Abstract
Background & Aims
Opioids and opiates inhibit gastrointestinal functions via μ, δ, and κ receptors. Although agonists of the δ opioid receptor (DOR) suppress motility and secretion, little is known about the localization and regulation of DOR in the gastrointestinal tract.
Methods
We studied mice in which the gene that encodes the enhanced green fluorescent protein (eGFP) was inserted into Oprd1, which encodes DOR, to express an ~80 kDa product (DOReGFP). We used these mice to examine how agonists of DOR regulate the subcellular distribution of the DOR.
Results
DOReGFP was expressed in all regions but confined to enteric neurons and fibers within the muscularis externa. In the submucosal plexus, DOReGFP was detected in neuropeptide Y-positive secretomotor and vasodilator neurons of the small intestine, but was rarely observed in the large bowel. In the myenteric plexus of the small intestine, DOReGFP was present in similar proportions of excitatory motoneurons and interneurons that expressed choline acetyltransferase and substance P, and in inhibitory motoneurons and interneurons that contained nitric oxide synthase. DOReGFP was mostly present in nitrergic myenteric neurons of colon. DOReGFP and μ opioid receptors were often co-expressed. DOReGFP-expressing neurons were associated with enkephalin-containing varicosities and enkephalin-induced, clathrin- and dynamin-mediated endocytosis and lysosomal trafficking of DOReGFP. DOReGFP replenishment at the plasma membrane was slow, requiring de novo synthesis, rather than recycling.
Conclusions
DOR localizes specifically to submucosal and myenteric neurons, which might account for the ability of DOR agonists to inhibit gastrointestinal secretion and motility. Sustained down-regulation of DOReGFP at the plasma membrane of activated could induce long-lasting tolerance to DOR agonists.
Keywords: Trafficking, opiate drug, constipation, morphine, transgenic mice
INTRODUCTION
Opioids and opiates inhibit gastrointestinal functions via G protein-coupled μ, δ, and κ opioid receptors (MOR, DOR, KOR) 1, 2. Opiates that activate MOR, DOR and KOR inhibit intestinal peristalsis in the guinea pig 3, 4, and opioid receptor antagonists disrupt peristaltic contractions in guinea pig, mouse and rat, suggesting that endogenous opioids modulate this reflex 3-6. Opioid receptor agonists also inhibit secretion from the rat jejunum 7. The mechanisms underlying these actions are of interest because they mediate constipation that is the major limiting side-effect of opiate analgesics.
Anatomical and pharmacological studies provide insight into the location and function of opioid receptors in the gut. However, it is unclear whether opioids and opiates exert their effects by activating receptors on enteric neurons or other cell types within the gut, or act centrally. Although the location and activation of MOR in enteric neurons have been extensively studied due to its importance in morphine-induced constipation 8, less is known about the distribution and activation of DOR and KOR in the gastrointestinal tract. Electrophysiological studies indicate that opioids regulate enteric neurons by DOR- and MOR-dependent mechanisms 9. However, it is not known whether these receptors are coexpressed by enteric neurons, where they may respond to the same agonists and could interact.
Agonists promote endocytosis of many G protein-coupled receptors in neurons, including opioid receptors, and redistribution of receptors to endosomes is a hallmark of activation. Endocytosis attenuates G protein-dependent signaling by depleting receptors from the cell surface, but also activates G protein-independent signaling by recruiting receptors and adaptor proteins, such as β-arrestins, to endosomes 10. Endosomal sorting targets receptors to the plasma membrane, where recycling mediates resensitization, or to lysosomes, where degradation down-regulates signaling. Activated DOR traffics to lysosomes 11, 12, but can also recycle 13. Nothing is known about the mechanism and pathway of DOR trafficking in enteric neurons.
We localized DOR in enteric neurons and determined the mechanism and pathway of agonist-stimulated trafficking of DOR. To enable specific detection and permit direct observation of receptor trafficking in real time, we studied mice in which enhanced GFP (eGFP) was knocked into the DOR gene Oprd1 14. We detected DOReGFP in specific populations of submucosal and myenteric neurons, some of which coexpressed MOR. Agonists stimulated clathrin- and dynamin-mediated endocytosis of DOR, which trafficked via early endosomes to lysosomes, and repletion of cell surface receptors required de novo synthesis.
MATERIALS AND METHODS
See Supplemental Information for materials and complete methodological details, and Supplemental Table 1 for sources and dilutions of antibodies.
Animals
Mice expressing DOR with C-terminal eGFP have been characterized 12, 14-16. Mice (male and female, 20-30 g) were anesthetized with tribromoethanol (Avertin™, 250 mg/kg i.p.) and killed by bilateral thoracotomy. The UCSF Institutional Animal Care and Use Committee approved all procedures.
Western blotting
Tissues extracts (60 μg protein) were fractionated by SDS-PAGE and DOReGFP was detected by Western blotting for GFP.
Immunofluorescence
Sections and wholemounts of enteric ganglia were incubated with primary antibodies, washed, and incubated with fluorescent secondary antibodies (1 h, room temperature). To facilitate detection of neuropeptides in the soma, mice were treated with colchicine (2.5 mg/kg, i.p.) 6 h before tissue collection. To facilitate detection of DOReGFP-positive submucosal neurons, mice were treated with the DOR agonist SNC80 (10 mg/kg, s.c.) 30 min before tissue collection, which concentrated the receptor in endosomes where it was readily detected. This strategy was not necessary for detecting DOReGFP in myenteric neurons.
DOReGFP trafficking
To examine DOR trafficking in vivo, mice were treated with SNC80 (10 mg/kg, s.c.), and whole mounts of myenteric and submucosal plexuses were prepared at various times. To examine trafficking in organotypic cultures, ileal segments were opened, pinned mucosa-down onto silicone-lined dishes, and equilibrated (1 h, 37°C) in Krebs’ buffer containing nicardipine (1 μM) and tetrodotoxin (1 μM). Tissues were stimulated with SNC80 or Met-enkephalin (10, 100 nM) for 1 h at 4°C, washed, incubated at 37°C for varying times to allow DOR trafficking to occur, and were fixed and processed for immunofluorescence. To induce endogenous opioid release, tissues were stimulated with KCl (50 mM, 2 min, wash, 30 min recovery) in buffer containing nicardipine, bestatin, captopril, leupeptin, phosphoramidon and thiorphan (10 μM). To examine trafficking in isolated neurons, myenteric neurons were enzymatically dispersed from the ileum or distal colon, and were cultured for 7-10 days 17. Cultured neurons were stimulated with DOR agonists, fixed and processed for immunofluorescence.
Imaging
Specimens were examined using a Zeiss LSM510 META confocal microscope. Expression of GFP relative to the total neuronal population and functional neuronal subtypes was determined by colabeling with neurochemical markers 18, 19.
Statistical analysis
Data are expressed as the mean±SEM and were analyzed using Student’s t-test or one-way ANOVA with Newman-Keuls or Bonferroni post-hoc test. p<0.05 was considered significant.
RESULTS
DOR is expressed in enteric neurons
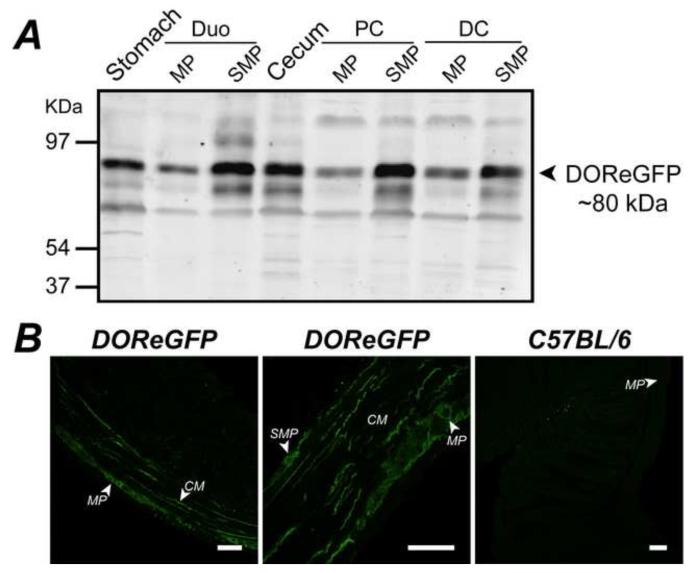
DOReGFP immunoreactivity (IR) was detected as a prominent band of ~80 kDa in all regions (Fig. 1A), consistent with the combined sizes of DOR and eGFP 12. Other immunoreactive proteins may correspond to oligomers or degradation products. No signal was detected in tissues from wild-type mice (not shown). We localized DOReGFP in sections by indirect immunofluorescence using GFP antibody to amplify the signal. DOReGFP-IR was detected in the esophagus, corpus, antrum, gall bladder, duodenum, ileum, cecum, proximal colon and distal colon, where it was localized to enteric ganglia and nerve fibers (Fig. 1B). Within ganglia, DOReGFP-IR colocalized with the neuronal marker PGP9.5 in a subset of myenteric and submucosal neurons, but it did not colocalize with glial fibrillary acidic protein, which identifies enteric glial cells (not shown). Nerve fibers containing DOReGFP-IR projected along the axes of the circular and longitudinal muscle layers of the muscularis externa (Fig. 1B), and contained nitric oxide synthase (NOS)-IR and vasoactive intestinal polypeptide (VIP)-IR (not shown), indicating origination from myenteric inhibitory motoneurons 19. DOReGFP-IR did not colocalize with calcitonin gene-related peptide (CGRP)-IR in nerve fibers innervating the mucosa (not shown), indicating that DOReGFP is not expressed in the terminals of intrinsic primary afferent neurons (IPANs) 19, 20. DOReGFP-IR was not detected in gastrointestinal or vascular smooth muscle, interstitial cells of Cajal or enterocytes. GFP antibody did not stain tissues from wild-type mice, confirming specificity (Fig. 1B).
Figure 1. Expression and localization of DOR in the gastrointestinal tract.
A. DOReGFP was detected as ~80 kDa protein in Western blots of stomach, duodenum (Duo), cecum, proximal colon (PC), and distal colon (DC), including muscularis externa/myenteric plexus (MP) and submucosa/submucosal plexus (SMP). B. DOReGFP-IR was localized to neurons of myenteric and submucosal plexuses and to nerve fibers within longitudinal and circular (CM) smooth muscle layers (left and middle panels) of colon. There was no detectable GFP-IR distal colon of wild-type mice (right panel), demonstrating specificity. Scale, 50 μm.
DOR is expressed by secretomotor/vasodilator submucosal neurons
Supplemental Table 2 reports the proportions of submucosal neurons coexpressing DOReGFP-IR and neurochemical markers. DOReGFPIR colocalized with PGP9.5-IR in submucosal neurons, but there were marked differences in the proportions of neurons expressing DOReGFP-IR in the small and large intestines. In the duodenum and ileum, DOReGFP-IR was detected in most submucosal neurons, whereas few neurons expressed DOReGFP-IR in the cecum and colon. We estimate by extrapolation from neurochemical coding studies18 that ~50% of all submucosal neurons of the ileum express DOReGFP. In the ileum, 90% of neurons expressing DOReGFP-IR coexpressed neuropeptide Y (NPY)-IR (identifies non-cholinergic secretomotor and vasodilator neurons 18) (Supplemental Fig. 1A). Of neurons expressing DOReGFP-IR, 8% coexpressed choline acetyl transferase (ChAT)-IR (identifies cholinergic secretomotor neurons) and 11% coexpressed somatostatin (SOM)-IR (localized to cholinergic submucosal neurons of the ileum) (Supplemental Fig. 1A), consistent with the minimal colocalization of ChAT and DOReGFP. In the distal colon, there was a smaller degree of overlap between DOReGFP-IR and NPY-IR (39%) than in the ileum, and DOReGFP-IR and ChAT-IR were rarely colocalized (10%, Supplemental Fig. 1B). A substantial proportion of submucosal neurons in the large intestine that expressed DOReGFP-IR coexpressed NOSIR (cecum 96%, proximal colon 33%, distal colon 79%, Fig. 2C). Although most submucosal neurons of the cecum expressed NOS-IR at a low level, those neurons expressing DOReGFP-IR had intense signals for NOS-IR (Supplemental Fig. 1C). The relative mean fluorescence intensity of NOS-IR in DOReGFP-negative neurons was 0.50±0.03 (n=188 neurons) and in DOReGFP-positive neurons was 1.00±0.03 (n=35 neurons). These neurons were unipolar.
Figure 2. Localization of DOR to nitrergic and cholinergic myenteric neurons of ileum and colon.
A. DOReGFP-IR was detected in ~50% of all PGP9.5-IR myenteric neurons (arrowheads indicate colocalization, arrowheads with asterisk indicate lack of colocalization). B, C. DOReGFP-IR extensively colocalized with NOS-IR (B) and ChAT-IR in small neurons (C) (arrowheads), but was absent from large ChAT-IR neurons (arrowheads with asterisks). D. DOReGFP-IR was not colocalized with NFM-IR. E. DOReGFP-IR and SSTR2A-IR were localized to distinct NOS-IR neurons in proximal colon (arrowheads with asterisks). Neurons positive only for NOS-IR are indicated by asterisk. Scale, 50 μm.
DOR is expressed by nitrergic and cholinergic myenteric neurons
Supplemental Table 3 reports the proportions of myenteric neurons coexpressing DOReGFP-IR and neurochemical markers. Intense DOReGFP-IR was detected at the plasma membrane of the soma and axons of a subset of myenteric neurons, with little intracellular signal (Fig. 2). In all regions (antrum to distal colon), ~50% of all PGP9.5-IR myenteric neurons coexpressed DOReGFP-IR, with the exception of the proximal colon where only ~38% of myenteric neurons were DOReGFP positive (Fig. 2A). There were marked differences in the neuronal subtypes that expressed DOReGFP in the small and large intestine. The proportion of DOReGFP-IR neurons coexpressing NOS-IR (identifies inhibitory motoneurons and interneurons) was 44-53% in the small intestine (Fig. 2B) and 66-95% in the large intestine (Supplemental Fig. 2A). The proportion of DOReGFP-IR neurons coexpressing ChAT-IR (identifies cholinergic excitatory motoneurons, interneurons and IPANs) was 41-44% in the small intestine (Fig. 2C) and only 9-30% in the large intestine (Supplemental Fig. 2B). In the small and large intestine, DOReGFPIR was not detected in large ChAT-IR neurons, which are probably IPANs. DOReGFP-IR was not detected in neurons with Dogiel type II morphology that expressed NFM-IR (identifies IPANs and other neurons in the mouse myenteric plexus 19) in any of the regions examined (Fig. 2D, Supplemental Fig. 2C). DOReGFP-IR did not colocalize with the IPAN marker CGRP-IR (not shown) 19, 20.
DOR and somatostatin receptor SSTR2A are expressed by distinct nitrergic myenteric neurons
Functional studies of peristalsis suggest that DOR is coexpressed with somatostatin (SOM) receptor SSTR2A by inhibitory motoneurons containing NOS and VIP in mouse colon 5. SSTR2A-IR colocalizes with NOS-IR and VIP-IR in myenteric neurons, suggesting overlap with DOReGFP-IR 21, 22. To examine this possibility, we simultaneously localized DOReGFP, SSTR2A and NOS in myenteric plexus wholemounts of large intestine. There was no detectable colocalization of DOReGFP-IR and SSTR2A-IR (Fig. 2E). Although DOReGFP-IR and SSTR2A-IR were extensively colocalized with NOS-IR, triple labeling indicated that the two receptors were present in distinct populations of NOS-IR neurons. Neurons coexpressing SSTR2A-IR and NOS-IR were larger (diameter 27.98±0.74 μm, n=56 neurons) than neurons coexpressing DOReGFP-IR and NOS-IR (diameter 20.80±0.36 μm, n=187 neurons; p<0.0001). Neurons expressing NOS-IR mostly coexpressed DOReGFP-IR (76% distal colon), with the remaining neurons either coexpressing SSTR2A-IR (17%) or only NOS-IR (7%). SOM-IR was detected in few myenteric neurons, which were characterized by intense labeling of Golgi-like structures and by filamentous morphology (Supplemental Fig. 2D). A small proportion (3-4%) of DOReGFP-IR neurons coexpressed SOM-IR in the ileum and distal colon, where SOM is present in a subset of descending interneurons 5, 19. The minimal overlap between DOR and SSTR2A or SOM is at variance with predictions of pharmacological studies.
DOR is coexpressed with substance P but not the neurokinin 1 receptor in myenteric neurons
Tachykinins are excitatory peristaltic neurotransmitters 5. Of DOReGFP-IR myenteric neurons, 33% coexpressed substance P (SP)-IR in the ileum and 25% coexpressed SP-IR in the distal colon of colchicine-treated mice (Supplemental Fig. 2E). In the distal colon, only 1% of DOReGFP-IR neurons coexpressed neurokinin 1 receptor (NK1R)-IR (Supplemental Fig. 2F). NK1R-IR neurons were mainly multipolar and larger than DOReGFP-IR neurons. The colocalization of DOR with SP in ascending interneurons and excitatory circular muscle motoneurons 19 suggests that DOR is appropriately located to inhibit SP release and indirectly modulate NK1R activation, but DOR could not directly regulate NK1R.
DOR and MOR are coexpressed by enteric neurons
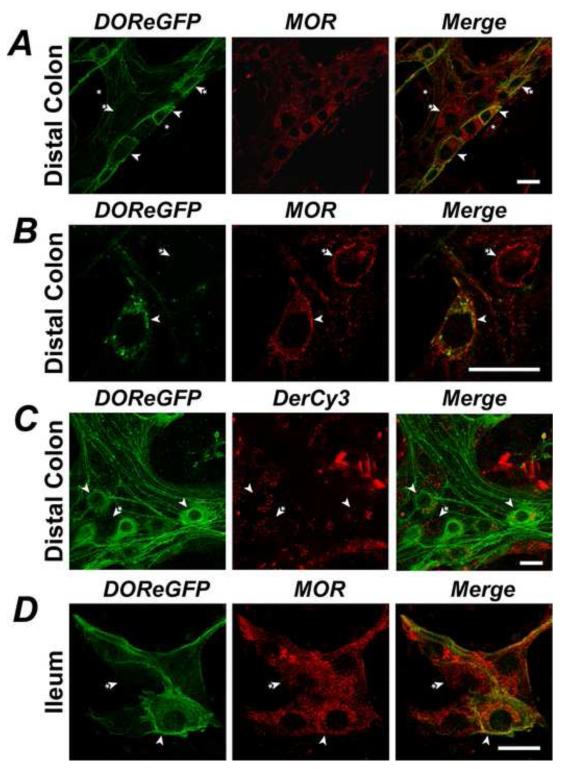
To examine DOR/MOR colocalization, we localized DOReGFP-IR and detected MOR by using a characterized MOR-specific antibody 8 that does not stain tissue from MOR-deficient mice 16, and by studying uptake of the MOR-selective agonist Cy3-[Lys7, Cys8]Dermorphin (Cy3-dermorphin). In myenteric ganglia of the distal colon, MOR-IR was localized to a subset of neurons and to myenteric interstitial cells of Cajal (Fig. 3A). Approximately 77-85% of all DOReGFP-IR myenteric neurons coexpressed MOR-IR, and 57-61% of MOR-IR neurons coexpressed DOReGFP (Fig. 3A, B). However, MOR-IR was also detected in large neurons, probably IPANs, that did not express DOReGFP-IR. Cy3-dermorphin (100 nM) bound and trafficked to endosomes of myenteric neurons, many of which also expressed DOReGFP-IR (Fig. 3C), although the extent of colocalization was not quantified. Naloxone (1 μM) abolished binding of Cy3-dermorphin, confirming specific interaction with MOR (not shown). MOR-IR was detected in submucosal neurons of ileum and distal colon, many of which also expressed DOReGFP-IR (Fig. 3D), although colocalization was not quantified. There were marked differences in the subcellular localization of DOR and MOR in some neurons where these receptors were coexpressed. In all neurons, DOReGFP was predominantly localized to the plasma membrane. However, MOR-IR was either mostly intracellular (Fig. 3A) or mostly at the plasma membrane (Fig. 3B).
Figure 3. Colocalization of DOR and MOR in a subpopulation of enteric neurons.
A. DOReGFP-IR colocalized in myenteric neurons of distal colon with MOR-IR (arrowheads), but MOR-IR was also detected in large neurons that did not express DOReGFP-IR (arrowhead with asterisk) and in interstitial cells of Cajal (asterisks). B. MOR-IR was present at the cell surface of some myenteric neurons, including those positive for DOReGFP-IR (arrowhead). C. Dermorphin-Cy3 (DerCy3) trafficked to endosomes of myenteric neurons of distal colon, including neurons expressing DOReGFP-IR (arrowheads). D. DOReGFP-IR colocalized with MOR-IR in some submucosal neurons of the ileum (arrowheads), but MOR-IR was also detected in neurons that did not express DOReGFP-IR (arrowhead with asterisk). Scale, 20 μm.
Enkephalinergic varicosities innervate DOR myenteric neurons
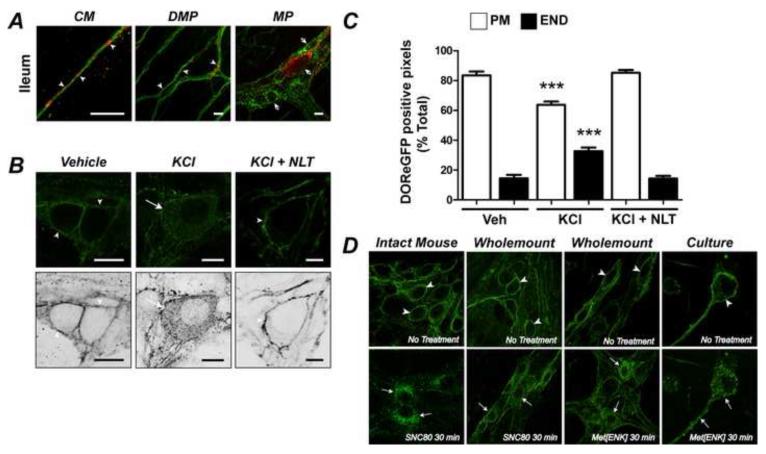
We examined the proximity between DOR and the endogenous agonist enkephalin, and determined whether endogenous agonists could activate DOR. Enkephalin-IR was detected in varicosities of fibers surrounding the soma and neurites of DOReGFP-IR neurons, as well as neurons that did not express DOReGFP-IR, in the myenteric (Fig. 4A panel) and submucosal (not shown) plexuses of ileum and colon. Enkephalinergic varicosities were also intimately associated with nerve fibers containing DOReGFP-IR in the circular muscle and deep muscular plexus (Fig. 4A, right panel). Enkephalin-IR and DOReGFP-IR did not colocalize in the soma of myenteric (Fig. 4A, right panel) or submucosal (not shown) neurons. To examine whether endogenous opioids activate DOR, we depolarized organotypic preparations of distal colon using KCl, which induces neuropeptide release 17, and examined the subcellular distribution of DOReGFP-IR. KCl induced endocytosis of DOReGFP-IR (Fig. 4B, C), indicating receptor activation. The DOR-selective antagonist naltrindole (100 nM) abolished KCl-induced endocytosis.
Figure 4. Localization of DOR and enkephalin (ENK), and opioid-induced activation of DOR in the myenteric plexus.
A. ENK-IR (red) varicosities were intimately associated with DOReGFP-IR (green) in nerve fibers in circular muscle (CM) and deep muscular plexus (DMP) (arrowheads). DOReGFP-IR was not colocalized with ENK-IR in myenteric neurons (MP, arrowheads with asterisks, right panel). B. KCl but not vehicle stimulated DOReGFP-IR endocytosis in organotypic myenteric plexus wholemounts from distal colon. Naltrindole (NLT, right panel) abolished DOR endocytosis. Lower panels are inverted images. Scale, 10 μm. C. Quantitative analysis confirmed KCl-induced depletion of DOReGFP-IR from plasma membrane (PM) and concomitant increase in DOReGFP-IR in endosomes (END), and that naltrindole abolished endocytosis. ***P < 0.0001 to vehicle (Veh). D. DOReGFP-IR endocytosis in myenteric neurons of distal colon after: SNC80 injection into the intact animal (left panels), treatment of organotypic wholemounts with SNC80 or Met-enkephalin (Met[ENK]) (middle panels), or incubation of cultured neurons with Met-enkephalin (right panels). Right panel shows eGFP fluorescence in the same cultured neuron.
Agonists induce clathrin- and dynamin-dependent DOR endocytosis
We examined agonist-induced DOR trafficking in myenteric neurons in vivo, in organotypic cultures and in isolated neurons.
Mice were treated with SNC80 (10 mg/kg s.c.) or vehicle (control), and DOReGFP-IR was localized in myenteric and submucosal plexus wholemounts. DOReGFP-IR was confined to the plasma membrane of myenteric and submucosal neurons from vehicle-treated mice (Fig. 4D, myenteric plexus). Within 10-30 min after SNC80, DOReGFP-IR was detected in endosomes in the soma and axons of myenteric (Fig. 4D) and submucosal (not shown) neurons.
To examine the precise timing and concentration-dependence of DOR trafficking, we incubated organotypic wholemounts of distal colon with SNC80 (10, 100 nM), Met-enkephalin (100 nM) or vehicle (0-120 min, 37°C). In vehicle-treated preparations, DOReGFP-IR was confined to the plasma membrane (Fig. 4D). SNC80 induced receptor clustering at the plasma membrane within 10 min and maximal internalization within 30-60 min (Fig. 4D). Quantitative analysis indicated that 90.4±0.7% (n=89 neurons) of total DOReGFP-IR was at the plasma membrane in unstimulated neurons, and that 42.7±5.1% (n=22 neurons) of total DOReGFP-IR was at the plasma membrane 60 min after SNC80 (100 nM, Supplemental Fig. 3A). Met-enkephalin also stimulated DOReGFP internalization (Fig. 4D). Both SNC80 and Met-enkephalin induced DOR-eGFP endocytosis in the presence of tetrodotoxin (100 nM), suggesting a direct action of these agonists on the DOReGFP receptor. Naltrindole abolished endocytosis (not shown), confirming selectivity.
By studying myenteric neurons in culture, we evaluated DOReGFP trafficking in the soma and neurites of the same neurons in real time. DOReGFP-IR was detected in 46% of PGP9.5-positive myenteric neurons in culture (n=26/57 neurons), indicating retained expression (Supplemental Fig. 3B). In unstimulated neurons, DOReGFP was uniformly distributed at the plasma membrane of the soma and neurites (Fig. 4D, Supplemental Fig. 3B). SNC80 and Met-enkephalin (100 nM) induced DOReGFP internalization, detected by live imaging of DOReGFP and by immunofluorescence detection of GFP. Within 10 min, DOReGFP was clustered at the plasma membrane of the soma and neurites, and after 30 min DOReGFP was redistributed to endosomes and depleted from the plasma membrane (Fig. 4D, Supplemental Fig. 3B). Naltrindole prevented SNC80- (Supplemental Fig. 3C) and Met-enkephalin-stimulated (not shown) trafficking, confirming specific activation of DOR.
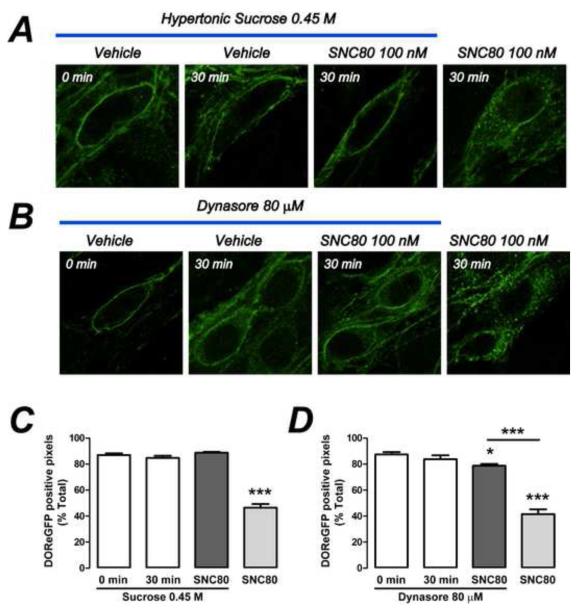
To examine the mechanism of DOR endocytosis, we treated organotypic cultures with hypertonic sucrose (0.45 M, blocks clathrin-mediated endocytosis 17), or Dynasore (80 μM, inhibits dynamin GTPase23). Sucrose and Dynasore inhibited SNC80-evoked endocytosis of DOReGFP-IR in wholemounts (Fig. 5). Sucrose or Dynasore alone were without effect. These findings were confirmed using live imaging of cultured neurons (not shown).
Figure 5. Clathrin- and dynamin-dependent endocytosis of DOR in myenteric neurons.
Hypertonic sucrose (A) or Dynasore (B) inhibited SNC80-induced endocytosis of DOReGFP-IR in myenteric neurons in organotypic cultures from distal colon. Quantitative analysis confirmed inhibitory effects of sucrose (C) and Dynasore (D). ***P < 0.0001 to control. n=30-39 neurons from ≥3 mice per data point.
DOR traffics via early endosomes to lysosomes and does not recycle
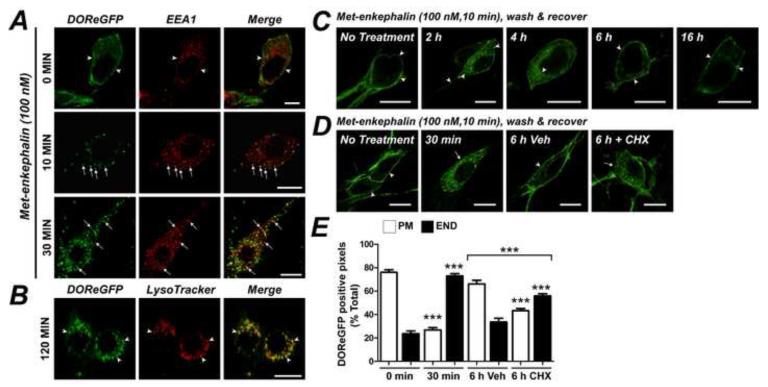
To examine the subcellular pathway of DOR trafficking, we simultaneously localized DOReGFP with early endosomal antigen 1 (EEA1, identifies early endosomes), and LAMP1 or LysoTracker (identify lysosomes). In cultured myenteric neurons, Met-enkephalin stimulated redistribution of DOReGFP-IR to EEA1-positive early endosomes within 10-30 min in the soma (Fig. 6A) and in neurites (Supplemental Fig. 3D). After 60-120 min, DOR-GFP had traversed the endosomal system and was detected in lysosomes of the soma (Fig. 6B). Although we did not detect lysosomes in the neurites using LysoTracker or LAMP1 antibodies (not shown), after 60 min DOReGFP was detected in vesicles in neurites that were distinct from early endosomes (Supplemental Fig. 3D). DOReGFP-IR also colocalized with LAMP1 in the soma of myenteric neurons of wholemounts 60-120 min after administration of SNC80 to the intact mouse, and DOReGFP was still detected in lysosomes after 16 h (Supplemental Fig. 3E). Since DOR traffics to lysosomes and there are no prominent intracellular stores, DOR replenishment at the plasma membrane probably requires receptor synthesis. To examine this process, cultured neurons were briefly incubated with Met-enkephalin (100 nM, 10 min) or vehicle, washed and recovered in agonist-free medium. Metenkephalin, but not vehicle, induced translocation of DOReGFP to endosomes and lysosomes within 0.5-2 h. DOReGFP-IR was replenished at the plasma membrane within 6-16 h (Fig. 6C). Cycloheximide (70 μM) did not affect DOReGFP-IR endocytosis, but prevented recovery of receptor at the plasma membrane (Fig. 6D, E), indicating a requirement for new receptor synthesis.
Figure 6. Intracellular trafficking of DOR in myenteric neurons.
A. Met-enkephalin induced DOReGFP-IR trafficking to EEA1-positive early endosomes (arrows) of cultured myenteric neurons after 10-30 min. Scale, 10 μm. B. After 120 min, DOReGFP-IR was detected in LysoTracker-positive lysosomes in the soma. Scale, 20 μm. C. Transient stimulation of cultured neurons with Met-enkephalin stimulated DOReGFP-IR endocytosis, which was replenished at the plasma membrane only after 6-16 h recovery. D. Cycloheximide (CHX) inhibited recovery of cell surface DOReGFP at 6 h, as confirmed by quantitative analysis (E). Scale, 10 μm, PM: plasma membrane-associated DOReGFP, END: endosome-associated DOReGFP, ***P < 0.0001, n=20-78 neurons from ≥3 mice per data point.
DISCUSSION
We report the first detailed examination of the expression and regulation of DOR in the enteric nervous system. DOReGFP knockin mice, previously used to study DOR in sensory and central neurons12, 14-16, enabled specific detection, avoiding concerns about antibody selectivity. DOR is expressed by secretomotor submucosal neurons, and inhibitory nitrergic and excitatory cholinergic/tachykinergic myenteric motoneurons. Agonists induce clathrin- and dynamin-mediated endocytosis of DOR in the soma and axons, although DOR traffics to lysosomes only in the soma, suggesting differences in the fate and regulation of the receptor in the soma and axons. Restoration of surface DOR is slow, requiring de novo synthesis. Agonists may activate DOR on myenteric neurons to suppress peristalsis 3-5, and the prolonged intracellular retention of activated DOR may induce sustained unresponsiveness of activated neurons.
Location and function of DOR in secretomotor neurons of the submucosal plexus
We report that most ileal submucosal neurons express DOR, in particular NPY-positive, non-cholinergic secretomotor neurons18. In contrast, DOR is rarely expressed in colonic submucosal neurons, where it is confined to nitrergic neurons. These findings support the localization of DOR to submucosal neurons of rat and pig 24. Although activation of opioid receptors inhibits electrolyte and fluid secretion 2, the mechanism of inhibition is not clear. Intracerebroventricular administration of DOR agonist inhibits cholera toxin-stimulated jejunal secretion in rats via stimulation of sympathetic postganglionic fibers and activation of α2 adrenoceptors on submucosal neurons 7. Our results suggest that such indirect mechanisms could operate in the large intestine, where the small number of DOR-expressing neurons may limit their influence on secretomotor activity. However, the extensive expression of DOR in secretomotor neurons of the small intestine indicates that agonists can inhibit secretion by direct action on submucosal neurons. Electrophysiological studies indicate that DOR agonists inhibit activity of submucosal neurons of guinea pig ileum and cecum 25, 26, thereby suppressing secretion 2. Enkephalinergic fibers innervating submucosal ganglia of the guinea pig small intestine originate in the myenteric plexus, with little evidence for expression by submucosal neurons 27. Our inability to detect enkephalin in submucosal neurons supports these findings and suggests interaction between the myenteric and submucosal plexuses in any DOR-dependent regulation of secretion.
Location and function of DOR in excitatory and inhibitory motoneurons and interneurons of the myenteric plexus
DOR agonists may inhibit motility by effects on central and enteric neurons. Peripheral SNC80 inhibits gastrointestinal propulsion in the mouse, and centrally-penetrant but not peripherally-restricted naloxone derivatives suppress this effect, suggesting a central mechanism 28. The observation that DOR knockdown in the rat brain inhibits the anti-propulsive actions in the colon of peripheral SNC80 supports a central mechanism 29. These reports are at variance with our demonstration that peripheral SNC80 directly activates (i.e. internalizes) DOR in myenteric neurons. DOR agonists hyperpolarize these neurons, causing dysmotility and suppression of propulsion 2. Intra-ventricular DOR agonists do not affect intestinal propulsion in rats 30, and DOR agonists affect contractility and peristalsis of isolated rat and mouse colon 31, supporting a direct, peripherally-mediated mechanism.
We detected DOR in excitatory (ChAT/SP-positive) and inhibitory (NOS/SOM-positive) myenteric motoneurons, and in nerve fibers innervating deep muscular plexus and circular muscle, supporting observations in rat and pig 24. DOR was intimately associated with enkephalin-containing varicosities, and endogenous opioids activated (i.e. internalized) DOR in myenteric neurons, suggesting that opioids suppress peristalsis by activating DOR on excitatory and inhibitory myenteric neurons. Opioid release from the rat colon declines during the inhibitory phase and increases during the excitatory phase of peristalsis 6. Met-enkephalin inhibits release of mediators of ascending contraction (acetylcholine) 32 and descending relaxation (VIP) 6 from isolated guinea pig ileum and rat colon, respectively. By releasing excitatory and inhibitory motoneurons from the inhibitory influence of endogenous opioids, DOR antagonists augment ascending contraction and descending inhibition of mouse colon 5. The effects of enkephalins on inhibitory junction potentials in the dog duodenum are neurogenic and DOR-dependent 9, suggesting that opioids activate DOR on inhibitory motoneurons that control neuromuscular transmission to circular muscle. The effects of opioids on inhibitory junction potentials are retained in preparations devoid of the myenteric plexus, supporting an action at inhibitory motor nerves within the circular muscle or deep muscular plexus 9, 33.
Coexpression of opioid receptors by enteric neurons
Most enteric neurons that express DOR also express MOR, detected immunochemically and by Cy3-dermorphin uptake. DOR and MOR are also coexpressed in myenteric neurons of rat ileum 34, and DOR colocalizes with KOR in myenteric neurons of the pig ileum 24. Electrophysiological 35 and pharmacological 36 studies support DOR/MOR coexpression by enteric neurons. In contrast to extensive DOR/MOR coexpression in enteric neurons, DOR and MOR are expressed by distinct neuronal populations of dorsal root ganglia neurons 16. Although the functional relevance of DOR/MOR coexpression in enteric neurons remains to be determined, enkephalins may coactivate these receptors, which could amplify their inhibitory effects on motoneurons 9. DOR/MOR heterodimerization also affects opioid affinity and G protein signaling 37. Whether these receptors dimerize in enteric neurons, and the functional relevance of their differential subcellular location (DOR mostly at the plasma membrane and MOR intracellular or at the plasma membrane), remain to be determined.
Regulation of DOR in the enteric nervous system
Neuronal responsiveness to extracellular agonists requires receptor localization at the plasma membrane. Endocytosis attenuates G protein-mediated signaling by depleting surface receptors, but some receptors interact in endosomes with β-arrestins, which transmit distinct signals 10. DOR internalizes in the soma and axons of enteric neurons by clathrin/dynamin-mediated mechanisms, and traffics via early endosomes to lysosomes in the soma but to unidentified vesicles in the neurites. Although DOR will be degraded in lysosomes in the soma, the fate of the receptor in axons is unknown. Activated DOR is depleted from the cell surface for prolonged periods and replenishment of DOR at the surface of enteric neurons requires synthesis of new receptors. These observations are consistent with ubiquitination-mediated trafficking of DOR to lysosomes in cell lines 11. Agonists that induce DOR endocytosis and down-regulation (SNC80) result in sustained tolerance to DOR-mediated analgesia, locomotor activation and anxiolysis, whereas non-internalizing agonists (ARM390) induce tolerance only to DOR-mediated analgesia 15. Thus, once activated by agonists that induce DOR endocytosis, enteric neurons would remain unresponsive to further challenges with DOR agonists for prolonged periods. DOR endocytosis and degradation could mediate tolerance to the antisecretory effects of enkephalins in the ileum after prolonged DOR activation 38. However, chronic morphine induces tolerance to the contractile effects of opioids in the ileum but not the colon 39. Further studies are required to define the mechanisms that regulate opioid receptor subtypes in enteric neurons of different intestinal regions after acute and chronic activation.
Supplementary Material
Acknowledgements
We thank Sarah Low for maintenance of the mouse colony and Allan Basbaum for providing the DOReGFP mice.
Grant Support: DK57850, DK39957, DK43207 (NWB); DK07573 (JCP and NWB), NIDA005010, Shirley and Stefan Hatos Neuroscience Research Foundation (BK); CJ Martin Fellowship NHMRC 454858 (DPP).
Abbreviations
- ChAT
choline acetyltransferase
- CGRP
calcitonin gene-related peptide
- DOR
δ opioid receptor
- EEA1
early endosomal antigen 1
- eGFP
enhanced green fluorescent protein
- GFAP
glial fibrillary acidic protein
- IPANs
intrinsic primary afferent neurons
- IR
immunoreactive/immunoreactivity
- KOR
κ opioid receptor
- MOR
μ opioid receptor
- NE
not examined
- NFM
neurofilament M
- NK1R
neurokinin 1 receptor
- NOS
nitric oxide synthase
- NPY
neuropeptide Y
- PBS
phosphate buffered saline
- SOM
somatostatin
- SP
substance P
- SSTR2A
somatostatin receptor 2A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
Author Contributions: DPP designed, performed and analyzed experiments and wrote the manuscript; JCP and GS performed and analyzed experiments; CJE provided critical reagents; BLK generated mice and provided critical revision of the manuscript; NWB wrote the manuscript and supervised the study.
REFERENCES
- 1.Sanger GJ, Tuladhar BR. The role of endogenous opioids in the control of gastrointestinal motility: predictions from in vitro modelling. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16(Suppl 2):38–45. doi: 10.1111/j.1743-3150.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- 2.Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl 2):17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 3.Waterman SA, Costa M, Tonini M. Modulation of peristalsis in the guinea-pig isolated small intestine by exogenous and endogenous opioids. British journal of pharmacology. 1992;106:1004–10. doi: 10.1111/j.1476-5381.1992.tb14448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahbazian A, Heinemann A, Schmidhammer H, Beubler E, Holzer-Petsche U, Holzer P. Involvement of mu- and kappa-, but not delta-, opioid receptors in the peristaltic motor depression caused by endogenous and exogenous opioids in the guinea-pig intestine. British journal of pharmacology. 2002;135:741–50. doi: 10.1038/sj.bjp.0704527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grider JR. Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J Pharmacol Exp Ther. 2003;307:460–7. doi: 10.1124/jpet.103.053512. [DOI] [PubMed] [Google Scholar]
- 6.Grider JR, Makhlouf GM. Role of opioid neurons in the regulation of intestinal peristalsis. Am J Physiol. 1987;253:G226–31. doi: 10.1152/ajpgi.1987.253.2.G226. [DOI] [PubMed] [Google Scholar]
- 7.Brown DR, Miller RJ. Adrenergic mediation of the intestinal antisecretory action of opiates administered into the central nervous system. J Pharmacol Exp Ther. 1984;231:114–9. [PubMed] [Google Scholar]
- 8.Sternini C, Spann M, Anton B, Keith DE, Jr., Bunnett NW, von Zastrow M, Evans C, Brecha NC. Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proc Natl Acad Sci U S A. 1996;93:9241–6. doi: 10.1073/pnas.93.17.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer AJ, Sarr MG, Szurszewski JH. Opioids inhibit neuromuscular transmission in circular muscle of human and baboon jejunum. Gastroenterology. 1991;101:970–6. doi: 10.1016/0016-5085(91)90723-x. [DOI] [PubMed] [Google Scholar]
- 10.Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci U S A. 2009;106:17615–22. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hislop JN, Henry AG, Marchese A, von Zastrow M. Ubiquitination regulates proteolytic processing of G protein-coupled receptors after their sorting to lysosomes. J Biol Chem. 2009;284:19361–70. doi: 10.1074/jbc.M109.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gaveriaux-Ruff C, Kieffer BL. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS One. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Wang F, Chen X, Chen Y, Ma L. Post-endocytic fates of delta-opioid receptor are regulated by GRK2-mediated receptor phosphorylation and distinct beta-arrestin isoforms. J Neurochem. 2008;106:781–92. doi: 10.1111/j.1471-4159.2008.05431.x. [DOI] [PubMed] [Google Scholar]
- 14.Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gaveriaux-Ruff C, Kieffer BL. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103:9691–6. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradhan AA, Walwyn W, Nozaki C, Filliol D, Erbs E, Matifas A, Evans C, Kieffer BL. Ligand-directed trafficking of the delta-opioid receptor in vivo: two paths toward analgesic tolerance. J Neurosci. 2010;30:16459–68. doi: 10.1523/JNEUROSCI.3748-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–59. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grady EF, Gamp PD, Jones E, Baluk P, McDonald DM, Payan DG, Bunnett NW. Endocytosis and recycling of neurokinin 1 receptors in enteric neurons. Neuroscience. 1996;75:1239–54. doi: 10.1016/0306-4522(96)00357-0. [DOI] [PubMed] [Google Scholar]
- 18.Mongardi Fantaguzzi C, Thacker M, Chiocchetti R, Furness JB. Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell Tissue Res. 2009;336:179–89. doi: 10.1007/s00441-009-0773-2. [DOI] [PubMed] [Google Scholar]
- 19.Qu ZD, Thacker M, Castelucci P, Bagyanszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147–61. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- 20.Grider JR, Jin JG. Distinct populations of sensory neurons mediate the peristaltic reflex elicited by muscle stretch and mucosal stimulation. J Neurosci. 1994;14:2854–60. doi: 10.1523/JNEUROSCI.14-05-02854.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen JP, Canty AJ, Schulz S, Humphrey PP, Emson PC, Young HM. Identification of cells expressing somatostatin receptor 2 in the gastrointestinal tract of Sstr2 knockout/lacZ knockin mice. J Comp Neurol. 2002;454:329–40. doi: 10.1002/cne.10466. [DOI] [PubMed] [Google Scholar]
- 22.Sternini C, Wong H, Wu SV, de Giorgio R, Yang M, Reeve J, Jr., Brecha NC, Walsh JH. Somatostatin 2A receptor is expressed by enteric neurons, and by interstitial cells of Cajal and enterochromaffin-like cells of the gastrointestinal tract. J Comp Neurol. 1997;386:396–408. [PubMed] [Google Scholar]
- 23.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–50. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Poonyachoti S, Kulkarni-Narla A, Brown DR. Chemical coding of neurons expressing delta- and kappa-opioid receptor and type I vanilloid receptor immunoreactivities in the porcine ileum. Cell Tissue Res. 2002;307:23–33. doi: 10.1007/s00441-001-0480-0. [DOI] [PubMed] [Google Scholar]
- 25.Mihara S, North RA. Opioids increase potassium conductance in submucous neurones of guinea-pig caecum by activating delta-receptors. Br J Pharmacol. 1986;88:315–22. doi: 10.1111/j.1476-5381.1986.tb10207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.North RA, Williams JT, Surprenant A, Christie MJ. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci U S A. 1987;84:5487–91. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furness JB, Costa M, Miller RJ. Distribution and projections of nerves with enkephalin-like immunoreactivity in the guinea-pig small intestine. Neuroscience. 1983;8:653–64. doi: 10.1016/0306-4522(83)90001-5. [DOI] [PubMed] [Google Scholar]
- 28.Broccardo M, Improta G, Tabacco A. Central effect of SNC 80, a selective and systemically active delta-opioid receptor agonist, on gastrointestinal propulsion in the mouse. Eur J Pharmacol. 1998;342:247–51. doi: 10.1016/s0014-2999(97)01470-2. [DOI] [PubMed] [Google Scholar]
- 29.Negri L, Broccardo M, Lattanzi R, Melchiorri P. Effects of antisense oligonucleotides on brain delta-opioid receptor density and on SNC80-induced locomotor stimulation and colonic transit inhibition in rats. Br J Pharmacol. 1999;128:1554–60. doi: 10.1038/sj.bjp.0702931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galligan JJ, Mosberg HI, Hurst R, Hruby VJ, Burks TF. Cerebral delta opioid receptors mediate analgesia but not the intestinal motility effects of intracerebroventricularly administered opioids. J Pharmacol Exp Ther. 1984;229:641–8. [PubMed] [Google Scholar]
- 31.Menzies JR, Glen T, Davies MR, Paterson SJ, Corbett AD. In vitro agonist effects of nociceptin and [Phe(1)psi(CH(2)-NH)Gly(2)]nociceptin(1-13)NH(2) in the mouse and rat colon and the mouse vas deferens. Eur J Pharmacol. 1999;385:217–23. doi: 10.1016/s0014-2999(99)00700-1. [DOI] [PubMed] [Google Scholar]
- 32.Vizi ES, Ono K, Adam-Vizi V, Duncalf D, Foldes FF. Presynaptic inhibitory effect of Met enkephalin on [14C] acetylcholine release from the myenteric plexus and its interaction with muscarinic negative feedback inhibition. J Pharmacol Exp Ther. 1984;230:493–9. [PubMed] [Google Scholar]
- 33.Bayguinov O, Sanders KM. Regulation of neural responses in the canine pyloric sphincter by opioids. Br J Pharmacol. 1993;108:1024–30. doi: 10.1111/j.1476-5381.1993.tb13500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray AC, Coupar IM, White PJ. Comparison of opioid receptor distributions in the rat ileum. Life Sci. 2006;78:1610–6. doi: 10.1016/j.lfs.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 35.Egan TM, North RA. Both mu and delta opiate receptors exist on the same neuron. Science. 1981;214:923–4. doi: 10.1126/science.6272393. [DOI] [PubMed] [Google Scholar]
- 36.Fox-Threlkeld JE, Daniel EE, Christinck F, Hruby VJ, Cipris S, Woskowska Z. Identification of mechanisms and sites of actions of mu and delta opioid receptor activation in the canine intestine. J Pharmacol Exp Ther. 1994;268:689–700. [PubMed] [Google Scholar]
- 37.Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vinayek R, Brown DR, Miller RJ. Tolerance and cross-tolerance to the antisecretory effects of enkephalins on the guinea-pig ileal mucosa. J Pharmacol Exp Ther. 1985;232:781–5. [PubMed] [Google Scholar]
- 39.Ross GR, Gabra BH, Dewey WL, Akbarali HI. Morphine tolerance in the mouse ileum and colon. J Pharmacol Exp Ther. 2008;327:561–72. doi: 10.1124/jpet.108.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.