral temporoparietal regions was present for the pitch that predicted the human voice, compared with the nonpredictive pitch.
In a subsequent study, Nakano et al. (2009) habituated 3-month-old infants to four identical blocks of a single repeating speech stimulus (e.g., the syllable/ba/), followed by either an identical (no change) block or a block with a different repeating stimulus (e.g., the syllable/pa/). As in many behavioral studies of speech discrimination using the habituation and recovery-to-novelty paradigm, they found a decrease across successive blocks for some prefrontal channels (but not for temporal channels), as well as recovery of activation in some prefrontal channels to the novel posthabituation test stimulus (e.g., from/ba/to/pa/). These results suggest that frontal regions are involved in differential activation to repeated (familiar) and novel stimuli.
Despite extensive behavioral evidence for the development of short-term memory (Blaser & Kaldy 2010) and working memory (Ross-Sheehy et al. 2004) in infants, paradigms that provide more sophisticated assessments of memory than simple habituation for understanding the neural correlates of memory are just being explored. Benavides-Varela et al. (2011) presented newborns with multiple repetitions of a single two-syllable word (e.g.,/pelu/) for 6 min, and then after a 2-min period of silence they heard either the same word or a different word. Results indicated that the previously heard word elicited a larger Oxy-Hb response than the novel word in bilateral temporoparietal and anterior areas. A subsequent study suggested that infants' encoding of the word-forms depended primarily on the vowels, and not the consonants (Benavides-Varela et al. 2012), consistent with previous behavioral research.
4.7. Resting State and Cortical Organization
By far the most common cognitive task studied in infancy using fMRI is no task at all: Numerous groups have investigated neural activity during rest, starting in the fetus (Thomason et al. 2013) and continuing through the first two postnatal years (e.g., Gao et al. 2011). While this literature follows in the footsteps of an interest in the default mode or resting state network in adults (e.g., Fox et al. 2006), the fact that most fMRI studies assess the resting state (or neural activity while sleeping) is largely the result of pragmatic constraints on collecting fMRI from infants (i.e., the need for rigid head restraint). In both infants and adults, recording fMRI during rest or sleep reveals that activity is correlated across regions of the brain at low frequencies (0.01–0.1 Hz, or periods of 100–10 s).
While correlated, large-scale neural activity exists at this temporal scale and is similar across individuals, it is unclear what a resting state network is. Two major theories are that it (a) is the neural correlate of the cognitive state that participants are engaging in while they are resting [e.g., recalling autobiographical memory or planning (Buckner et al. 2008)], or (b) reflects the endogenous cortical connectivity of the functional brain. The latter is the dominant perspective in the field of developmental cognitive neuroscience and is supported by the developmental trajectory of resting state networks that appear to differentiate in the first few postnatal years (Gao et al. 2011) and to be disrupted by early white matter injury (Smyser et al. 2013). However, a divergent finding in adults suggests that there is no direct relationship between structural connectivity [assessed using diffusion tensor imaging (DTI)] and functional connectivity (Damoiseaux & Greicius 2009).
Resting state networks in newborns, defined by temporally correlated fluctuations in neural activity across cortical regions during sleep, are quite different from adults (Fransson 2005). The default mode network in adults is characterized by strong inter- and intrahemispheric correlations that encompass a number of higher-level or associative cortical regions (e.g., posterior cingulate, medial prefrontal, medial temporal including the hippocampus, parietal cortex). This network is not present in infants, at least not including this breadth of regions and in both hemispheres. Instead, the infant resting state is dominated by correlated activity in sensorimotor regions (Smyser et al. 2010; Fransson et al. 2007, 2009, 2011). These regions exhibit very restricted and local connectivity within hemispheres (i.e., encompassing adjacent voxels within the same lobe or basic cortical structure) and, in some cases, the homologous region in the other hemisphere [homotopic regions (Smyser et al. 2010)]. Thus, the infant resting state is characterized by multiple networks mostly in basic sensorimotor regions and restricted to a single cortical region within and across hemispheres. Indeed, increases in age are associated with increased long-range and interhemispheric functional connectivity (Gao et al. 2011). Smyser et al. (2010) noted that there is greater homologous interhemispheric correlation in regions closer to the mid-line, suggesting that immaturity of white matter tracts traveling long distances limits the spatial extent of the resting state network in early infancy (see also Lee et al. 2013, Thomason et al. 2013).
While the majority of resting state studies have been conducted with fMRI, a recent combined fNIRS-fMRI study provided evidence that the resting state networks identified using fMRI can be detected using fNIRS in adults (Sasai et al. 2012). Moreover, three high-density fNIRS studies have provided estimates of functional connectivity among nearly 100 channels arranged around the circumference of the infant head (Homae et al. 2010, Sasai et al. 2011, Taga et al. 2011). These studies provide clear evidence of changes in resting state connectivity (during sleep) between newborns, 3-month-olds, and 6-month-olds (i.e., an increase in interhemispheric correlation), as well as changes from the resting state when infants are awake and presented with auditory stimuli.
Finally, changes in resting state functional connectivity have been observed in infants at risk for poor developmental outcomes associated with preterm birth (Smyser et al. 2013) even at term equivalence and without major health complications associated with prematurity [e.g., white matter injury (Damaraju et al. 2010, Smyser et al. 2010)]. Because preterm infants are at risk for poorer developmental outcomes, there are differences in resting state networks that parallel findings from adults with neuropsychiatric disorders (Greicius 2012). This is an important finding in two aspects: First, it deepens our understanding of the role and function of the resting state network, and second, it has the potential to provide a neurobiomarker for infants likely to exhibit poor developmental outcomes.
5. Open Questions and Future Directions
Two broad conclusions that emerge from our review of the literature on fMRI and fNIRS recordings from infants are (a) the difficulty associated with obtaining reliable fMRI data from awake infants, and (b) the encouraging prospects for fNIRS to reveal important aspects of the neural mechanisms underlying infant cognition. We conclude by considering a variety of new approaches that could enable fNIRS to become an even more powerful tool in the search for a better understanding of cognitive development (see sidebar Using fNIRS for a Brain Computer Interface).
5.1. New Kinds of Questions and New Designs
Just as in the first decade of research using fMRI with adults, the first decade of research using fNIRS with infants struggled to establish robust paradigms, to overcome technical problems, and to create a suite of statistical packages that are open-source and validated across labs. For example, in adults the hemodynamic response is approximately linear in the temporal domain (Boynton et al. 1996). Thus, it is possible to present discrete stimulus events at intervals shorter than the 10-s impulse response function and still detect reliable, albeit smaller amplitude, hemodynamic responses (Dale & Buckner 1997). It is now common to use such brief stimulus presentations in an event-related design, with randomly jittered interstimulus intervals, to accelerate the data-collection process compared with blocked designs that require the hemodynamic response to return to baseline before each stimulus event. Although standard in the fMRI literature, these event-related designs are still quite rare in the fNIRS literature, in part because of spurious (i.e., non-neural) vascular noise that reduces the S/N ratio of the neurovascular signal of interest (e.g., from cortex). Because of artifacts created by motion, hair, and optical probe design, infant fNIRS studies began with block designs, sleeping newborns, and simple stimulus versus no-stimulus contrasts. The field has now progressed beyond these constraints to include older infants, and like fMRI, to employ event-related paradigms (Plichta et al. 2007), thereby enabling more complex multifactorial designs within the time limits of a typical infant experiment (e.g., 5– 15 min).
Three relatively new experimental designs that have been described in the adult/child fMRI literature are now ready to be implemented in infant fNIRS studies. The first design employs computational models to isolate brain regions that are correlated with the model's predictions. For example, den Ouden et al. (2010) used model predictions about the rate of learning to seek average fMRI time course data consistent with these predictions, and Summerfield et al. (2008) compared estimates of repetition-based familiarity with estimates of expectation errors to determine whether these estimation processes share the same neural substrate. A particularly intriguing application of this model-based fMRI approach, described by Park & Friston (2013), involves constraining the types of functional connectivity present during the resting state. This approach could be applied to functional connectivity analyses in infants, thereby charting the progression of developmental changes in the strength of functional interactions among various brain regions.
The second design employs moment-to-moment predictions about a cognitive process (e.g., variations in attention) as indexed by detailed behavioral measures such as visual fixations assessed with an eye-tracker or value decisions made in a rating task (Büchel et al. 1998). Given that visual attention in infants under some conditions is characterized by a U-shaped function (Kidd et al. 2012), one could use fNIRS to examine cortical areas whose activation is modulated by the predicted quadratic function or alternatively is segregated into separate familiarity and novelty subregions (see Kafkas & Montaldi 2014).
A third design employs continuous stimuli (e.g., a movie) within which discrete events are embedded at intermittent intervals (Hasson et al. 2009). These events are irregular, as in an event-related design, but there is no contrast with the absence of a stimulus or with a second type of stimulus. A key advantage of this continuous stimulus design for use with infants is that the movie serves to engage their attention for long periods of time that otherwise would be interrupted by a no stimulus baseline. The fact that fMRI data collected from children as young as 4 years of age using such continuous stimulus designs match data from traditional block designs (Cantlon & Li 2013) suggest that they could be particularly useful in extending the time (and amount) of data collected in fNIRS studies with infants.
A final prospect for the future is for fNIRS to become robust enough that it can be implemented on a behaving infant. Atsumori et al. (2010) and Piper et al. (2014) describe such a mobile fNIRS apparatus and their success in extracting reliable multichannel fNIRS signals from adults in the context of locomotor activity without the presence of intractable motion artifacts. Safaie et al. (2013) describe a wireless version that combines both fNIRS and EEG. If further technical developments enable a similarly robust system for use with infants, it could be used in the future to gather neural correlates of cognition during active behavioral tasks such as reaching, crawling, and walking.
5.2. New Instrumentation and New Methods of Analysis
The initial decade of fNIRS studies relied primarily on simple statistical analyses rather than the general linear model (GLM)-based regression analyses typical of fMRI studies. A suite of standardized analysis tools (HOMER 2.0) has been available for several years to perform these non-GLM analyses, including principal components analysis (PCA) to eliminate between-channel correlations indicative of surface vascular noise.1 Several recent fNIRS studies with infants have employed adaptations of standard GLM statistical packages (e.g., Minagawa-Kawai et al. 2011, Southgate et al. 2014), including a fNIRS version of the statistical parametric mapping package (Ye et al. 2010, Fekete et al. 2014). A recent comprehensive review of these statistical packages by Tak & Ye (2014) charts their history of use, pros and cons, and prospects for the future of fNIRS analyses of infant data.
Aglaring weakness of most fNIRS studies is the absence of a map of estimated functional activity projected onto the underlying brain anatomy of each participant. This spatial mapping is one of the powerful aspects of fMRI and only requires a brief anatomical scan before or after the functional scan. While there are certainly distortions in taking a particular anatomical scan and transforming it to a standard brain, these distortions are relatively small in adults because the average brain template is proportionally quite similar. Based on recent anatomical scans from infants (Sanchez et al. 2012), it is clear that transforming the infant brain to adult templates results in large errors because the growth of the cortex is not uniform between birth and adolescence. Thus, age- or headsize-specific brain templates (see Figure 4) are required to localize the fNIRS channels over similar anatomical areas across development (see Akiyama et al. 2013 for 6-month-old infants2, and Lloyd-Fox et al. 2014 and Sanchez et al. 2012 for a wide range of ages starting at 2 weeks3). However, the spatial resolution of fNIRS is an order of magnitude less than fMRI, so it has been argued that variability in fNIRS channel placement does not require subject-specific anatomy, at least in adults, but only approximate channel placement based on skull landmarks [e.g., the 10– 20 system used with EEG (see Ferradal et al. 2014)]. A recent review by Tsuzuki & Dan (2014) provides guidelines for how to evaluate the need for coregistration of fNIRS to the underlying brain anatomy, particularly as it applies to studies of infants.
Figure 4.

Spatial coregistration of functional near-infrared spectroscopy (fNIRS) channels with age- and headsize-appropriate magnetic resonance (MR) templates courtesy of John Richards and Sarah Lloyd-Fox, with permission (to appear in Lloyd-Fox et al. 2014).
Another approach to understanding brain function using fMRI—multivariate pattern analysis (MVPA)—has not yet been applied to fNIRS data. The basic idea underlying MVPA is that local clusters of voxels may not provide aggregate activations that differ for two stimulus conditions, but the detailed pattern of voxel activations within a local cluster could differ reliably between the two stimulus conditions. Thus, rather than smoothing across clusters of voxels and transforming voxels to an average brain template (which also introduces blur in the spatial location of activation), the raw voxel activations are entered into a regression analysis or a machine-learning algorithm (Norman et al. 2006) to determine for a given set of data how well one can predict which stimulus was present based on the pattern of voxel activations. This is typically done by withholding some of the data, training the classifier so that it finds the voxel activations that best predict the stimulus, and then asking whether this same classifier performs above chance on the withheld data (Tong & Pratte 2012). As noted by Turk-Browne (2013), the MVPA approach is limited to seeking a reliable classifier in a local subregion, and therefore is unable to determine whether the failure of a classifier is because a given brain region has insufficient information for classification, or because the functional connectivity of that brain region with other brain regions is insufficient. Thus, an exciting goal for the future is to meld the MVPA approach with estimates of resting-state and stimulus-driven functional connectivity (see sidebar The Potential of Time-of-Flight fNIRS).
In conclusion, research on infant cognition using fNIRS is ripe for extensive deployment of event-related and continuous-presentation designs, for the use of anatomical atlases to determine the precise placement of recording channels, for studies of functional connectivity (while asleep or awake), and for the application of MVPA methods to gather distributed activations that predict specific cognitive processes. As with any technique, fNIRS is only as informative as the questions asked and the designs employed to answer them, but the prospects are encouraging that a greater level of sophistication will push the field of infant fNIRS forward in new and interesting ways.
Using Fnirs for a Brain Computer Interface.
In the past five years, there has been substantial interest in creating brain computer interfaces (BCIs) that enable a user to control an external device, such as a computer keyboard or a cursor. Although much of this interest stems from the desire to enable individuals with disabilities to interact with a computer or other device (e.g., a wheelchair), there is also a basic science question that focuses on how various features of signals from the brain can be brought under user control. Typical BCI systems rely on fast neural signals such as the EEG, but because their spatial resolution and source localization are underspecified, newer BCI systems have attempted to use fNIRS (Sitaram et al. 2009) or fNIRS in conjunction with EEG (Fazli et al. 2012). Because infants initially have immature motor systems, the use of fNIRS as a BCI method to enable activation of visual displays, much like reaching to a touch screen, could provide the infant with visual-motor experiences that are under their control months before they master their own motor movements.
The Potential of Time-of-Flight fNIRS.
It has been known since the late 1990s that rather than injecting continuous near-infrared illumination into the brain and measuring the attenuation at the surface due to absorption by Oxy-Hb and deOxy-Hb, it is feasible to measure the time-of-flight of photons as they travel into the brain and back to the detector. This time-of-flight signal varies with the predicted absorption properties of Oxy-Hb and deOxy-Hb depending on the wavelength of the picosecond pulse that delivers the photons to the brain. As summarized in a recent comprehensive review by Torricelli et al. (2014), the time-of-flight approach has three distinct advantages over the continuous wave fNIRS systems now in use with infants. First, because the pulse of photons is so brief and the speed of photons in the media is so fast, there is essentially no possibility of motion artifacts. Second, the canonical banana-shaped pathway through which the majority of photons travel in a continuous wave system does not apply because some photons will return to the surface of the scalp at the same location where they were emitted. This allows a much higher density of channels on the scalp. Third, the detector that records the returning photons can be temporally gated so that only photons that travel to at least a depth of X mm will be sampled. This means that a depth-dependent signal can be obtained if the timing of the gated detector is systematically varied.
Summary Points.
Recording fMRI signals from infants is extremely challenging because of the need to reduce head motion. Thus, developmental fMRI studies are conducted with sleeping subjects. This requirement is eliminated by having fNIRS signals recorded from optical fibers attached to the scalp, but fNIRS is limited to recordings from the cortical surface and not from deeper brain structures.
The fNIRS signal is contaminated by non-neural vascular noise and infant studies are typically completed in 5–15 min to accommodate infant attention spans. This requires the use of experimental designs and corrections to the fNIRS signal that reduce the effects of these impediments to estimating neural activations that are correlated with infant cognition.
Both fMRI and fNIRS require linking hypotheses between the recorded hemodynamic signal and the underlying cognitive process. These assumptions should be clearly defined to avoid making unwarranted inferences about brain mechanisms correlated with cognitive development, especially given substantial developmental differences in task difficulty and effectiveness of task instructions.
The majority of fMRI and fNIRS studies in infants are confirmatory of standard findings in the field of adult cognitive neuroscience rather than establishing novel principles by which the immature brain is qualitatively different from the adult brain. In fact, most infant studies target a single age rather than charting the development of the neural correlates of a cognitive process across age.
Technical improvements in the methods of gathering fNIRS data from infants are poised to reveal new insights about functional brain development. These improvements include the extensive use of event-related designs, more sophisticated statistical analyses, broad-scale estimates of functional connectivity, and the implementation of time-of-flight fNIRS systems that have much higher spatial resolution.
Acknowledgments
The writing of this article was supported by research grant HD-037082 (R.N.A.), Center for Visual Science Core grant EY-01319, a Canadian Institute for Health Research grant 201210MFE-290131-231192, and a NIH K99 research grant (HD-076166) (L.L.E.). We are grateful for the helpful comments and corrections on an earlier draft provided by David Boas, Jessica Cantlon, Charles Nelson, Sarah Lloyd-Fox, and Raj Raizada. The first author was on leave at MIT during preparation of this article and gratefully acknowledges the Department of Brain and Cognitive Sciences and especially the members of the Kanwisher Lab.
Glossary
- fMRI
functional magnetic resonance imaging
- fNIRS
functional near-infrared spectroscopy
- deOxy-Hb
deoxygenated hemoglobin
- Oxy-Hb
oxygenated hemoglobin
- EEG
electroen-cephalography
- MEG
magnetoen-cephalography
- PET
positron emission tomography
- BOLD
blood oxygen-level-dependent response
- Event-related design
presentation of single events (e.g., single stimuli of different types), often with uneven temporal spacing, to model hemodynamic responses to individual stimuli
- Diffusion tensor imaging (DTI)
an MRI-based method that estimates white-matter fiber tracts using diffusion of water molecules
Footnotes
HemOdynaMic Evoked Response (HOMER) NIRS data analysis GUI at http://www.nmr.mgh.harvard.edu/DOT/resources/homer/documentation.htm.
Typical 6-month-old head templates/atlas at http://ilabs.washington.edu/6-m-templates-atlas.
Neurodevelopmental MRI Database at http://jerlab.psych.sc.edu/NeurodevelopmentalMRIDatabase/.
The Annual Review of Psychology is online at psych.annualreviews.org
Disclosure Statement: The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Richard N. Aslin, Email: aslin@cvs.rochester.edu.
Mohinish Shukla, Email: mohinish.shukla@umb.edu.
Lauren L. Emberson, Email: lemberson@bcs.rochester.edu.
Literature Cited
- Aguiar A, Baillargeon R. 2.5-month-old infants' reasoning about when objects should and should not be occluded. Cogn Psychol. 1999;39:116–57. doi: 10.1006/cogp.1999.0717. [DOI] [PubMed] [Google Scholar]
- Akiyama L, Richards T, Imada T, Dager S, Wroblewski L, Kuhl P. Age-specific average head template for typically developing 6-month-old infants. PLoS ONE. 2013;8:e73821. doi: 10.1371/journal.pone.0073821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman NR, Bernal B. Brain activation in sedated children: auditory and visual functional MR imaging. Radiology. 2001;221(1):56–63. doi: 10.1148/radiol.2211010074. [DOI] [PubMed] [Google Scholar]
- Anderson AW, Marois R, Colson ER, Peterson BS, Duncan CC, et al. Neonatal auditory activation detected by functional magnetic resonance imaging. Magn Reson Imaging. 2001;19(1):1–5. doi: 10.1016/s0730-725x(00)00231-9. [DOI] [PubMed] [Google Scholar]
- Arichi T, Fagiolo G, Varela M, Melendez-Calderon A, Allievi A, et al. Development of BOLD signal hemodynamic responses in the human brain. NeuroImage. 2012;63:663–673. doi: 10.1016/j.neuroimage.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arichi T, Moraux A, Melendez A, Doria V, Groppo M, et al. Somatosensory cortical activation identified by functional MRI in preterm and term infants. NeuroImage. 2010;49:2063–2071. doi: 10.1016/j.neuroimage.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Arimitsu T, Uchida-Ota M, Yagihashi T, Kojima S, Watanabe S, et al. Functional hemispheric specialization in processing phonemic and prosodic auditory changes in neonates. Front Psychol. 2011;2:202. doi: 10.3389/fpsyg.2011.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslin RN. Questioning the questions that have been asked about the infant brain using near-infrared spectroscopy. Cogn Neuropsychol. 2012;29:7–33. doi: 10.1080/02643294.2012.654773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslin RN, Fiser J. Methodological challenges for understanding cognitive development in infants. Trends Cogn Sci. 2005;9:92–98. doi: 10.1016/j.tics.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Atsumori H, Kiguchi M, Katura T, Funane T, Obata A, et al. Noninvasive imaging of prefrontal activation during attention-demanding tasks performed while walking using a wearable optical topography system. J Biomed Opt. 2010;15:046002. doi: 10.1117/1.3462996. [DOI] [PubMed] [Google Scholar]
- Baird AA, Kagan J, Gaudette T, Walz KA, Hershlag N, Boas DA. Frontal lobe activation during object permanence: data from near-infrared spectroscopy. NeuroImage. 2002;16:1120–25. doi: 10.1006/nimg.2002.1170. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Beurlot MR, Fava E, Nath AR, Parikh NA, et al. The developmental trajectory of brain-scalp distance from birth through childhood: implications for functional neuroimaging. PLoS ONE. 2011;6:e24981. doi: 10.1371/journal.pone.0024981. A detailed analysis of the anatomy of the developing brain using structural MRI, showing that the distance from the surface of the scalp, where fNIRS signals are sampled, to underlying cortex varies across the skull and across age. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides-Varela S, Gómez DM, Mehler J. Studying neonates' language and memory capacities with functional near-infrared spectroscopy. Front Psychol. 2011;2:64. doi: 10.3389/fpsyg.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides-Varela S, Hochmann JR, Macagno F, Nespor M, Mehler J. Newborn's brain activity signals the origin of word memories. Proc Natl Acad Sci USA. 2012;109:17908–13. doi: 10.1073/pnas.1205413109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser E, Kaldy Z. Infants get five stars on iconic memory tests: a partial-report test of 6-month-old infants' iconic memory capacity. Psycholog Sci. 2010;21:1643–45. doi: 10.1177/0956797610385358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi A, Mercure E, Lloyd-Fox S, Thomson A, Brammer M, et al. Early specialization for voice and emotion processing in the infant brain. Curr Biol. 2011;21:1220–24. doi: 10.1016/j.cub.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Boas DA, Elwell CE, Ferrari M, Taga G. Twenty years of functional near-infrared spectroscopy: introduction for the special issue. NeuroImage. 2014;85(Pt. 1):1–5. doi: 10.1016/j.neuroimage.2013.11.033. [DOI] [PubMed] [Google Scholar]
- Born A, Law I, Lund T, Rostrup E, Hanson L, et al. Cortical deactivation induced by visual stimuliation in human slow-wave sleep. NeuroImage. 2002;17:1325–35. doi: 10.1006/nimg.2002.1249. [DOI] [PubMed] [Google Scholar]
- Born A, Miranda M, Rostrup E, Toft P, Peitersen B, et al. Functional magnetic resonance imaging of the normal and abnormal visual system in early life. Neuropediatrics. 2000;31:24–32. doi: 10.1055/s-2000-15402. [DOI] [PubMed] [Google Scholar]
- Bortfeld H, Fava E, Boas DA. Identifying cortical lateralization of speech processing in infants using near-infrared spectroscopy. Dev Neuropsychol. 2009;34:52–65. doi: 10.1080/87565640802564481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortfeld H, Wruck E, Boas DA. Assessing infants' cortical response to speech using near-infrared spectroscopy. NeuroImage. 2007;34:407–15. doi: 10.1016/j.neuroimage.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human v1. J Neurosci. 1996;16:4207–21. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Holmes A, Rees G, Friston K. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. NeuroImage. 1998;8:140–48. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cantlon J, Li R. Neural activity during natural viewing of Sesame Street statistically predicts test scores in early childhood. PLoS Biol. 2013;11(1):e1001462. doi: 10.1371/journal.pbio.1001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon J, Pinel P, Dehaene S, Pelphrey K. Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cereb Cortex. 2011;21:191–99. doi: 10.1093/cercor/bhq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheour M, Imada T, Taulu S, Ahonen A, Salonen J, Kuhl P. Magnetoencephalography is feasible for infant assessment of auditory discrimination. Exp Neurol. 2004;190(Suppl. 1):S44–51. doi: 10.1016/j.expneurol.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–97. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Cohen D, Halgren E. Magnetoencephalography. In: Squire LR, editor. Encyclopedia of Neuroscience. Vol. 5. Oxford, UK: Acad. Press; 2009. pp. 615–22. [Google Scholar]
- Cui X, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage. 2011;54:2808–21. doi: 10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp. 1997;5:329–40. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Damaraju E, Phillips JR, Lowe JR, Ohls R, Calhoun VD, Caprihan A. Resting-state functional connectivity differences in premature children. Front Syst Neurosci. 2010;4:0023. doi: 10.3389/fnsys.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213:525–33. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–15. doi: 10.1126/science.1077066. The first fMRI study with human infants that reported differential cortical activation to speech and speech-like sounds. [DOI] [PubMed] [Google Scholar]
- den Ouden H, Daunizeau J, Roiser J, Friston K, Stephan K. Striatal prediction error modulates cortical coupling. J Neurosci. 2010;30:3210–19. doi: 10.1523/JNEUROSCI.4458-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endress AD, Bonatti LL. Rapid learning of syllable classes from a perceptually continuous speech stream. Cognition. 2007;105:247–99. doi: 10.1016/j.cognition.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Erberich SG, Panigrahy A, Friedlich P, Seri I, Nelson MD, Gilles F. Somatosensory lateralization in the newborn brain. NeuroImage. 2006;29:155–61. doi: 10.1016/j.neuroimage.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Farroni T, Johnson MH, Menon E, Zulian L, Faraguna D, Csibra G. Newborns' preference for face-relevant stimuli: effects of contrast polarity. Proc Natl Acad Sci USA. 2005;102:17245–50. doi: 10.1073/pnas.0502205102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazli S, Mehnert J, Steinbrink J, Curio G, Villringer A, et al. Enhanced performance by a hybrid NIRS–EEG brain computer interface. NeuroImage. 2012;59:519–29. doi: 10.1016/j.neuroimage.2011.07.084. [DOI] [PubMed] [Google Scholar]
- Fekete T, Rubin D, Carlson JM, Mujica-Parodi LR. The NIRS analysis package: noise reduction and statistical inference. PLoS ONE. 2014;6(9):e24322. doi: 10.1371/journal.pone.0024322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferradal S, Eggebrecht A, Hassanpour M, Snyder A, Culver J. Atlas-based head modeling and spatial normalization for high-density diffuse optical tomography: in vivo validation against fMRI. NeuroImage. 2014;85:117–26. doi: 10.1016/j.neuroimage.2013.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage. 2012;63:921–35. doi: 10.1016/j.neuroimage.2012.03.049. [DOI] [PubMed] [Google Scholar]
- Fox M, Corbetta M, Snyder A, Vincent J, Raichle M. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–51. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S, Wagner J, Shrock C, Tager-Flusberg H, Nelson C. Neural processing of facial identity and emotion in infants at high-risk for autism spectrum disorders. Front Hum Neurosci. 2013;7:89. doi: 10.3389/fnhum.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Åden U, Blennow M, Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex. 2011;21:145–54. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiöld B, Engström M, Hallberg B, Mosskin M, et al. Spontaneous brain activity in the newborn brain during natural sleep—an fMRI study in infants born at full term. Pediatr Res. 2009;66:301–5. doi: 10.1203/PDR.0b013e3181b1bd84. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiöld B, Horsch S, Nordell A, Blennow M, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci. 2007;104:15531–36. doi: 10.1073/pnas.0704380104. A pioneering study of spontaneous brain activation in early infancy using fMRI during sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon L, Yucel M, Boas D, Cooper R. Further improvement in reducing superficial contamination in NIRS using double short separation measurements. NeuroImage. 2014;85:127–35. doi: 10.1016/j.neuroimage.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Giovanello KS, Smith JK, Shen D, et al. Temporal and spatial evolution of brain network topology during the first two years of life. PLoS ONE. 2011;6:e25278. doi: 10.1371/journal.pone.0025278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Tarr M, Moylan J, Skudlarski P, Gore J, Anderson A. The fusiform “face area” is part of a network that processes faces at the individual level. J Cogn Neurosci. 2000;12:495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- Gerken L, Dawson C, Chatila R, Tenenbaum J. Surprise! Infants consider possible bases of generalization for a single input example. Dev Sci. 2014 doi: 10.1111/desc.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain J, Berent I, Werker JF. Binding at birth: the newborn brain detects identity relations and sequential position in speech. J Cogn Neurosci. 2012;24:564–74. doi: 10.1162/jocn_a_00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain J, Macagno F, Cogoi S, Peña M, Mehler J. The neonate brain detects speech structure. Proc Natl Acad Sci USA. 2008;105:14222–27. doi: 10.1073/pnas.0806530105. A fNIRS study showing that newborns can extract temporal repetition structure embedded in sequences of speech. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain J, Mehler J, Werker JF, Nelson CA, Csibra G, et al. Near-infrared spectroscopy: a report from the McDonnell infant methodology consortium. Dev Cogn Neurosci. 2011;1:22–46. doi: 10.1016/j.dcn.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goense JBM, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol. 2008;18:631–40. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- Gómez RL. Variability and detection of invariant structure. Psychol Sci. 2002;13:431–36. doi: 10.1111/1467-9280.00476. [DOI] [PubMed] [Google Scholar]
- Goren CC, Sarty M, Wu PY. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics. 1975;56:544–49. [PubMed] [Google Scholar]
- Graham AM, Fisher PA, Pfeifer JH. What sleeping babies hear: a functional MRI study of interparental conflict and infants' emotion processing. Psychol Sci. 2013;24:782–89. doi: 10.1177/0956797612458803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2012;4:424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH, Lloyd-Fox S, Blasi A, Deligianni F, et al. Early cortical specialization for face-to-face communication in human infants. Proc Biol Sci. 2008;275:2803–11. doi: 10.1098/rspb.2008.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Parise E, Friederici AD. The detection of communicative signals directed at the self in infant prefrontal cortex. Front Hum Neurosci. 2010;4:201. doi: 10.3389/fnhum.2010.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Malach R, Heeger D. Reliability of cortical activity during natural stimulation. Trends Cogn Sci. 2009;14:40–48. doi: 10.1016/j.tics.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Otobe T, Nakano T, Go T, et al. Development of global cortical networks in early infancy. J Neurosci. 2010;30:4877–82. doi: 10.1523/JNEUROSCI.5618-09.2010. The first fNIRS study to use a large number of channels around the entire skull to record functional connectivity during sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde DC, Boas DA, Blair C, Carey S. Near-infrared spectroscopy shows right parietal specialization for number in pre-verbal infants. NeuroImage. 2010;53:647–52. doi: 10.1016/j.neuroimage.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa H, Otsuka Y, Kanazawa S, Yamaguchi MK, Kakigi R. Contrast reversal of the eyes impairs infants' face processing: a near-infrared spectroscopic study. Neuropsychologia. 2013;51(13):2556–61. doi: 10.1016/j.neuropsychologia.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Imada T, Zhang Y, Cheour M, Taulu S, Ahonen A, Kuhl PK. Infant speech perception activates Broca's area: a developmental magnetoencephalography study. NeuroReport. 2006;17:957–62. doi: 10.1097/01.wnr.0000223387.51704.89. [DOI] [PubMed] [Google Scholar]
- Jöbsis FF. Non-invasive, infra-red monitoring of cerebral O2 sufficiency, blood volume, HbO2-Hb shifts and blood flow. Acta Neurol Scand Suppl. 1977;64:452–53. [PubMed] [Google Scholar]
- Kafkas A, Montaldi D. Two separate, but interacting, neural systems for familiarity and novelty detection: a dual route mechanism. Hippocampus. 2014;24:516–27. doi: 10.1002/hipo.22241. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Wagner JB, Tager-Flusberg H, Nelson CA. Functional connectivity in the first year of life in infants at-risk for autism: a preliminary near-infrared spectroscopy study. Front Hum Neurosci. 2013;7:444. doi: 10.3389/fnhum.2013.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrer M, Schöning M. A longitudinal study of cerebral blood flow over the first 30 months. Pediatr Res. 2009;66:560–64. doi: 10.1203/PDR.0b013e3181ba1a29. [DOI] [PubMed] [Google Scholar]
- Keil B, Blau JN, Biber S, Hoecht P, Tountcheva V, et al. A 64-channel 3T array coil for accelerated brain MRI. Magn Reson Med. 2013;70:248–58. doi: 10.1002/mrm.24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd C, Piantadosi S, Aslin R. The Goldilocks effect: human infants allocate attention to visual sequences that are neither too simple nor too complex. PLoS ONE. 2012;7:e36399. doi: 10.1371/journal.pone.0036399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Otsuka Y, Kanazawa S, Yamaguchi MK, Kakigi R. Size-invariant representation of face in infant brain: an fNIRS-adaptation study. NeuroReport. 2012;23:984–88. doi: 10.1097/WNR.0b013e32835a4b86. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Otsuka Y, Nakato E, Kanazawa S, Yamaguchi MK, Kakigi R. Do infants represent the face in a viewpoint-invariant manner? Neural adaptation study as measured by near-infrared spectroscopy. Front Hum Neurosci. 2011;5:153. doi: 10.3389/fnhum.2011.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotilahti K, Nissilä I, Huotilainen M, Mäkelä R, Gavrielides N, et al. Bilateral hemodynamic responses to auditory stimulation in newborn infants. NeuroReport. 2005;16:1373–77. doi: 10.1097/01.wnr.0000175247.35837.15. [DOI] [PubMed] [Google Scholar]
- Kozberg MG, Chen BR, DeLeo SE, Bouchard MB, Hillman EM. Resolving the transition from negative to positive blood oxygen level-dependent responses in the developing brain. Proc Natl Acad Sci. 2013;110:4380–85. doi: 10.1073/pnas.1212785110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Chugani HT. Functional imaging: PET. Handb Clin Neurol. 2013;111:767–76. doi: 10.1016/B978-0-444-52891-9.00079-8. [DOI] [PubMed] [Google Scholar]
- Kusaka T, Isobe K, Miki T, Ueno M, Koyano K, et al. Functional lateralization of sensorimotor cortex in infants measured using multichannel near-infrared spectroscopy. Pediatr Res. 2011;69:430–35. doi: 10.1203/PDR.0b013e3182125cbd. [DOI] [PubMed] [Google Scholar]
- Lee W, Donner EJ, Nossin-Manor R, Whyte HE, Sled JG, Taylor MJ. Visual functional magnetic resonance imaging of preterm infants. Dev Med Child Neurol. 2012;54:724–29. doi: 10.1111/j.1469-8749.2012.04342.x. [DOI] [PubMed] [Google Scholar]
- Lee W, Morgan BR, Shroff MM, Sled JG, Taylor MJ. The development of regional functional connectivity in preterm infants into early childhood. Neuroradiology. 2013;55:105–11. doi: 10.1007/s00234-013-1232-z. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A, Poldrack RA. Brain imaging. In: Koob GF, Le Moal M, Thompson RF, editors. Encyclopedia of Behavioral Neuroscience. Amsterdam: Elsevier; 2010. pp. 187–93. [Google Scholar]
- Liao S, Ferradal S, White B, Gregg N, Inder T, Culver J. High-density diffuse optical tomography of term infant visual cortex in the nursery. J Biomed Opt. 2012;17(8):081414. doi: 10.1117/1.JBO.17.8.081414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Harris A, Kanwisher A. Perception of face parts and face configurations: an fMRI study. J Cogn Neurosci. 2009;22:203–11. doi: 10.1162/jocn.2009.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Elwell CE. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci Biobehav Rev. 2010;34:269–84. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Elwell CE, Charman T, Murphy D, Johnson MH. Reduced neural sensitivity to social stimuli in infants at risk for autism. Proc Biol Sci. 2013;280:20123026. doi: 10.1098/rspb.2012.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Volein A, Everdell N, Elwell CE, Johnson MH. Social perception in infancy: a near infrared spectroscopy study. Child Dev. 2009;80:986–99. doi: 10.1111/j.1467-8624.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Richards JE, Blasi A, Murphy DGM, Elwell CE, Johnson MH. Coregistering functional near-infrared spectroscopy with underlying cortical areas in infants. Neurophotonics. 2014;1:025006–16. doi: 10.1117/1.NPh.1.2.025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S. An Introduction to The Event-Related Potential Technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Mahmoudzadeh M, Dehaene-Lambertz G, Fournier M, Kongolo G, Goudjil S, et al. Syllabic discrimination in premature human infants prior to complete formation of cortical layers. Proc Natl Acad Sci USA. 2013;110:4846–51. doi: 10.1073/pnas.1212220110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science. 1996;272:551–54. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- Marchetto E, Bonatti LL. Words and possible words in early language acquisition. Cogn Psychol. 2013;67:130–50. doi: 10.1016/j.cogpsych.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Marcus GF, Vijayan S, Bandi Rao S, Vishton PM. Rule learning by seven-month-old infants. Science. 1999;283:77–80. doi: 10.1126/science.283.5398.77. [DOI] [PubMed] [Google Scholar]
- Matuz T, Govindan RB, Preissl H, Siegel ER, Muenssinger J, et al. Habituation of visual evoked responses in neonates and fetuses: a MEG study. Dev Cogn Neurosci. 2012;2:303–16. doi: 10.1016/j.dcn.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek JH, Firbank M, Elwell CE, Atkinson J, Braddick O, Wyatt JS. Regional hemodynamic responses to visual stimulation in awake infants. Pediatr Res. 1998;43:840–43. doi: 10.1203/00006450-199806000-00019. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, Matsuoka S, Dan I, Naoi N, Nakamura K, Kojima S. Prefrontal activation associated with social attachment: facial-emotion recognition in mothers and infants. Cereb Cortex. 2009;19:284–92. doi: 10.1093/cercor/bhn081. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, Mori K, Hebden JC, Dupoux E. Optical imaging of infants' neurocognitive development: recent advances and perspectives. Dev Neurobiol. 2008;68:712–28. doi: 10.1002/dneu.20618. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, van der Lely H, Ramus F, Sato Y, Mazuka R, Dupoux E. Optical brain imaging reveals general auditory and language-specific processing in early infant development. Cereb Cortex. 2011;21:254–61. doi: 10.1093/cercor/bhq082. A comprehensive fNIRS study that estimated the hemodynamic response function (HRF) in newborns and used SPMfor analysis of the fNIRS signals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Homae F, Watanabe H, Taga G. Anticipatory cortical activation precedes auditory events in sleeping infants. PLoS ONE. 2008;3:e3912. doi: 10.1371/journal.pone.0003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Watanabe H, Homae F, Taga G. Prefrontal cortical involvement in young infants' analysis of novelty. Cereb Cortex. 2009;19:455–63. doi: 10.1093/cercor/bhn096. The first fNIRS study to use the habituation and recovery-to-novelty paradigm to study speech sound discrimination. [DOI] [PubMed] [Google Scholar]
- Nakato E, Otsuka Y, Kanazawa S, Yamaguchi MK, Kakigi R. Distinct differences in the pattern of hemodynamic response to happy and angry facial expressions in infants—a near-infrared spectroscopic study. NeuroImage. 2011;54:1600–6. doi: 10.1016/j.neuroimage.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Nelson CA, 3rd, McCleery JP. Use of event-related potentials in the study of typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2008;47:1252–61. doi: 10.1097/CHI.0b013e318185a6d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Ferrier IN, Coventry K, Bester A, Finkelmeyer A. Negative BOLD response in the hippocampus during short-term spatial memory retrieval. J Cogn Neurosci. 2013;25:1358–71. doi: 10.1162/jocn_a_00396. [DOI] [PubMed] [Google Scholar]
- Norman K, Polyn S, Detre G, Haxby J. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10:424–30. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Otsuka Y, Nakato E, Kanazawa S, Yamaguchi MK, Watanabe S, Kakigi R. Neural activation to upright and inverted faces in infants measured by near infrared spectroscopy. NeuroImage. 2007;34:399–406. doi: 10.1016/j.neuroimage.2006.08.013. One of the first fNIRS studies to record activations in the lateral occipital-temporal areas to face stimuli. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128:e488–95. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342:6158. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- Peña M, Maki A, Kovacić D, Dehaene-Lambertz G, Koizumi H, et al. Sounds and silence: an optical topography study of language recognition at birth. Proc Natl Acad Sci USA. 2003;100:11702–5. doi: 10.1073/pnas.1934290100. The first fNIRS study to record cortical activations from newborns in temporal and frontal areas to speech and speech-like sounds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper S, Krueger A, Koch S, Mehnert J, Habermehl C, et al. A wearable multi-channel fNIRS system for brain imaging in freely moving subjects. NeuroImage. 2014;85:64–71. doi: 10.1016/j.neuroimage.2013.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta M, Heinzel S, Ehlis A, Pauli P, Fallgatter A. Model-based analysis of rapid event-related functional near-infrared spectroscopy (NIRS) data: a parametric validation study. NeuroImage. 2007;35:625–34. doi: 10.1016/j.neuroimage.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Poeppel D. The analysis of speech in different temporal integration windows: cerebral lateralization as ‘asymmetric sampling in time.’. Speech Commun. 2003;41:245–55. [Google Scholar]
- Redcay E, Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biol Psychiatry. 2008;64:589–98. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Haist F, Courchesne E. Functional neuroimaging of speech perception during a pivotal period in language acquisition. Dev Sci. 2008;11:237–52. doi: 10.1111/j.1467-7687.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- Redcay E, Kennedy DP, Courchesne E. fMRI during natural sleep as a method to study brain function during early childhood. NeuroImage. 2007;38:696–707. doi: 10.1016/j.neuroimage.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Cortical source localization of infant cognition. Dev Neuropsychol. 2009;34:312–29. doi: 10.1080/87565640902801890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche-Labarbe N, Fenoglio A, Aggarwal A, Dehaes M, Carp SA, et al. Near-infrared spectroscopy assessment of cerebral oxygen metabolism in the developing premature brain. J Cereb Blood Flow Metab. 2012;32:481–88. doi: 10.1038/jcbfm.2011.145. A recent study that provides evidence of substantial developmental change in neurovascular coupling of the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Sheehy S, Oakes L, Luck S. The development of visual short-term memory capacity in infants. Child Dev. 2004;74:1807–22. doi: 10.1046/j.1467-8624.2003.00639.x. [DOI] [PubMed] [Google Scholar]
- Saager R, Berger A. Direct characterization and removal of interfering absorption trends in two-layer turbid media. J Opt Soc Am A Opt Image Sci Vis. 2005;22:1874–82. doi: 10.1364/josaa.22.001874. [DOI] [PubMed] [Google Scholar]
- Saager R, Berger A. Measurement of layer-like hemodynamic trends in scalp and cortex: implications for physiological baseline suppression in functional near-infrared spectroscopy. J Biomed Opt. 2008;13:034017. doi: 10.1117/1.2940587. [DOI] [PubMed] [Google Scholar]
- Saager R, Telleri N, Berger A. Two-detector corrected near infrared spectroscopy (c-NIRS) detects hemodynamic activation responses more robustly than single-detector NIRS. NeuroImage. 2011;55:1679–85. doi: 10.1016/j.neuroimage.2011.01.043. Evaluation of a scheme for using a short-distance fNIRS channel to obtain a direct measure of surface vascular signals that can be regressed out of the longer-distance fNIRS signals obtained from the cortex. [DOI] [PubMed] [Google Scholar]
- Safaie J, Grebe R, Moghaddam H, Wallois F. Toward a fully integrated wireless wearable EEG-NIRS bimodal acquisition system. J Neural Eng. 2013;10:056001. doi: 10.1088/1741-2560/10/5/056001. [DOI] [PubMed] [Google Scholar]
- Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month-old infants. Science. 1996;274:1926–28. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Chen S, Lichty W, Zuo H, Wang YP. Cerebral blood oxygenation changes induced by auditory stimulation in newborn infants measured by near infrared spectroscopy. Early Hum Dev. 1999;55:229–36. doi: 10.1016/s0378-3782(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Richards J, Almli R. Neurodevelopmental MRI brain templates for children from 2 weeks to 4 years of age. Dev Psychobiol. 2012;37:379–399. doi: 10.1002/dev.20579. The first comprehensive brain atlas obtained at 3T from structural MRI data across infancy and early childhood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai S, Homae F, Watanabe H, Sasaki A, Tanabe H, et al. A NIRS-fMRI study of resting state network. NeuroImage. 2012;63:179–93. doi: 10.1016/j.neuroimage.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Sasai S, Homae F, Watanabe H, Taga G. Frequency-specific functional connectivity in the brain during resting state revealed by NIRS. NeuroImage. 2011;56:252–57. doi: 10.1016/j.neuroimage.2010.12.075. [DOI] [PubMed] [Google Scholar]
- Scholkmann F, Kleiser S, Jaakko Metz A, Zimmermann R, Mata Pavia J, et al. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. NeuroImage. 2014;85:6–27. doi: 10.1016/j.neuroimage.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, Huppi PS. Functional MRI of the newborn. Semin Fetal Neonatal Med. 2006;11:479–88. doi: 10.1016/j.siny.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Chaimow D, Logothetis NK, Ugurbil K. Spatio-temporal point-spread function of fMRI signal in human gray matter at 7 Tesla. NeuroImage. 2007;35:539–52. doi: 10.1016/j.neuroimage.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram R, Caria A, Birbaumer N. Hemodynamic brain–computer interfaces for communication and rehabilitation. Neural Netw. 2009;22:1320–28. doi: 10.1016/j.neunet.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–62. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Shimony JS, Blazey TM, Inder TE, Neil JJ. Effects of white matter injury on resting state fMRI measures in prematurely born infants. PLoS ONE. 2013;8:e68098. doi: 10.1371/journal.pone.0068098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V, Begus K, Lloyd-Fox S, Gangi V, Hamilton A. Goal representation in the infant brain. NeuroImage. 2014;85:294–301. doi: 10.1016/j.neuroimage.2013.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Nunez PL. Electroencephalography. In: Ramachandran VS, editor. Encyclopedia of Human Behavior. 2nd Burlington, MA: Acad. Press; 2012. pp. 15–23. [Google Scholar]
- Stiles J. The Fundamentals of Brain Development: Integrating Nature and Nurture. Cambridge, MA: Harvard Univ. Press; 2008. [Google Scholar]
- Strangman GE, Li Z, Zhang Q. Depth sensitivity and source-detector separations for near infrared spectroscopy based on the colin27 brain template. PLoS ONE. 2013;8:e66319. doi: 10.1371/journal.pone.0066319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Trittschuh E, Monti J, Mesulam M, Egners T. Neural repetition suppression reflects fulfilled perceptual expectations. Nat Neurosci. 2008;11:1004–6. doi: 10.1038/nn.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga G, Asakawa K, Hirasawa K, Konishi Y. Hemodynamic responses to visual stimulation in occipital and frontal cortex of newborn infants: a near-infrared optical topography study. Early Hum Dev. 2003;75(Suppl):S203–10. doi: 10.1016/j.earlhumdev.2003.08.023. The first comprehensive fNIRS study of awake infants as they viewed a variety of visual stimuli. [DOI] [PubMed] [Google Scholar]
- Taga G, Homae F, Watanabe H. Effects of source-detector distance of near infrared spectroscopy on the measurement of the cortical hemodynamic response in infants. NeuroImage. 2007;38:452–60. doi: 10.1016/j.neuroimage.2007.07.050. [DOI] [PubMed] [Google Scholar]
- Taga G, Watanabe H, Homae F. Spatiotemporal properties of cortical haemodynamic response to auditory stimuli in sleeping infants revealed by multi-channel near-infrared spectroscopy. Philos Trans A Math Phys Eng Sci. 2011;369:4495–511. doi: 10.1098/rsta.2011.0238. [DOI] [PubMed] [Google Scholar]
- Tak S, Ye J. Statistical analysis of fNIRS data: a comprehensive review. NeuroImage. 2014;85:72–91. doi: 10.1016/j.neuroimage.2013.06.016. An up-to-date review of software packages used to analyze fNIRS data. [DOI] [PubMed] [Google Scholar]
- Telkemeyer S, Rossi S, Koch S, Nierhaus T, Steinbrink J, et al. Sensitivity of newborn auditory cortex to the temporal structure of sounds. J Neurosci. 2009;29:14726–33. doi: 10.1523/JNEUROSCI.1246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telkemeyer S, Rossi S, Nierhaus T, Steinbrink J, Obrig H, Wartenburger I. Acoustic processing of temporally modulated sounds in infants: evidence from a combined near-infrared spectroscopy and EEG study. Front Psychol. 2011;1:62. doi: 10.3389/fpsyg.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Dassanayake MT, Shen S, Katkuri Y, Alexis M, et al. Cross-hemispheric functional connectivity in the human fetal brain. Sci Transl Med. 2013;5:173ra24. doi: 10.1126/scitranslmed.3004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong F, Pratte M. Decoding patterns of human brain activity. Annu Rev Psychol. 2012;63:483–509. doi: 10.1146/annurev-psych-120710-100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli A, Contini D, Pifferi A, Caffini M, Re R, et al. Time domain functional NIRS imaging for human brain mapping. NeuroImage. 2014;85:28–50. doi: 10.1016/j.neuroimage.2013.05.106. [DOI] [PubMed] [Google Scholar]
- Tsuzuki D, Dan I. Spatial registration for functional near-infrared spectroscopy: from channel position on the scalp to cortical location in individual and group analyses. NeuroImage. 2014;85:92–103. doi: 10.1016/j.neuroimage.2013.07.025. A comprehensive evaluation of the issues involved in spatial co-registration of fNIRS channels to the underlying brain anatomy. [DOI] [PubMed] [Google Scholar]
- Turk-Browne N. Functional interactions as big data in the human brain. Science. 2013;342:580–84. doi: 10.1126/science.1238409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk-Browne N, Scholl B, Chun M. Babies and brains: habituation in infant cognition and functional neuroimaging. Front Hum Neurosci. 2008;2:1–11. doi: 10.3389/neuro.09.016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JB, Fox SE, Tager-Flusberg H, Nelson CA. Neural processing of repetition and non-repetition grammars in 7- and 9-month-old infants. Front Psychol. 2011;2:168. doi: 10.3389/fpsyg.2011.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell B, Dumoulin S, Brewer A. Visual field maps in visual cortex. Neuron. 2007;55:366–83. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Wang G, Tanaka K, Tanifuji M. Optical imaging of functional organization in the monkey inferotemporal cortex. Science. 1996;262:1665–68. doi: 10.1126/science.272.5268.1665. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Homae F, Nakano T, Taga G. Functional activation in diverse regions of the developing brain of human infants. NeuroImage. 2008;43:346–57. doi: 10.1016/j.neuroimage.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Homae F, Nakano T, Tsuzuki D, Enkhtur L, et al. Effect of auditory input on activations in infant diverse cortical regions during audiovisual processing. Hum Brain Mapp. 2013;34:543–65. doi: 10.1002/hbm.21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Homae F, Taga G. General to specific development of functional activation in the cerebral cortexes of 2- to 3-month-old infants. NeuroImage. 2010;50:1536–44. doi: 10.1016/j.neuroimage.2010.01.068. [DOI] [PubMed] [Google Scholar]
- Watson JD. Images of the working brain: understanding human brain function with positron emission tomography. J Neurosci Methods. 1997;74:245–56. doi: 10.1016/s0165-0270(96)02253-4. [DOI] [PubMed] [Google Scholar]
- White BR, Culver JP. Phase-encoded retinotopy as an evaluation of diffuse optical neuroimaging. NeuroImage. 2010;49:568–77. doi: 10.1016/j.neuroimage.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T. Object individuation: infants' use of shape, size, pattern, and color. Cognition. 1999;72:125–66. doi: 10.1016/s0010-0277(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Wilcox T, Bortfeld H, Woods R, Wruck E, Armstrong J, Boas D. Hemodynamic changes in the infant cortex during the processing of featural and spatiotemporal information. Neuropsychologia. 2009;47:657–62. doi: 10.1016/j.neuropsychologia.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Bortfeld H, Woods R, Wruck E, Boas DA. Hemodynamic response to featural changes in the occipital and inferior temporal cortex in infants: a preliminary methodological exploration. Dev Sci. 2008;11:361–70. doi: 10.1111/j.1467-7687.2008.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Chapa C. Priming infants to attend to color and pattern information in an individuation task. Cognition. 2004;90:265–302. doi: 10.1016/s0010-0277(03)00147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Haslup JA, Boas DA. Dissociation of processing of featural and spatiotemporal information in the infant cortex. NeuroImage. 2010;53:1256–63. doi: 10.1016/j.neuroimage.2010.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Hirshkowitz A, Hawkins L, Boas DA. The effect of color priming on infant brain and behavior. NeuroImage. 2014;85(Pt. 1):302–13. doi: 10.1016/j.neuroimage.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Woods R, Chapa C. Color-function categories that prime infants to use color information in an object individuation task. Cogn Psychol. 2008;57:220–61. doi: 10.1016/j.cogpsych.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn K. Addition and subtraction by human infants. Nature. 1992;358:749–50. doi: 10.1038/358749a0. [DOI] [PubMed] [Google Scholar]
- Ye J, Tak S, Jang K, Jung J, Jang J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. NeuroImage. 2010;44:428–47. doi: 10.1016/j.neuroimage.2008.08.036. [DOI] [PubMed] [Google Scholar]
- Zaramella P, Freato F, Amigoni A, Salvadori S, Marangoni P, et al. Brain auditory activation measured by near-infrared spectroscopy (NIRS) in neonates. Pediatr Res. 2001;49:213–19. doi: 10.1203/00006450-200102000-00014. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brooks D, Franceschini M, Boas D. Eigenvector-based spatial filtering for reduction of physiological interference in diffuse optical imaging. J Biomed Opt. 2005;10:11014. doi: 10.1117/1.1852552. [DOI] [PubMed] [Google Scholar]