Abstract
Rationale
The neuropeptide pituitary adenylyl cyclase-activating peptide (PACAP) and its receptors (PAC1 and VPAC2) are expressed in the ventral tegmental area and nucleus accumbens, raising the possibility that PACAP could be a potential modulator of the mesolimbic dopaminergic system.
Objective
The present study was designed to determine if PACAP plays a role in acute motor stimulatory and rewarding actions of morphine.
Methods
The effect of intracerebroventricular PACAP administration (0, 0.03, 0.3, 1.0, or 3.0 μg/3 μL) was studied on basal motor activity as well as on morphine (5 mg/kg)-stimulated motor activity. Motor stimulation and conditioned place preference (CPP) induced by morphine (5 or 10 mg/kg) were also determined in mice lacking PACAP and their wild-type controls.
Results
Intracerebroventricular PACAP dose-dependently suppressed basal motor activity and PACAP-deficient mice exhibited higher basal motor activity than control mice, providing evidence that the action of endogenous PACAP on basal motor activity is inhibitory. Paradoxically, low doses of PACAP which did not alter basal motor activity were found to enhance the motor stimulatory action of morphine. Furthermore, morphine-induced motor stimulation was blunted in PACAP-deficient mice. Additionally, morphine-induced CPP following a single, but not repeated, alternate-day saline/morphine (10 mg/kg) conditioning was blunted in PACAP-deficient mice compared to their wild-type littermates/controls.
Conclusion
The present results suggest that endogenous PACAP, at low doses, positively modulates the acute motor stimulatory and rewarding actions of morphine.
Keywords: Pituitary adenylyl cyclase-activating peptide (PACAP), Morphine, Motor activity, Conditioned place preference, Reward, Intracerebroventricular administration, PACAP-deficient mice
Introduction
Pituitary adenylyl cyclase-activating peptide (PACAP) was isolated in 1989 as a hypothalamic factor that strongly caused accumulation of cyclic-5′-adenosine-monophosphate (cAMP) in the pituitary, but was later found to exhibit widespread expression in the central and peripheral nervous systems (reviewed by Vaudry et al. 2000). PACAP, a 38 amino acid peptide, is a member of the secretin family with closest homology to vasoactive intestinal peptide (VIP). Three different high affinity PACAP receptors have been characterized in terms of sequence, peptide affinity profiles, and tissue expression. Two of the receptors, VPAC1 and VPAC2, recognize both VIP and PACAP with equal high affinity. The third, PAC1, is a PACAP-preferring receptor (reviewed in Harmar et al. 2004). Each receptor belongs to the seven-transmembrane regulatory protein-coupled receptor family and is coupled primarily via Gs to adenylyl cyclase and stimulates cAMP production (reviewed in Vaudry et al. 2000).
PACAP concentrations in different parts of the brain have been determined by radioimmunoassay. The ventral tegmental area (VTA) and nucleus accumbens (N Ac) are among the brain regions that express highest levels of PACAP (Ghatei et al. 1993; Palkovits et al. 1995). High affinity [125I]-PACAP binding has been demonstrated in the VTA and N Ac by receptor autoradiography (Masuo et al. 1992). In situ hybridization revealed that these binding sites in the N Ac correspond to VPAC2 receptors, whereas both VPAC2 and PAC1 receptors are present in the VTA (Hashimoto et al. 1996; Sheward et al. 1996). These data suggest that PACAP might act in the VTA and/or N Ac to alter the function of the mesolimbic dopaminergic system.
The PACAP system and its downstream signaling molecules have previously been associated with the actions of alcohol. The Drosophila mutant cheapdate, shows enhanced sensitivity to ethanol, and harbors a mutation that maps to a PACAP-like neuropeptide gene (Moore et al. 1998). Furthermore, mice with a targeted mutation in the regulatory subunit of cAMP-dependent protein kinase A show high ethanol consumption and low sensitivity to ethanol-induced sedation (Thiele et al. 2000). Despite the anatomical data in rodents and genetic data in flies, there are limited reports in the literature that address possible actions of PACAP on the mesolimbic dopaminergic system. Importantly, the role of endogenous PACAP in the modulatory action of opioids on this circuitry has not been determined. Thus, using pharmacological tools and mice with a targeted deletion in the PACAP gene, the present study was designed to determine the role of endogenous PACAP in morphine-induced motor stimulation and conditioned place preference (CPP).
Materials and methods
Subjects
Pharmacological experiments were performed using male and female C57BL/6 mice (3 to 4 months old) obtained from a colony that has been maintained at the University of California, Los Angeles (UCLA) for the last several years. We recently described the generation and partial characterization of mice with a targeted deletion in the PACAP gene (Colwell et al. 2004). We showed by radioimmunoassay that PACAP was undetectable in the brain of these mice, and that no overt abnormalities in brain morphology could be detected. These mice, initially generated on a mixed (C57BL/6×129) background, were crossed on C57BL/6 background for at least six generations. The C57BL/6 and 129 mice were originally obtained from the Jackson Laboratory (Bar Harbor, MA, USA) and/or Charles River Laboratories (Wilmington, MA, USA). In experiments where responses in PACAP-deficient and control mice were compared, the latter were either wild-type littermates or age-matched wild-type mice of the same genetic background generated in the same breeding colony. Mice were kept two to four mice per cage under a 12-h light/12-h dark cycle with free access to laboratory food and water. The light was on from 7:00 A.M. to 7:00 P.M. All experiments were conducted during the light cycle and approved by the Institutional Animal Care and Use Committee at UCLA (Los Angeles, CA, USA) or Western University of Health Sciences (Pomona, CA, USA).
Drugs
Morphine sulfate was obtained from the NIDA drug research supply (Research Triangle Institute, Triangle Park, NC, USA) and administered subcutaneously (s.c.). PACAP was purchased from Calbiochem (San Diego, CA, USA) and administered intracerebroventricularly (ICV). The choice of the doses of morphine to induce motor stimulation and CPP was based on our previous findings (Marquez et al. 2006, 2008).
Measurement of motor activity
Distance traveled (cm) was used as a measure of motor activity and recorded using a Videomex-V system (Columbus Instruments, OH, USA) as described previously (Lutfy et al. 2001). All experiments were conducted during the light cycle between 11:00 A.M. and 5:00 P.M. Motor activity testing chambers (14 cm length × 14 cm width × 22 cm height) were painted black in order to prevent noise due to light reflection.
The effect of exogenous PACAP on basal motor activity and the motor stimulatory action of morphine
Mice were habituated to the testing chambers for 1 h, then lightly anesthetized with halothane and injected ICV with artificial cerebroventricular fluid (aCSF) or PACAP (0, 0.03, 0.3, 1.0, and 3.0 μg in 3 μL aCSF). The composition of aCSF in mM was NaCl (125); KCl (2.5); NaH2PO4 (0.9); Na2HPO4 (5); MgCl2 (1); d-glucose (2.5); CaCl2 (1.2); bovine serum albumin (0.025%). Mice were then immediately injected with saline or morphine (5 mg/kg, s.c.), and motor activity was recorded at 15-min intervals for 1 h. A lower dose of morphine was used in this experiment to allow us to observe both increases and decreases in morphine-stimulated motor activity by PACAP. In a separate experiment, we also determined the effect of PACAP on basal and morphine-stimulated motor activity in mice implanted with an indwelling guide cannula. Briefly, mice were anesthetized with isoflurane (5% for the induction and 1–2% for the maintenance of anesthesia) and implanted with a guide cannula in the right lateral ventricle and then tested for motor activity a week later. On the test day, mice were habituated to the motor activity chambers for 1 h, then injected with aCSF or PACAP (1.0 μg/ 3.0 μL) through the guide cannula, and motor activity was recorded for 1 h. Mice were then treated with morphine (5 mg/kg, s.c.), and their motor activity was recorded for another hour.
The role of endogenous PCAPC in basal motor activity and morphine-induced motor stimulation
Mice lacking PACAP and their wild-type littermates/controls were habituated to the activity chambers for 1 h. Mice were then injected with morphine (5 or 10 mg/kg, s.c.), and motor activity was recorded at 15-min bins for 1 h.
The role of endogenous PACAP in morphine-induced CPP
The CPP paradigm was according to our previously published data (Marquez et al. 2006, 2007, 2008). We first determined morphine-induced CPP after a single alternate-day saline/morphine or morphine/saline conditioning. Mice were initially tested for preconditioning place preference, in which each mouse was placed in the neutral chamber of the CPP apparatus and allowed to freely explore all three CPP chambers for 15 min. The amount of time that each mouse spent in each conditioning chamber was recorded and used for analysis of the preconditioning data. On day 2 (first conditioning day), mice were randomly assigned to receive saline or morphine (5 or 10 mg/kg, s.c.) and confined to the vehicle-paired or drug-paired chamber for 1 h, respectively. The next day (second conditioning day), mice were injected with the alternate treatment and confined to the opposite conditioning chamber for 1 h. The pairing of the treatments with the conditioning chambers was balanced. Moreover, half of the group of mice received one of the treatments in the almond-scented chamber and the other half of the group received the treatments in the citrus-scented chamber. Also, half of the group of mice received morphine on the first conditioning day and the other half on the second conditioning day. Furthermore, two mutant mice and two wild-type littermates/controls mice were run side-by-side. On day 4 (postconditioning session), mice were placed in the neutral chamber and allowed to freely explore all the CPP chambers for 15 min. The amount of time that the mice spent in each chamber was recorded and used for analysis of postconditioning data. Following the testing on day 4, mice received their respective treatment (saline or morphine) and confined to the designated conditioning chamber for 1 h. The alternate-day saline/morphine or morphine/saline conditioning session continued on days 5– 9. Mice were then tested for postconditioning place preference on day 10.
Verification of the site of injection
All ICV injections were conducted according to our previously described method (Yoburn et al. 1990). The injections were made unilaterally into the right lateral ventricle (free handed) over 20 s. The needle was left in place for an additional 10 s. The coordinates (AP=0.0 mm; ML=+2.0 mm, and DV= −2.0 mm) were according to the atlas of Franklin and Paxinos (1997) with bregma as the point of reference. At the end of each experiment, 3 μL of the dye was injected ICV to verify the site of PACAP injection.
Data analysis
The motor activity data were analyzed using a two-way analysis of variance (ANOVA) followed by the post hoc Newman–Keuls test to reveal significant differences between various groups. The CPP data were expressed as the CPP score (difference in the amount of time that the mice spent in the morphine-paired versus saline-paired chamber on postconditioning day 4 or day 10) minus the difference in the amount of time that the mice spent in the two chambers on preconditioning (day 1) and analyzed using an unpaired Student's t test. A p<0.05 was considered significant.
Results
Intracerebroventricular administration of low doses of PACAP-enhanced morphine-induced motor stimulation in C57BL/6 mice
The effect of exogenous PACAP on basal and morphine-stimulated motor activity is shown in Fig. 1. A two-way ANOVA of the total distance traveled during the 1-h test period revealed a significant effect of treatment (saline versus morphine; F1,61=54.21; p<0.001), a significant effect of PACAP dose (F4,61=27.05; p<0.001), and a significant interaction between treatment and PACAP dose (F4,61 =12.06; p<0.001). Further analysis of the data showed that ICV administration of PACAP (0, 0.03, 0.3, 1, or 3 μg) dose-dependently suppressed locomotor activity (Fig. 1a, compare mice treated with aCSF versus those that received higher doses of PACAP followed by saline). Indeed, PACAP at the highest dose (3 μg) completely blocked basal motor activity (Fig. 1a). Doses of PACAP which reduced basal motor activity (1 and 3 μg) also suppressed the motor stimulatory action of morphine. In contrast, lower doses of PACAP (0.03 and 0.3 μg) which did not alter basal motor activity enhanced the action of morphine (p<0.05; compare mice treated with these doses of PACAP to their aCSF-treated control group). The stimulatory action of PACAP occurred after the first 15-min interval, i.e., morphine-induced motor stimulation was enhanced during the last three 15-min bins. On the other hand, the locomotor suppressant action of high-dose PACAP occurred immediately after PACAP administration and lasted for the entire testing period (Fig. 1b). As these experiments were conducted using free-hand ICV drug administration, we also determined the effect of PACAP on basal and morphine-stimulated motor activity in mice implanted with permanently indwelling guide cannulae. Our results showed that mice treated with ICV PACAP (1.0 μg/3.0 μL) had reduced basal and morphine-stimulated motor activity compared to their vehicle-treated controls (Table 1), confirming our results of free-hand ICV PACAP administration.
Fig 1.
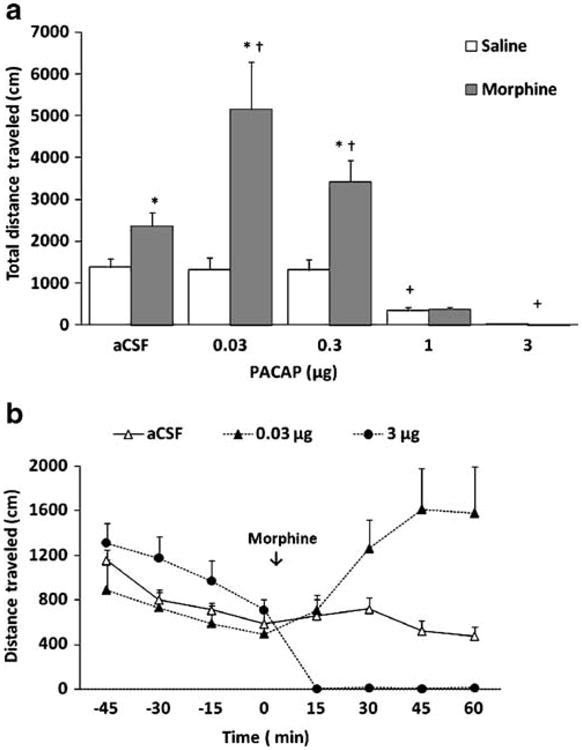
Effects of intracerebroventricular PACAP administration on basal (white bars) and morphine-stimulated (black bars) motor activity in C57BL/6 mice. Data are mean (±SEM) of the total distance traveled during the entire 1-h test period (a) or the distance traveled at 15-min bins of mice (n=6–12 mice/dose for each treatment) treated with aCSF, low or high PACAP dose (b). Asterisk indicates a significant increase compared to their respective saline-treated control group (open bars; p<0.05); Dagger indicates a significant increase compared to aCSF-morphine group (p<0.05). Plus sign indicates a significant decrease compared to their respective aCSF-treated control group (p<0.05)
Table 1. Total distance traveled during 1-h test period in mice treated with PACAP (1.0 μg/3.0 μL; ICV) or vehicle before and following morphine (5 mg/kg) administration.
| Treatment/animal ID | Vehicle | PACAP |
|---|---|---|
| Before morphine | ||
| 1 | 710 | 114 |
| 2 | 460 | 150 |
| 3 | 642 | 0 |
| 4 | 488 | 201 |
| 5 | 336 | 183 |
| 6 | 513 | 84 |
| Mean (±SEM) | 532±58 | 122±30* |
| After morphine | ||
| 1 | 3,742 | 2,218 |
| 2 | 4,520 | 3,023 |
| 3 | 7,010 | 1.5 |
| 4 | 5,549 | 1,982 |
| 5 | 2,648 | 2,331 |
| 6 | 3,585 | 6.2 |
| Mean (±SEM) | 5,369± 1,067 | 1,577±518* |
p<0.05, unpaired Student's t test
Data represent mean (±SEM) of six mice per treatment
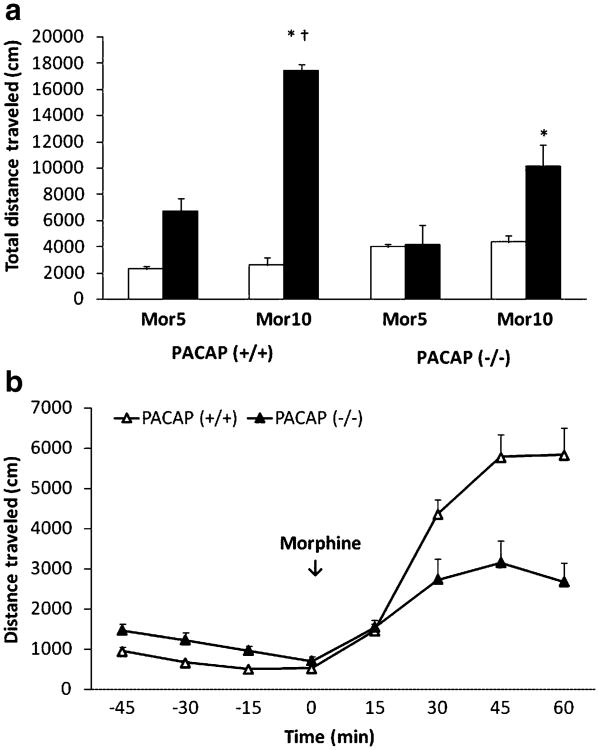
PACAP-deficient mice exhibited reduced morphine-induced motor stimulation
The motor stimulatory action of morphine in mice lacking PACAP and their wild-type litter-mates is shown in Fig. 2. Basal motor activity was approximately 50% greater in PACAP-deficient mice compared to the wild-type littermates during the habituation period (Fig. 2a; open bars). The increased activity in mutant mice was not due to the lack of habituation in these mice because they habituated to the same level as their wild-type littermates by the end of the 1-h habituation period (Fig. 2b). Despite the fact that basal motor activity was greater in PACAP-deficient mice, the response to morphine was markedly blunted in mutant mice (Fig. 2a; closed bars). A two-way ANOVA revealed a significant effect of genotype (F1,17=11.78; p<0.01), a significant effect of morphine dose (F1,17=37.82; p<0.001), but no significant interaction between genotype and dose (F1,17=2.63; p>0.05). Further analysis of the data showed that the higher dose of morphine (10 mg/kg) increased motor activity to a significantly greater extent compared to its lower dose (5 mg/kg) in both mutant mice and their wild-type littermates (p<0.05). Additionally, the effect of morphine was significantly higher in the wild-type mice compared to PACAP-deficient mice (p<0.05). The blunted response to morphine (10 mg/kg) in mutant mice was evident during the last three 15-min bins (Fig. 2b).
Fig 2.
Motor stimulation induced by morphine in PACAP-deficient mice and their wild-type littermates/controls. Data are mean (±SEM) of the total distance traveled before (open bars) or following morphine treatment (a) in PACAP-deficient mice and their wild-type littermates/ controls (n=5–6 mice/dose per genotype). Distance traveled at 15-min bins before and following high dose morphine is shown in (b). Asterisk indicates a significant increase compared to their respective low-dose morphine (5 mg/kg)-treated group (p<0.05); Dagger indicates a significant increase compared to PACAP-deficient mice (p<0.05). Mor5 and Mor10 stand for 5 and 10 mg/kg dose of morphine, respectively
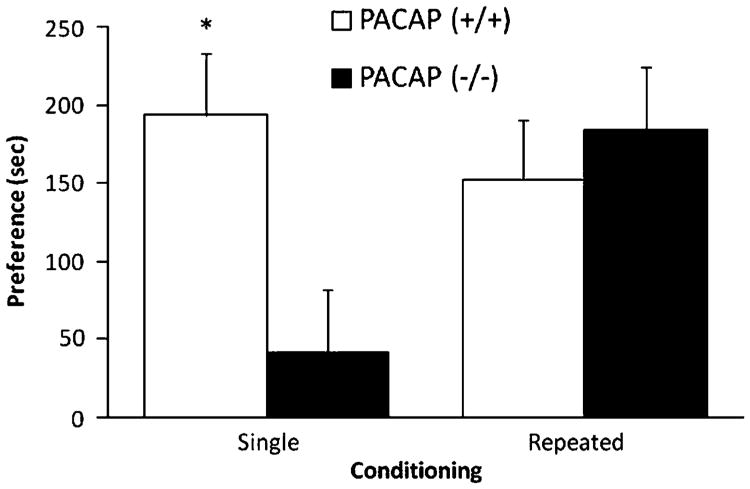
Mice lacking PACAP displayed blunted CPP following single conditioning with morphine
Morphine (5 mg/kg) did not induce CPP in PACAP-deficient mice and their wild-type littermates (data not shown). On the other hand, a higher dose of morphine (10 mg/kg) induced a significant CPP (Fig. 3). There was no initial basal (preconditioning) preference toward the drug-paired over vehicle-paired chamber for either genotype (Table 2). However, a single morphine conditioning shifted the preference toward the drug-paired chamber in wild-type, but not in mutant, mice (Table 2). Analysis of the CPP scores revealed a significant difference between the two groups (t(d.f.=12)=7.19; p<0.02), suggesting that CPP induced by single conditioning with morphine was attenuated in PACAP-deficient mice. The CPP score was mainly due to a greater reduction in the amount of time that the mutant mice spent in the saline-paired compared to morphine-paired chamber (Table 2). On the other hand, the CPP score was due to decreased preference toward the vehicle-paired chamber and increased preference toward the drug-paired chamber in wild-type mice (Table 2, compare the amount of time that the mutant and wild-type mice spent in each conditioning chamber following single conditioning with morphine). We then determined whether CPP following repeated conditioning with morphine would be altered in PACAP-deficient mice. Analysis of the CPP score following repeated conditioning did not reveal any significant difference between the two groups (t(d.f.=12)=0.33; p>0.05), showing that PACAP-deficient mice displayed comparable CPP to that of their wild-type controls (Fig. 3).
Fig 3.
CPP induced by single or repeated conditioning with morphine in PACAP-deficient mice and their wild-type littermates/controls. Data represent mean (±SEM) of preference score in mutant mice and their wild-type littermates/ controls (n=7 mice per genotype) following single and repeated conditioning with morphine (10 mg/kg). *p<0.05 compared to mutant mice following single conditioning
Table 2.
The amount of time that mutant mice (PACAP−/−) and their wild-type littermates/controls (PACAP+/+) spent in the morphine-paired chamber (MOR-paired) and saline-paired chamber (SAL-paired) prior to (preconditioning) and following single or repeated conditioning with morphine
| Genotype/test day | SAL-paired | MOR-paired |
|---|---|---|
| PACAP (+/+) | ||
| Preconditioning | 302±22 | 311 ±32 |
| Single conditioning | 193 ±16 | 396±32 |
| Repeated conditioning | 202±13 | 366±25 |
| PACAP (−/−) | ||
| Preconditioning | 301 ± 16 | 302±23 |
| Single conditioning | 246±24 | 289±27 |
| Repeated conditioning | 187 ±15 | 373 ±24 |
Data represent mean (±SEM) of seven mice per genotype
Discussion
The main findings reported here are that low doses of PACAP enhanced morphine-induced motor activity in C57BL/6 mice, and mice with a targeted deletion in the gene encoding for PACAP exhibited decreased morphine-induced CPP and motor stimulation. Together, the results of the present study provide both pharmacological and genetic evidence that endogenous PACAP functions to enhance the motor stimulation and CPP induced by acute morphine in mice.
Previous studies have shown several types of activity in an open field to be increased in mice with a different-targeted PACAP gene mutation (Hashimoto et al. 2001). Consistent with these, we observed that PACAP-deficient mice expressed enhanced basal motor activity. Furthermore, we discovered that high doses of PACAP suppressed basal motor activity. Because mice with a targeted deletion in the PAC1 receptor gene were shown to exhibit elevated levels of basal motor activity (Otto et al. 2001), it seems likely that the PAC1 receptor mediates this generalized inhibitory effect of PACAP on basal motor activity. However, it is not known whether PACAP-induced motor suppression is due to an inhibitory action of PACAP on the mesolimbic dopaminergic system or due to nonspecific effects, such as actions on anxiety-like behaviors or arousal.
The ability of PACAP to modulate the actions of morphine, however, is novel and clearly distinct from the actions of PACAP on basal motor activity. Low doses of PACAP, which had no effect on basal motor activity, enhanced morphine-induced motor stimulation. Furthermore, the motor stimulatory action of morphine was attenuated in mice lacking PACAP. Interestingly, the blunted morphine-induced motor stimulation in mutant mice was evident during the second 15-min bin and lasted until the end of the observation period. Likewise, the enhanced motor stimulatory action of morphine by lowest dose of exogenous PACAP displayed a similar time-course, raising the possibility that morphine may cause the release of endogenous PACAP, and this response may play a functional role in the acute motor stimulatory action of morphine. However, PACAP itself has no stimulatory effects on locomotor activity (Fig. 1a); thus, PACAP may act as a modulator of the mesolimbic dopamine system to enhance the action of morphine (see below, for details).
The CPP paradigm is used to assess the rewarding/ incentive value of a drug (Bardo and Bevins 2000). Thus, using this paradigm, we determined the role of the endogenous PACAP in the rewarding action/incentive value of morphine and discovered that a single conditioning session with morphine (10 but not 5 mg/kg) induced a robust CPP in wild-type mice. However, no CPP was observed in mutant mice under an identical conditioning paradigm, raising the possibility that endogenous PACAP may be released in response to acute morphine which contributes to the incentive value of acute morphine. This lack of CPP following single conditioning with morphine in PACAP-deficient mice was not due to inability of the mutant mice to express CPP because PACAP-deficient mice were found to express a comparable CPP to that of their wild-type littermates following repeated morphine conditioning. Morphine has been shown to increase accumbal dopamine (Di Chiara and Imperato 1988), a response that may play a functional role in morphine-induced motor stimulation and CPP. It is thought that morphine increases dopaminergic neurotransmission via inhibition of GABA-ergic interneurons in the VTA (Fibiger and Phillips 1988; Johnson and North 1992; Koob and Swerdlow 1988; Wise 1989). Together, the current results implicate endogenous PACAP in the incentive value of acute morphine and probably as a modulator of the mesolimbic dopaminergic neurons.
Although the mechanism of this modulatory action of PACAP on morphine-induced motor stimulation and CPP is not known, PACAP has been shown to stimulate tyrosine hydroxylase activity in homogenates of rat N Ac (Moser et al. 1999). Thus, one potential mechanism for this modulatory action of PACAP would be to increase dopamine synthesis, thereby leading to increased levels of accumbal dopamine in response to morphine administration and a resultant increase in motor activity and CPP in wild-type mice compared to PACAP-deficient mice. However, how high doses of PACAP caused motor suppression and how PACAP-deficient mice exhibited enhanced basal motor activity cannot be explained by this mechanism. Thus, other mechanisms may be involved. PACAP-binding sites in the N Ac correspond to VPAC2 receptors, whereas both VPAC2 and PAC1 receptors are present in the VTA (Hashimoto et al. 1996; Sheward et al. 1996), raising the possibility that PACAP could have acted via different classes of PACAP receptors to elicit these distinct pharmacological effects. Furthermore, PACAP have been shown to alter the activity of the hypothalamic–pituitary–adrenal (HPA) axis (Nussdorfer and Malendowicz 1998). As the HPA axis has been implicated in the actions of morphine (Marinelli et al. 1998), another possibility is that endogenous PACAP modulate the motor stimulatory and rewarding effects of morphine by altering the function of the HPA axis.
PACAP and its receptors are expressed during development (Sheward et al. 1998; Waschek et al. 1998). Thus, the behavioral differences observed in PACAP-deficient mice could be due to morphological or other compensatory changes. Although we have not observed any obvious morphological abnormalities in the adult brain of PACAP-deficient mice (Colwell et al. 2004), it remains possible that such developmental effects could explain some of the behavioral differences observed in mutant mice. However, monoaminergic neuronal development is not affected in PACAP-deficient mice (Ogawa et al. 2005). Importantly, the fact that the facilitatory action of PACAP on morphine-induced motor stimulation was mimicked by low doses of exogenous PACAP suggests that the observed behavioral deficits in mutant mice were due to the absence of the peptide in the adult brain reward and stress circuits rather than a defect in the circuitry per se.
In summary, we observed that the motor stimulatory and rewarding action of acute morphine was blunted in mice lacking PACAP, implicating that this neuropeptide may function as a novel facilitator of morphine reinforcement.
Acknowledgments
The authors wish to thank Mr. Rishi Talwar for technical assistance. This work was supported in part by the State of California funds for medical research on alcohol and substance abuse through the University of California, San Francisco and in part by National Institutes of Health grants HD06576, HD34475, and HD0461 and (J.A.W.), DA05010 (C.J.E.), DA016682, and an intramural grant at Western University of Health Sciences (K.L.)
Contributor Information
Paul Marquez, Department of Pharmaceutical Sciences, Western University of Health Sciences, 309 East 2nd Street, Pomona, CA 91766, USA.
David Bebawy, Department of Pharmaceutical Sciences, Western University of Health Sciences, 309 East 2nd Street, Pomona, CA 91766, USA.
Vincent Lelièvre, Department of Psychiatry and Biobehavioral Sciences, Neuropsychiatric Institute, David Geffen School of Medicine, University of California, Los Angeles, 760 Westwood Plaza, Los Angeles, CA 90024, USA; Mental Retardation Research Center, University of California, Los Angeles, 760 Westwood Plaza, Los Angeles, CA 90024, USA.
Anne-Claire Coûté, Department of Psychiatry and Biobehavioral Sciences, Neuropsychiatric Institute, David Geffen School of Medicine, University of California, Los Angeles, 760 Westwood Plaza, Los Angeles, CA 90024, USA; Mental Retardation Research Center, University of California, Los Angeles, 760 Westwood Plaza, Los Angeles, CA 90024, USA.
Christopher J. Evans, Department of Psychiatry and Biobehavioral Sciences, Neuropsychiatric Institute, David Geffen School of Medicine, University of California, Los Angeles, 760 Westwood Plaza, Los Angeles, CA 90024, USA
James A. Waschek, Department of Psychiatry and Biobehavioral Sciences, Neuropsychiatric Institute, David Geffen School of Medicine, University of California, Los Angeles, 760 Westwood Plaza, Los Angeles, CA 90024, USA; Mental Retardation Research Center, University of California, Los Angeles, 760 Westwood Plaza, Los Angeles, CA 90024, USA
Kabirullah Lutfy, Email: klutfy@westernu.edu, Department of Pharmaceutical Sciences, Western University of Health Sciences, 309 East 2nd Street, Pomona, CA 91766, USA.
References
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Waschek JA. Selective deficits in the circadian light response in mice lacking PACAP. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1194–R1201. doi: 10.1152/ajpregu.00268.2004. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibiger HC, Phillips AG. Mesocorticolimbic dopamine systems and reward. Ann N Y Acad Sci. 1988;537:206–215. doi: 10.1111/j.1749-6632.1988.tb42107.x. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press, Inc.; San Diego, California, USA: 1997. [Google Scholar]
- Ghatei MA, Takahashi K, Suzuki Y, Gardiner J, Jones PM, Bloom SR. Distribution, molecular characterization of pituitary adenylate cyclase-activating polypeptide and its precursor encoding messenger RNA in human and rat tissues. J Endocrinol. 1993;136:159–166. doi: 10.1677/joe.0.1360159. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Sheward WJ, Morrison CF, Waser B, Gugger M, Reubi JC. Distribution of the VPAC2 receptor in peripheral tissues of the mouse. Endocrinology. 2004;145:1203–1210. doi: 10.1210/en.2003-1058. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K, Matsuda T, Mizuno N, Nagata S, Baba A. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J Comp Neurol. 1996;371:567–577. doi: 10.1002/(SICI)1096-9861(19960805)371:4<567::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanaka K, Mori W, Hirose M, Matsuda T, Sakaue M, Miyazaki J, Niwa H, Tashiro F, Yamamoto K, Koga K, Tomimoto S, Kunugi A, Suetake S, Baba A. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP) Proc Natl Acad Sci USA. 2001;98:13355–13360. doi: 10.1073/pnas.231094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Swerdlow NR. The functional output of the mesolimbic dopamine system. Ann N Y Acad Sci. 1988;537:216–227. doi: 10.1111/j.1749-6632.1988.tb42108.x. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Do T, Maidment NT. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl) 2001;154:1–7. doi: 10.1007/s002130000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Aouizerate B, Barrot M, Le MM, Piazza PV. Dopamine-dependent responses to morphine depend on glucocorticoid receptors. Proc Natl Acad Sci USA. 1998;95:7742–7747. doi: 10.1073/pnas.95.13.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Gajawada N, Friedman TC, Lutfy K. Differential involvement of enkephalins in analgesic tolerance, locomotor sensitization, and conditioned place preference induced by morphine. Behav Neurosci. 2006;120:10–15. doi: 10.1037/0735-7044.120.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Kieffer BL, Lutfy K. The mu opioid receptor is involved in buprenorphine-induced locomotor stimulation and conditioned place preference. Neuropharmacology. 2007;52:1336–1341. doi: 10.1016/j.neuropharm.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Dabaja I, Gajawada N, Lutfy K. The role of beta-endorphin in the acute motor stimulatory and rewarding actions of cocaine in mice. Psychopharmacology (Berl) 2008;197:443–448. doi: 10.1007/s00213-007-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuo Y, Ohtaki T, Masuda Y, Tsuda M, Fujino M. Binding sites for pituitary adenylate cyclase activating polypeptide (PACAP): comparison with vasoactive intestinal polypeptide (VIP) binding site localization in rat brain sections. Brain Res. 1992;575:113–123. doi: 10.1016/0006-8993(92)90430-h. [DOI] [PubMed] [Google Scholar]
- Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- Moser A, Scholz J, Gansle A. Pituitary adenylate cyclase-activating polypeptide (PACAP-27) enhances tyrosine hydroxylase activity in the nucleus accumbens of the rat. Neuropeptides. 1999;33:492–497. doi: 10.1054/npep.1999.0768. [DOI] [PubMed] [Google Scholar]
- Nussdorfer GG, Malendowicz LK. Role of VIP, PACAP, and related peptides in the regulation of the hypothalamo–pituitary– adrenal axis. Peptides. 1998;19:1443–1467. doi: 10.1016/s0196-9781(98)00102-8. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Nakamachi T, Ohtaki H, Hashimoto H, Ndummyra S, Baba A, Watanabe J, Kikuyama S, Shioda S. Monoaminergic neuronal development is not affected in PACAP-gene-deficient mice. Regul Pept. 2005;126:103–108. doi: 10.1016/j.regpep.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schutz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res. 2001;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Somogyvari-Vigh A, Arimura A. Concentrations of pituitary adenylate cyclase activating polypeptide (PACAP) in human brain nuclei. Brain Res. 1995;699:116–120. doi: 10.1016/0006-8993(95)00869-r. [DOI] [PubMed] [Google Scholar]
- Sheward WJ, Lutz EM, Harmar AJ. Expression of pituitary adenylate cyclase activating polypeptide receptors in the early mouse embryo as assessed by reverse transcription polymerase chain reaction and in situ hybridisation. Neurosci Lett. 1996;216:45–48. doi: 10.1016/0304-3940(96)13002-0. [DOI] [PubMed] [Google Scholar]
- Sheward WJ, Lutz EM, Copp AJ, Harmar AJ. Expression of PACAP, and PACAP type 1 (PAC1) receptor mRNA during development of the mouse embryo. Brain Res Dev Brain Res. 1998;109:245–253. doi: 10.1016/s0165-3806(98)00086-8. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci. 2000;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Waschek JA, Casillas RA, Nguyen TB, Cicco-Bloom EM, Carpenter EM, Rodriguez WI. Neural tube expression of pituitary adenylate cyclase-activating peptide (PACAP) and receptor: potential role in patterning and neurogenesis. Proc Natl Acad Sci USA. 1998;95:9602–9607. doi: 10.1073/pnas.95.16.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Opiate reward: sites and substrates. Neurosci Biobehav Rev. 1989;13:129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- Yoburn BC, Lutfy K, Azimuddin S, Sierra V. Differentiation of spinal and supraspinal opioid receptors by morphine tolerance. Life Sci. 1990;46:343–350. doi: 10.1016/0024-3205(90)90013-h. [DOI] [PubMed] [Google Scholar]