Abstract
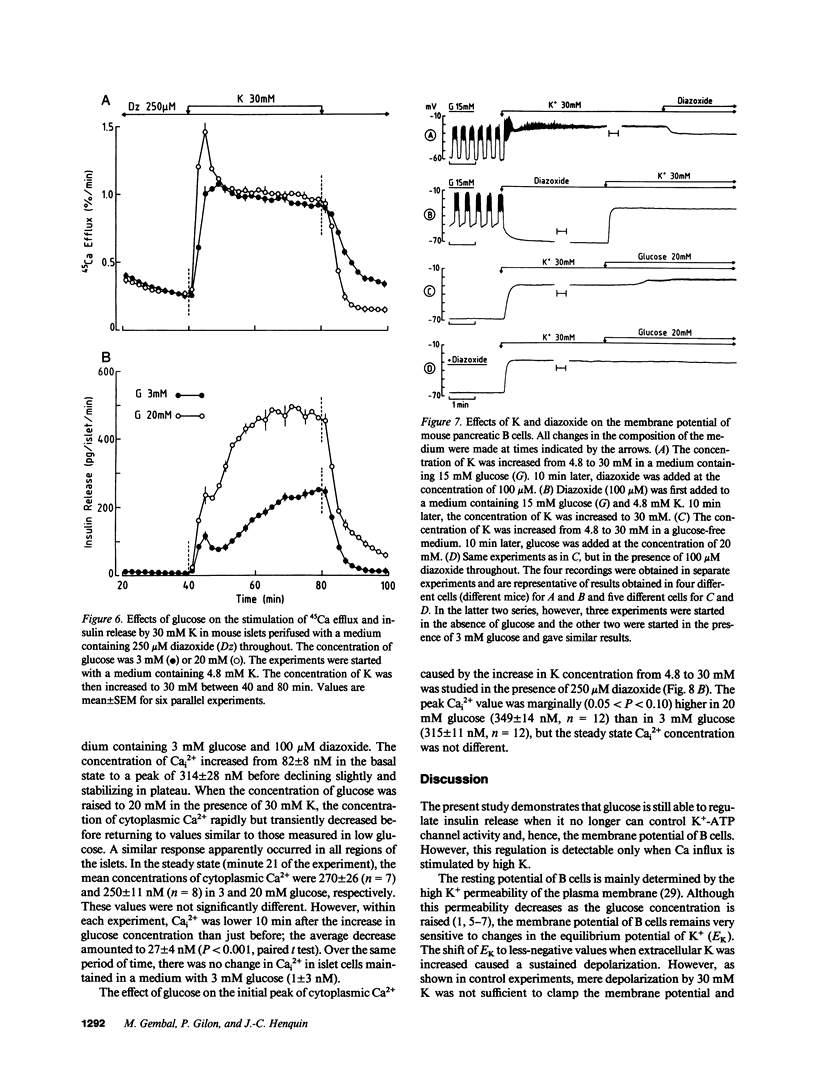
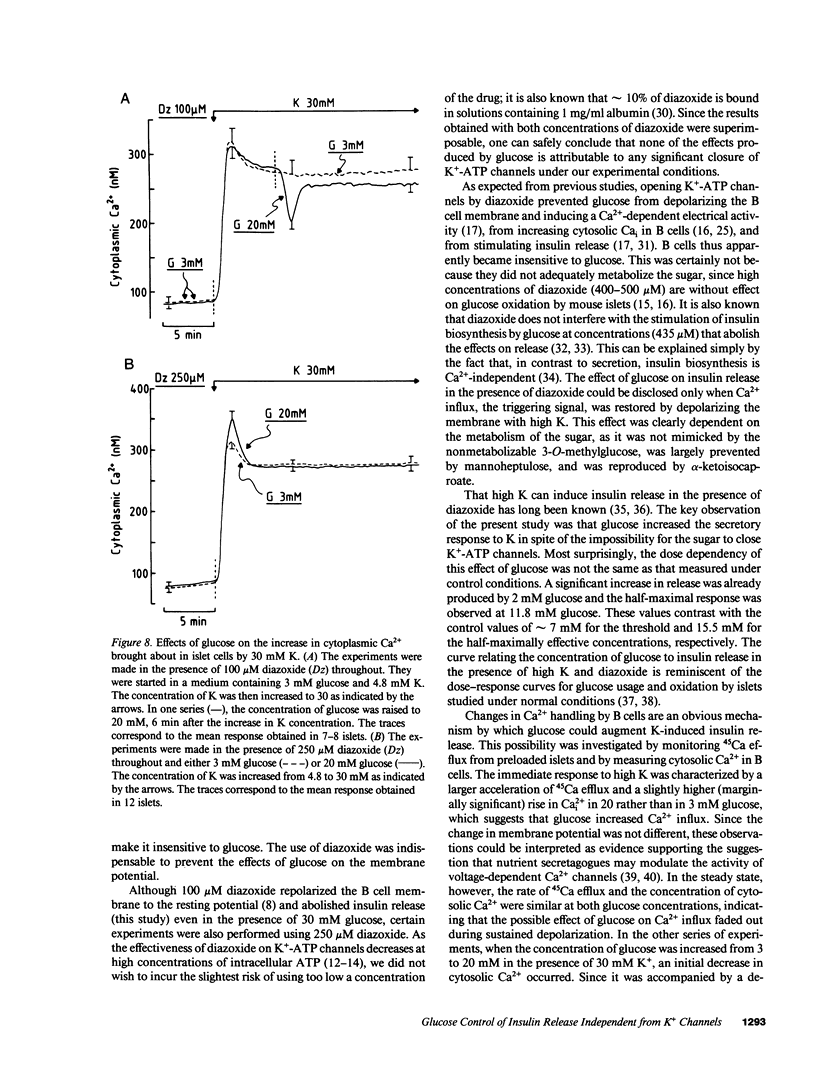
Glucose stimulation of insulin release involves closure of ATP-sensitive K+ channels, depolarization, and Ca2+ influx in B cells. Mouse islets were used to investigate whether glucose can still regulate insulin release when it cannot control ATP-sensitive K+ channels. Opening of these channels by diazoxide (100-250 mumol/liter) blocked the effects of glucose on B cell membrane potential (intracellular microelectrodes), free cytosolic Ca2+ (fura-2 method), and insulin release, but it did not prevent those of high K (30 mmol/liter). K-induced insulin release in the presence of diazoxide was, however, dose dependently increased by glucose, which was already effective at concentrations (2-6 mmol/liter) that are subthreshold under normal conditions (low K and no diazoxide). This effect was not accompanied by detectable changes in B cell membrane potential. Measurements of 45Ca fluxes and cytosolic Ca2+ indicated that glucose slightly increased Ca2+ influx during the first minutes of depolarization by K, but not in the steady state when its effect on insulin release was the largest. In conclusion, there exists a mechanism by which glucose can control insulin release independently from changes in K(+)-ATP channel activity, in membrane potential, and in cytosolic Ca2+. This mechanism may serve to amplify the secretory response to the triggering signal (closure of K(+)-ATP channels--depolarization--Ca2+ influx) induced by glucose.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkhammar P., Nilsson T., Rorsman P., Berggren P. O. Inhibition of ATP-regulated K+ channels precedes depolarization-induced increase in cytoplasmic free Ca2+ concentration in pancreatic beta-cells. J Biol Chem. 1987 Apr 25;262(12):5448–5454. [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Hedeskov C. J., Randle P. J. Glucose metabolism in mouse pancreatic islets. Biochem J. 1970 Jun;118(1):143–154. doi: 10.1042/bj1180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Dawson C. M., Eddlestone G. T., Rojas E. Voltage noise measurements across the pancreatic beta-cell membrane: calcium channel characteristics. J Physiol. 1981 May;314:195–212. doi: 10.1113/jphysiol.1981.sp013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsten P., Gylfe E., Wesslén N., Hellman B. Diazoxide unmasks glucose inhibition of insulin release by counteracting entry of Ca2+. Am J Physiol. 1988 Oct;255(4 Pt 1):E422–E427. doi: 10.1152/ajpendo.1988.255.4.E422. [DOI] [PubMed] [Google Scholar]
- Cerasi E. Mechanisms of glucose stimulated insulin secretion in health and in diabetes: some re-evaluations and proposals. Diabetologia. 1975 Feb;11(1):1–13. doi: 10.1007/BF00422811. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Ikeuchi M. Tolbutamide as mimic of glucose on beta-cell electrical activity. ATP-sensitive K+ channels as common pathway for both stimuli. Diabetes. 1989 Apr;38(4):416–421. doi: 10.2337/diab.38.4.416. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Satin L. S., Ashford M. L., Hales C. N. ATP-sensitive K+ channels in pancreatic beta-cells. Spare-channel hypothesis. Diabetes. 1988 May;37(5):495–498. doi: 10.2337/diab.37.5.495. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Illot M. C., Peterson O. H. Interaction of diazoxide, tolbutamide and ATP4- on nucleotide-dependent K+ channels in an insulin-secreting cell line. J Membr Biol. 1987;99(3):215–224. doi: 10.1007/BF01995702. [DOI] [PubMed] [Google Scholar]
- Grapengiesser E., Gylfe E., Hellman B. Sulfonylurea mimics the effect of glucose in inducing large amplitude oscillations of cytoplasmic Ca2+ in pancreatic beta-cells. Mol Pharmacol. 1990 Mar;37(3):461–467. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gylfe E. Glucose-induced early changes in cytoplasmic calcium of pancreatic beta-cells studied with time-sharing dual-wavelength fluorometry. J Biol Chem. 1988 Apr 15;263(11):5044–5048. [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Lernmark A., Sehlin J., Täljedal I. B. The pancreatic beta-cell recognition of insulin secretagogues. Effects of calcium and sodium on glucose metabolism and insulin release. Biochem J. 1974 Jan;138(1):33–45. doi: 10.1042/bj1380033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C. ATP-sensitive K+ channels may control glucose-induced electrical activity in pancreatic B-cells. Biochem Biophys Res Commun. 1988 Oct 31;156(2):769–775. doi: 10.1016/s0006-291x(88)80910-0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Charles S., Nenquin M., Mathot F., Tamagawa T. Diazoxide and D600 inhibition of insulin release. Distinct mechanisms explain the specificity for different stimuli. Diabetes. 1982 Sep;31(9):776–783. doi: 10.2337/diab.31.9.776. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. D-glucose inhibits potassium efflux from pancreatic islet cells. Nature. 1978 Jan 19;271(5642):271–273. doi: 10.1038/271271a0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem Pharmacol. 1982 Apr 1;31(7):1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia. 1984 Oct 15;40(10):1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Role of voltage- and Ca2(+)-dependent K+ channels in the control of glucose-induced electrical activity in pancreatic B-cells. Pflugers Arch. 1990 Jul;416(5):568–572. doi: 10.1007/BF00382691. [DOI] [PubMed] [Google Scholar]
- Hermans M. P., Schmeer W., Henquin J. C. The permissive effect of glucose, tolbutamide and high K+ on arginine stimulation of insulin release in isolated mouse islets. Diabetologia. 1987 Aug;30(8):659–665. doi: 10.1007/BF00277325. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Effects of diazoxide on insulin secretion in vitro. Lancet. 1966 Jan 15;1(7429):128–129. doi: 10.1016/s0140-6736(66)91263-3. [DOI] [PubMed] [Google Scholar]
- Leinweber K. P., Schatz H. Long-term and short-term effects of calcium, verapamil and diazoxide on biosynthesis and release of (pro-) insulin in isolated islets of rat pancreas. Acta Endocrinol (Copenh) 1982 Jan;99(1):94–100. doi: 10.1530/acta.0.0990094. [DOI] [PubMed] [Google Scholar]
- Lenzen S., Panten U. 2-oxocarboxylic acids and function of pancreatic islets in obese-hyperglycaemic mice. Insulin secretion in relation to 45Ca uptake and metabolism. Biochem J. 1980 Jan 15;186(1):135–144. doi: 10.1042/bj1860135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B. J., Haist R. E. Effects of some modifiers of insulin secretion on insulin biosynthesis. Endocrinology. 1973 Mar;92(3):735–742. doi: 10.1210/endo-92-3-735. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A., Herchuelz A., Hutton J. C. Insulin release: the fuel hypothesis. Metabolism. 1979 Apr;28(4):373–386. doi: 10.1016/0026-0495(79)90111-2. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. New perspectives on pancreatic islet glucokinase. Am J Physiol. 1984 Jan;246(1 Pt 1):E1–13. doi: 10.1152/ajpendo.1984.246.1.E1. [DOI] [PubMed] [Google Scholar]
- Meissner H. P., Henquin J. C., Preissler M. Potassium dependence of the membrane potential of pancreatic B-cells. FEBS Lett. 1978 Oct 1;94(1):87–89. doi: 10.1016/0014-5793(78)80912-0. [DOI] [PubMed] [Google Scholar]
- Meissner H. P., Schmelz H. Membrane potential of beta-cells in pancreatic islets. Pflugers Arch. 1974;351(3):195–206. doi: 10.1007/BF00586918. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Hales C. N. The interaction of various inhibitors and stimuli of insulin release studied with rabbit pancreas in vitro. Biochem J. 1969 Jul;113(3):473–479. doi: 10.1042/bj1130473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panten U., Burgfeld J., Goerke F., Rennicke M., Schwanstecher M., Wallasch A., Zünkler B. J., Lenzen S. Control of insulin secretion by sulfonylureas, meglitinide and diazoxide in relation to their binding to the sulfonylurea receptor in pancreatic islets. Biochem Pharmacol. 1989 Apr 15;38(8):1217–1229. doi: 10.1016/0006-2952(89)90327-4. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. G., Marichal M., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. XV. Participation of cations in the recognition of glucose by the beta-cell. Endocrinology. 1973 Nov;93(5):1012–1018. doi: 10.1210/endo-93-5-1012. [DOI] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F. M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987 Oct;67(4):1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Hellman B. Voltage-activated currents in guinea pig pancreatic alpha 2 cells. Evidence for Ca2+-dependent action potentials. J Gen Physiol. 1988 Feb;91(2):223–242. doi: 10.1085/jgp.91.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. A., Rorsman P., Ashcroft F. M. Modulation of dihydropyridine-sensitive Ca2+ channels by glucose metabolism in mouse pancreatic beta-cells. Nature. 1989 Nov 30;342(6249):550–553. doi: 10.1038/342550a0. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Velasco J. M., Petersen J. U., Petersen O. H. Single-channel Ba2+ currents in insulin-secreting cells are activated by glyceraldehyde stimulation. FEBS Lett. 1988 Apr 25;231(2):366–370. doi: 10.1016/0014-5793(88)80851-2. [DOI] [PubMed] [Google Scholar]
- Wang J. L., McDaniel M. L. Secretagogue-induced oscillations of cytoplasmic Ca2+ in single beta and alpha-cells obtained from pancreatic islets by fluorescence-activated cell sorting. Biochem Biophys Res Commun. 1990 Jan 30;166(2):813–818. doi: 10.1016/0006-291x(90)90882-n. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Biden T. J. Signal transduction in insulin secretion: comparison between fuel stimuli and receptor agonists. Ann N Y Acad Sci. 1986;488:317–333. doi: 10.1111/j.1749-6632.1986.tb46568.x. [DOI] [PubMed] [Google Scholar]
- Zünkler B. J., Lenzen S., Männer K., Panten U., Trube G. Concentration-dependent effects of tolbutamide, meglitinide, glipizide, glibenclamide and diazoxide on ATP-regulated K+ currents in pancreatic B-cells. Naunyn Schmiedebergs Arch Pharmacol. 1988 Feb;337(2):225–230. doi: 10.1007/BF00169252. [DOI] [PubMed] [Google Scholar]



