Abstract
Pancreatic cancer is a major oncological challenge due to its aggressive growth and metastasis. In the current study, we investigated the role of anterior gradient 2 (AGR2) in these processes. AGR2 mRNA, as assessed by quantitative real-time reverse transcription–PCR (Q-RT-PCR), was 14-fold higher in pancreatic cancer compared with normal and pancreatitis tissues. Immunohistochemistry revealed high expression of AGR2 in neoplastic cells with 98% (56 of 57) positivity on pancreatic cancer and minimal staining in normal and pancreatitis tissues. AGR2 was also expressed in early pancreatic intraepithelial neoplastic lesions. RT-PCR and Western blotting showed elevated AGR2 expression in seven of nine pancreatic cancer cell lines. AGR2, as detected in conditioned media from cancer cells, indicated that it was secreted. The influence of AGR2 on pancreatic cancer cells was evaluated by silencing with small interfering RNA and short hairpin RNA. Silencing of AGR2 significantly reduced cell proliferation (MTS assay) and invasion (Boyden chamber assay) and improved gemcitabine sensitivity (fluorescence-activated cell sorting analysis). Conditioned media from cells in which AGR2 was silenced had a reduced ability to stimulate proliferation of pancreatic cancer cells, suggesting that secreted AGR2 was active. In vivo, silencing of AGR2 in MPanc-96 cells led to a significant reduction of tumor growth and increased the effectiveness of gemcitabine treatments in orthotopic tumor models evaluated by noninvasive bioluminescence imaging. In summary, AGR2 is expressed and secreted during pancreatic cancer development and plays an important role in cancer cell growth and survival. These observations suggest that AGR2 may be a useful molecular target in pancreatic cancer.
Introduction
Pancreatic cancer remains one of the most deadly tumor types. The 5-year survival rate after diagnosis is <3.5% (1). One reason for the poor prognosis is that the molecular mechanisms underlying pancreatic oncogenesis remain poorly understood. Altered levels of gene products are thought to be associated with the changed behavior of cancer cells (2). Large-scale analysis of gene expression has been widely proposed as a powerful method for malignancy diagnosis and predicting invasion and metastasis through the identification of biomarkers. Pancreatic cancer has previously been the focus for studies of cancer-specific gene expression (3–5). In our previous study, we identified genes expressed specifically in pancreatic cancer cells by comparing microarray generated expression profiles of resected adenocarcinoma with those of normal pancreas and chronic pancreatitis (6). Among the genes, highly specifically expressed in pancreatic cancer was anterior gradient 2 (AGR2).
AGR2, also known as hAG-2 (7) or Gob-4 (8), is the human orthologue of the Xenopus laevis AGR protein, XAG-2. XA G-2 has a putative role in ectodermal patterning of the frog embryo and is regulated by a number of fundamental embryonic molecules like noggin and chordin (9). In the frog embryo, XAG-2 is secreted and induces cement gland differentiation and expression of neural marker genes in a fibroblast growth factor–dependent way (9). The function of AGR2 in humans is unclear. Some evidence suggests that, in humans, the AGR2 protein may also be secreted (10). Others have suggested, based on Basic Local Alignment Search Tool Analysis (11), that AGR2 may represent a novel member of the protein disulfide isomerase family involved in protein maturation in the endoplasmic reticulum (12, 13). Overexpression of AGR2 was found to attenuate p53 activation in UV-damaged cancer cells, leading to the suggestion that AGR2 functions as a survival factor through inhibition of p53 (14). In other studies, rat mammary tumor cells overexpressing AGR2 showed enhanced adhesion to substratum and increased metastatic potential (10). Gene expression profiling of circulating tumor cells in peripheral blood from metastatic cancers (colorectal, prostate, and breast cancers) showed the presence of AGR2 (15). AGR2 has been shown previously to be expressed in breast (7, 16), prostate (17), and lung (18) cancers, although its role in these diseases is unknown. Recently, AGR2 was shown to act as an oncogene when overexpressed in NIH3T3 cells (19).
The aim of our present study was to investigate the presence and function of AGR2 in pancreatic cancer. We found that AGR2 was highly expressed in precancerous lesions and in neoplastic cells of pancreatic tumors and cancer cell lines. Pancreatic cancer cell lines secreted AGR2 into the culture media. Silencing of AGR2 decreased cancer cell proliferation and invasion and increased gemcitabine sensitivity of resistant cells in vitro and reduced tumor growth and increased gemcitabine effectiveness in vivo. These data support the further investigation of AGR2 as a potential target for pancreatic cancer therapy.
Materials and Methods
Pancreatic tissues and cell lines
Paraffin-embedded tissue slides of matched pancreatic adenocarcinoma/normal tissue micro array (n = 57) and pancreatic intraepithelial neoplasia (PanIn) slides (n = 10) were obtained from the Department of Pathology, University of Texas M.D. Anderson Cancer. Pancreatic cancer cell lines BxPC3, MiaPaCa-2, CFPAC-1, HPAC, Panc-1, Aspc-1, and SU.86.86 were obtained from the American Type Culture Collection. MPanc-96 pancreatic adenocarcinoma cell lines were originally established by Dr. Timothy J. Eberlein (20). BxPC3 and SU.86.86 cells were cultured in RPMI 1640 with 10% fetal bovine serum (FBS). Panc-1, MiaPaCa-2, CFPAC-1, HPAC, Aspc-1, and MPanc-96 cells were routinely cultured in DMEM with 10% FBS. All cells were maintained at 37°C in a humidified atmosphere of 5% CO2. Antibody (rabbit polyclonal) against AGR2 was a kind gift from Dr. Devon A. Thompson, Department of Surgery, Stanford University.
Immunohistochemical staining
Unstained 5.0-µm sections of clinical specimens or a matched tumor/benign pancreatic tissue microarray were deparaffinized with xylene and rehydrated with ethanol. Immunohistochemistry was performed using RTU Vectastain Elite ABC Universal kit (Vector Laboratories), as described previously (21). Primary antibody against AGR2, diluted 1:250 in 2% bovine serum albumin/0.2% Triton in PBS was added, and samples were incubated overnight at 4°C followed by biotinylated secondary antibody. Finally, slides were developed with 3,3-diaminobenzidine substrate counterstained with hematoxylin, dehydrated with ethanol, fixed with xylene, and mounted. Immunohistochemistry was analyzed using Olympus microscope (Olympus). Images were captured using a chilled, charge-coupled device camera (Photometrics) and SmartCapture software (Digital Scientific). Images were further processed with Adobe Photoshop software (Adobe Systems). The staining results were evaluated by a gastrointestinal pathologist (Dr. Huamin Wang) to determine the intensity and the percentage of positive tumor cells. The expression of ARG2 was categorized as positive (cytoplasmic staining of ≥10% of the tumor cells) and negative (cytoplasmic staining of <10% of the tumor cells). The staining intensity of positive cells was further graded as either strong, moderate, or low.
Quantitative real-time reverse transcription-PCR and reverse transcription-PCR
Total RNA was isolated from normal pancreas (n = 5), pancreatic adenocarcinoma (n = 6), and chronic pancreatitis (n = 5) tissues and pancreatic cancer cell lines (n = 8), and the quality of RNA was tested as mentioned previously (21). Primers designed for human AGR2 (Genbank NM_006408) were forward 5′ATG GAG AAA ATT CCA GTG 3′ and reverse 5′ TTA CAA TTC AGT CTT CAG 3′. The AGR2 primers were also specifically compared with the sequence of the related gene AGR3 (16) and showed no significant homology. Quantitative real-time reverse transcription-PCR (Q-RT-PCR) was performed for the tissue samples with Syber green as the tag on the I-cycler machine (Bio-Rad). Levels of mRNA expression were expressed in terms of fold difference. Pancreatic cancer cell lines were analyzed for AGR2 expression, and amplified products were separated on 1.5% agarose gels and visualized by ethidium bromide. Primers designed for β-actin (Genbank BC_016045), which was used as a loading control for the PCR reactions, were forward 5′ ATG ATA TCG CCG CGC TCG TCG TC 3′ and reverse 5′ CGC TCG GCC GTG GTG GTG AA 3′.
Collection of conditioned media
For collection of conditioned media, wild-type cell lines, including SU.86.86 and MPanc-96, were grown to 80% confluence, washed with PBS, and then cultured for 24 h in serum-free media. Media were collected and concentrated 10× using Centricon YM-3 filter devices (Millipore Corporation). Protein concentrations were determined using Bio-Rad reagent (Bio-Rad Laboratories).
Transient transfection of small interfering RNA
Pancreatic cancer cells (BxPC3, Su.86.86, CFPAC-1, and MPanc-96) were plated on 100-mm dishes and transiently transfected with prevalidated On-Target plus Smart Pool siRNAs-siControl and siAGR2 at a final concentration of 10 nmol/L (Dharmacon, Inc.) with Hiperfect transfection reagent (Qiagen, Inc.), and lysates were prepared for Western blot after 72 h.
Development of short hairpin RNA stable cell lines
We examined the effects of stable short hairpin RNA (shRNA) silencing of AGR2 in pancreatic cancer cells using BLOCK-iT-RNAi Entry vector kit and pBLOCK-iT 3-DEST vector (Invitrogen Corporation). MPanc-96 cells, which express relatively high levels of AGR2, were transfected with an shRNA designed for AGR2 or with a LacZ control shRNA (shControl, 5′ GCT ACA CAA ATC AGC GAT TT 3′). Two different shRNAs were created for AGR2 using the Invitrogen’s BLOCK-iT RNAi Designer protocol [Invitrogen Corporation; AGR2 target sequence 1 (shAGR2-1) 5′ GCT TAC GAA CCT GCA GAT ACA 3′ and AGR2 target sequence 2 (shAGR2-2) 5′ GCC CAC ACA GTC AAG CTT TAA 3′]. The shRNAs were annealed and cloned into a BLOCK-iT U6 RNAi entry vector containing the human U6 pol III type promoter. For stable silencing, the shControl, shAGR2-1, and shAGR2-2 were cloned into DEST vectors, according to the manufacturer’s protocols. Stable expression of shControl, shAGR2-1, and shAGR2-2 were established in MPanc-96 cells by selecting for neomycin resistance (1 mg/mL) and silencing of AGR2 in the lysates were verified by Western blotting.
Western blotting
Cell lysates and conditioned media were prepared, and protein concentrations were measured by Bio-Rad reagent. Protein (50 µg) was loaded onto 10% SDS-PAGE gels, and Western blot was conducted using primary antibody against AGR2 (rabbit polyclonal, 1:1,000 dilution). The same blot was reprobed for β-actin (1:200 dilution; Sigma), which served as loading control for the experiment. Precision plus protein standards (Bio-Rad) served as molecular weight markers. Western blot imaging and processing were done with Odyssey machine (LiCor BioScience).
Cell growth studies
Cell growth was analyzed using MTS reagent (Promega) according to the manufacturer’s directions. Pancreatic cancer cells transiently transfected with siControl or siAGR2 for 24 h and MPanc-96 cells stably transfected with shControl or shAGR2-1 or shAGR2-2 vectors (1,000 cells) were plated on 96-well plates and grown on 0.5% serum containing media. Cell numbers were estimated after 0, 24, 48, and 72 h by adding MTS to the wells 1 h before taking the photometric reading, as described previously (21).
Invasion assays
For studies of cell invasiveness, BIOCOAT Matrigel invasion chambers (BD Biosciences) were used. Briefly, pancreatic cancer cells transiently transfected with siControl or siAGR2 for 48 h and MPanc-96 cells stably transfected with shControl or shAGR2-1 or shAGR2-2 vectors (2 × 105) were suspended in 100 µL serum-free media and were added to the upper chamber and 0.5% serum containing DMEM was added into the lower chamber. The cells were allowed to invade the Matrigel for 24 h at 37°C in a 5% CO2 atmosphere. The invaded cells were analyzed, as described previously (21).
Apoptosis study by fluorescence-activated cell sorting analysis in vitro
Standard propidium iodide staining by the hypotonic lysis method was used for apoptosis studies based on fluorescence-activated cell sorting (FACS). Pancreatic cancer cells transiently transfected with siControl and siAGR2 for 24 h and MPanc-96 cells stably transfected with shControl or shAGR2-1 or shAGR2-2 were seeded in 100-mm plates. Apoptosis was induced by treating with gemcitabine (1 µmol/L) for 72 h, and cells were collected by trypsinization, washed once with cold PBS, mixed with 500 µL of hypotonic solution (0.1% sodium citrate, 0.1% Triton X-100, 20 µg/mL RNase, 50 µg/mL propidium iodide) for 30-min incubation and analyzed by flow cytometry using EPICS-XO (Beckman Coulter). Cells undergoing apoptosis (due to the DNA fragmentation) were detected as the population of cells with sub-G1 DNA contents.
Apoptosis study by TUNEL assay in vivo
Analysis of apoptotic cells in tumor tissue was done by using a commercially available TUNEL kit (Promega), as described in detail previously (22). Paraffin sections from tumor tissue treated with and without gemcitabine were analyzed for apoptosis. Immunohistochemistry was analyzed using Olympus microscope (Olympus). Images were captured using a chilled, charge-coupled device camera (Photometrics) and SmartCapture software (Digital Scientific). Images were further processed with Adobe Photoshop software (Adobe Systems). To quantify the apoptotic events, the number of cells undergoing apoptosis in 10 random fields at 100× magnification was counted.
Tumor growth study in vivo
Orthotopic tumors were formed in 4-wk-old male athymic nude nu/nu mice with MPanc-96 cells stably expressing shControl or shAGR2-1. Both AGR2 silenced cell lines showed the same effects in vitro; therefore, we choose one cell line (AGR2-1) for further in vivo studies compared with the shControl cells. These cells were further modified to stably express the firefly luciferase gene by lentivirus transfection (23). Cells were then grown to 80% confluence, harvested by trypsinization, washed twice in PBS, and resuspended to a final concentration of 1 × 106 cells/mL. Cell suspensions (0.1 mL) were injected into the pancreas of five mice per test group. From the second week onwards, these mice were treated with or without the chemotherapeutic drug gemcitabine (100 mg/kg body weight twice a week i.p.; group 1, shControl; group 2, shControl + gemcitabine; group 3, shAGR2-1; group 4, shAGR2-1 + gemcitabine).
Tumor growth was assessed by bioluminescence using a cryogenically cooled imaging system coupled to a data acquisition computer running LivingImage software (Xenogen Corp.) as mentioned previously (21). Before imaging, animals were anesthetized in an acrylic chamber with 1.5% isofluorane/air mixture and injected i.p. with 40 mg/mL of luciferin potassium salt in PBS at a dose of 150 mg/kg body weight. A digital grayscale animal image was acquired followed by acquisition and overlay of a pseudocolor image representing the spatial distribution of detected photons emerging from active luciferase within the animal. Signal intensity was quantified as the sum of all detected photons within the region of interest per second. After the final tumor imaging, the pancreas was removed and the animals were reimaged to visualize and count cancer cell metastases. Tissues were also fixed with formaldehyde, and histology was conducted to verify the accuracy of the bioluminescence data.
Statistical analysis
All the in vitro experiments were conducted in triplicate and carried out on three or more separate occasions. Data presented are means of the three or more independent experiments ± SE. Statistically significant differences were determined by two-tailed unpaired Student’s t test and were defined as *P < 0.05.
Results
AGR2 is highly expressed in pancreatic cancer tissues and cell lines and is secreted
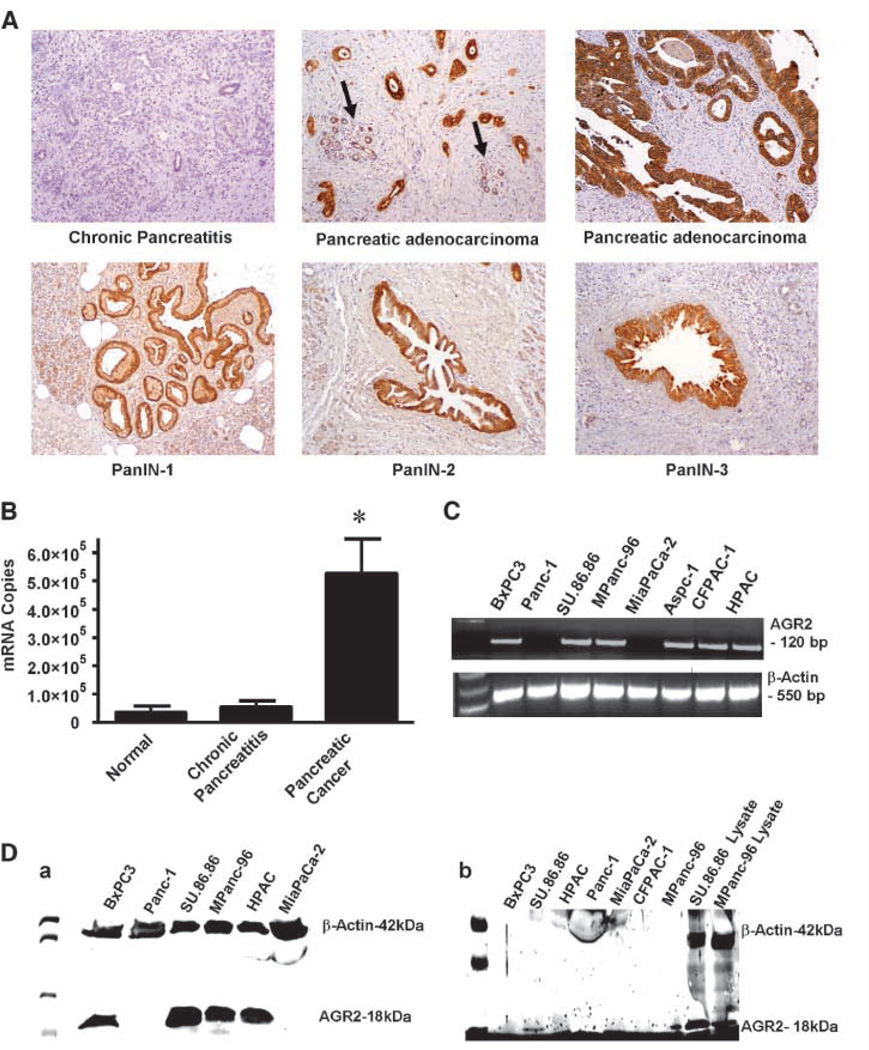
In profiling studies, AGR2 mRNA levels have previously been found to be elevated in pancreatic tumor (6, 24), pancreatic cancer cell lines (25), and preneoplastic pancreatic intraepithelial lesions (26). To identify the specific cells expressing this molecule, we conducted immunohistochemistry. AGR2 was difficult to detect in normal pancreas, although low levels of expression were sometimes observed in normal duct but not in acinar cells or islets (Fig. 1A). In tumor tissues, AGR2 levels were very high in pancreatic cancer cells. AGR2 was not expressed in the stroma of tumors or in foci of chronic pancreatitis. AGR2 was also expressed in the dysplastic duct cells in all grades of pancreatic intraepithelial neoplastic (PanIN) lesions (Fig. 1A). To evaluate the frequency of AGR2 expression in pancreatic tumors, we analyzed a tissue microarray and observed that 98% (56 of 57) of pancreatic cancer tissues were positive, of which staining was high in 60%, moderate in 22%, and low in 18%. These data confirm that AGR2 is highly expressed during pancreatic cancer development and in the neoplastic cells of pancreatic adenocarcinoma. For quantitation, we used Q-RT-PCR analysis to compare levels of AGR2 mRNA in samples of pancreatic cancer, normal pancreas, and chronic pancreatitis (Fig. 1B). AGR2 mRNA levels were 14-fold higher in pancreatic cancer compared with normal pancreas and chronic pancreatitis.
Figure 1.
AGR2 is expressed in pancreatic cancer tissues and cell lines and secreted. A, AGR2 was analyzed in samples of chronic pancreatitis, pancreatic adenocarcinoma, and PanIN lesions. Chronic pancreatitis samples did not stain for AGR2. In contrast, pancreatic adenocarcinomas showed strong positive staining for AGR2 (the arrow indicates low level of AGR2 expression in adjacent benign pancreatic tissue). High levels of AGR2 expression were also found in PanIN-1, PanIN-2, and PanIN-3. B, Q-RT-PCR results are shown for AGR2 mRNA in normal, chronic pancreatitis and pancreatic adenocarcinoma tissues. C, RT-PCR showing the expression of AGR2 mRNA in human pancreatic cancer cell lines. AGR2 mRNA was present in all cell lines except Panc-1 and MiaPaCa-2 (lanes 2 and 5). Water served as blank, and β-actin served as loading control. A full-length gel is presented in Supplementary Fig. S1. D, a, Western blot showing the presence of AGR2 protein in cell lysates of all human pancreatic cancer cell lines, except Panc-1 and MiaPaCa-2 (lanes 2 and 6). AGR2 was not present in normal pancreas tissue. β-Actin was used as a loading control. AGR2 was identified as an 18-kDa protein. A full-length gel is presented in Supplementary Fig. S2. b, Western blot showing the presence of AGR2 protein in conditional media and lysates of human pancreatic cancer cell lines. Significant levels of AGR2 were observed in the conditioned media from SU.86.86 and MPanc-96 (lanes 1 and 7, respectively) but not other cell lines. Lysates from SU.86.86 and MPanc-96 served as positive controls (lanes 8 and 9, respectively). β-Actin was detected in the lysates but not in the conditioned media that was positive for AGR2. Micrograph shown is representative of three independent experiments. A full-length gel is presented in Supplementary Fig. S3.
Further investigation of AGR2 mRNA levels in pancreatic cancer cell lines indicated that six of eight pancreatic cancer cell lines were positive for AGR2 (Fig. 1C; full-length gel is presented in Supplementary Fig. S1). This was further confirmed at the protein level by Western blot analysis (Fig. 1D, a; full-length gel is presented in Supplementary Fig. S2). AGR2 was recognized as an 18-kDa protein, similar to what has been previously reported (17). As a loading control, β-actin was used and recognized as a 42-kDa protein. To determine whether AGR2 was secreted, we analyzed conditioned media from pancreatic cancer cells by Western blot (Fig. 1D, b; full-length gel is presented in Supplementary Fig. S3). AGR2 was identified in the media bathing the cells of SU.86.86 and MPanc-96 and in their respective lysates, whereas in rest of the cell lines, it could not be detected in the media. To control for protein spilling from damaged cells, we probed for β-actin, as a nonsecreted control. Actin was found in the lysates but not in the conditioned media that was positive for AGR2, supporting its status as a secreted molecule.
AGR2 levels correlate with rates of pancreatic cancer cell proliferation, invasion, and survival in vitro
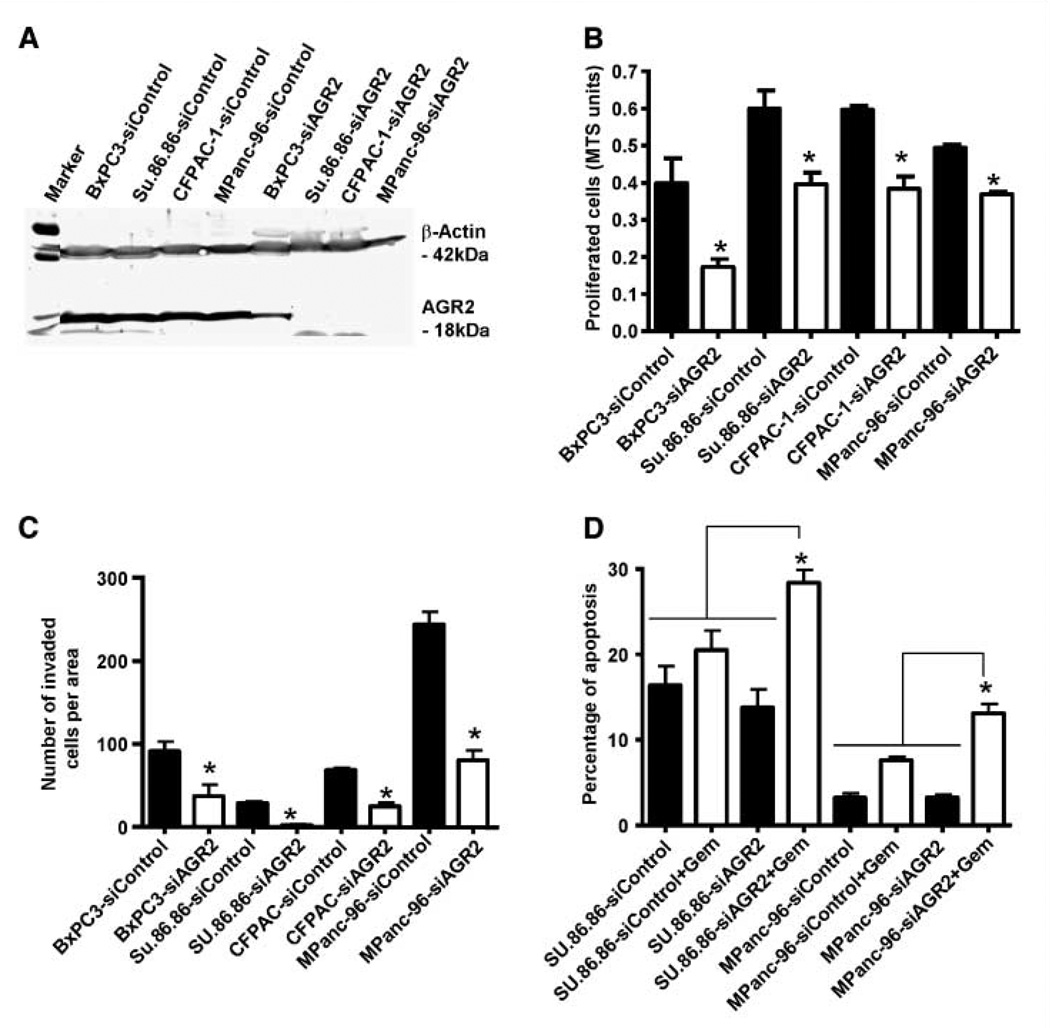
To evaluate the functional role of AGR2, four different pancreatic cancer cells were transiently transfected with siControl and siAGR2, and silencing was confirmed by Western blot (Fig. 2A; full-length gel is presented in Supplementary Fig. S4). Cells were then analyzed for changes in cell proliferation, invasion, and resistance to treatment with the cytotoxic agent gemcitabine. Proliferation was significantly reduced at 48 hours in AGR2 silenced cells when compared with their respective siControl transfected cells (Fig. 2B). Silencing of AGR2 also significantly reduced the invasion of pancreatic cancer cells that expressed AGR2 natively (Fig. 2C). To analyze the effects on cell survival and resistance to chemotherapy, cells with high levels of AGR2 secretion, SU.86.86 and MPanc-96, were treated with gemcitabine (1 µmol/L) in the presence of either siControl or siAGR2. Silencing of AGR2 more than doubled the apoptotic response to gemcitabine in both cell lines (P < 0.05; Fig. 2D).
Figure 2.
Transient silencing of AGR2 reduces pancreatic cancer cell proliferation, invasion, and apoptosis. A, to access the effectiveness of siAGR2, AGR2 levels were determined in pancreatic cancer cells after being transfected with siControl or siAGR2 at a concentration of 10 nmol/L. Cell lysates were prepared after 72 h, and Western blotting was performed with an anti-AGR2 antibody and anti–β-actin as loading control. AGR2 was either partially or completely silenced in pancreatic cancer cell lines. The micrograph shown is representative of three independent experiments. A full-length gel is presented in Supplementary Fig. S4. B, to determine the effects of AGR2 on cell proliferation, pancreatic cancer cell lines transfected with siControl or siAGR2 were plated into 96-well plates, and cell numbers were estimated by MTS assay after 48 h. AGR2 silencing resulted in significant reduction in cell proliferation. C, for investigation of the effects of AGR2 on cell invasion, cells were transfected with siControl or siAGR2 and plated into Boyden chambers for invasion studies. After 24 h, the invaded cells in 10 random fields at 100× magnification were counted. AGR2 silenced cells showed a significant reduction in cell invasion. D, for apoptosis studies, cell lines were transfected with siControl or siAGR2 and were then treated with or without gemcitabine (1 µmol/L). After 72 h, the percentage of cells with a sub G0/G1 DNA content was identified by FACS analysis. AGR2 silencing resulted in increasing the sensitivity of the cells to gemcitabine and, therefore, resulted in increased apoptosis. Columns, mean for three experiments; bars, SE (*, P < 0.05 versus control).
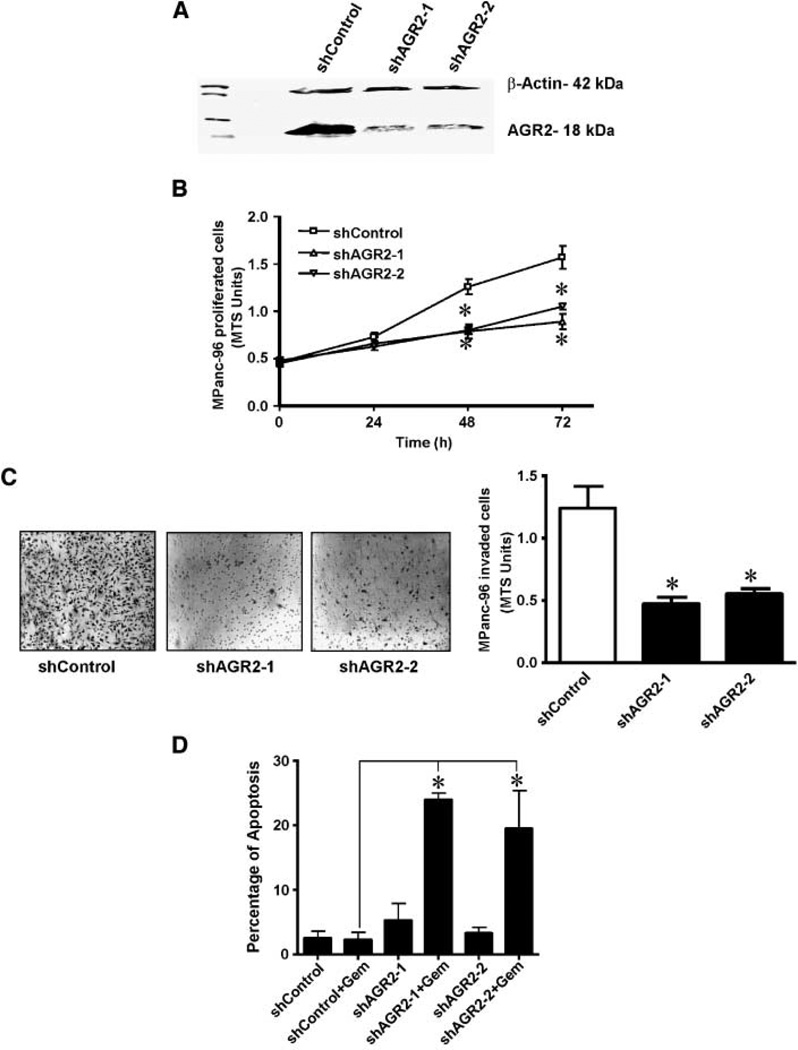
To further investigate the influence of AGR2, the MPanc-96 pancreatic cancer cell line that had a relatively high level of endogenous AGR2 was genetically modified using shRNA constructs to stably reduce AGR2 levels. Western blotting verified shRNA silencing, and AGR2 protein levels were reduced by >90% with either shAGR2-1 or shAGR2-2 (Fig. 3A; full-length gel is presented in Supplementary Fig. S5). The effects of stably reducing AGR2 expression levels were then evaluated on MPanc-96 cell proliferation, invasion, and survival in vitro. There was a significant decrease in proliferation of AGR2 silenced MPanc-96 cells when compared with MPanc-96 cells stably expressing shControl after 48 h (Fig. 3 B). Silencing of AGR2 in MPanc-96 cells also significantly decreased the extent of cell invasion when compared with shControl transfected cells (Fig. 3 C). Likewise, silencing of AGR2 had a dramatic effect on the resistance of MPanc-96 cells to treatment with gemcitabine. MPanc-96 cells are gemcitabine resistant and gemcitabine at 1 µmol/L had no significant effect on rates of apoptosis in wild-type cells. However, after stable silencing of AGR2 gemcitabine-induced cell killing was significantly increased (23-fold) compared with cells expressing shControl (Fig. 3D).
Figure 3.
Cellular levels of AGR2 correlate with pancreatic cancer cell proliferation, invasion, and survival in vitro. A, Western blot showing the effects of AGR2 silencing in cell lysates of MPanc-96 cells stably transfected with shControl or shAGR2-1, or shAGR2-2 vector. AGR2 was silenced in MPanc-96 cells. The micrograph is representative of three independent experiments. A full-length gel is presented in Supplementary Fig. S5. Effects of stable silencing of AGR2 on MPanc-96 cells were determined. B, MPanc-96 cells stably transfected with shControl or shAGR2-1 or shAGR2-2 (1,000 cells) were plated on 96-well plates with 0.5% serum containing DMEM, and cell numbers were estimated at 48 h by MTS assay. Stably silenced AGR2 cells showed a significant reduction in cell proliferation. Points, mean for three experiments; bars, SE (*, P < 0.05 versus control). C, effects of stable silencing of AGR2 on the invasiveness of MPanc-96 cells were analyzed. MPanc-96 cells stably transfected with shControl or shAGR2-1 or shAGR2-2 vectors were added into Biocoat Matrigel invasion upper chamber, and cells invading the lower chamber were estimated after 24 h using MTS reagent. Cellular invasiveness was significantly reduced on stably silenced AGR2 cells. Columns, means for three experiments; bars, SE (*, P < 0.05 versus control). Representative micrographs of invading cells in microscope fields for each membrane were pictured at 20× magnification. D, the effects of stable silencing of AGR2 on cell survival after gemcitabine treatment were examined. MPanc-96 cells stably transfected with shControl or shAGR2-1 or shAGR2-2 vectors were plated on 100-mm dish and gemcitabine (1 µmol/L) was added, and after 72 h, apoptotic cells were analyzed by FACS. Stable silencing of AGR2 showed significant increase in the gemcitabine sensitivity, and therefore, increase in apoptosis was observed. Columns, mean for three experiments; bars, SE (*, P < 0.05 versus control).
AGR2 silencing reduces pancreatic cancer in vivo
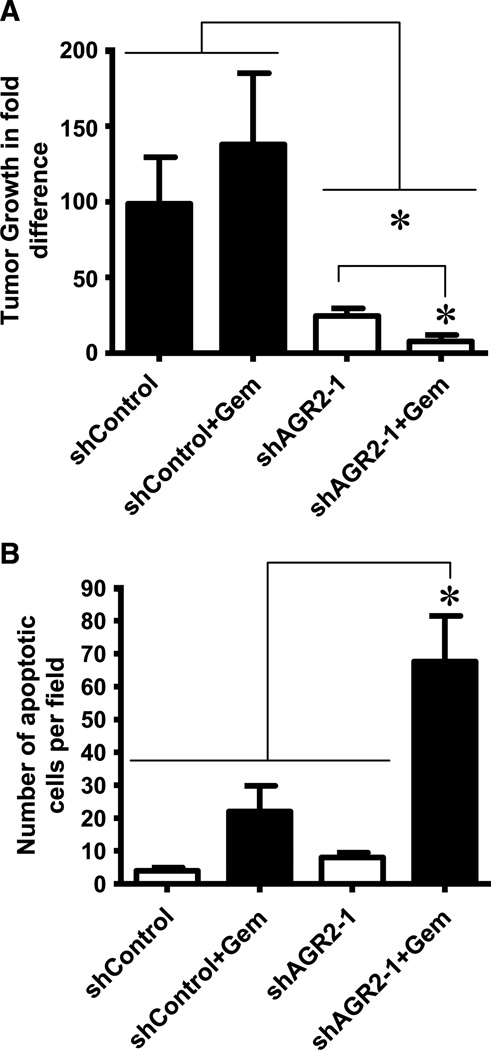
Based on our observations on the effects of AGR2 on pancreatic cancer cell growth, invasion, and survival in vitro, we further wished to analyze its effect on tumors developed in vivo in immune-deficient mice. Orthotopic tumors were developed with MPanc-96 cells stably expressing shControl or shAGR2-1 and followed using noninvasive bioluminescence imaging. MPanc-96 cells with silenced AGR2 expression showed a dramatic 4-fold reduction in tumor volume compared with shControl transfected cells (Fig. 4A; P < 0.05). As expected, there was no statistical effect of gemcitabine treatment on shControl groups of these highly resistant cells. However, after silencing of AGR2 in this cell, gemcitabine treatment caused a significant reduction in tumor volume compared with controls. Supporting the sensitization of the MPanc-96 cells by silencing of AGR2, TUNEL analysis indicated significant increase of apoptotic cells on tissues silenced with AGR2 and treated with gemcitabine when compared with all other tissues (Fig. 4B).
Figure 4.
AGR2 silencing decreases pancreatic cancer growth and metastasis in vivo. A, tumor growth of MPanc-96 cells stably expressing shControl or shAGR2-1 and also expressing the luciferase gene with and without gemcitabine treatment was assessed in nude mice. Bioluminescent imaging was used to estimate tumor volume. AGR2 silencing with and without gemcitabine significantly reduced the tumor volume. B, in vivo apoptosis was measured by TUNEL assay on paraffin sections from shControl and shAGR2-1 animals treated with gemcitabine. AGR2 silencing along with gemcitabine treatment significantly increased the apoptosis in tumor tissues. Columns, mean for five animals; bars, SE (*, P < 0.05 versus control).
We also evaluated metastasis at the end of the study. AGR2 silencing alone did not reduce the incidence of metastases, but in combination with gemcitabine, there was a complete reduction of lung metastasis (100%) and 60% reduction of liver metastasis (Table 1). The number of metastatic foci was reduced by silencing AGR2 itself and gemcitabine treatment further significantly reduced the numbers of metastases in lung and liver (Table 2).
Table 1.
Metastasis incidence
| shControl | shControl + Gem | ShAGR2-1 | shAGR2-1 + Gem | |
|---|---|---|---|---|
| Lung | 100% (5/5) | 40% (2/5) | 100% (5/5) | 0% (0/5)* |
| Liver | 100% (5/5) | 100% (5/5) | 100% (5/5) | 60% (3/5)* |
NOTE: AGR2 silencing combined with gemcitabine treatment reduces the metastasis incidence in vivo. Tumors were developed in nude mice with MPanc-96 cells stably expressing shControl or shAGR2-1 and also expressing the luciferase gene, and these animals were treated with and without gemcitabine. At the end of the experiment, animals were analyzed by bioluminescence imaging, and the incidence of metastasis was calculated in lung and liver. AGR2 silencing in combination with gemcitabine significantly reduced the lung and liver metastasis incidence. Data shown are mean ± SE for five animals.
Abbreviation: Gem, gemcitabine.
P < 0.05 versus control.
Table 2.
Number of metastatic foci
| shControl (per animal) |
shAGR2-1 (per animal) |
Percentage of reduction |
shControl + Gem (per animal) |
shAGR2-1 + Gem (per animal) |
Percentage of reduction |
|
|---|---|---|---|---|---|---|
| Lung | 3.8 ± 0.7 | 1.8 ± 0.5* | 47% | 0.6 ± 0.18 | 0* | 100% |
| Liver | 10.2 ± 0.9 | 6.5 ± 1.2* | 64% | 2.0 ± 1.0 | 0.4 ± 0.2* | 80% |
NOTE: AGR2 silencing combined with gemcitabine treatment reduces the number of metastatic foci in vivo. Tumors were developed in nude mice with MPanc-96 cells stably expressing shAGR2-1 or shControl and also expressing the luciferase gene, and these animals were treated with and without gemcitabine. At the end of the experiment, cancer cells were quantitated by bioluminescence imaging and numbers of metastatic foci were counted in lung and liver. AGR2 silencing significantly reduced the lung and liver metastatic foci, whereas combination with gemcitabine further reduced them. Data shown are mean ± SE for five animals.
Abbreviation: Gem, gemcitabine.
P < 0.05 versus control.
Secreted AGR2 is functionally active
To examine whether the AGR2 secreted by pancreatic cancer cell lines was functionally active, proliferation studies were performed on MiaPaCa-2 cells, which do not secrete AGR2. Conditioned media (2× concentrated) from MPanc-96 cells stably bearing shControl or shAGR2 were added to MiaPaCa-2 cells, and the effects on cell numbers was determined (Supplementary Fig. 6). Condition media from control MPanc-96 cells significantly stimulated the growth of MiaPaCa-2 cells. In comparison, conditioned media from MPanc-96 cells silenced for AGR2 (shAGR2-1 and shAGR2-2) stimulated significantly less growth of MiaPaCa-2 cells. This supports the suggestion that the secreted AGR2 is functionally active.
Discussion
AGR2 has been previously noted as overexpressed in pancreatic cancer based on expression profiles (6, 24–26). This is the first report validating these observations in a large number of tumors. Furthermore, this study showed for the first time the secretion of AGR2 in pancreatic cancer cell lines and elucidated some of the effects of this molecule in cancer cells and tumors. AGR2 silencing significantly inhibited cell proliferation, invasion, and survival in vitro; inhibited tumor growth in vivo; and sensitized cancer cells to gemcitabine both in vitro and in vivo. Taken together, these data suggest that AGR2 may be a reasonable target for the development of future therapies.
AGR2 was markedly increased at both mRNA and protein levels in pancreatic cancer cells compared with their benign counterparts, indicating that AGR2 expression might be involved in the malignant progression of pancreatic cancer. Our data show that, even in the early stages of pancreatic cancer development, in PanIn, AGR2 was found to be highly expressed. In other cancers, like breast cancer (27) and prostate cancer (28), the expression of AGR2 was correlated with the poor survival of patients. However, in our analysis of pancreatic tumors by immunohistochemistry, we could not find any correlation between AGR2 levels and patient outcomes likely due to its prevalence in virtually all patient samples of cancer. It may be that a quantitative analysis would indicate a prognostic significance but such a study has not yet been conducted. It was also reported that AGR2 was coexpressed with estrogen receptor (7) and its expression was induced by estrogen in breast cells (29) and by androgen in prostate cells (17). However, the expression and functions of AGR2 in pancreatic cancer cells suggest that AGR2 is not hormone-inducible in this tissue.
Previous literature suggested that AGR2 could be a secretory molecule. In Xenopus laevis, translation of the single open reading frame revealed a protein of 185 amino acids with a putative hydrophobic signal sequence from amino acid positions 1–18 as calculated by the signal sequence prediction method (9). In breast cancer tissues, the presence of NH2 terminal cleavable secretory signal sequence was predicted (16) and the presence of AGR2 was shown as granular cytoplasmic appearance by immunohistochemistry (10, 16, 27). In another study, it was shown that AGR2 was likely a secretory molecule as the signal peptide was cleaved from this molecule when performing rabbit reticulocyte transcription/translation coupled with microsomal vesicle processing (17). In a recent publication, it was shown that conditioned media from an esophageal adenocarcinoma cell line expressing higher levels of AGR2 enhanced the migration of cells in which AGR2 was knocked down (19). The current study is the first to provide direct evidence in human pancreatic cancer cells that AGR2 is secreted. We found that AGR2 was present in the media of pancreatic cancer cells expressing high levels of AGR2, whereas a cytoplasmic protein, actin, was not. These data strongly support its identity as a secreted molecule. That we did not observe the presence of AGR2 in the media from all of the cell lines probably relates to variations in the levels of production and to the sensitivity of our assay. This secreted AGR2 seemed to be biologically active as suggested by the observation that condition media from cells expressing AGR2 was significantly better at stimulating pancreatic cancer cell growth than that from cells silenced for AGR2.
That AGR2 is secreted suggests that it may generate some of its biological effects by acting in an autocrine or paracrine manner. Previously, yeast two-hybrid cloning analysis suggested that AGR2 can interact with metastasis gene (C4.4a) and with dystroglycan (DAG-1; ref. 16). However, whether these interactions are involved in the effects of AGR2 in cancer remain uncertain. Neither of these molecules is highly expressed in pancreatic cancer. Identification of the receptor through which the functions of AGR2 are mediated will provide another potential target for therapeutic development.
Our current observation that silencing AGR2 sensitized the cells to cell killing by gemcitabine may be of particular translational significance. Previously, AGR2 has been suggested to be involved in cell survival based on its specific up-regulation in breast cancer cells subjected to serum and oxygen depletion (30). Also, AGR2 was reported to inhibit p53 tumor suppressor–mediated effects in response to UV exposure (14). Molecules that provide a survival benefit may protect cells from chemotherapeutic treatments. Pancreatic cancer is well recognized as being highly resistant to therapeutics. In the current study, transient silencing of AGR2 increased the effectiveness of gemcitabine treatment in two cancer cell lines in vitro, and stable silencing had a more profound effect, probably related to a greater reduction in cellular levels of AGR2. Furthermore, in vivo experiments indicated that the effects of AGR2 on gemcitabine resistance were also important in vivo. These data suggest that the inhibition of AGR2-regulated pathways could be of clinical benefit in pancreatic cancer treatment. Future efforts will be devoted to understanding the mechanisms and pathways involved in the effects of AGR2 in chemoresistance and the possible use of inhibitors of these pathways to sensitize cancer cells to chemotherapy.
In the current study, it seemed that AGR2 might also influence metastasis. We observed a significant reduction in invasiveness in vitro after AGR2 silencing. We also observed a reduction in the incidence and volume of metastases in vivo. However, in the current study, it was not possible to determine if this was a direct effect on metastasis or simply due to the great reduction in the primary tumor. Previously, it was reported that AGR2 was involved in developing lung metastasis in breast cancer as ectopic expression increased metastasis but did not show any effect on primary tumor growth (10). Further research will be necessary to understand all of the roles of this important new regulator of pancreatic cancer.
In summary, the current study describes the expression and function of AGR2 in pancreatic cancer. This molecule was found to stimulate growth, invasiveness, and survival in vitro and tumor growth and sensitivity to chemotherapy in vivo. Future studies to identify the mechanisms involved in these effects may lead to useful diagnostic and therapeutic approaches.
Supplementary Material
Acknowledgments
Grant support: Lockton Endowment, Lustgarten Foundation, and MDACC Pancreatic Cancer Specialized Programs of Research Excellence.
Footnotes
Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Schneider G, Schmid RM. Genetic alterations in pancreatic carcinoma. Mol Cancer. 2003;2:15–21. doi: 10.1186/1476-4598-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin K, Kritzman DM, Price LM, et al. Linking gene expression patterns to therapeutic groups in breast cancer. Cancer Res. 2000;60:2232–2238. [PubMed] [Google Scholar]
- 3.Iacobuzio-Donahue CA, Maitra A, Olsen M, et al. Exploration of global gene expression patterns in pancreatic adenocarcinoma using cDNA microarrays. Am J Pathol. 2003;162:1151–1162. doi: 10.1016/S0002-9440(10)63911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan ZJ, Hu XG, Cao GS, et al. Analysis of gene expression profile of pancreatic carcinoma using cDNA microarray. World J Gastroenterol. 2003;9:818–823. doi: 10.3748/wjg.v9.i4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crnogorac-Jurcevic T, Efthimiou E, Nielsen T, et al. Expression profiling of microdissected pancreatic adenocarcinomas. Oncogene. 2002;21:4587–4594. doi: 10.1038/sj.onc.1205570. [DOI] [PubMed] [Google Scholar]
- 6.Logsdon CD, Simeone DM, Binkley C, et al. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 7.Thompson DA, Weigel RJ. hAG-2, the human homologue of the Xenopus laevis cement gland gene XAG-2, is coexpressed with estrogen receptor in breast cancer cell lines. Biochem Biophys Res Commun. 1998;251:111–116. doi: 10.1006/bbrc.1998.9440. [DOI] [PubMed] [Google Scholar]
- 8.Komiya T, Tanigawa Y, Hirohashi S. Cloning of the gene gob-4, which is expressed in intestinal goblet cells in mice. Biochim Biophys Acta. 1999;1444:434–438. doi: 10.1016/s0167-4781(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 9.Aberger F, Weidinger G, Grunz H, et al. Anterior specification of embryonic ectoderm: the role of the Xenopus cement gland-specific gene XAG-2. Mech Dev. 1998;72:115–130. doi: 10.1016/s0925-4773(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 10.Liu D, Rudland PS, Sibson DR, et al. Human homologue of cement gland protein, a novel metastasis inducer associated with breast carcinomas. Cancer Res. 2005;65:3796–3805. doi: 10.1158/0008-5472.CAN-04-3823. [DOI] [PubMed] [Google Scholar]
- 11.Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persson S, Rosenquist M, Knoblach B, et al. Diversity of the protein disulfide isomerase family: identification of breast tumor induced Hag2 and Hag3 as novel members of the protein family. Mol Phylogenet Evol. 2005;36:734–740. doi: 10.1016/j.ympev.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Sevier CS, Kaiser CA. Formation and transfer of disulphide bonds in living cells. Nat Rev Mol Cell Biol. 2002;3:836–847. doi: 10.1038/nrm954. [DOI] [PubMed] [Google Scholar]
- 14.Pohler E, Craig AL, Cotton J, et al. The Barrett’s antigen anterior gradient-2 silences the p53 transcriptional response to DNA damage. Mol Cell Proteomics. 2004;3:534–547. doi: 10.1074/mcp.M300089-MCP200. [DOI] [PubMed] [Google Scholar]
- 15.Smirnov DA, Zweitzig DR, Foulk BW, et al. Global gene expression profiling of circulating tumor cells. Cancer Res. 2005;65:4993–4997. doi: 10.1158/0008-5472.CAN-04-4330. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher GC, Patel S, Tyson K, et al. hAG-2 and hAG-3, human homologues of genes involved in differentiation, are associated with oestrogen receptor-positive breast tumours and interact with metastasis gene C4.4a and dystroglycan. Br J Cancer. 2003;88:579–585. doi: 10.1038/sj.bjc.6600740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang JS, Gong A, Cheville JC, et al. AGR2, an androgen-inducible secretory protein overexpressed in prostate cancer. Genes Chromosomes Cancer. 2005;43:249–259. doi: 10.1002/gcc.20188. [DOI] [PubMed] [Google Scholar]
- 18.Zhu H, Lam DC, Han KC, et al. High resolution analysis of genomic aberrations by metaphase and array comparative genomic hybridization identifies candidate tumour genes in lung cancer cell lines. Cancer Lett. 2007;245:303–314. doi: 10.1016/j.canlet.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Hao Y, Lowe AW. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Res. 2008;68:492–497. doi: 10.1158/0008-5472.CAN-07-2930. [DOI] [PubMed] [Google Scholar]
- 20.Peiper M, Nagoshi M, Patel D, et al. Human pancreatic cancer cells (MPanc-96) recognized by autologous tumor-infiltrating lymphocytes after in vitro as well as in vivo tumor expansion. Int J Cancer. 1997;71:993–999. doi: 10.1002/(sici)1097-0215(19970611)71:6<993::aid-ijc15>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Ramachandran V, Arumugam T, Hwang RF, et al. Adrenomedullin is expressed in pancreatic cancer and stimulates cell proliferation and invasion in an autocrine manner via the adrenomedullin receptor, ADMR. Cancer Res. 2007;67:2666–2675. doi: 10.1158/0008-5472.CAN-06-3362. [DOI] [PubMed] [Google Scholar]
- 22.Langley RR, Fan D, Tsan RZ, et al. Activation of the platelet-derived growth factor receptor enhances survival of murine bone endothelial cells. Cancer Res. 2004;64:3727–3730. doi: 10.1158/0008-5472.CAN-03-3863. [DOI] [PubMed] [Google Scholar]
- 23.Arumugam T, Simeone DM, Van Golen K, et al. S100 P promotes pancreatic cancer growth, survival, and invasion. Clin Cancer Res. 2005;11:5356–5364. doi: 10.1158/1078-0432.CCR-05-0092. [DOI] [PubMed] [Google Scholar]
- 24.Ryu B, Jones J, Blades NJ, et al. Relationships and differentially expressed genes among pancreatic cancers examined by large-scale serial analysis of gene expression. Cancer Res. 2002;62:819–826. [PubMed] [Google Scholar]
- 25.Missiaglia E, Blaveri E, Terris B, et al. Analysis of gene expression in cancer cell lines identifies candidate markers for pancreatic tumorigenesis and metastasis. Int J Cancer. 2004;112:100–112. doi: 10.1002/ijc.20376. [DOI] [PubMed] [Google Scholar]
- 26.Buchholz M, Braun M, Heidenblut A, et al. Transcriptome analysis of microdissected pancreatic intraepithelial neoplastic lesions. Oncogene. 2005;24:6626–6636. doi: 10.1038/sj.onc.1208804. [DOI] [PubMed] [Google Scholar]
- 27.Fritzsche FR, Dahl E, Pahl S, et al. Prognostic relevance of AGR2 expression in breast cancer. Clin Cancer Res. 2006;12:1728–1734. doi: 10.1158/1078-0432.CCR-05-2057. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Forootan SS, Liu D, et al. Increased expression of anterior gradient-2 is significantly associated with poor survival of prostate cancer patients. Prostate Cancer Prostatic Dis. 2007;10:293–300. doi: 10.1038/sj.pcan.4500960. [DOI] [PubMed] [Google Scholar]
- 29.Wilson CL, Sims AH, Howell A, et al. Effects of oestrogen on gene expression in epithelium and stroma of normal human breast tissue. Endocr Relat Cancer. 2006;13:617–628. doi: 10.1677/erc.1.01165. [DOI] [PubMed] [Google Scholar]
- 30.Zweitzig DR, Smirnov DA, Connelly MC, et al. Physiological stress induces the metastasis marker AGR2 in breast cancer cells. Mol Cell Biochem. 2007;306:255–260. doi: 10.1007/s11010-007-9562-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.